方案详情
文
使用纳米颗粒(NPs)制备薄膜镀层材料日益受到了人们的重视,并且被广泛应用到如显示器、传感器、医疗器械、储能和能量收集材料等各种现代产品和研究领域。纳米粒子的合成方法已经广为人知,但为了能够在上述应用中使用它们,需要将纳米颗粒从溶液相转移到基材表面。为此,需要一种可控的沉积方法。
附件白皮书回顾了在气-液界面处形成纳米颗粒单层膜的方法以及使用Langmuir-Blodgett和Langmuir-Schaefer方法制备薄膜后,将其转移到固体基底上的方法。如果您对如何沉积单层纳米球感兴趣,请下载附件的白皮书。
方案详情

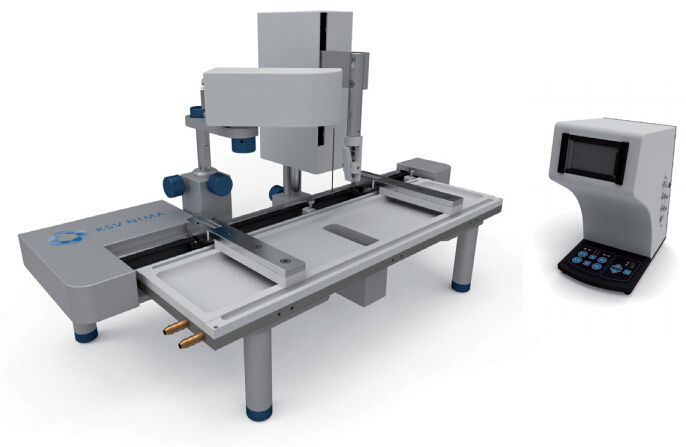
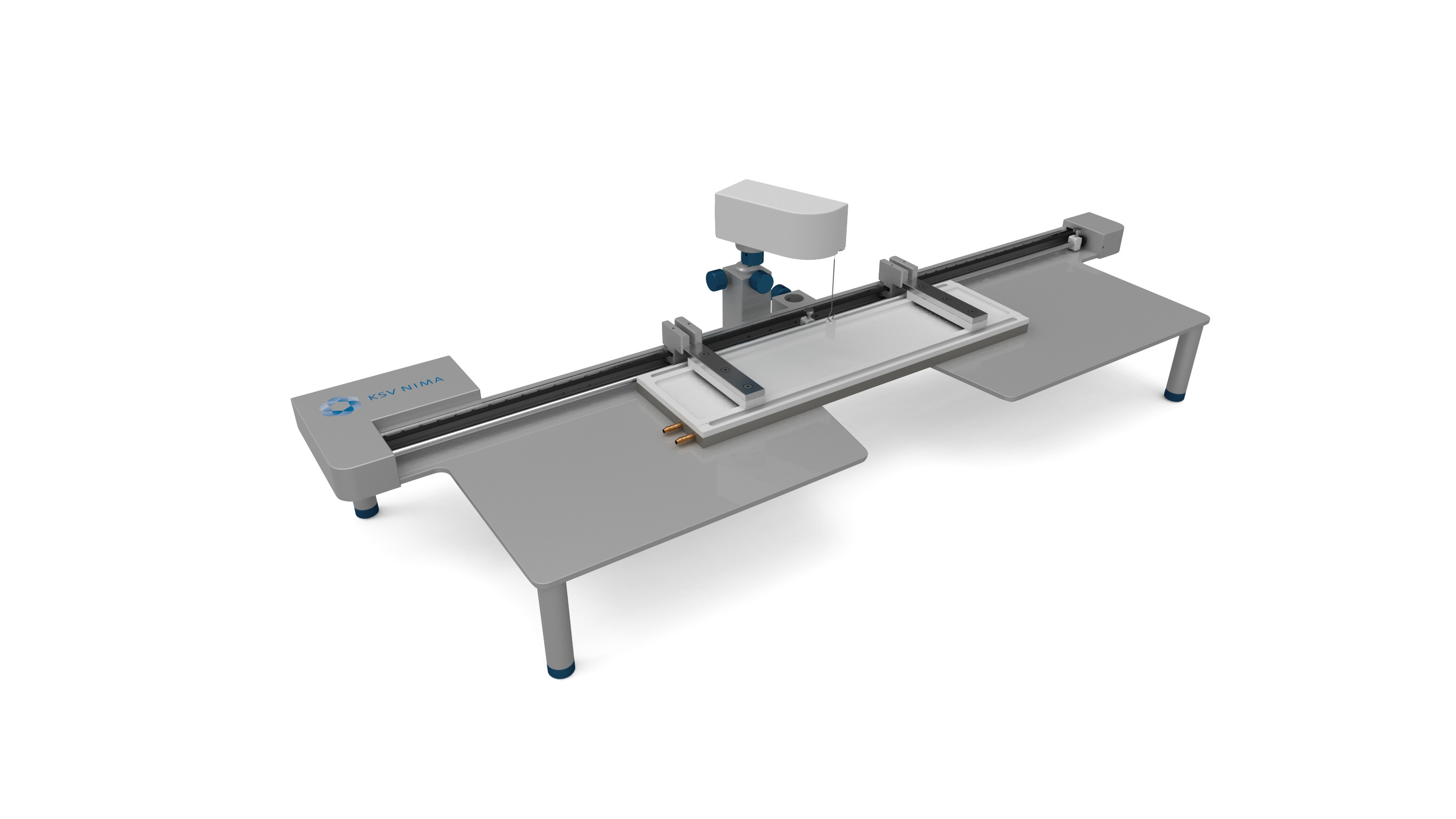
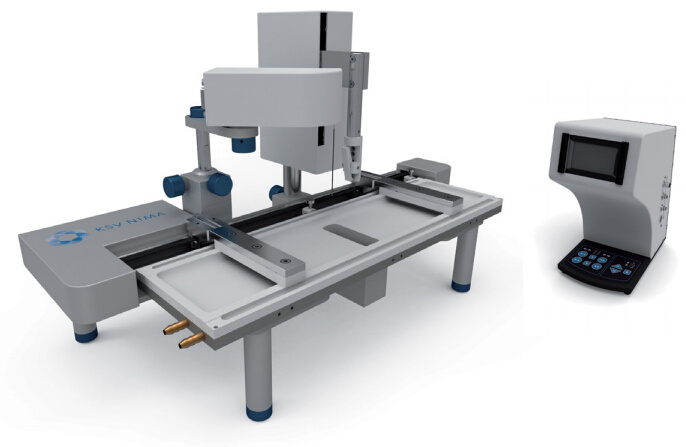
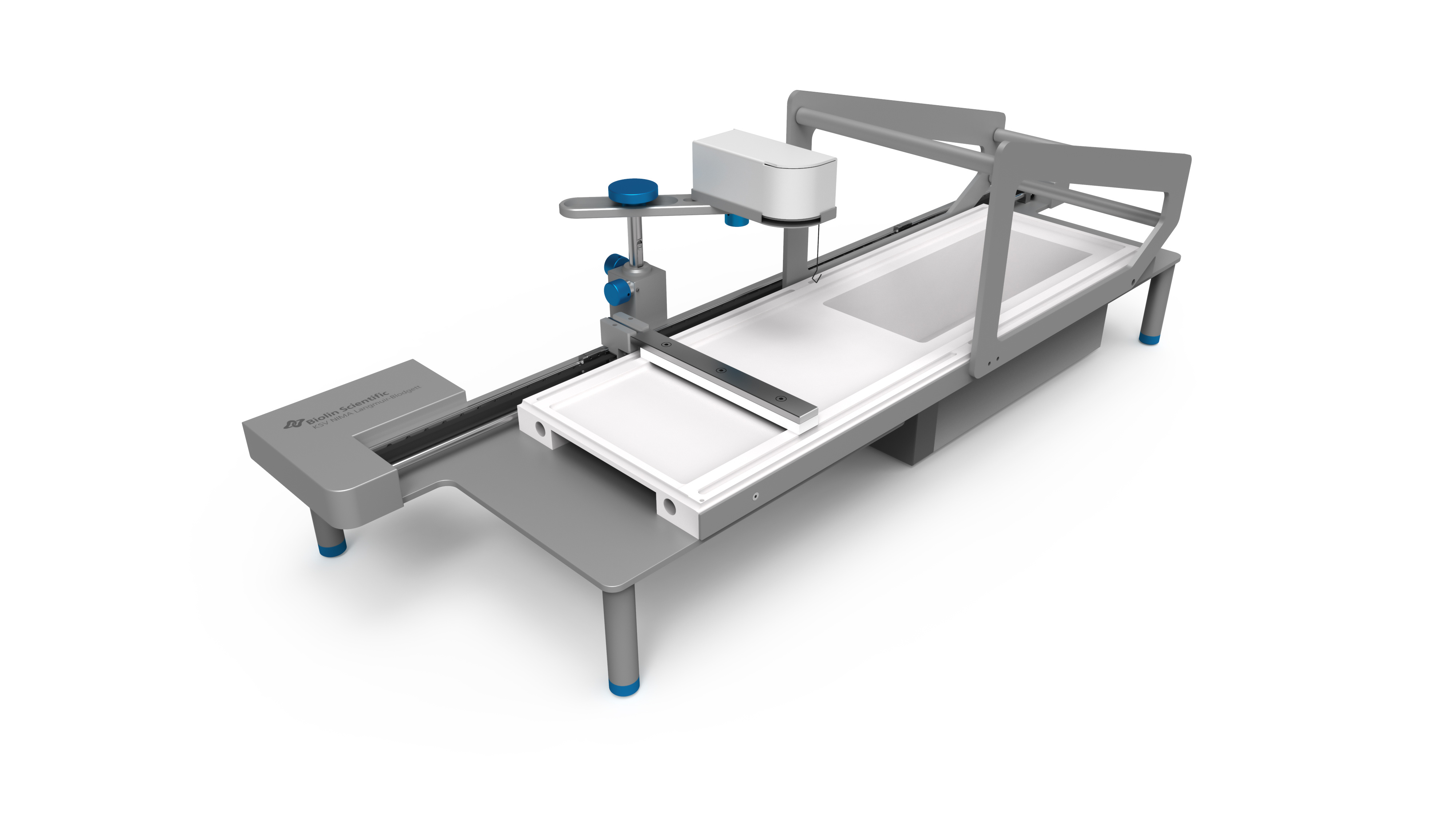
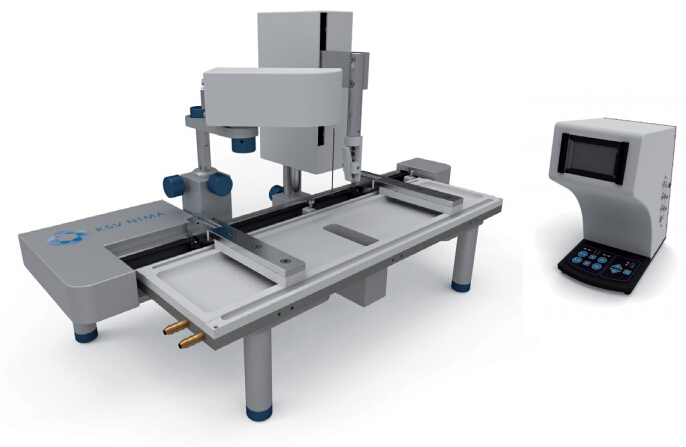
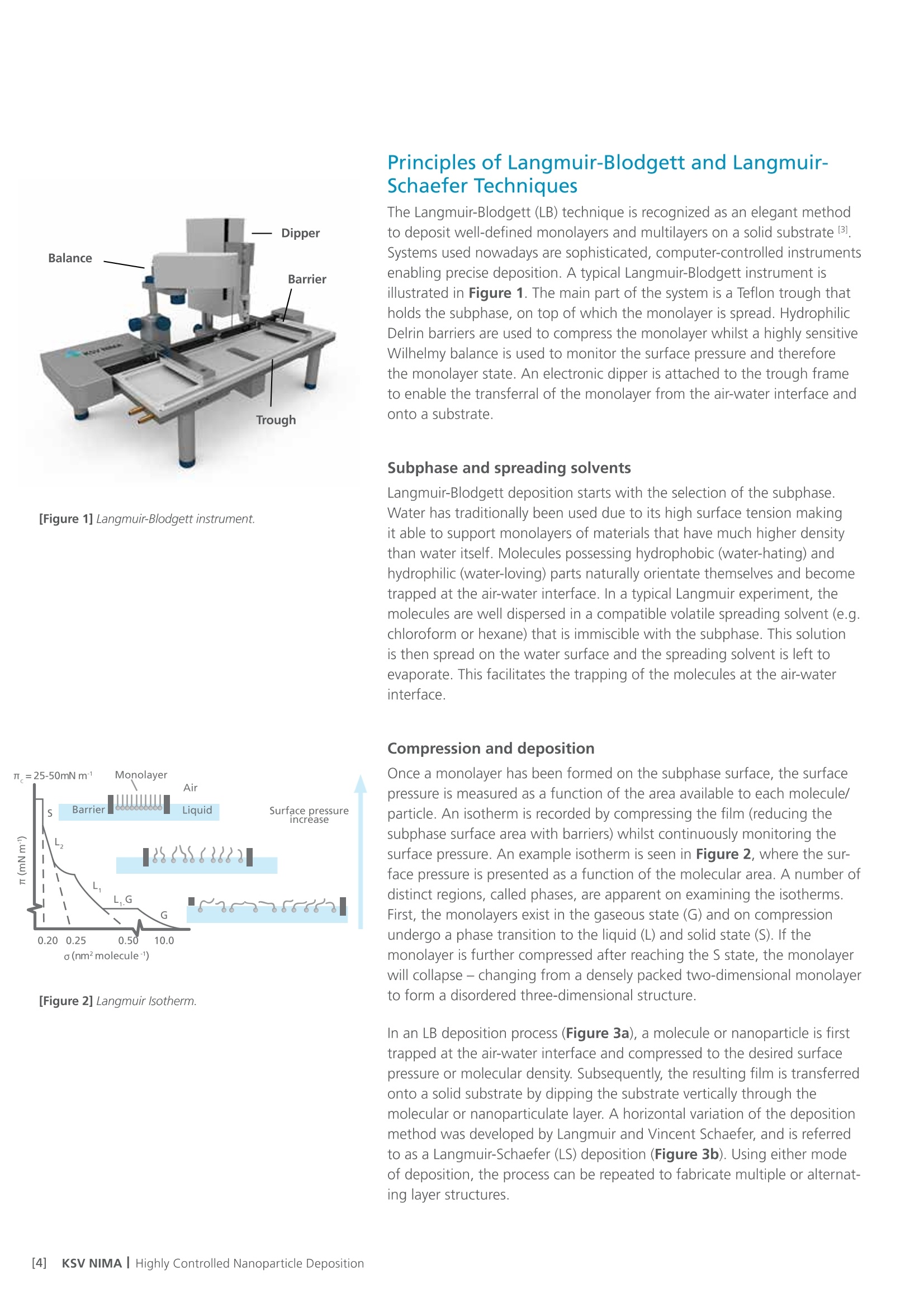
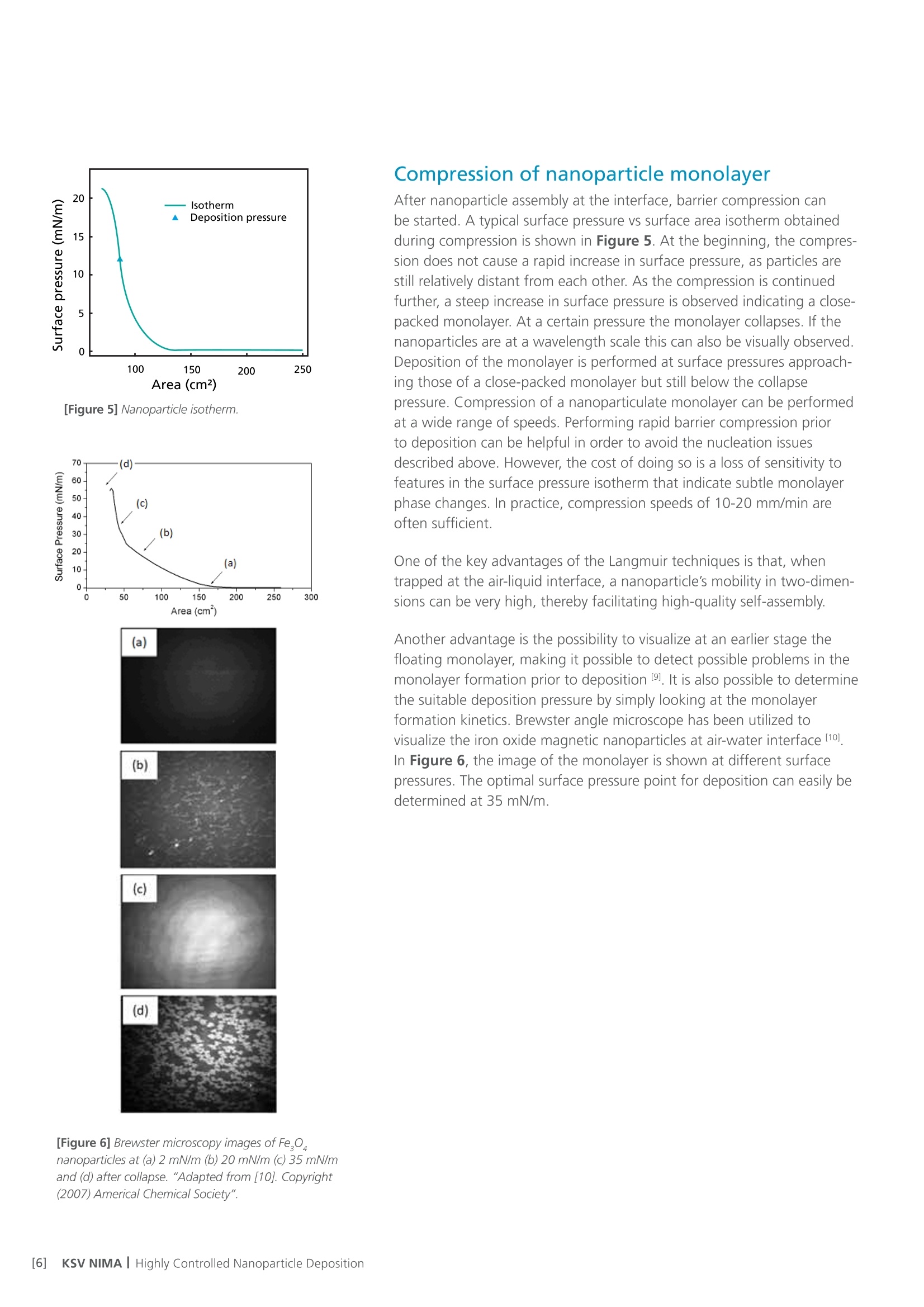
White Paper KSV NIMAAWhite Paper A Highly Controlled Nanoparticle Depositionusing the Langmuir-Blodgett Method Authors: Alaric Taylor, PhD, EPSRC Prize Fellow, University College LondonSusanna Lauren, PhD, Biolin Scientific [Progress Together] Highly Controlled Nanoparticle Depositionusing the Langmuir-Blodgett Method Coatings and thin films made from nanoparticles (NPs) aregaining recognition and use in various products and appli-cations including displays, sensors, medical devices, energystorage devices and energy harvesting. Synthesis methodsfor nanoparticles Ill are already relatively well known, but tobe able to utilize them in the applications mentioned above,NPs need to be transferred from the solution phase to asubstrate's surface -often as strict monolayer or definedthickness multilayer. For this, controlled deposition methodsare required. This white paper reviews the methods forforming nanoparticle monolayers at the air-liquid interfaceand their subsequent transfer onto solid substrates usingthe Langmuir-Blodgett and Langmuir-Schaefer methods.These methods can form high-density (poly-crystalline)monolayers on a wide variety of solid substrates, includingconventionally challenging non-flat surfaces. Methods for Nanoparticle Deposition Common methods to fabricate nanoparticle thin films include Lang-muir-Blodgett (LB) and Langmuir-Schaefer (LS) depositions 12], self-assemblyduring solvent evaporation, dip coating and spin coating. These methodsare summarized in Table 1. As this white paper discusses only depositionof nanoparticles from suspension, the chemical vapor deposition methodscommonly utilized with e.g. nanotubes and -wires, are not included in thecomparison. Most technologies that employ self-assembled nanoparticle monolayersrequire dense packing arrangements and, in many cases, that the particlesare organized in regular arrays. Therefore, it is vital for a successful fab-rication method to demonstrate the precise control over the particulateassembly kinetics. The simplest method for nanoparticle deposition is the solvent evaporationtechnique. In this method the colloidal nanoparticle solution is first spreadon the substrate. The solvent is then evaporated and the nanoparticlesare thus stabilized on the substrate and held in place by a Van der Waalsattraction. Marangoni flow during the evaporation of the solvent liquidleads to the so-called coffee ring effect, whereby more nanoparticles aredeposited at the meniscus pinning line, often leading therefore to multilay-ered regions at the droplet edge. Method Control of packing Langmuir-Blodgett/Lang-muir-Schaefer High Solvent evaporation LOW Dip coating LOW Spin coating Intermediate Doctor blade LOW Another relatively simple method is known as the Doctor blade method.With this technique, a nanoparticle solution is dispensed on the surfaceand a blade is used to spread the solution. The speed at which the bladetracks across the surface defines a drying front and influences the way inwhich the nanoparticles convectively assemble. This technique is also proneto multilayered assembly and non-flat substrate topologies can pose aproblem. [Table 1] Common methods to fabricate nanoparticle thinfilms and their contro/ of packing. The spin and dip coating methods are easy to perform and can avoid theoccurrence of multilayer formation. However, strict environmental controlis necessary and high density monolayers can prove elusive. By nature, thevolume of nanoparticle suspension required for both spin coating and dipcoating can render the technique unattractive when using rare or expensivematerials, as a large portion of the suspension goes to waste. The LB and LS deposition methods offer the possibility for excellent controlover packing density; readily achieving close-packed monolayers whosecrystallinity can extend over millimeters and centimeters. Lower volumes ofcolloidal solution are required and deposits can be made on conventionallychallenging substrates (curved or hierarchical). As a summary, with LB and LS methods, it is possible to: · Precisely control the monolayer (thin film) thickness and packing density Achieve homogenous deposition over large areas Intentionally form multilayer assemblies with specific layer compositions Deposit to any kind of rigid, or even semi-rigid, substrate Efficiently use the available nanoparticle suspension The key principles of LB and LS deposition methods are discussed in moredetail in the next section. [Figure 1] Langmuir-Blodgett instrument. [Figure 2] Langmuir lsotherm. Principles of Langmuir-Blodgett and Langmuir-Schaefer Techniques The Langmuir-Blodgett (LB) technique is recognized as an elegant methodto deposit well-defined monolayers and multilayers on a solid substrate 3]Systems used nowadays are sophisticated, computer-controlled instrumentsenabling precise deposition. A typical Langmuir-Blodgett instrument isillustrated in Figure 1. The main part of the system is a Teflon trough thatholds the subphase, on top of which the monolayer is spread. HydrophilicDelrin barriers are used to compress the monolayer whilst a highly sensitiveWilhelmy balance is used to monitor the surface pressure and thereforethe monolayer state. An electronic dipper is attached to the trough frameto enable the transferral of the monolayer from the air-water interface andonto a substrate. Subphase and spreading solvents Langmuir-Blodgett deposition starts with the selection of the subphase.Water has traditionally been used due to its high surface tension makingit able to support monolayers of materials that have much higher densitythan water itself. Molecules possessing hydrophobic (water-hating) andhydrophilic (water-loving) parts naturally orientate themselves and becometrapped at the air-water interface. In a typical Langmuir experiment, themolecules are well dispersed in a compatible volatile spreading solvent (e.g.chloroform or hexane) that is immiscible with the subphase. This solutionis then spread on the water surface and the spreading solvent is left toevaporate. This facilitates the trapping of the molecules at the air-waterinterface. Compression and deposition Once a monolayer has been formed on the subphase surface, the surfacepressure is measured as a function of the area available to each molecule/particle. An isotherm is recorded by compressing the film (reducing thesubphase surface area with barriers) whilst continuously monitoring thesurface pressure. An example isotherm is seen in Figure 2, where the sur-face pressure is presented as a function of the molecular area. A number ofdistinct regions, called phases, are apparent on examining the isotherms.First, the monolayers exist in the gaseous state (G) and on compressionundergo a phase transition to the liquid (L) and solid state (S). If themonolayer is further compressed after reaching the S state, the monolayerwill collapse- changing from a densely packed two-dimensional monolayerto form a disordered three-dimensional structure. In an LB deposition process (Figure 3a), a molecule or nanoparticle is firsttrapped at the air-water interface and compressed to the desired surfacepressure or molecular density. Subsequently, the resulting film is transferredonto a solid substrate by dipping the substrate vertically through themolecular or nanoparticulate layer. A horizontal variation of the depositionmethod was developed by Langmuir and Vincent Schaefer, and is referredto as a Langmuir-Schaefer (LS) deposition (Figure 3b). Using either modeof deposition, the process can be repeated to fabricate multiple or alternat-ing layer structures. Formation of Langmuir Monolayer ofNanoparticles In addition to being an excellent method for amphiphilic moleculeassembly, the value of the LB technique for nanoparticle deposition hasbecome apparent. The challenge of trapping non-amphiphilic particles, orthose that are soluble in aqueous solution, at the air-water interface canbe overcome by careful attention to the spreading method. Spreading solvent plays a major role in nanoparticle deposition. Thetraditional method applied to form nanoparticle monolayers at theair-water interface has been droplet casting with an subphase-immisciblespreading solvent. However, some of the most commonly used spreadingimmiscible solvents such as chloroform or hexane can dissolve polymericnanoparticles or their ligand shells and must therefore be avoided. Insituations where a nanoparticle-compatible and a subphase-immisciblesolvent is not available, low surface tension subphase-miscible solventscan be used, such as ethanol or methanol. When subphase-miscible spreading solvents are used, extra care must betaken whilst adding the nanoparticle solution to the subphase surface inorder to avoid sedimentation of the nanoparticles into the subphase bulkOne such approach with miscible spreading solvents is to use a flumeaddition method (as shown in Figure 4) [4.5. The use of a hydrophilicflume for subphase-miscible spreading solvents promotes the formationof a surface monolayer over sedimentation into the bulk, as verticalmomentum of the colloidal solution is minimized and the three-phasecontact line maximized. When a low surface tension nanoparticle solutionmeets a high surface tension subphase, Gibbs-Marangoni mass transferat the interface leads to the formation of a two-dimensional monolayer.Over a short period of time the miscible components of the nanoparticlesolution diffuse into the bulk subphase leaving behind the immisciblenanoparticle components trapped at the interface. One drawback of the flume method is a loss of monolayer coverage, asthe flume is withdrawn prior to compression. Nonetheless, by using thismethod with water-immiscible spreading solvents, the monolayer yieldcan remain high and well-ordered nanoparticle layers are formed. Varia-tions of the methods described above include the use of syringe pumpsor electrospraying to introduce an ethanolic nanoparticle solution to thesubphase interface [6,7]. Subphase composition also plays a role. As withtraditional Langmuir experiments, water is the most popular subphaseliquid. In some cases, however, the aqueous subphase can be an issue, asnanoparticles tend to agglomerate and later sink due to their compara-tively high density. To reduce agglomeration, other polar subphases suchas ethylene glycol and diethylene glycol have proven to be successful [8,9] [Figure 3a] Langmuir-Blodgett deposition. [Figure 3b] Langmuir-Schaefer deposition. [Figure 4] Floating nanoparticle monolayer formationwith the help of a glass slide. Area (cm2) [Figure 5] Nanoparticle isotherm. (a) [Figure 6] Brewster microscopy images ofFe,0,nanoparticles at (a) 2 mN/m (b) 20 mN/m (c) 35 mN/mand (d) after collapse. “Adapted from [10]. Copyright(2007) Americal Chemical Society" Compression of nanoparticle monolayer After nanoparticle assembly at the interface, barrier compression canbe started. A typical surface pressure vs surface area isotherm obtainedduring compression is shown in Figure 5. At the beginning, the compres-sion does not cause a rapid increase in surface pressure, as particles arestill relatively distant from each other. As the compression is continuedfurther, a steep increase in surface pressure is observed indicating a close-packed monolayer. At a certain pressure the monolayer collapses. If thenanoparticles are at a wavelength scale this can also be visually observed.Deposition of the monolayer is performed at surface pressures approach-ing those of a close-packed monolayer but still below the collapsepressure. Compression of a nanoparticulate monolayer can be performedat a wide range of speeds. Performing rapid barrier compression priorto deposition can be helpful in order to avoid the nucleation issuesdescribed above.However, the cost of doing so is a loss of sensitivity tofeatures in the surface pressure isotherm that indicate subtle monolayerphase changes. In practice, compression speeds of 10-20 mm/min areoften sufficient. One of the key advantages of the Langmuir techniques is that, whentrapped at the air-liquid interface, a nanoparticle's mobility in two-dimen-sions can be very high, thereby facilitating high-quality self-assembly. Another advantage is the possibility to visualize at an earlier stage thefloating monolayer, making it possible to detect possible problems in themonolayer formation prior to deposition [9]. It is also possible to determinethe suitable deposition pressure by simply looking at the monolayerformation kinetics. Brewster angle microscope has been utilized tovisualize the iron oxide magnetic nanoparticles at air-water interface [10]ln Figure 6, the image of the monolayer is shown at different surfacepressures. The optimal surface pressure point for deposition can easily bedetermined at 35 mN/m. Nanoparticle monolayer deposition When the monolayer is compressed to a desired surface pressure, it canbe transferred onto a solid substrate. Traditional methods are the previ-ously discussed Langmuir-Blodgett and Langmuir-Schaefer depositions.If hydrophilic substrates such as glass or silicon are used, the substrate isimmersed into the dipping well prior to the spreading of the monolayer.For hydrophobic substrates, the dipping can be started with the substrateabove the interface. A dipping speed of 1mm/min is recommended but,if surface pressure measurements remain stable, then higher speeds arepossible. Counterintuitively, higher dipping speeds of up to 20 mm/mincan yield good quality films. The barriers are programmed to move freelyduring deposition to maintain the target surface pressure over the courseof the deposition. This is one of the advantages of the LB technique, as itensures a uniform packing density over the entire deposition area. Figure7 shows nanoparticle arrays deposited with LB technique. ConclusionsThe Langmuir-Blodgett and Langmuir-Schaefer depositionmethods are widely used to fabricate controlled monolayersand multilayers of nanoparticulate materials. Unlike any otherdeposition method, LB and LS methods offer the possibility tocontrol the packing density of the monolayers prior to theirdeposition. For this reason alone, the LB technique has a criticalrole in current advanced material science and nanotechnology.In addition, LB can be utilized with a vast selection of differ-ent types of nanoparticles on practically any solid substrateincluding non-flat ones. Due to its long history, the methodis relatively well established and thousands of high qualityacademic publications have been written on it. However, asthe Langmuir-Blodgett technique is still relatively new in thefield of advanced material science and nanotechnology, there isplenty of room for new innovate research in this field. [Figure 7] AFM image of a monolayer of 200nm-diameterpolystyrene nanospheres deposited on a quartz substrateusing the automated Langmuir-Blodgett technique. Copyright Dr. Alaric Taylor. [References and further reading] [1] Kruis, F.E., Fissan, H. and Peled, A., "Synthesis of nanoparticlesin the gas phase for electronic, optical and magnetic applica-tions - a review", Journal of Aerosol Science 29 (1998) 511. [2] Ariga, K., Yamauchi, Y., Mori, T. and Hill, J.P., "25th Anniver-sary Article: What can be done with the Langmuir-BlodgettMethod? Recent developments and its critical role in materialsscience., Advanced Materials 25 (2013) 6477. [3] Giles, C.H., Forrester, S.D. and Roberts, G.G., "Historical intro-duction", In a Langmuir-Blodgett films edited by G. Roberts,Chapter 1, New York, Springer US, 1990. [4] Vogel, N., de Viguerie, L., Jonas, U., Weiss, C.K. and Landfester,K., "Wafer-scale fabrication of ordered binary colloidal mono-layers with adjustable stoichiometries", Advanced functionalmaterials, 21 (2011)3064. [5] Weekes, S.M., Ogrin, F.Y., Murray, W.A. and Keatley, P.S., "Mac-roscopic arrays of magnetic nanostructures from self-assem-bled nanosphere templates", Langmuir 23 (2007)1057. [6] Nie, H-L., Dou, X., Tang, Z., Jang, H.D. and Huang, J.,"High-Yield spreading of water-miscible solvents on water for Lang-muir-Blodgett assembly", Journal of the American chemicalsociety 137 (2015)10683 [7] Gao, P., He, J., Zhou, S., Yang, X., Li,S., Sheng, J., Wang,D., Yu, T., Ye,j. and Cui, Y. (2015). Large-Area NanosphereSelf-Assembly by a Micro-Propulsive Injection Method for HighThroughput Periodic Surface Nanotexturing. Nano Letters, 15.(2015)4591. [8] Aleksandrovic, V., Greshnykh, D., Randjelovic, I., Fromsdorf, A.,Kornowski, A., Roth,S.V., Klinke, C. and Weller, H., "Prepara-tion and electrical properties of cobolt-platinum nanoparticlemonolayers deposited by the Langmuir-Blodgett technique",ACS Nano 2 (2008) 1123. [9] Pohjalainen, E., Pohjakallio, M.,Johans, C., Kontturi, K., Timo-nen, J. V. I., Ikkala,O., Ras, R. H. A., Viitala, T., Heino, M. T.and Seppala, E. T., "Cobalt nanoparticle Langmuir-Schaeferfilms on ethylene glycol suphase", Langmuir 26 (2010) 13937. [10] Lee, D.K., Kim, Y.H., Kim, C.W., Cha, H.G. and Kang,Y.S.,"Vast magnetic monolayer film with surfactant-stabilizedFe304 nanoparticles using Langmuir-Blodgett technique",Journal of Physical Chemistry B, 111 (2007)9288. About us Biolin Scientific is a leading Nordic instrumentation company with rootsin Sweden, Denmark and Finland. Our customers include companiesworking with pharmaceuticals, energy, chemicals, and advanced materials,as well as academic and governmental research institutes. Our precisioninstruments help discover better drugs faster, develop better solutionsfor energy and materials, and perform research at the frontiers of scienceand technology. Contact us at info@biolinscientific.com Biolin Scientific [] KSV NIMAI Highly Controlled Nanoparticle Deposition 纳米颗粒光俘获层对薄膜太阳能电池的优化 随着能源需求和环境问题的不断增加,太阳能成为可再生能源中最受关注的来源之一。人们对包括晶圆、薄膜和有机光电等多种太阳能电池技术进行了研究,以实现低成本和高效率。基于晶圆技术的硅太阳能电池作为最有效的太阳能电池,占世界太阳能光伏市场的90%。然而,与传统的基于硅片技术的太阳能设备相比,薄膜太阳能电池减少了活性材料的使用,更具成本效益,因此是更好的选择。 薄膜太阳能电池的材料 薄膜太阳能电池可以通过在玻璃、塑料或金属的基板上沉积一个或多个薄层或光电材料薄膜来制造。最常用的光伏材料是非晶硅(a-Si),碲化镉(CdTe)和铜铟镓二硒化物(CIGS)。其中,非晶硅在如钟表和计算器等商业应用中较为常见。非晶硅不像碲化镉那样有毒性,但碲化镉的其他技术在能量转换效率方面比非晶硅更高。 提高薄膜太阳能电池的效率 基于晶片技术的硅太阳能电池通常通过图案化活性材料即硅以增加光俘获来提升效率。然而,对于活性材料层相对较薄的薄膜太阳能电池,通过图案化或刻蚀对吸收层进行的任何物理改性,都会导致材料质量下降而降低效率。因此,急需开发一种不影响电子性能的加工薄膜太阳能电池纹理的工艺。 具有相对均匀直径的六边形填充纳米球,可以提供最佳的几何形状,可通过光子晶体共振提高电池的效率。这种方法所面临的主要挑战是如何沉积一个大而密集的球体单层。 使用纳米颗粒(NPs)制备薄膜镀层材料日益受到了人们的重视,并且被广泛应用到如显示器、传感器、医疗器械、储能和能量收集材料等各种现代产品和研究领域。纳米粒子的合成方法已经广为人知,但为了能够在上述应用中使用它们,需要将纳米颗粒从溶液相转移到基材表面。为此,需要一种可控的沉积方法。 附件白皮书回顾了在气-液界面处形成纳米颗粒单层膜的方法以及使用Langmuir-Blodgett和Langmuir-Schaefer方法制备薄膜后,将其转移到固体基底上的方法。如果您对如何沉积单层纳米球感兴趣,请下载附件的白皮书。
确定





还剩6页未读,是否继续阅读?
瑞典百欧林科技有限公司为您提供《太阳能电池中薄膜沉积检测方案(LB膜分析仪)》,该方案主要用于太阳能中薄膜沉积检测,参考标准--,《太阳能电池中薄膜沉积检测方案(LB膜分析仪)》用到的仪器有KSV NIMA常规 交替型LB膜分析仪
推荐专场
相关方案
更多