方案详情
文
高分辨率的对完全原态下(fully native)的单克隆抗体的电荷变异体的分离,对 mAb 和 ADC (抗体药物结合物)的分析均能提供非常好的准确度和灵敏度
方案详情

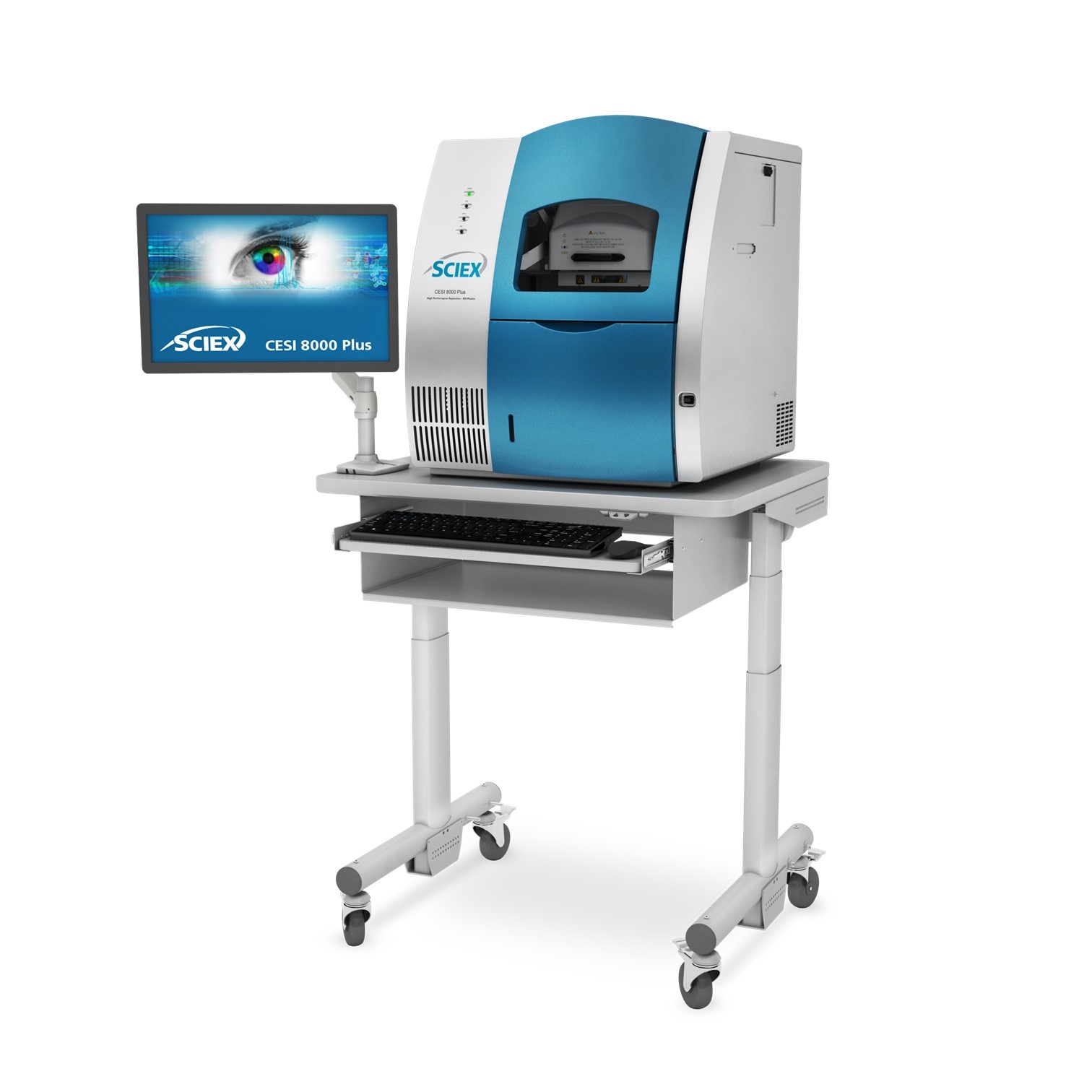
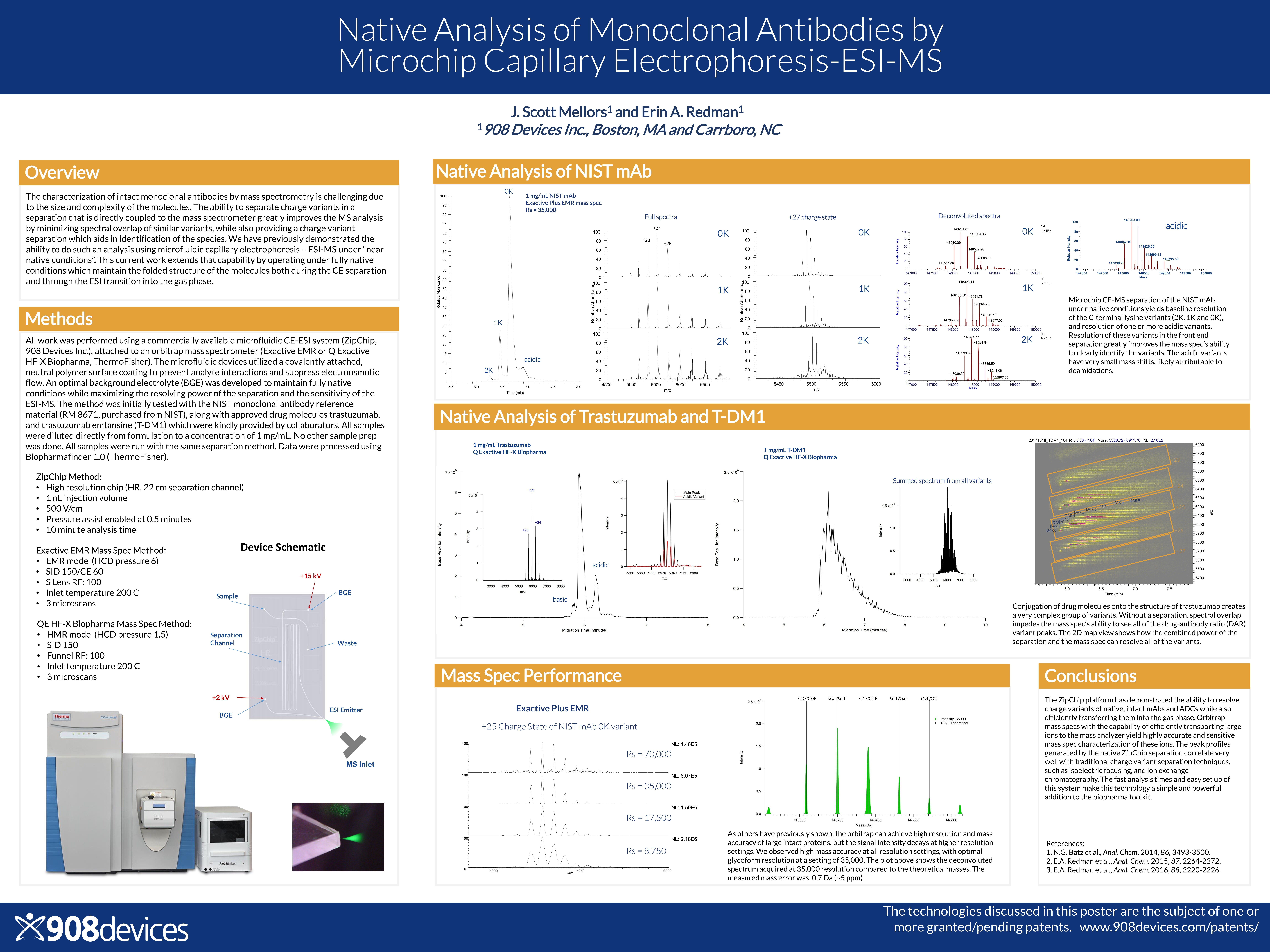
The technologies discussed in this poster are the subject ofone ormore granted/pending patents. www.908devices.com/patents/X908devices Overview The characterization of intact monoclonal antibodies by mass spectrometry is challenging dueto the size and complexity of the molecules. The ability to separate charge variants in aseparation that is directly coupled to the mass spectrometer greatly improves the MS analysisby minimizing spectral overlap of similar variants, while also providing a charge variantseparation which aids in identification of the species. We have previously demonstrated theability to do such an analysis using microfluidic capillary electrophoresis-ESI-MS under"nearnative conditions". This current work extends that capability by operating under fully nativeconditions which maintain the folded structure of the molecules both during the CE separationand through the ESl transition into the gas phase. Methods All work was performed using a commercially available microfluidic CE-ESI system (ZipChip,908 Devices Inc.), attached to an orbitrap mass spectrometer (Exactive EMR or Q ExactiveHF-XBiopharma,ThermoFisher). The microfluidic devices utilized a covalently attached,neutral polymer surface coating to prevent analyte interactions and suppress electroosmoticflow. An optimal background electrolyte (BGE) was developed to maintain fully nativeconditions while maximizing the resolving power of the separation and the sensitivity of theESI-MS. The method was initially tested with the NIST monoclonal antibody referencematerial (RM 8671, purchased from NIST), along with approved drug molecules trastuzumab,and trastuzumab emtansine (T-DM1) which were kindly provided by collaborators. All sampleswere diluted directly from formulation to a concentration of 1 mg/mL. No other sample prepwas done. All samples were run with the same separation method. Data were processed usingBiopharmafinder 1.0 (ThermoFisher). ZipChip Method: · High resolution chip (HR, 22 cm separation channel) ·1nLinjection volume 。 500V/cm · Pressure assist enabled at 0.5 minutes · 10 minute analysis time Exa ct iv e E MR Ma ss Sp ec MethodD evice Schema :tic ·EMR mode (HCD pressure 6)· SID 150/CE60 · S Lens RF: 100 · Inlet temperature 200C · 3 microscans QE HF-XBiopharma Mass Spec Method: · HMR mode (HCD pressure 1.5) · SID 150 · Funnel RF: 100 · Inlettemperature 200C · 3 microscans ESIEmitter Native Analysis of Monoclonal Antibodies byMicrochip Capillary Electrophoresis-ESI-MS J.Scott Mellors1 and Erin A. Redman1 1908 Devices Inc.,Boston,MA and Carrboro, NC Native Analysis of NIST mAb 0K 1mg/mL NIST mAb 1K Microchip CE-MS separation of the NIST mAbunder nativeconditions yields baseline resolutionof the C-terminal lysine variants (2K, 1K and OK),and resolution of one or more acidic variants.Resolution of these variants in the frontendseparation greatly improves the mass spec’s abilityto clearly identify the variants.The acidic variantshave very small mass shifts, likely attributable to 2K 20171018_TDM1_104 RT:5.53-7.84 Mass: 5328.72-6911.70 NL:2.16E5 630 60 75 mmm Conjugation of drug molecules onto the structure of trastuzumab creates a very complex group of variants. Without a separation,spectral overlap impedes the mass spec’s ability to see all of the drug-antibodyratio (DAR) variant peaks. The 2D map view shows how the combined power of the separation and the mass spec can resolve all of the variants. Conclusions 2.53 GOF/GOF 10F/G1iF1F G1F/G1F G1F/G2F G2F/G2F The ZipChip platform has demonstrated the ability to resolve Exactive Plus EMR charge variants of native, intact mAbs and ADCs while also efficiently transferring them into the gas phase.Orbitrap mass specs with the capability of efficiently transporting large ions to the mass analyzer yield highly accurate and sensitive mass spec characterization of these ions. The peak profiles generated by the native ZipChipseparation correlate very well with traditional charge variant separation techniques, such as isoelectric focusing,and ion exchange chromatography. The fast analysis times and easy set up o this system make this technology a simple and powerfuljl 05- addition to the biopharma toolkit. 107 As others have previously shown, the orbitrap can achieve high resolution and massaccuracy of large intact proteins, but the signalintensity decays at higher resolutionsettings. We observed high mass accuracy at all resolution settings, with optimalglycoform resolution at a setting of 35,000. The plot above shows the deconvolutedspectrum acquired at 35,000 resolution compared to the theoretical masses. Themeasuredmass error was 0.7 Da(~5 ppm) References: 1.N.G. Batz et al., Anal. Chem.2014,86,3493-3500.2.E.A. Redman et al.,Anal. Chem.2015,87,2264-2272.3.E.A. Redman et al., Anal. Chem.2016,88,2220-2226. 质谱用于鉴定完整的单克隆抗体由于样品分子的大小和复杂性而具有很大的挑战性。在质谱仪前端增加可以分离电荷变体的前端分离装置可以大大改善了质谱分析,减少了电荷变异体在质谱图中的重叠,有助于样品或药物分子的鉴定。 先前的解决方案中证明了在近似原态的条件下(near native)使用微流控毛细管电泳-电喷雾质谱进行这种分析的能力。本方案中进一步拓展了这种能力,在完全原态(fully native)的条件下工作,在电泳过程以及电喷雾过程中均可以保持样品分子的折叠结构,有助于用户掌握跟多的样品分子的结构信息。 本方案展示了ZipChip解决native、完整mAb和ADC的电荷变异体的能力,同时可以有效地将它们转移到气相中实现后续的质谱仪中,得到高准确度和高灵敏度的质谱分析。 此外,原态的分析环境与等电聚焦、离子交换色谱等传统电荷变异分离技术有很好的相关性。ZipChip的快速、高效的分析和简单易用的特性,使这项技术成为一个简单和强大的生物制药领域的分析工具包。
确定
还剩1页未读,是否继续阅读?
908 Devices为您提供《NIST 单克隆抗体标准物, RM 8671中电荷变异体检测方案(自动进样器)》,该方案主要用于治疗类生物药品中微生物相关及生化特性检测,参考标准--,《NIST 单克隆抗体标准物, RM 8671中电荷变异体检测方案(自动进样器)》用到的仪器有908Devices ZipChip 自动进样器、908Devices ZipChip AS 毛细管电泳质谱联用系统 (自动进样款)、ZipChip 完整抗体分析试剂盒
推荐专场
相关方案
更多