采用266 nm飞秒激光烧蚀(fLA)系统连接多集电极ICP-MS (MC-ICP-MS),通过严格控制分析程序,获得了具有良好精度和准确性的铅同位素比值数据。266nm飞秒激光烧蚀诱导的质量分馏率约比193nm准分子激光烧蚀(eLA)诱导的质量分馏率低28%。摘要采用调优Tl比的Tl归一化指数律校正方法,获得了具有较好精度和准确度的Pb同位素数据。NIST SRM 610、612、614玻璃参考材料的Pb同位素比值;USGS bhvog - 2g、BCR-2G、GSD-1G、bir1 g;采用fa - mc - icp - ms法测定MPI-DING GOR132-G、KL2-G、T1-G、StHs60/80-G、ATHO-G、ML3B-G。在2s测量不确定度范围内,测得的铅同位素比值与参考值或公布值吻合较好。利用飞秒激光消融MC-ICP-MS分析获得了GSE- 1G、GSC-1G、GSA-1G、CGSG-1、CGSG-2、CGSG-4、CGSG-5玻璃基准材料的高精度铅同位素资料技术。
方案详情

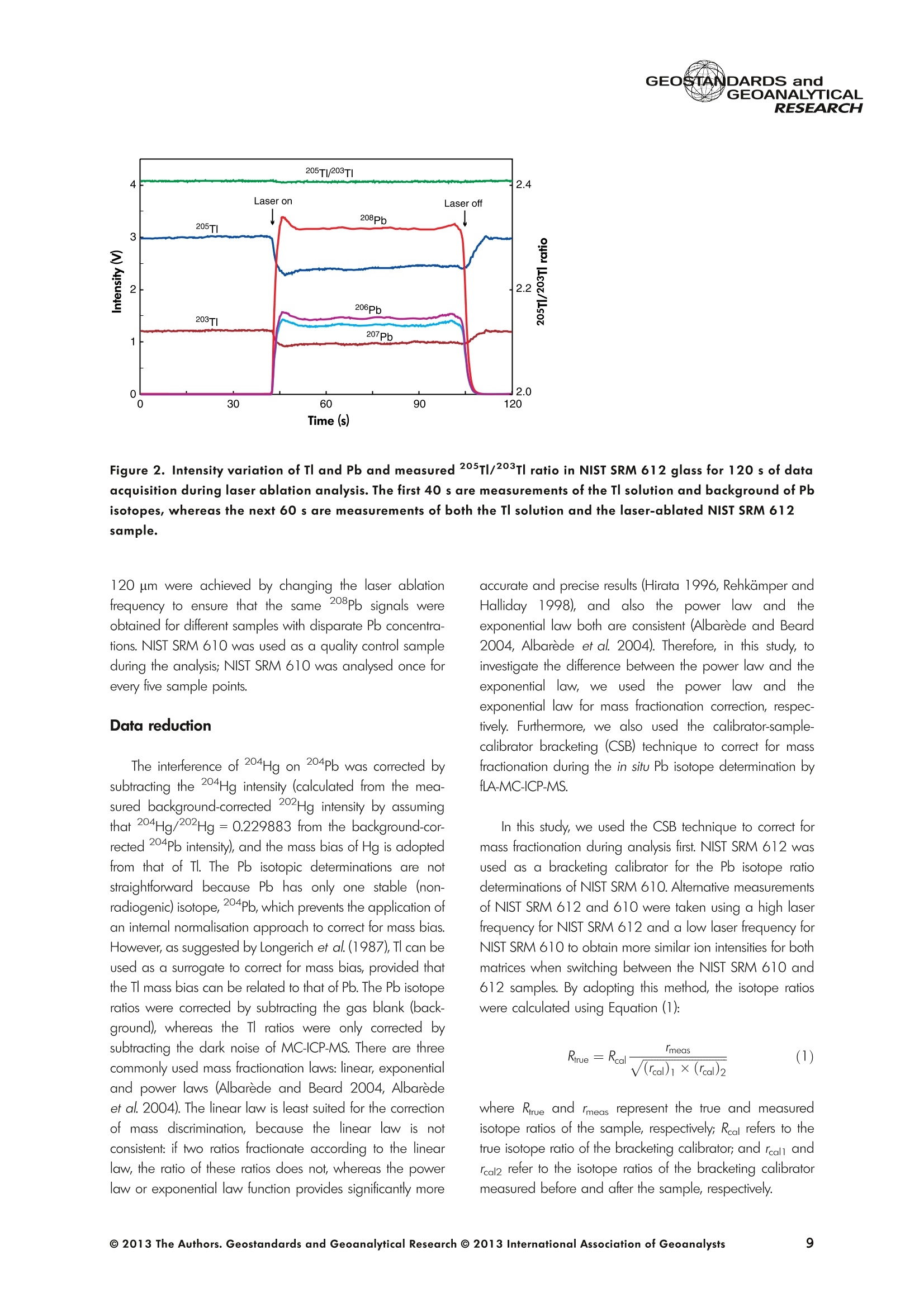
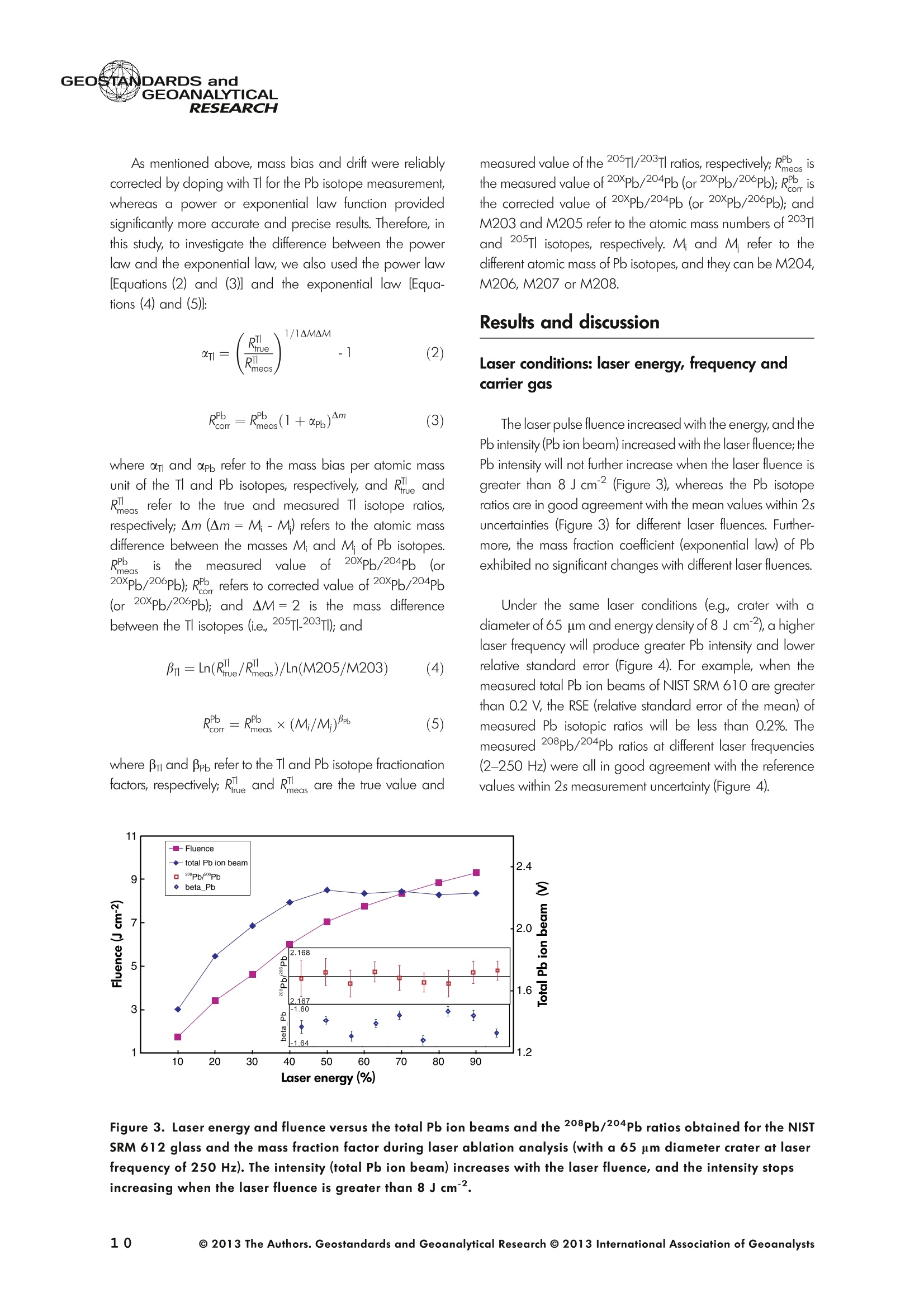
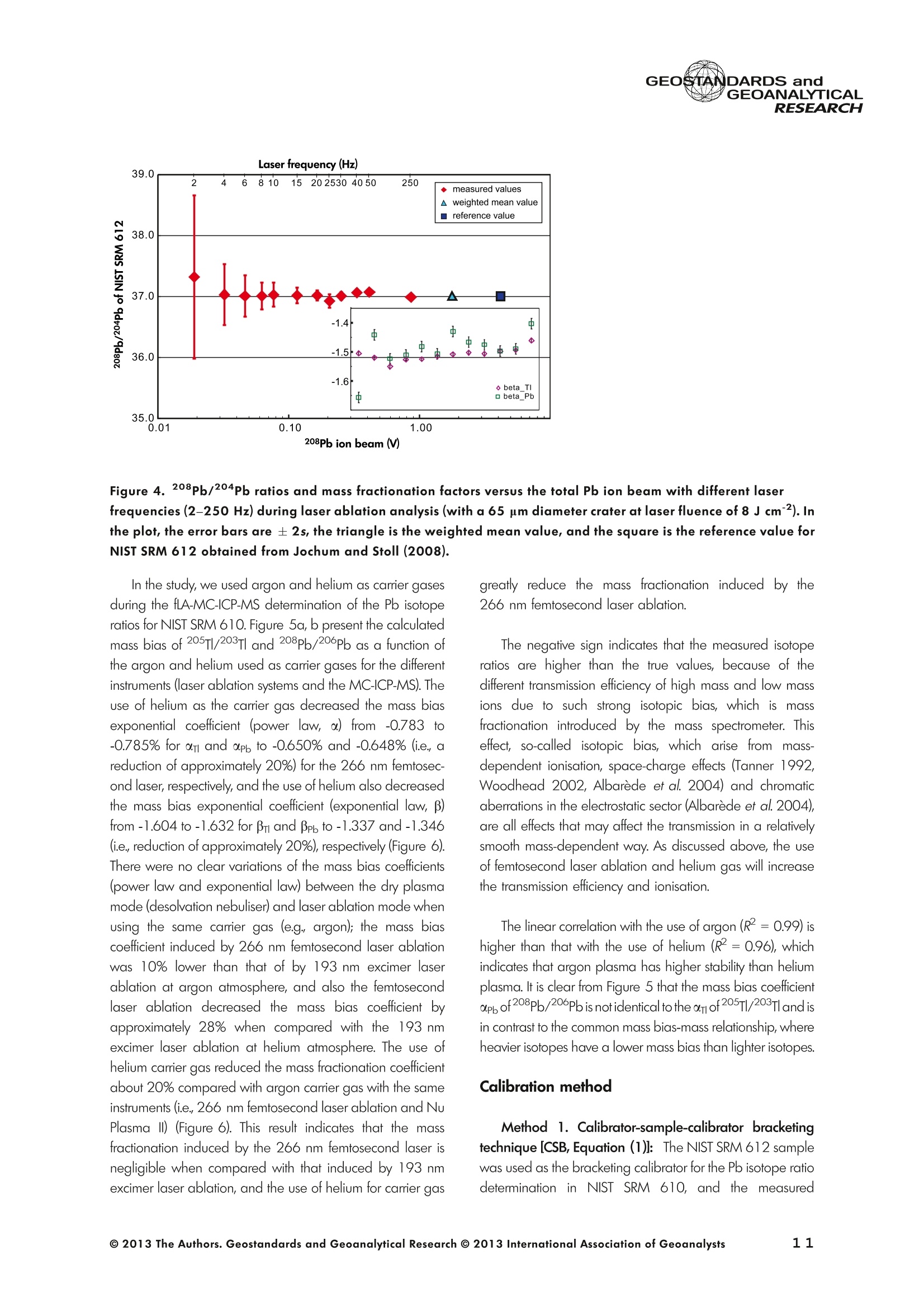
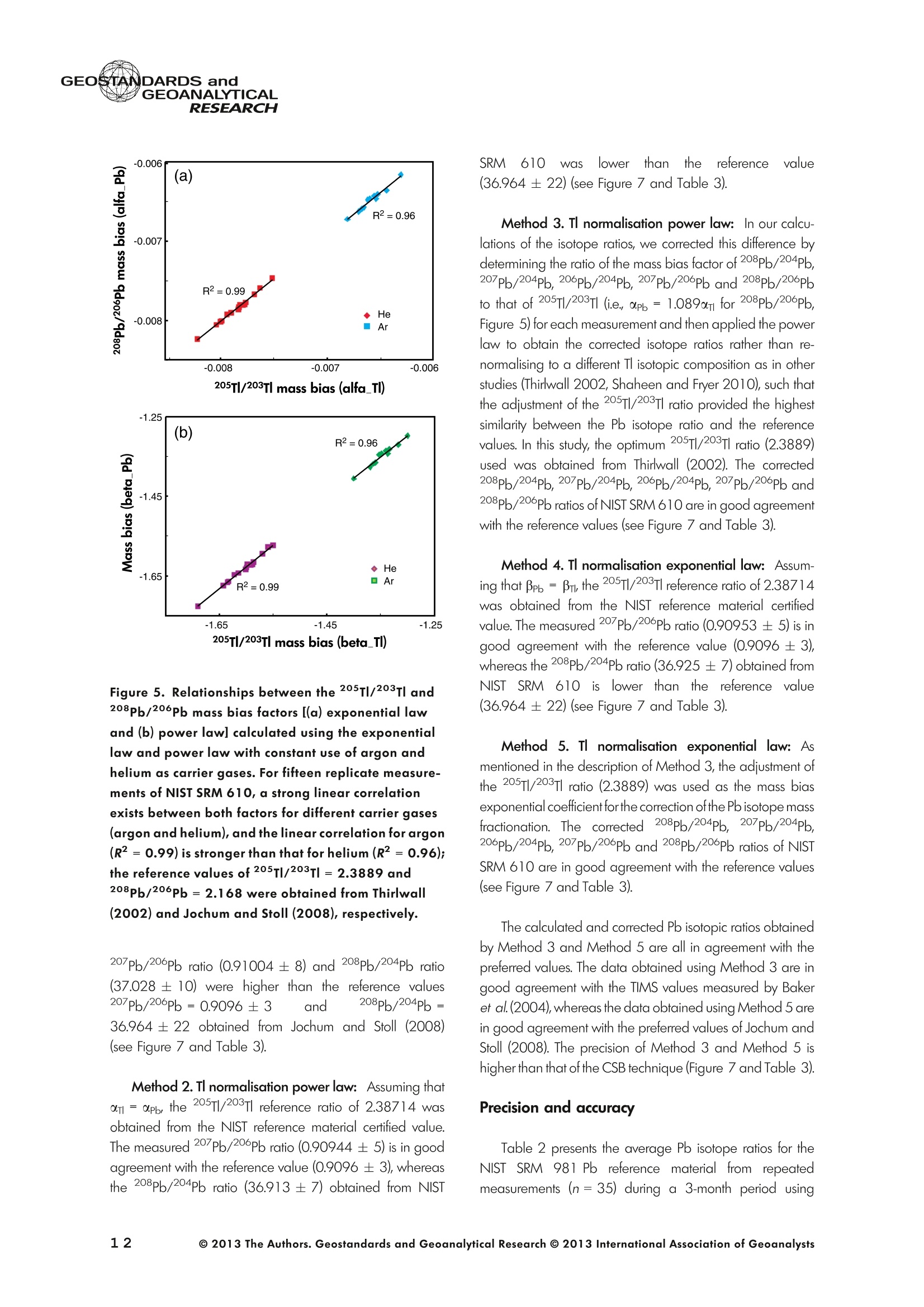
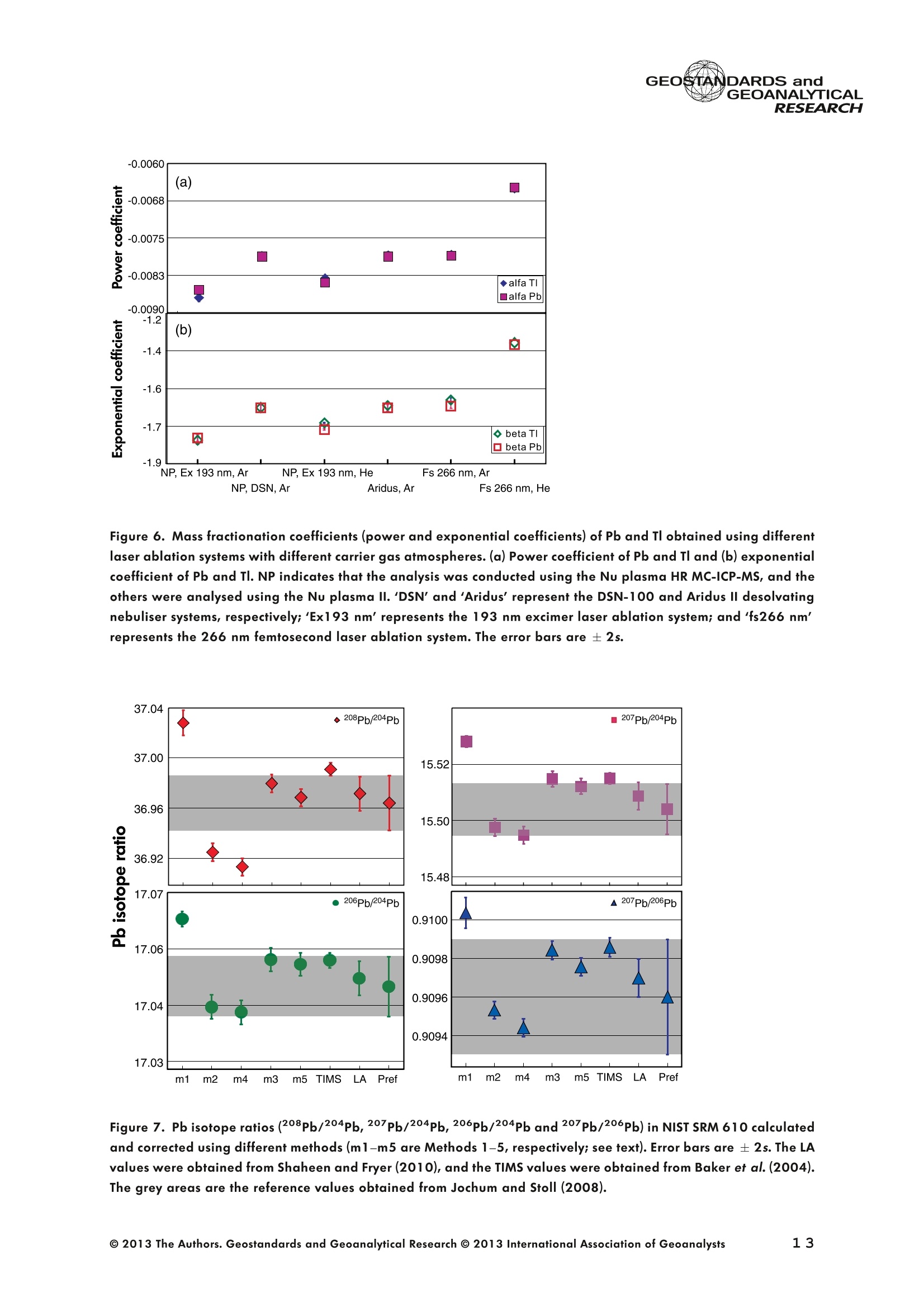
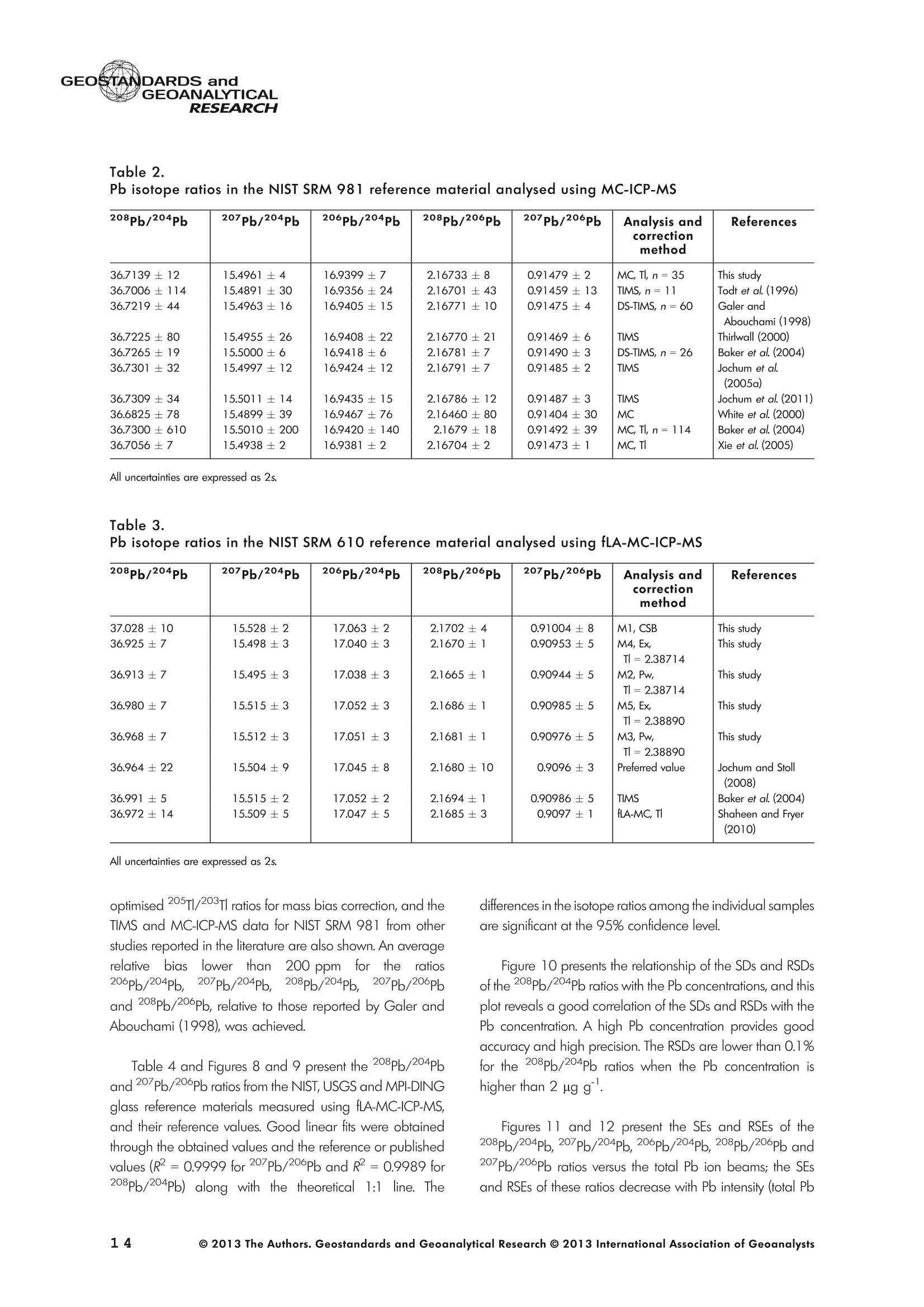
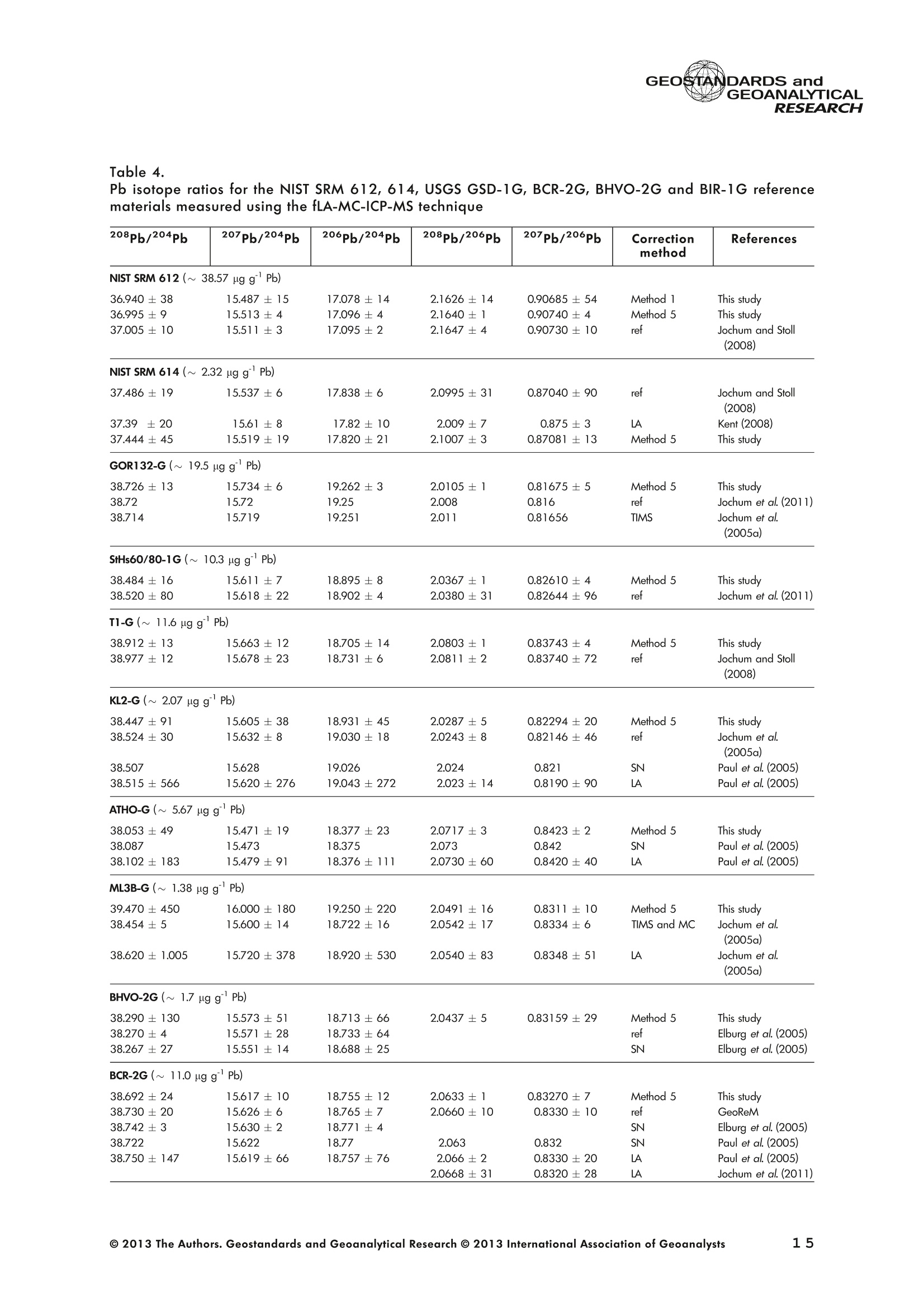
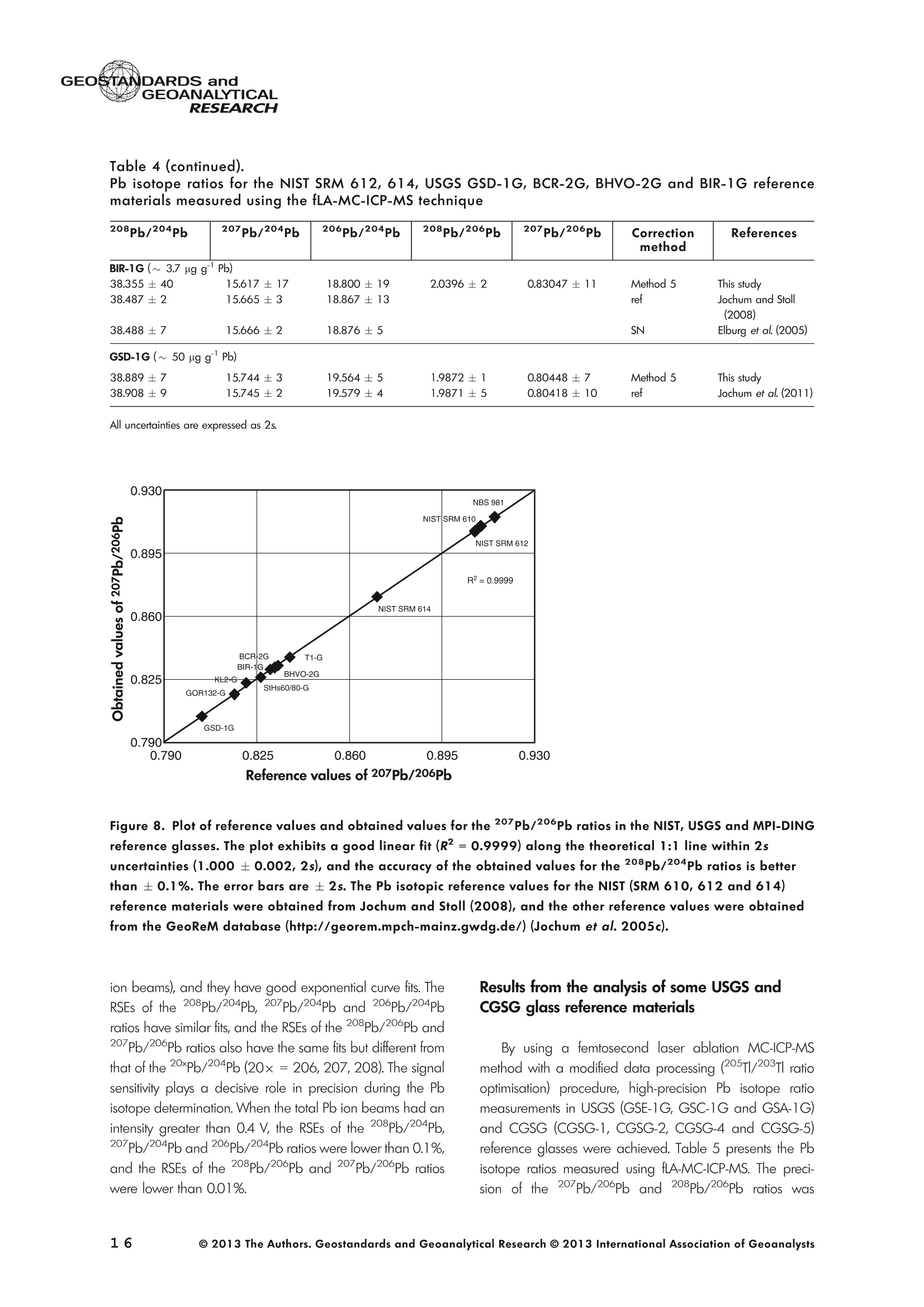
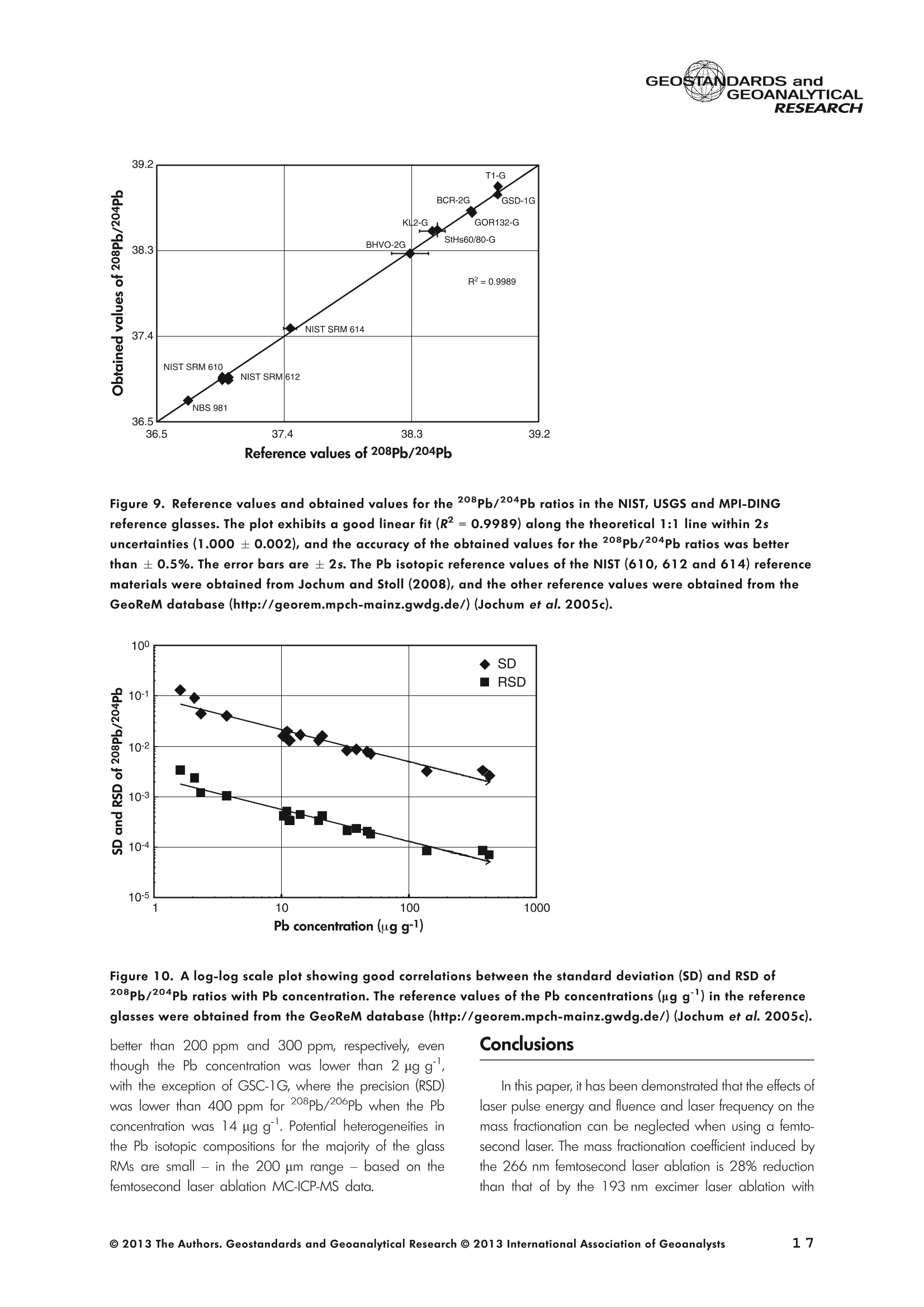
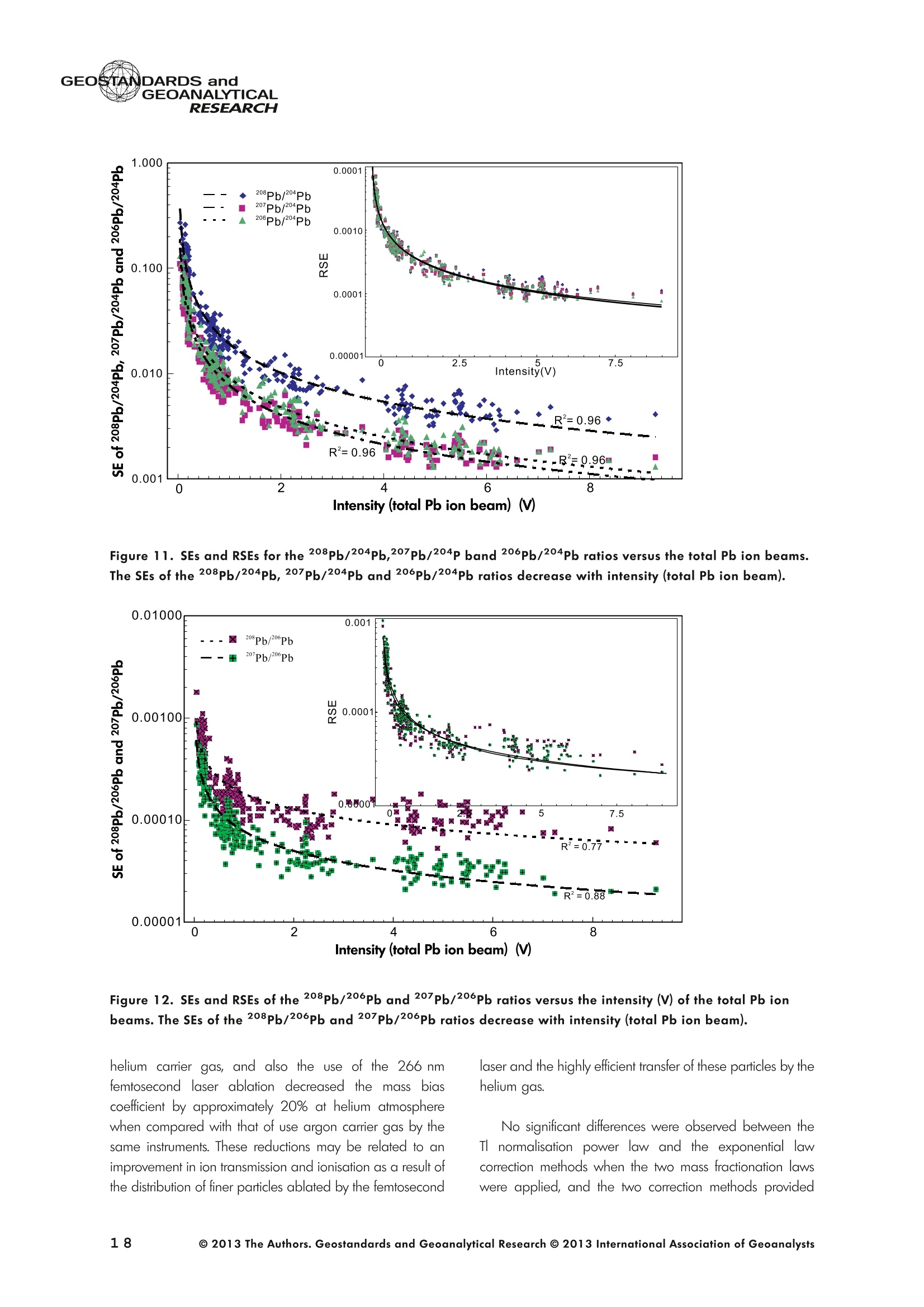
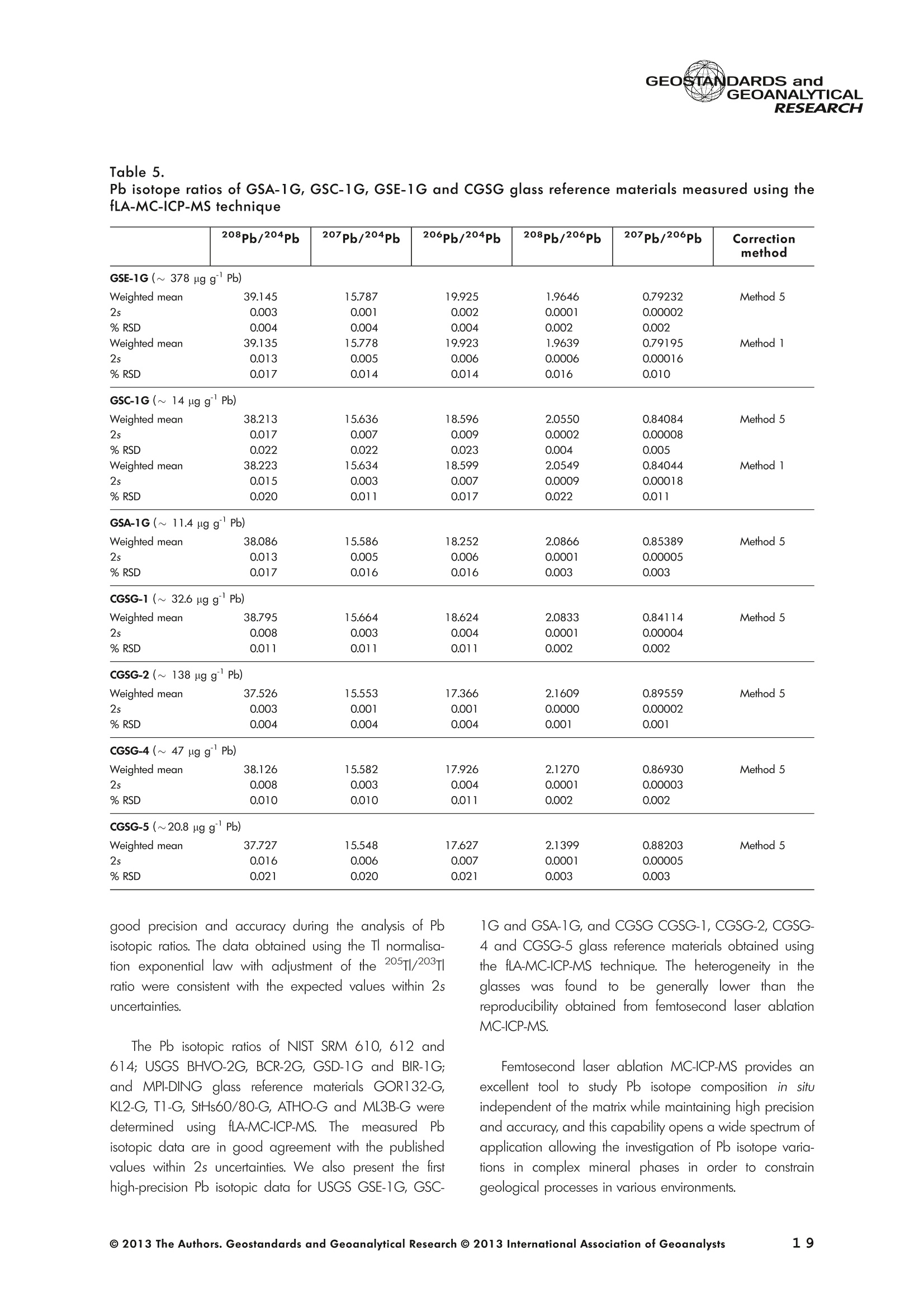
GEOSTANDARDS andVol. 38-N°103P. 5- 2 1RESEARCH STANBBRSandicALRESEARCH GEOANALYTICAL Precise and Accurate In Situ Determination of Lead IsotopeRatios in NIST, USGS, MPI-DING and CGSG Glass ReferenceMaterials using Femtosecond Laser Ablation MC-ICP-MS Chen Kaiyun, Yuan Honglin*,Bao Zhian, Zong Chunlei and Dai Mengning State Key Laboratory of Continental Dynamics, Department of Geology, Northwest University, Xi'an, 710069, China* Corresponding author. e-mail: sklcd@nwu.edu.cn Lead isotope ratio data were obtained with goodprecision and accuracy using a 266 nm femtosecondlaser ablation (fLA) system connected to a multi-collectorICP-MS (MC-ICP-MS) and through careful control ofanalytical procedures. The mass fractionation coefficientinduced by 266 nm femtosecond laser ablation wasapproximately 28% lower than that by 193 nm excimerlaser ablation (eLA) with helium carrier gas. The expo-nential law correction method for Tl normalisation withoptimum adjusted Tl ratio was utilised to obtain Pbisotopic data with good precision and accuracy. The Pbisotopic ratios of the glass reference materials NIST SRM610, 612, 614; USGS BHVO-2G, BCR-2G, GSD-1G,BIR-1G; and MPI-DING GOR132-G, KL2-G, T1-G,StHs60/80-G, ATHO-G and ML3B-G were determinedusing fLA-MC-ICP-MS. The measured Pb isotopic ratioswere in good agreement with the reference or publishedvalues within 2s measurement uncertainties. We alsopresent the first high-precision Pb isotopic data for GSE-1G, GSC-1G, GSA-1G and CGSG-1, CGSG-2, CGSG-4and CGSG-5 glass reference materials obtained usingthe femtosecond laser ablation MC-ICP-MS analysistechnique. Keywords: Pb isotopes, femtosecond laser, MC-ICP-MS, glassreterence materials, matrix-independent. Received 09 Nov 12 -Accepted 07 Feb 13 The introduction of MC-ICP-MS has provided a tech-nique that is competitive with thermal ionisation massspectrometry (TIMS), which has been considered to be thereference technique for high-precision measurements of Pb Des rapports isotopiques du Plomb ont éte obtenus avecune bonne precision et une bonne exactitude en utilisantun systeme d'ablation laser 266 nm femtoseconde (fLA)relie a un multicollecteur ICP-MS (MC-ICP-MS) et cecigrace a un controle rigoureux des procedures analy-tiques. Le coefficient de fractionnement de masse induitpar le systeme d'ablation laser femtoseconde 266 nmetait d'environ 28% inferieur a celui induit par unsysteme d'ablation laser de type excimer 193 nm (eLA)avec de Ihelium comme gaz porteur. La methode decorrection en loi exponentielle pour la normalisation du Tlavec le rapport Tl ajuste de facon optimale a éte utiliseepour obtenir des donnees isotopiques du Pb avec unebonne precision et une bonne exactitude. Les rapportsisotopiques du Pb des NIST SRM 610, 612 et 614; desverres de reference de IUSGS: BHVO-2G,BCR-2G, GSD-1G et BIR-1G et des verres de reference MPI-DING:GOR132-G, KL2-G, T1-G, StHs60/80-G,ATHO-G etML3B-G ont ete determines a laide du systeme fLA-MC-ICP-MS. Les rapports isotopiques du Pb mesurés sont enbon accord avec les valeurs de reference ou cellespubliees dans un intervalle d'incertitude de mesure de 2s.Nous presentons egalement les premieres donneesisotopiques du Pb de haute precision pour les verres dereference GSE-1G,CGC-1G, GSA-1G et CGSG (CGSG-1,CGSG-2, CGSG-4 et CGSG-5) obtenues en utilisant latechnique d'analyse d'ablation laser femtosecondeMC-ICP-MS. Mots-cles :isotopes du Pb, laser femtoseconde, MC-ICP-MS,verres de reference, independance de la matrice. isotope ratios (Hirata 1996, Belshaw et al. 1998, Albaredeand Beard 2004, Baker et al.2004, Ortega et al. 2012).The simultaneous detection of ions using Faraday detectorsminimises the effects of ion beam instability and allows highly precise and accurate measurements of isotope ratios(Paul et al.2005, Shaheen and Fryer 2010). The MC-ICP-MS technique provides the potential toreliably correct for mass discrimination and drift by dopingwith thallium (iwo natural isotopes: 205Tl and 203Tl) (Hirata1996). Various articles and abstracts have been publishedsubsequently that describe different approaches to Tl-dopingusing different MC-ICP-MS instruments (Hirata 1996, Bel-shaw et al. 1998,Rehkamper andHallidayv1998,Rehkamper and Mezger 2000, White et al. 2000, Thirlwall2002,Woodhead 2002, Baker et al. 2004, Ortega et al.2012), but where the 205Tl/203Tl ratio is acquired simulta-neously with the Pb during Pb isotopic determination.Because of the relative constancy of the mass bias ofisotopes of similar mass over the course of a run analysisduring plasma ionisation (Longerich et al. 1987), it washypothesised that this method may yield mass bias-correctedPb isotopic data that approach the data obtained using thebenchmark double-spike (DS) or triple-spike (TS) TIMStechniques. However, the assumption that the two elementsfractionate to the same extent is incorrect and results insystematic errors of a few hundred ppm or more (Albaredeet al.2004). Fontaine et al. (2009) reported the presence ofsystematic variations in mass bias in MC-ICP-MS withchanges in operating conditions (e.g., carrier gas and flowrate, sampling depth and extraction lens voltage), sampleintroduction and matrix effects. Therefore, a suitable correc-tion strategy for mass bias must be sought. During the in situ determination of Pb isotope compo-sition, laser ablation produces aggravated mass fraction-ation such that high spatial resolution is achieved; however,isotope ratio measurements by LA-MC-ICP-MS are affectedby fractionation and mass bias effects. The principallimitations of the ablation physics introduced by thetraditionally employed nanosecond lasers (elemental andisotope fractionation) have prevented the routine measure-ment of bias-free stable isotope ratios (Shaheen and Fryer2010. Many processes involved in LA-ICP-MS (particle forma-tion, particle transport and atomisation and ionisation in theICP) can contribute to interelemental and isotopic fraction-ation (Koch et al. 2004, 2007). The proper choice of laserparameters (wavelength, pulse width, fluence and fre-quency) (Koch et al.2007), ICP-MS operating conditions(RF power, gas flow and sampling depth) and carrier gas(helium and argon) helps to minimise the extent offractionation and mass bias (Mank and Mason 1999, Kochet al. 2008). Recently, femtosecond laser ablation has beenreported to improve the analytical capability of LA-ICP-MS through the reduction in fractionation and matrix effects(Russo et al. 2002, Horn et al. 2006, Fernandez et al.2007, Koch et al. 2007, Shaheen et al. 2008, Garcia et al.2009, Steinhoefel et al. 2009, Shaheen and Fryer 2010,Koch and Gunther 2011). Mass bias is an important factorthat affects the precision and accuracy of isotope ratiodeterminations by LA-MC-ICP-MS, and it must be minimisedand robustly corrected to obtain the most precise andaccurate isotope ratio results (Shaheen and Fryer 2010). The purpose of this study was to investigate the effects oflaser conditions (wavelength, pulse width, energy or fluenceand frequency), ICP conditions (e.g., carrier gas) andcalculation/correction methods (calibrator-sample-calibratorbracketing, Tl normalisation power law and exponential law)on the bias-free determination of Pb isotope ratios. Inaddition, the different mass fractionation laws for Pb isotopedetermination by fLA-MC-ICP-MS were compared. Finally, Pbisotope ratios were determined in the glass referencematerials NIST SRM 610, 612 and 614 (Woodhead andHergt 2001, Baker et al. 2004), USGS BCR-2G, BHVO-2GBIR-1G and GSD-1G (Jochum et al. 2011); and MPI-DINGT1-G, KL-G, GOR132-G, StHs60/80-G, ATHO-G andML3B-G (Jochum et al. 2005a, 2011). The purpose of thiscontribution is also to present the first high-precision Pbisotopic data for GSE-1G, GSC-1G, GSA-1G (Jochum et al.2005b) and CGSG (CGSG-1, CGSG-2, CGSG-4 andCGSG-5) reference glasses (Hu et al. 2011) obtained usingfemtosecond laser ablation MC-ICP-MS. Analytical method General A Pb isotope-certified reference material NIST SRM 981(NBS 981) solution and Tl isotope-certified reference mate-rial NIST SRM 997 solution were prepared using ultra-pureMilli-Q water produced by a Millipore (Millipore Corpora-tion, Billerica, MA, USA) pure water system with a resistivity of18.2 MQ cm and subsequently purified by sub-boilingdistillation. Hydrochloric acid, nitric acid and hydrobromicacid were also purified twice at least by sub-boilingdistillation. All of the gases (argon and helium) used in thisstudy were of high purity (99.9995%). Samples and reference materials Twenty reference glasses/materials for microanalysiscalibration were analysed in the present study, includingNIST SRM 610, 612 and 614; USGS GSA-1G, GSC-1G,GSD-1G,GSE-1G,BCR-2G, BHVO-2G and BIR-1G; CGSGCGSG-1,CGSG-2, CGSG-4 and CGSG-5; and MPI-DING GOR132-G, KL2-G, T1-G, StHs60/80-G, ATHO-G andML3B-G. Test portions were attached to double-sided tapeand itself subsequently attached to a glass slide. A PVC ringwas then placed around the samples, and a mixture ofepoxy and hardener was poured into the PVC ring and leftuntil it hardened. The mount was then peeled off of the glassslide, and the target was polished to a flat surface. Thesample target was carefully cleaned using anhydrousethanol to eliminate any potential contamination of thesample surface prior to laser ablation analysis. Instrumentation All of the experiments were conducted using instrumentslocated at the State Key Laboratory of Continental Dynamics,Northwest University, Xi’an, China. The Nu Plasma multi-collector ICP-MS (Nu Instruments, Wrexham, UK) representsthe latest generation of double-focussing mass spectrometers.The new collector has sixteen Faraday detectors for greaterflexibility, and its five full-size discrete dynode multipliers enablethe determination of precise isotope ratios, even for isotopeswith very low abundance. Its zoom optics system allows theobservation of instant changes in dispersion and perfect peakoverlap without slow and potentially unreliable detectormovement. The instrumental parameters are summarised inTable 1. The CETAC Aridus IIdesolvating nebuliser system hasenhanced sensitivity (at least ten-fold) for ICP-MS and a PTFEmembrane desolvator for extremely low oxide (CeO*/Ce*<0.03%) and hydride levels. The Aridus ll desolvatoroperating conditions are shown in Table 1. The NWR UP Femto femtosecond laser ablation systemconsisted of a Quantronix Ti:sapphire femtosecond laseramplifier Integra-HE and ESI NWR Femto laser ablationsystem components. This system is a regenerative andmultipass Ti:sapphire laser ablation system based on thechirped pulse amplification (CPA) technique. The laser outputultraviolet wavelength was 266 nm and had a Gaussianpeak produced by the 3rd harmonic; a pulse duration of lessthan 130 fs (at 800 ± 10 nm); output energy greater than600 pJ (at2250 Hz)Inthe ultraviolet region(UV,A=266 nm); samplesurfacefluencegreater tthan10 J cm; repetition frequency of 1-50 Hz, 125 Hz and250 Hz; and thirteen adjustable apertures (laser ablationspot size of 1-65 um). The instrument was equipped with acolour CCD camera and a two-volume chamber thatconsists of a large, efficient sample chamber (5000 cm)ands a super-high-sensitiviy sample cell (1.6 cm). Thespecifications of the laser ablation system and the operatingconditions are presented in Table 1. Table 1. Instrumental parameters for the MC-ICP-MS andfemtosecond laser ablation system Nu Plasma II MC-ICP-MS Cool gas 17I minAr Auxiliary gas 0.8| min Ar Sample gas 0.25l min Ar RF generator power 1300 W Acceleration voltage 6010V Sample cone Ni orifice, diameter 1.1 mm Skimmer cone Ni orifice, diameter 0.8 mm Amplifiers 101 Q Faraday cup voltage 0-50V Sensitivity 45 V/ug ml for wet plasma 600 V/ug ml for dry plasma Faraday cup setup 208Pb (H2),207Pb(H1),206Pb (Ax),205TI (L1),204Pb+Hg(L2),203Tl (L3),202Hg(L4) Gas blank time 30 s for OPZ at TRA mode Sample integration time 10 s for 1 cycle, total: 50 measurements for static 0.2 s, total time of 50 s for TRA mode Aridus II desolvation nebuliser system Membrane temperature Spray chamber temperature Sweep gas flow Sample uptake rate Nebuliser flow 160°C 110°℃ 3.25 minAr 100 ul min PFA nebuliser 0.96l min' New wave UP Femto 266 nm fs laser system Laser type Quantronix Integra-HE Ti/sapphire 3rd harmonic Output wavelength 266 nm (ultraviolet) Beam profile > 95% fit to Gaussian Beam diameter 1.0 mm Pulse duration 130 fs at 800 nm Pulse energy output 0.6 mJ Energy density 6 J cm Irradiance 1013 W cm2 Laser beam 30 um Pulse repetition rate 1-50 Hz, 125 Hz, 250 Hz Ablation cell volume Two volume ablation cells Carrier gas and flow rate into the Ar or He, 0.9I min ablation cell Transport from ablation cell to ICP Tygon tube (100 cm in length, 5 mm id) We also employed the excimer 193 nm laser ablationsystem and Nu plasma HR MC-ICP-MS for Pb isotopedetermination; the Hg, Tl and Pb ions were also collected bythe Faraday cups; and the specifications and operatingconditions of the laser system and MC-ICP-MS are similar tothose of Yuan et al.(2008). Measurement procedure The Nu PlasmalM MC-ICP-MS was coupled to thefemtosecond laser ablation system (fLA-MC-ICP-MS) and prepared for the introduction of the TI solution through theCETAC Aridus IIM desolvation nebuliser system. The thalliumsolution was self-aspirated at an uptake rate of 100 ul minthrough the PFA nebuliser and desolvated by the AridusM.Argon and helium were used as the carrier gases for laserablation. The aerosol from the ablation cell was mixed withTl (argon withTl) in a glass aerosol homogeniser and thenintroduced into the ICP for atomisation and ionisation(Figure 1). During the instrumental analysis, the intensities of the ionbeams of 202Hg, 203Tl, 204pb+Hg, 205Tl, 206Pb, 207Pb and208Pb were simultaneously monitored with the Faradaycollectors L4, L3, L2, L1, Ax, H1 and H2, respectively, all ofwhich utilise 10 resistors. Amplifier board gains of thecollector were calibrated on a daily basis using an internal40 V reference signal, and the RSDs of the gain factors wereless than 0.001%. The concentrations of lead and mercury inthe gas blank used in the procedure were lower than10 ng I and 20 pg 1,respectively; therefore, their contri-bution to the measurement was negligible. The 202Hg signalwas examined to determine the isobaric interference of204Hg on 204Pb. Thallium was used to monitor and correctfor instrumental mass discrimination, and 202Hg was used tocorrect for the isobaric overlap of 204Hg on 204Pb. Thecalculated and determined interference of 204Hgon 204PbiWaSachieved usingthenaturallabundance ratio204Hg/202Hg=0.229883 (202Hg=0.29863 and204Hg=0.06865) adjusted for instrumental mass fraction-ation asiSmonitored by the,205Tl/203Tl ratio. The204Hg/204Pb ratios varied from day to day, but they wereless than 20 ppm in the experimental system when the ionbeam of 204Pb had an intensity greater than 0.25 V. To improve the repeatability of the analytical conditionsfor the determination of the Pb isotopic compositions andconsequently improve the precision and the accuracy (i.e.,better precision for 205TL203Tl and less interference for 204Pb)(Figure 2), all samples were analysed using the same Pb/TImole ratio (Pbrotal/Ttotal=~3), and the Tl solution wascontinuously sampled by the Aridus II desolvation nebulisersystem to reduce changes in the instrumental conditionsresulting from switching between the injection of the sampleand the wash. The decreased intensity for the Tl isotopeswhen the laser was fired may be related to changes in theplasma conditions as a result of ablated material beingintroduced into the ICP (Shaheen and Fryer 2010). However,the 205Tl/203Tl ratios were not affected by changes in thesignal intensity or the source of Tl, and it was stablethroughout the entire data acquisition period. The acquisition of the MC-ICP-MS data employed thetime-resolved analysis (TRA) mode with an integration time of0.2 s, and laser ablation was performed in the line scanablation mode at a speed of 5 um s’with the laser beamfocused on the sample surface. Each line scan analysisconsisted of background collection for 40 s followed by anadditional 50 s of ablation for signal collection and 40 s ofwash time to reduce memory effects and to allow theinstrument to stabilise after each gas addition. All of therecorded Pb and Hg signals were corrected for backgroundby subtracting the background signals (gas blank and darknoisy signals) from the corresponding gross signals (signalsobtained after firing the laser), whereas the Tl signals werecorrected for background by subtracting the average darknoisy signals (stability <25 ppm at 10 min). A laser spotsize of 30-65 um and line scan length of approximately Figure 2. Intensity variation of Tl and Pb and measured 205TI/203Tl ratio in NIST SRM 612 glass for 120 s of dataacquisition during laser ablation analysis. The first 40 s are measurements of the Tl solution and background of Pbisotopes, whereas the next 60 s are measurements of both the Tl solution and the laser-ablated NIST SRM 612sample. 120 um were achieved by changing the laser ablationfrequency to ensure that the same 208Pb signals wereobtained for different samples with disparate Pb concentra-tions. NIST SRM 610 was used as a quality control sampleduring the analysis; NIST SRM 610 was analysed once forevery five sample points. Data reduction The interference of 204Hg on 204Pb was corrected bysubtracting the 204Hg intensity (calculated from the mea-sured background-corrected202Hg intensity by assumingthat 204Hg/202Hg=0.229883 from the background-cor-rected 204Pb intensity), and the mass bias of Hg is adoptedfrom that of Tl. The Pb isotopic determinations are notstraightforward because Pb has only one stable (non-radiogenic) isotope, 204Pb, which prevents the application ofan internal normalisation approach to correct for mass bias.However, as suggested by Longerich et al. (1987), TI can beused as a surrogate to correct for mass bias, provided thatthe Tl mass bias can be related to that of Pb. The Pb isotoperatios were corrected by subtracting the gas blank (back-ground), whereas the Tl ratios were only corrected bysubtracting the dark noise of MC-ICP-MS. There are threecommonly used mass fractionation laws: linear, exponentialand power laws (Albarede and Beard 2004, Albaredeet al. 2004). The linear law is least suited for the correctionof mass discrimination, because the linear law is notconsistent: if two ratios fractionate according to the linearlaw, the ratio of these ratios does not, whereas the powerlaw or exponential law function provides significantly more accurate and precise results (Hirata 1996, Rehkamper andHalliday 1998), and also thepower law and theexponential law both are consistent (Albarède and Beard2004, Albarede et al. 2004). Therefore, in this study, toinvestigate the difference between the power law and theexponential law, we used the power law andtheexponential law for mass fractionation correction, respec-tively. Furthermore, we also used the calibrator-sample-calibrator bracketing (CSB) technique to correct for massfractionation during the in situ Pb isotope determination byfLA-MC-ICP-MS. In this study, we used the CSB technique to correct formass fractionation during analysis first. NIST SRM 612 wasused as a bracketing calibrator for the Pb isotope ratiodeterminations of NIST SRM 610. Alternative measurementsof NIST SRM 612 and 610 were taken using a high laserfreauency for NIST SRM 612 and a low laser frequency forNIST SRM 610 to obtain more similar ion intensities for bothmatrices when switching between the NIST SRM 610 and612 samples. By adopting this method, the isotope ratioswere calculated using Equation (1): where Rrue and fmeas represent the true and measuredisotope ratios of the sample, respectively; Rcal refers to thetrue isotope ratio of the bracketing calibrator; and feali andcal2 refer to the isotope ratios of the bracketing calibratormeasured before and after the sample, respectively. As mentioned above, mass bias and drift were reliablycorrected by doping with Tl for the Pb isotope measurement,whereas a power or exponential law function providedsignificantly more accurate and precise results. Therefore, inthis study, to investigate the difference between the powerlaw and the exponential law, we also used the power law[Equations (2) and (3)] and the exponential law [Equa-tions (4) and (5)]: where ar and op refer to the mass bias per atomic massunit of the Tl and Pb isotopes, respectively, and Rile andRmeas refer to the true and measured Tl isotope ratios,respectively; Am(Am=M-M) refers to the atomic massdifference between the masses M and M of Pb isotopes.pPbmeas isthemeasuredvaluee of20XPb/204pb(or20XPb/206Pb); RPb refers to corrected value of 20Xpb/204Pb(or 20Xpb/206Pb); and AM=2 is the mass differencebetween the Tl isotopes (i.e., 205T|_203Tl); and where ri and Bp refer to the Tand Pb isotope fractionationfactors, respectively; Riue and Rieas are the true value and measured value of the 205Tl/203Tl ratios, respectively; RPleas isthe measured value of 20XPb/204Pb (or 20XPb/206Pb); RPb isthe corrected value of20Xpb/204Pb (or 20Xpb/206Pb); andM203 and M205 refer to the atomic mass numbers of 203T|and 205Tl isotopes, respectively. M. and M refer to thedifferent atomic mass of Pb isotopes, and they can be M204,M206, M207 or M208. Results and discussion Laser conditions: laser energy, frequency andcarrier gas The laser pulse fluence increased with the energy, and thePb intensity (Pb ion beam) increased with the laser fluence;thePb intensity will not further increase when the laser fluence isgreater than 8 J cm²(Figure 3), whereas the Pb isotoperatios are in good agreement with the mean values within 2suncertainties (Figure 3) for different laser fluences. Further-more, the mass fraction coefficient (exponential law) of Pbexhibited no significant changes with different laser fluences. Under the same laser conditions (e.g., crater with adiameter of 65 um and energy density of 8 J cm?), a higherlaser frequency will produce greater Pb intensity and lowerrelative standard error (Figure4). For example, when themeasured total Pb ion beams of NIST SRM 610 are greaterthan 0.2 V, the RSE (relative standard error of the mean) ofmeasured Pb isotopic ratios will be less than 0.2%. Themeasured 208Pb/204Pb ratios at different laser frequencies(2-250 Hz) were all in good agreement with the referencevalues within 2s measurement uncertainty (Figure 4). Figure 3. Laser energy and fluence versus the total Pb ion beams and the 208Pb/204Pb ratios obtained for the NISTSRM 612 glass and the mass fraction factor during laser ablation analysis (with a 65 um diameter crater at laserfrequency of 250 Hz). The intensity (total Pb ion beam) increases with the laser fluence, and the intensity stopsincreasing when the laser fluence is greater than 8 J cm. Figure 4. 208Pb/204Pb ratios and mass fractionation factors versus the total Pb ion beam with different laserfrequencies (2-250 Hz) during laser ablation analysis (with a 65 um diameter crater at laser fluence of 8 J cm2). Inthe plot, the error bars are ±2s, the triangle is the weighted mean value, and the square is the reference value forNIST SRM 612 obtained from Jochum and Stoll (2008). In the study, we used argon and helium as carrier gasesduring the fLA-MC-ICP-MS determination of the Pb isotoperatios for NIST SRM 610. Figure 5a, b present the calculatedmass bias of 205Tl/203Tl and 208pb/206Pb as a function ofthe argon and helium used as carrier gases for the differentinstruments (laser ablation systems and the MC-ICP-MS). Theuse of helium as the carrier gas decreased the mass biasexponential coefficient (power law, a) from -0.783 to-0.785% for ar and oplto -0.650% and -0.648% (i.e., areduction of approximately 20%) for the 266 nm femtosec-ond laser, respectively, and the use of helium also decreasedthe mass bias exponential coefficient (exponential law, B)from -1.604 to-1.632 for Bri and Bpb to-1.337 and -1.346(i.e., reduction of approximately 20%), respectively (Figure 6).There were no clear variations of the mass bias coefficients(power law and exponential law) between the dry plasmamode (desolvation nebuliser) and laser ablation mode whenusing the same carrier gas (e.g, argon); the mass biascoefficient induced by 266 nm femtosecond laser ablationwas 10% lower than that of by 193 nm excimer laserablation at argon atmosphere, and also the femtosecondlaser ablation decreased the mass bias coefficient byapproximately 28% when compared with the 193 nmexcimer laser ablation at helium atmosphere. The use ofhelium carrier gas reduced the mass fractionation coefficientabout 20% compared with argon carrier gas with the sameinstruments (i.e.,266 nm femtosecond laser ablation and NuPlasma ll) (Figure6). This result indicates that the massfractionation induced by the 266 nm femtosecond laser isnegligible when compared with that induced by 193 nmexcimer laser ablation, and the use of helium for carrier gas greatly reduce the mass fractionation induced by the266 nm femtosecond laser ablation. The negative sign indicates that the measured isotoperatios are higher than the true values, because of thedifferent transmission efficiency of high mass and low massions due to such strong isotopic bias, which is massfractionation introduced by the mass spectrometer. Thiseffect, so-called isotopic bias, which arise from mass-dependent ionisation, space-charge effects (Tanner 1992,Woodhead 2002, Albarede et al. 2004) and chromaticaberrations in the electrostatic sector (Albarede et al. 2004),are all effects that may affect the transmission in a relativelysmooth mass-dependent way. As discussed above, the useof femtosecond laser ablation and helium gas will increasethe transmission efficiency and ionisation. The linear correlation with the use of argon (R2=0.99) ishigher than that with the use of helium (R2=0.96), whichindicates that argon plasma has higher stability than heliumplasma. It is clear from Figure 5 that the mass bias coefficientapp of208pb/206Pb is not identical to the om of 205Tl/203Tland isin contrast to the common mass bias-mass relationship, whereheavier isotopes have a lower mass bias than lighter isotopes. Calibration method Method 1. Calibrator-sample-calibrator bracketingtechnique [CSB, Equation (1)]::The NIST SRM 612 samplewas used as the bracketing calibrator for the Pb isotope ratiodetermination in NIST SRM 610, and the measured Figure 5. Relationships between the 205TI/203Tl and208Pb/206Pb mass bias factors [(a) exponential lawand (b) power law] calculated using the exponentiallaw and power law with constant use of argon andhelium as carrier gases. For fifteen replicate measure-ments of NIST SRM 610, a strong linear correlationexists between both factors for different carrier gases(argon and helium), and the linear correlation for argon(R²= 0.99) is stronger than that for helium (R²=0.96);the reference values of 205TI/203TI=2.3889 and208Pb/206Pb=2.168 were obtained from Thirlwall(2002) and Jochum and Stoll (2008), respectively. 207Pb/206Pb ratio (0.91004±8) and 208Pb/204Pb ratio(37.028±10) were higher than the reference values207Pb/206Pb=0.9096±3 and 208Pb/204Pb=36.964 ±22 obtained from Jochum and Stoll (2008)(see Figure 7 and Table 3). Method 2. Tl normalisation power law: Assuming thatom= opl, the 205Tl/203Tl reference ratio of 2.38714 wasobtained from the NIST reference material certified value.The measured 207Pb/206Pb ratio (0.90944 ± 5) is in goodagreement with the reference value (0.9096 ±3), whereasthe 208pb/204Pb ratio (36.913±7) obtained from NIST SRM 610l waslower tthainntherreferenceevalue(36.964±22) (see Figure7 and Table 3). Method 3. Tl normalisation power law: In our calcu-lations of the isotope ratios, we corrected this difference bydetermining the ratio of the mass bias factor of 208pb/204pb,207Pb/204Pb,206pb/204Pb,207Pb/206Pb and 208Pb/206Pbto that of 205T1/203Tl (i.e., opb= 1.089om for 208pb/206pb,Figure 5) for each measurement and then applied the powerlaw to obtain the corrected isotope ratios rather than re-normalising to a different Tl isotopic composition as in otherstudies (Thirlwall 2002, Shaheen and Fryer 2010), such thatthe adjustment of the 205Tl/203Tl ratio provided the highestsimilarity between the Pb isotope ratio and the referencevalues. In this study, the optimum 205Tl/203Tl ratio (2.3889)used was obtained from Thirlwall (2002). The corrected208pb/204pb,207pb/204Pb,206pb/204pb,207pb/206Pband208Pb/206Pb ratios of NIST SRM 610 are in good agreement with the reterence values (see Figure 7 and Table 3). Method 4. Tl normalisation exponential law: Assum-ing that Bpo =ni, the 205Tl/203Tl reference ratio of 2.38714was obtained from the NIST reference material certifiedvalue. The measured 207pb/206pb ratio (0.90953 ±5) is ingood agreement with the reference value (0.9096±3),whereas the 208pb/204Pb ratio (36.925 ±7) obtained fromNIST SRM 610 is lower than the reference value(36.964±22) (see Figure 7 and Table 3). Method 5. T normalisation exponential law: Asmentioned in the description of Method 3, the adjustment ofthe 205Tl/203Tl ratio (2.3889) was used as the mass biasexponential coefficient forthe correction of the Pb isotope massfractionation. The corrected208pb/204pb, 207Pb/204Pb,206pb/204Pb, 207Pb/206Pb and 208pb/206pb ratios of NISTSRM 610 are in good agreement with the reference values(see Figure 7 and Table 3). The calculated and corrected Pb isotopic ratios obtainedby Method 3 and Method 5 are all in agreement with thepreferred values. The data obtained using Method 3 are ingood agreement with the TIMS values measured by Bakeret al (2004), whereas the data obtained using Method 5 arein good agreement with the preferred values of Jochum andStoll (2008). The precision of Method 3 and Method 5 ishigherthan that of the CSB technique (Figure 7 and Table 3). Precision and accuracy Table2 presents the average Pb isotope ratios for theNIST SRM 981 Pb reference material from repeatedmeasurements (n=35) during a 3-month period using Figure 6. Mass fractionation coefficients (power and exponential coefficients) of Pb and Tl obtained using differentlaser ablation systems with different carrier gas atmospheres. (a) Power coefficient of Pb and Tl and (b) exponentialcoefficient of Pb and Tl. NP indicates that the analysis was conducted using the Nu plasma HR MC-ICP-MS, and theothers were analysed using the Nu plasma ll. 'DSN'and 'Aridus’ represent the DSN-100 and Aridus II desolvatingnebuliser systems, respectively;‘Ex193 nm’represents the 193 nm excimer laser ablation system; and 'fs266 nm’represents the 266 nm femtosecond laser ablation system. The error bars are ± 2s. Figure 7. Pb isotope ratios (208Pb/204Pb,207Pb/204Pb,206Pb/204Pb and 207Pb/206Pb) in NIST SRM 610 calculatedand corrected using different methods (m1-m5 are Methods 1-5, respectively; see text). Error bars are ± 2s. The LAvalues were obtained from Shaheen and Fryer(2010), and the TIMS values were obtained from Baker et al.(2004).The grey areas are the reference values obtained from Jochum and Stoll (2008). Table 2. Pb isotope ratios in the NIST SRM 981 reference material analysed using MC-ICP-MS 208pb/204Pb 207Pb/204Pb 206Pb/204Pb 208pb/206pb 207Pb/206Pb Analysis andcorrectionmethod References 36.7139±1236.7006±11436.7219±44 36.7225±80 36.7265±1936.7301±32 36.7309±3436.6825±7836.7300±610 36.7056±7 15.4961±415.4891 ±3015.4963±16 15.4955±2615.5000±615.4997 ±12 15.5011±1415.4899±3915.5010±20015.4938±2 16.9399±716.9356±2416.9405±15 16.9408±2216.9418±616.9424±12 16.9435±1516.9467±7616.9420±14016.9381±2 2.16733±82.16701±432.16771±10 2.16770±212.16781±72.16791±7 2.16786±122.16460±802.1679±182.16704±2 0.91479±20.91459±130.91475±4 0.91469±60.91490±30.91485±2 0.91487±30.91404±300.91492±390.91473±1 MC, Tl, n=35TIMS,n= 11DS-TIMS, n=60 TIMS DS-TIMS, n=26TIMS TIMS MC MC, Tl, n=114MC, TI This study Todt et al. (1996) Galer and Abouchami (1998) Thirlwall (2000) Baker et al. (2004) Jochum et al. (2005a) Jochum et al.(2011) White et al. (2000) Baker et al.(2004) Xie et al. (2005) All uncertainties are expressed as 2s. Table 3.Pb isotope ratios in the NIST SRM 610 reference material analysed using fLA-MC-ICP-MS 208Pb/204Pb 207Pb/204Pb 206Pb/204Pb 208pb/206Pb 207Pb/206Pb Analysis andcorrectionmethod References 37.028±1036.925±7 36.913±7 36.980±7 36.968±7 36.964±22 36.991±5 36.972±14 15.528±215.498±3 15.495±3 15.515±3 15.512±3 15.504±9 15.515±215.509±5 17.063±217.040±3 17.038±3 17.052±3 17.051±3 17.045±8 17.052±217.047±5 2.1702±42.1670±1 2.1665±1 2.1686±1 2.1681±1 2.1680±10 2.1694±12.1685±3 0.91004±80.90953±5 0.90944±5 0.90985±5 0.90976±5 0.9096±3 0.90986±50.9097±1 M1, CSBM4, Ex, TI=2.38714M2, Pw,TI=2.38714M5, Ex,TI=2.38890M3, Pw, TI=2.38890 Preferred value TIMS fLA-MC, TI This studyThis study This study This study This study Jochum and Stoll(2008)Baker et al. (2004) Shaheen and Fryer (2010) All uncertainties are expressed as 2s. optimised 205Tl/203Tl ratios for mass bias correction, and theTIMS and MC-ICP-MS data for NIST SRM 981 from otherstudies reported in the literature are also shown. An averagerelativebiasISlowertthan200 ppm for theeratios206Pb/204Pb,207Pb/204pb,208Pb/20?44PPbb,, 220077;Pb/206pband 208pb/206Pb, relative to those reported by Galer andAbouchami (1998), was achieved. Table 4 and Figures 8 and 9 present the 208pb/204pband 207Pb/206Pb ratios from the NIST, USGS and MPI-DINGglass reference materials measured using fLA-MC-ICP-MS,and their reference values. Good linear fits were obtainedthrough the obtained values and the reference or publishedvalues (R²=0.9999 for 207Pb/206Pb and R2=0.9989 for208pb/204Pb) along with the theoretical11:1line. The differences in the isotope ratios among the individual samplesare significant at the 95% confidence level. Figure 10 presents the relationship of the SDs and RSDsof the 208pb/204Pb ratios with the Pb concentrations, and thisplot reveals a good correlation of the SDs and RSDs with thePb concentration. A high Pb concentration provides goodaccuracy and high precision. The RSDs are lower than 0.1%for the, 208pb/204Pb ratios when the Pb concentration ishigher than 2 ug g. Figures 11 and 12 present the SEs and RSEs of the208pb/204Pb,207pb/204Pb,206pb/204pb,208pb/206Pb and207Pb/206pb ratios versus the total Pb ion beams; the SEsand RSEs of these ratios decrease with Pb intensity (total Pb Table 4. Pb isotope ratios for the NIST SRM 612, 614, USGS GSD-1G, BCR-2G, BHVO-2G and BIR-1G referencematerials measured using the fLA-MC-ICP-MS technique Table 4 (continued). Pb isotope ratios for the NIST SRM 612, 614, USGS GSD-1G, BCR-2G, BHVO-2G and BIR-1G referencematerials measured using the fLA-MC-ICP-MS technique 208Pb/204Pb 207Pb/204Pb 206Pb/204Pb 208Pb/206Pb 207Pb/206pb Correctionmethod References Pb) 15.617±17 BIR-1G (~ 3.7 ug g 38.355±40 18.800±19 2.0396±2 0.83047 ± 11 Method 5 This study 38.487±2 15.665±3 18.867±13 ref Jochum and Stoll (2008) 38.488±7 15.666±2 18.876±5 SN Elburg et al. (2005) GSD-1G (~ 50 ug gPb) 38.889±7 15.744±3 19.564±5 1.9872±1 0.80448±7 Method 5 This study 38.908±9 15.745±2 19.579±4 1.9871±5 0.80418±10 ref Jochum et al. (2011) All uncertainties are expressed as 2s. Figure 8. Plot of reference values and obtained values for the 207Pb/206Pb ratios in the NIST, USGS and MPI-DINGreference glasses. The plot exhibits a good linear fit (R²= 0.9999) along the theoretical 1:1 line within 2suncertainties (1.000±0.002, 2s), and the accuracy of the obtained values for the 208pb/204Pb ratios is betterthan ± 0.1%. The error bars are ±2s. The Pb isotopic reference values for the NIST (SRM 610, 612 and 614)reference materials were obtained from Jochum and Stoll (2008), and the other reference values were obtainedfrom the GeoReM database (http://georem.mpch-mainz.gwdg.de/) (Jochum et al. 2005c). ion beams), and they have good exponential curve fits. TheRSEs of the 208Pb/204Pb, 207Pb/204Pb and 206pb/204Pbratios have similar fits, and the RSEs of the 208Pb/206Pb and207Pb/206Pb ratios also have the same fits but different fromthat of the 20xpb/204pb (20x=206,207,208). The signalsensitivity plays a decisive role in precision during the Pbisotope determination. When the total Pb ion beams had anintensity greater than 0.4V, the RSEs of the 208pb/204pb,207Pb/204Pb and 206pb/204Pb ratios were lower than 0.1%,and the RSEs of the 208pb/206pb and 207Pb/206Pb ratioswere lower than 0.01%. Results from the analysis of some USGS andCGSG glass reference materials By using a femtosecond laser ablation MC-ICP-MSmethod with a modified data processing (205Tl/203Tl ratiooptimisation) procedure, high-precision Pb isotope ratiomeasurements in USGS (GSE-1G, GSC-1G and GSA-1G)and CGSG (CGSG-1, CGSG-2, CGSG-4 and CGSG-5)reference glasses were achieved. Table 5 presents the Pbisotope ratios measured using fLA-MC-ICP-MS. The preci-sion of the207Pb/206Pb andI 208pb/206Pb ratios was Figure 9. Reference values and obtained values for the 208Pb/204Pb ratios in the NIST, USGS and MPI-DINGreference glasses. The plot exhibits a good linear fit (R²= 0.9989) along the theoretical 1:1 line within 2suncertainties (1.000 ±0.002), and the accuracy of the obtained values for the 208Pb/204Pb ratios was betterthan ±0.5%. The error bars are ± 2s. The Pb isotopic reference values of the NIST (610, 612 and 614) referencematerials were obtained from Jochum and Stoll (2008), and the other reference values were obtained from the GeoReM database (http://georem.mpch-mainz.gwdg.de/)(Jochum et al. 2005c). Figure 10. A log-log scale plot showing good correlations between the standard deviation (SD) and RSD of208Pb/204Pb ratios with Pb concentration. The reference values of the Pb concentrations (ug g) in the referenceglasses were obtained from the GeoReM database (http://georem.mpch-mainz.gwdg.de/) (Jochum et al.2005c). Conclusions better than 200 ppm and 300 ppm, respectively, eventhough the Pb concentration was lower than 2 ug g,with the exception of GSC-1G, where the precision (RSD)was lower than 400 ppm for 208pb/206pb when the Pbconcentration was 14 ug g. Potential heterogeneities inthe Pb isotopic compositions for the majority of the glassRMs are small - in the 200 um range - based on thefemtosecond laser ablation MC-ICP-MS data. In this paper, it has been demonstrated that the effects oflaser pulse energy and fluence and laser frequency on themass fractionation can be neglected when using a femto-second laser. The mass fractionation coefficient induced bythe 266 nm femtosecond laser ablation is 28% reductionthan that of by the 193 nm excimer laser ablation with Intensity (total Pb ion beam) (V Figure 11. SEs and RSEs for the 208Pb/204Pb,207Pb/204P band 206Pb/204Pb ratios versus the total Pb ion beams. The SEs of the 208Pb/204Pb, 207Pb/204Pb and 206Pb/204Pb ratios decrease with intensity (total Pb ion beam). Figure 12. SEs and RSEs of the 208Pb/206Pb and 207Pb/206Pb ratios versus the intensity (v) of the total Pb ionbeams. The SEs of the 208pb/206Pb and 207Pb/206Pb ratios decrease with intensity (total Pb ion beam). laser and the highly efficient transfer of these particles by thehelium gas. helium carrier gas, and also the use of the 266 nmfemtosecond| laser ablation decreased the mass biascoefficient by approximately 20% at helium atmospherewhen compared with that of use argon carrier gas by thesame instruments. These reductions may be related to animprovement in ion transmission and ionisation as a result ofthe distribution of finer particles ablated by the femtosecond No significant differences were observed between theTl normalisation power law and the exponential lawcorrection methods when the two mass fractionation lawswere applied, and the two correction methods provided Table 5. Pb isotope ratios of GSA-1G, GSC-1G, GSE-1G and CGSG glass reference materials measured using thefLA-MC-ICP-MS technique good precision and accuracy during the analysis of Pbisotopic ratios. The data obtained using the Tl normalisa-tion exponential law with adjustment of the 205Tl/203TIratio were consistent with the expected values within 2suncertainties. The Pb isotopic ratios of NIST SRM 610, 612 and614; USGS BHVO-2G, BCR-2G, GSD-1G and BIR-1G;and MPI-DING glass reference materials GOR132-G,KL2-G, T1-G,StHs60/80-G, ATHO-G and ML3B-G weredetermined usinafLA-MC-ICP-MS.). Theemeasured:Pbisotopic data are in good agreement with the publishedvalues within 2s uncertainties. We also present the firsthigh-precision Pb isotopic data for USGS GSE-1G, GSC- 1G and GSA-1G, and CGSG CGSG-1, CGSG-2, CGSG-4 and CGSG-5 glass reference materials obtained usingthe fLA-MC-ICP-MS technique. The heterogeneity in theglasses was found to be generally lower than thereproducibility obtained from femtosecond laser ablationMC-ICP-MS. Femtosecond laser ablation MC-ICP-MS provides anexcellent tool to study Pb isotope composition in situindependent of the matrix while maintaining high precisionand accuracy, and this capability opens a wide spectrum ofapplication allowing the investigation of Pb isotope varia-tions in complex mineral phases in order to constraingeological processes in various environments. Acknowledgements This work was co-supported by the National NaturalScience Foundation of China (Grants 40973011), the StateKey Laboratory of independent research key topics of specialfunds from Ministry of Science and Technology (CJ11070)and the MOST Research Foundation from the State KeyLaboratory of Continental Dynamics. We thank K.P. Jochumand Zhan Xiuchun for providing the reference glasses. Reterences Albarede F. and Beard B. (2004) Analytical methods for non-traditional isotopes. In: Rosso J.J.(ed.), Reviews in mineralogy and geochemistry:Geo-chemistry of non-traditional stable isotopes. MineralogicalSociety of America (Washington DC), 133-152. Albarede F., Telouk P., Blichert-Toft J., Boyet M., AgranierA. and Nelson B. (2004) Precise and accurate isotopic measurements using multi-ple-collector ICP-MS. Geochimica et Cosmochimica Acta,68,2725-2744. Baker J., Peate D., Waight T. and Meyzen C. (2004) ( P b isotopic analysis of standards an d samples using a 2 07Pb- 2 04Pb d o uble spike a n d thallium to correct for mass bias with a double-focusing MC-ICP-MS. Chemical Geol- ogy, 211,275-303. ) ( Belshaw N . S., F reedman P.A., O'Nions R.K., Frank M. and Guo Y. (1998) ) ( A new variable dispersion double-focusin g plasma mass s pectrometer with performance illustrated for Pb i sot o pes. International Journal of Mass Spectrometry, 181,51- 5 8. ) ( E lburg M ., V roon P . , v an der Wagt B. and Tchalikian A. (2005) ) ( Sr and Pb isotopic composition of five USGS glasses (BHVO-2G, BIR-1G, BCR-2G, T B-1G,NKT-1G). Chemical Geology, 223, 196- 2 07. ) ( Fernandez B . , Claverie F ., Pecheyran C. and Donard O.F.X. (2007) D irect analysis of solid samples by fs-LA-ICP-MS. TrAC T rends in Analytical Chemistry, 26, 951- 96 6. ) ( F ontaine G.H.,Hattendorf B., Bourdon B. and Gunther D. (2009) ) ( E ffects of operating c onditions and m a trix on m a ss bias inMC-ICP-MS. Journal o f Analytical Atomic Spectrometry, 24,637- 6 48. ) ( Galer S.J.G. and Abouchami W. (1998) P ractical application of lead t r iple s piking for c orrection of i nstrumental m ass discrimination. Mineralogical M aga- zine, 62A, 491 -4 92. ) Garcia C.C., Lindner H. and Niemax K. (2009)Laser ablation inductively coupled plasma-mass spec-trometry: Current shortcomings, practical suggestions forimproving performance, and experiments to guide futuredevelopment. Journal of Analytical Atomic Spectrometry,24,14-26. Hirata T.(1996) Lead isotopic analyses of NIST standard reference mate-rials using multiple collector inductively coupled plasma-mass spectrometry coupled with a modified externalcorrection method for mass discrimination effect. Analyst,121,1407-1411. Horn l., von Blanckenburg F., Schoenberg R., Steinhoefel G. and Markl G. (2006) ( I n situ iron isotope r atio d e termination us i ng UV-femtosec- o nd l a ser ablation with a pplication t o h ydrothermal o r e f ormation processes. Geochimica et Cosmochimica Acta, 70, 3677- 3 688. ) ( Hu M . -Y., F a n X.-T., S t oll B., Kuzmin D., Liu Y., Liu Y., Sun W., Wang G., Zhan X.-C. and Jochum K . P. (2011) Preliminary characterisation of new reterence materials for m icroanalysis: Chinese geological s t andard gl a sses CGSG-1, CGSG-2, CGSG-4 and CGSG-5. Geostan-dards and Geoanalytical Research, 35, 235 - 251. ) ( Jochum K.P. and Stoll B. (2008) R eterence materials for e l emental and isotopic analyses by L A-(MC)-ICP-MS: Successes and outstanding needs. In: Sylvester P. (ed.) , Laser ablation ICP-MS in the Earth sciences: C urrent practices and outstanding issues. Mi n eralogical Association of Canada (Vancouver, B.C.), 147- 16 8. ) ( Jochum K . P., Pfander J., Woodhead J . D., Willbold M., Stoll B., Herwig K., Amini M. , Abouchami W. and Hofmann A.W.(2005a) M PI-DING glasses:New geological refe r ence materials for i n situ Pb isotope analysis . Geochemistry Geophysics Geosystems,6, Q10008. ) ( Jochum K.P., Willbold M . , R a czek l., Stoll B. and H erwigK. (2005b) Chemical c h aracterisation of the USGS reference glasses GSA-1G,GSC-1G, GSD-1G, GSE-1G, BCR-2G, B HVO- 2G and BIR-1G u s ing EPMA, ID-TIMS, ID-ICP-MS and LA- ICP-MS. Geostandards and Geoanalytical Research, 29, 285-302. ) ( Jochum K.P., Nohl U., Herwig K., Lammel E., Stoll B. and Hofmann A.W. (2005c) GeoReM: A new geochemical database for reference materials and isotopic standards. Geostandards and Geoanalytical Research, 29, 333- 3 38. ) ( Jochum K . P., Wilson S.A., Abouchami W., Amini M., Chmeleff J., Eisenhauer A., Hegner E., laccheri L .M.,Kieffer B. , Krause J., McDonough W.F., Mertz-Kraus R., R aczek l., Rudnick R.L., Scholz D., S t einhoefel G. , Stoll B., Stracke A., Tonarini S. , Weis D., Weis U. and Woodhead J.D.(2011) ) GSD-1G and MPI-DING reference glasses for in situ and bulk isotopic determination. Geostandards and Geoana- lytical Research, 35, 193-226. Kent A.J.R. (2008) ( I n-situ analysis of P b isotope r atios using laser ablation MC- ICP-MS: Controls on precision an d accuracy and com- parison between Faraday cup and io n counting systems. Journal of Analytica l Atomic Spectrometry, 23, 968-975. ) Koch J. and Gunther D. (2011) Review of the state-of-the-art of laser ablation inductivelycoupled plasma-mass spectrometry. Applied Spectroscopy,65,155A-162A. Koch J., von Bohlen A., Hergenroder R. and Niemax K.(2004) Particle size distributions and compositions of aerosolsproduced by near-lR femto- and nanosecond laser abla-tion of brass. Journal of Analytical Atomic Spectrometry,19,267-272. Koch J., Schlamp S., Rosgen T., Fliegel D. and Gunther D.(2007) Visualization of aerosol particles generated by nearinfrared nano- and femtosecond laser ablation. Spectro-chimica Acta Part B, 62, 20-29. KochJ., Walle M., Schlamp S., Rosgen T. and Gunther D.(2008) Expansion phenomena of aerosols generated by laserablation under helium and argon atmosphere. Spectro-chimica Acta Part B, 63, 37-41. Longerich H.P., Fryer B.J. and Strong D.F. (1987)Determination of lead isotope ratios by inductively coupledplasma-mass spectrometry (ICP-MS). Spectrochimica ActaPart B, 42, 39-48. Mank A.J.G. and Mason P.R.D.(1999) A critical assessment of laser ablation ICP-MS as ananalytical tool for depth analysis in silica-based glasssamples. Journal of Analytical Atomic Spectrometry, 14,1143-1153. Ortega G.S., Pecheyran C., Berail S. and Donard O.F.X.(2012) A fit-for purpose procedure for lead isotopic ratio deter-mination in crude oil, asphaltene and kerogen samples byMC-ICP-MS. Journal of Analytical Atomic Spectrometry,27, 1447-1456. Paul B., Woodhead J.D. and Hergt J. (2005)Improved in situ isotope analysis of low-Pb materials usingLA-MC-ICP-MS with parallel ion counter and Faradaydetection. Journal of Analytical Atomic Spectrometry, 20,1350-1357. Rehkamper M. and Halliday A.N.(1998) ( Accuracy and long-term re p roducibility of lead i s otopic m easurements by mu l tiple-collector inductively coupled plasma-mass s p ectrometry using an external m ethod f o r correction of mass discrimination. International Journal o f Mass Spectrometry, 181, 123-133. ) Rehkamper M. and Mezger K. (2000) Investigation of matrix effects for Pb isotope ratio mea-surements by multiple collector ICP-MS: Verification andapplication of optimized analytical protocols. Journal ofAnalytical Atomic Spectrometry, 15, 1451-1460. Russo R.E., Mao X., Gonzalez J.J. and Mao S.S. (2002)Femtosecond laser ablation ICP-MS. Journal of AnalyticalAtomic Spectrometry, 17,1072-1075. Shaheen M. and Fryer B.J. (2010)Improving the analytical capabilities of femtosecond laser ablation multicollector ICP-MS for high precision Pbisotopic analysis: The role of hydrogen and nitrogen.Journal of Analytical Atomic Spectrometry, 25,1006-1913 Shaheen M., Gagnon J.E., Yang Z. and Fryer B.J.(2008)Evaluation of the analytical performance of femtosecondlaser ablation inductively coupled plasma-mass spectrom-etry at 785 nm with glass reference materials. Journal ofAnalytical Atomic Spectrometry, 23,1610-1621. Steinhoefel G., Horn l. and von Blanckenburg F. (2009). Matrix-independent Fe isotope ratio determination insilicates using UV femtosecond laser ablation. ChemicalGeology, 268, 67-73. Tanner S.D. (1992)Space charge in ICP-MS: Calculation and implications.Spectrochimica Acta Part B, 47, 809-823. Thirlwall M.F.(2000)Inter-laboratory and other errors in Pb isotope analysesinvestigated using a 207pb_204Pb double spike. ChemicalGeology, 163, 299-322. Thirlwall M.F. (2002) Multicollector ICP-MS analysis of Pb isotopes using a20/Pb-204Pb double spike demonstrates up to 400 ppm/amu systematic errors in Tl-normalization. Chemical Geol-ogy, 184,255-279. Todt W., Cliff R., Hanser A. and Hofmann A.W. (1996)Evaluation of a 202pb-205pb double spike for high-precision lead isotope analysis. In: Hart S.R. and Basu A(eds), Earth processes: Reading the isotopic code. AGU(Washington, DC), 429-437. White W.M., Albarede F. and Telouk P. (2000)High-precision analysis of Pb isotope ratios by multi-collector ICP-MS. Chemical Geology, 167, 257-270 Woodhead J. (2002) A simple method for obtaining highly accurate Pb isotope data by MC-ICP-MS. Journal of Analytical Atomic Spec- trometry, 17, 1381-1385. Woodhead J.D. and Hergt J.M. (2001) Strontium, neodymium and lead isotope analyses of NIST glass certified reference materials: SRM 610, 612, 614. Geostandards Newsletter: The Journal of Geostandards and Geoanalysis, 25, 261-266. Xie L, Yang J., Yang Y. and Wu F.(2005)High-precision analysis of Pb isotope ratios using MC-ICP-MS. Acta Geoscientica Sinica, 26 (Supplement), 23. ( Yuan H . -L, Gao S., Dai M.-N., Zong C.-L., Gunther D., Fontaine G.H., Liu X.-M. and Diwu C . (2008) Simultaneous determinations of U-Pb age, Hf isotopes and t race e lement compositions o f z i rcon b y e x cimer laser- a blation quadrupole and mu l tiple-collector I CP-MS. Chemical G eology, 247, 1 00-118. ) doi: j..xC The Authors. Geostandards and Geoanalytical Research C International Association of Geoanalysts 采用266 nm飞秒激光烧蚀(fLA)系统连接多集电极ICP-MS (MC-ICP-MS),通过严格控制分析程序,获得了具有良好精度和准确性的铅同位素比值数据。266nm飞秒激光烧蚀诱导的质量分馏率约比193nm准分子激光烧蚀(eLA)诱导的质量分馏率低28%。摘要采用调优Tl比的Tl归一化指数律校正方法,获得了具有较好精度和准确度的Pb同位素数据。NIST SRM 610、612、614玻璃参考材料的Pb同位素比值;USGS bhvog - 2g、BCR-2G、GSD-1G、bir1 g;采用fa - mc - icp - ms法测定MPI-DING GOR132-G、KL2-G、T1-G、StHs60/80-G、ATHO-G、ML3B-G。在2s测量不确定度范围内,测得的铅同位素比值与参考值或公布值吻合较好。利用飞秒激光消融MC-ICP-MS分析获得了GSE- 1G、GSC-1G、GSA-1G、CGSG-1、CGSG-2、CGSG-4、CGSG-5玻璃基准材料的高精度铅同位素资料技术。
确定






还剩15页未读,是否继续阅读?
上海凯来仪器有限公司为您提供《玻璃基准材料中铅同位素比值检测方案(激光剥蚀进样)》,该方案主要用于建筑玻璃中铅同位素比值检测,参考标准--,《玻璃基准材料中铅同位素比值检测方案(激光剥蚀进样)》用到的仪器有ESLfemto 飞秒激光剥蚀系统
推荐专场
相关方案
更多















