The results presented demonstrate the successful
development of an accurate, precise, and selective
SPE-LC-MS/MS method for the analysis of desmopressin
from human plasma. Use of micro-elution SPE allowed
for concentration of the sample prior to analysis without
the need for post-extraction processing. Utilizing SOLAµ
mixed-mode SPE provided both a clean and repeatable
extract across the concentration range.
方案详情

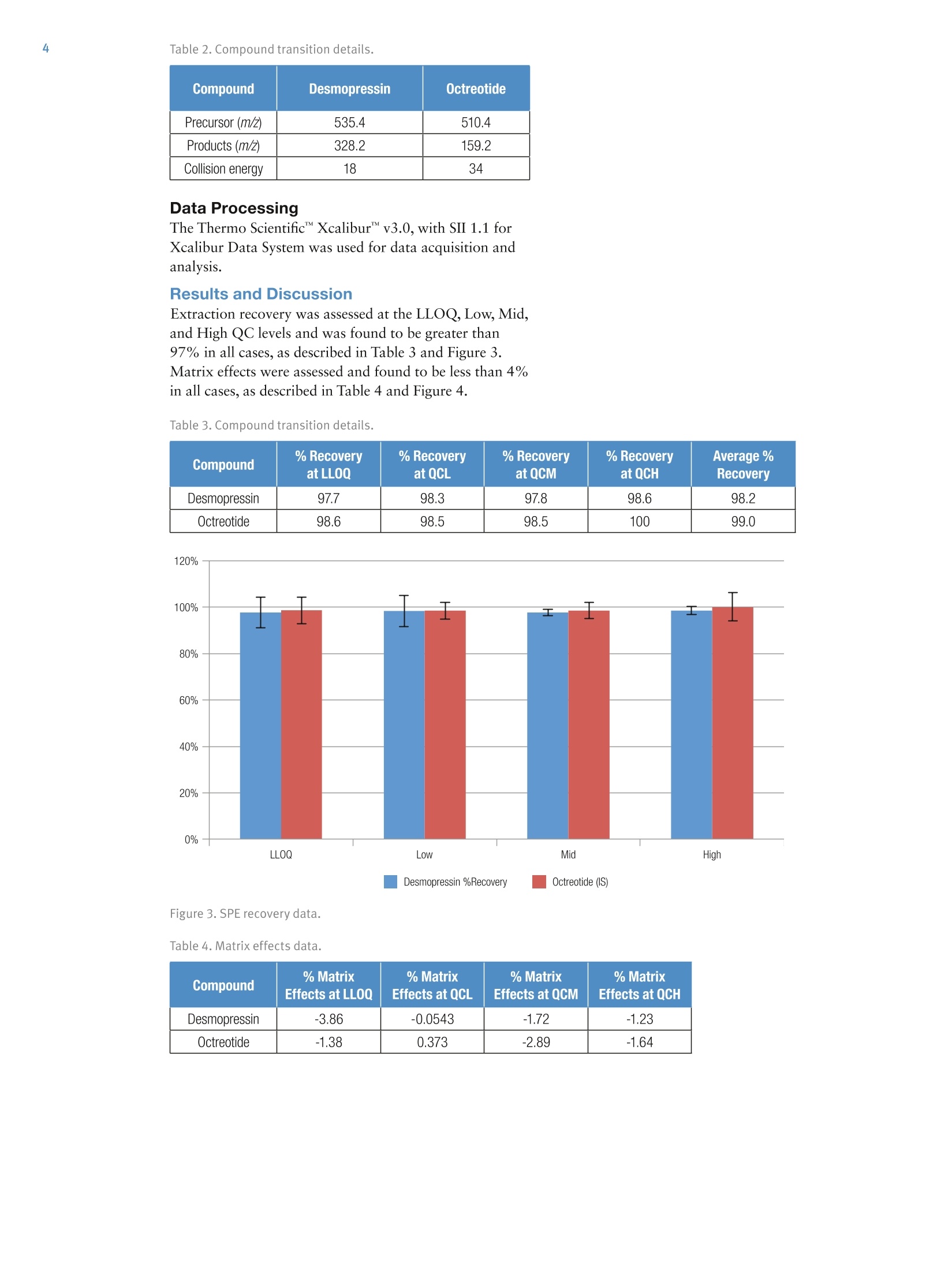
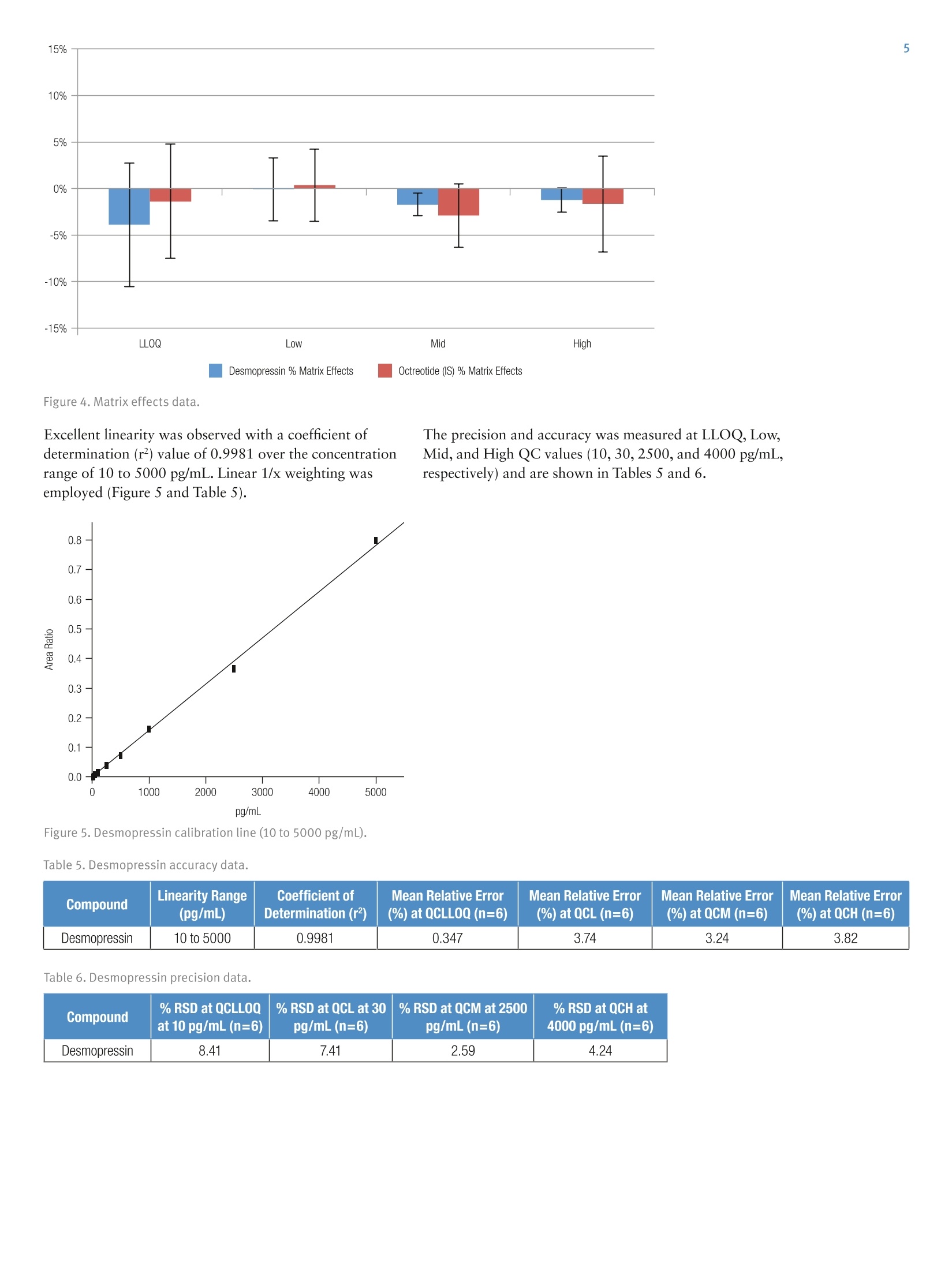
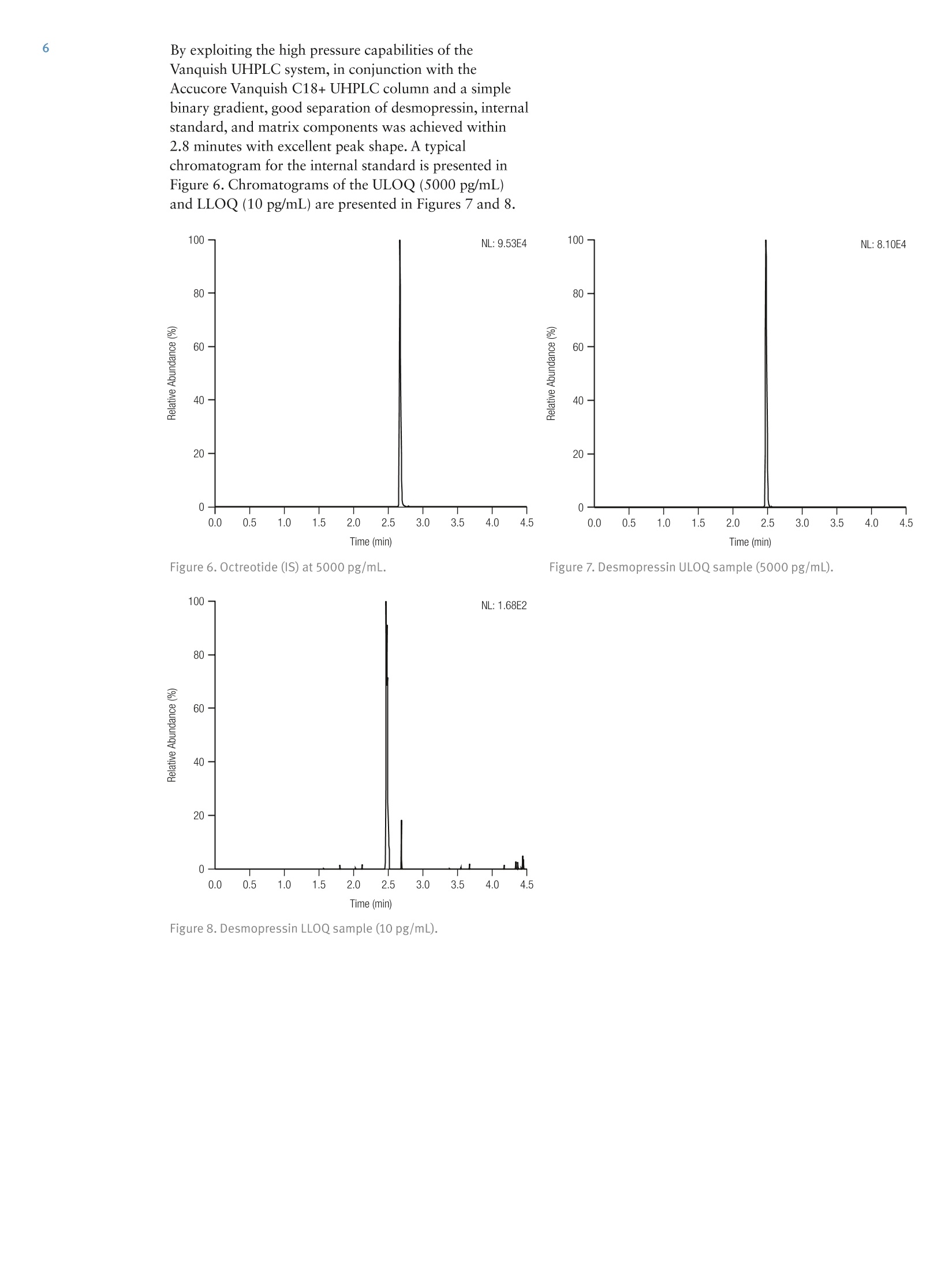
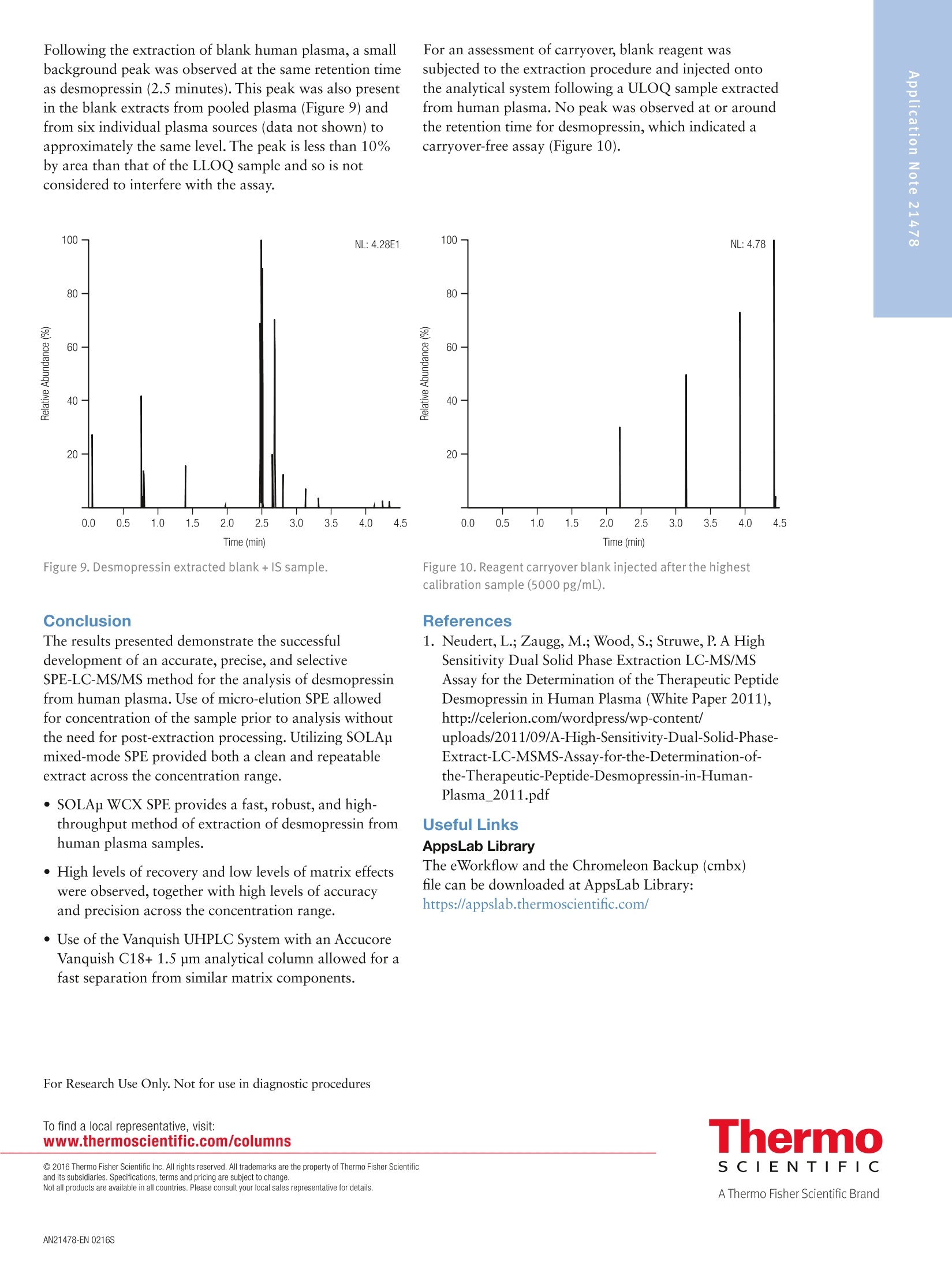
2 4 Jon Bardsley, Thermo Fisher Scientific, Runcorn, UK Key Words SOLAp WCX, Accucore Vanquish, Vanquish, desmopressin, LC-MS/MS,UHPLC, mixed-mode SPE, solid-phase extraction, micro-elution, peptides,bioanalysis, biopharma Goal To describe an accurate, precise, high-throughput workflow for the analysisof desmopressin from human plasma utilizing micro-elution solid-phaseextraction (SPE), followed by liquid chromatography separation coupled totriple quadrupole mass spectrometry detection (LC-MS/MS). Introduction Analysis of peptides presents very specific challenges forthe bioanalyst: analyte solubility, non-specific binding tolabware, and the ability to selectively detect a specificpeptide in the presence of a complex matrix. Analysis cantypically require long gradients and extensive samplepreparation, which can result in low recovery levels forthe peptide in question. Issues with system or columncarryover can also be challenging. Desmopressin (Figure 1) is a synthetic peptide consistingof nine amino acids and is very similar to endogenouspeptides present in human plasma. Selective analysis canbe achieved by combining micro-elution solid-phaseextraction (SPE) with ultra-high pressure liquidchromatography (UHPLC) and ultra fast selectivereaction monitoring (SRM) mass spectrometry. Micro-elution SPE provides quick and selective extraction ofpeptides from biological matrices without the need forpost-extraction processing. Utilizing UHPLC technologyallows for fast and reproducible separation,and massspectrometry SRM provides robust,selective, and sensitivedetection. Sample preparation was performed using ThermoScientific SOLApWCX, a weak cation exchange,mixed-mode, micro-elution SPE product. SOLAp productsprovide reproducibility, robustness, and ease of use at lowelution volumes by utilizing the revolutionary ThermoScientific"" SOLA"" solid-phase extraction technology. Thisremoves the need for frits, delivering a robust,reproducible format that ensures highly consistent resultsat low elution volumes. Selective and Highly Accurate Analysisof Desmopressin from Human Plasma SOLAp products deliver: · Lower sample failures due to high reproducibility atlow elution volumes · Increased sensitivity due to lower elution volumes · The ability to process samples which are limited involume ·Improved stability of bio-molecules by reduction ofadsorption and solvation issues This technique selectively extracts and concentratesdesmopressin from human plasma with high recovery andlow matrix effects. Octreotide (Figure 2) was used as ananalogue internal standard (IS) and was added to theplasma before processing. Separation was achieved on a Thermo Scientific"VanquishUHPLC system with a Thermo Scientific"Accucore" Vanquish C18+ analytical column. AccucoreVanquish C18+ UHPLC columns use Solid CoreTechnology to facilitate fast and highly efficientseparations. This next-generation column features 1.5 pmsolid core particles, which are not totally porous butinstead have a solid core and a porous outer layer. Theoptimized phase bonding creates a high-coverage, robustphase. This coverage results in a significant reduction insecondary interactions and delivers highly efficient peaks.The tightly controlled 1.5 um diameter of AccucoreVanquish particles, in combination with controlledmanufacturing processes, results in a column that deliversthe increased chromatographic performance required forrapid screening methods. The Accucore Vanquish UHPLC column and VanquishUHPLC system were designed in combination to achievethe best possible chromatographic performance. Thesystem is optimized to reduce extra column banddispersion and allow users to significantly improve theseparation power in their analytical assays. By exploitingthe 1500 bar high-pressure capability of the VanquishUHPLC system,the flow rate used with the AccucoreVanquish column can be increased while maintainingpeak capacity, resulting in shorter method times andincreased assay throughput. Selective reaction monitoring was used for detection on aThermo ScientificTSQ Quantivatriple quadrupolemass spectrometer with positive electrospray ionization.A linear range of 10 to 5000 pg/mL was achieved. Qualitycontrol (QC) samples were prepared at 10, 30,2500, and4000 pg/mL levels and demonstrated excellent accuracyand precision. Figure 1. Desmopressin structure. Experimental Consumables ·SOLAp WCX plate (P/N 60209-004) · Accucore Vanquish C18+1.5 pm, 100×2.1 mm(P/N27101-102130) ·Thermo Scientific"Webseal 96-well square wellmicroplate (P/N 60180-P212) ( · Webseal mat (P/N 60180-M120) ) ·Fisher Scientific"LC-MS grade water (P/N 10095164) ·Fisher Scientific LC-MS grade acetonitrile (ACN)(P/N 10055454) ·Fisher Scientific LC-MS grade methanol (MeOH)(P/N 10636545) ·Fisher Scientific analytical grade formic acid (HCOOH)(P/N 10063427) Fisher Scientific OptimaLC-MS trifluoroacetic acid(TFA) (P/N A116-50) Fisher Scientific 2,2,2-trifluoroethanol (TFE)(Peptide Synthesis)(P/N BP622-100) Fisher Scientific phosphoric acid (H,PO)(P/NA365-1) ·Fisher Scientific acetic acid (CH,COOH)(P/N A465-50) ·Fisher Scientific ammonium bicarbonate (NHHCO,)(P/N BP2413500) Sample Handling Equipment Thermo Scientific"" HyperSep" 96 well vacuummanifold (P/N 60103-351) · Thermo Scientific vacuum pump, European version(P/N 60104-241) ·Thermo Scientific vacuum pump, North Americanversion (P/N 60104-243) Sample Pretreatment Desmopressin and octreotide were obtained from areputable source in accurately pre-weighed ampoules.An appropriate volume of methanol/deionized water/acetic acid (30:60:10 v/v/v) was added in order to make a1 mg/mL stock solution of each. Further dilutions ofdesmopressin were prepared in the same solvent toproduce working solutions at the appropriate concentrations. To prepare calibration standards in the range of 10 to5000 pg/mL, 5 pL of individual working solutions ofdesmopressin were added to 295 pL of human plasma.The same approach was used to prepare QC samples at10, 30, 2500, and 4000 pg/mL to be used as the LLOQ,Low, Mid, and High QC, respectively. A working solutionof octreotide was prepared in methanol/deionized water/acetic acid (30:60:10 v/v/v) at a concentration of250 ng/mL. Then, 10 pL of this was added to each standard and QC sample. Zero standard samples were prepared by adding 5 pL ofsolvent and 10 pL of octreotide working solution to295 pL of blank plasma. Double blank samples wereprepared by adding 15 pL of solvent to 295 pL of humanplasma. Prior to SPE application each standard, QC sample, zerostandard,and double blank were diluted with 300 pL 4%phosphoric acid and mixed well. Sample Preparation Compound: Desmopressin Internal standard: OctreotideMatrix: Human plasmaPlate type: SOLAu WCX Flow rate: 1 drip per second SPE Procedure Condition Add 200 pL of ACN to each well.Apply vacuum to draw all liquid through the plate. Equilibrate Add 200 pL of 4% HgP0 (aq) to each well.Apply vacuum to draw all liquid through the plate. SampleLoad Transfer 600 pL of each sample into individual wells.Apply vacuum to draw all the liquid through the plate. Wash 1 Add 200 pL of 10 mM NH HCO, to each well.Apply vacuum to draw all liquid through the plate. Wash 2 Add 200 pL of 20:80 (ACN/10 mM NH4HC03) to each well.Apply vacuum to draw all liquid through the plate. ManifoldSet-up Discard all effluent collected and place a fresh collectionplate under the SPE device. Elution Add 25 pL of 60:39:1 (ACN/water/TFA) to each well.Apply a slow vacuum until all liquid has passed through the plate.Repeat this step once (total volume 50 pL) Dilution Remove the collection plate from the manifold and add 50 pLof water to each well. Cap and mix well. Method Optimization Multiple SPE chemistries were screened during methoddevelopment. WCX was chosen, as the results indicatedthe highest analyte recovery. Small amounts of carryoverwere initially observed during chromatographydevelopment,however, the addition of 1% TFE in themobile phase and autosampler wash reduced thecarryover to zero. Optimization of the ions used in the SRM detectionmethod was performed. Sensitivity increased by a factorof two when methanol was used in the mobile phaseinstead of acetonitrile. Upon investigation it was foundthat in the presence of acetonitrile, desmopressin wasobserved in both +1 and +2 charge states in roughly equalproportions. In the presence of methanol almost all of thedesmopressin existed in the +2 charge state,increasing thesensitivity of the chosen precursor ion. Other publicationsof this analysis have achieved additional sensitivity bysummation of ions; however, this was not adopted in ourapproach. Separation Conditions Instrumentation Analyses were performed using a Vanquish UHPLC System consisting of: Column · System base (P/NVH-S01-A) ·Binary pump H (P/NVH-P10-A) ·Split sampler HT (P/N VH-A10-A) ·Column compartment H (P/NVH-C10-A) Accucore Vanquish C18+, 1.5 um, 100×2.1 mm Mobile Phase A 0.1% formic acid in water Mobile Phase B 0.1% formic acid in acetonitrile/methanol/TFE (49:50:1 v/v/v) Gradient See Table 1 Flow Rate 0.4 mL/min lemp. 50°C Mobile Phase 50 °℃ Pre-heater njection Details 10 pL Injection Wash Acetonitrile/water/acetic acid/TFE Solvent (60:30:9:1v/v/v/v) Table 1. LC gradient conditions. Time (min) A (%) B (%) 0 85 15 0.5 85 15 3.5 40 60 3.5 10 90 4 10 90 4 85 15 5 85 15 MS Conditions nstrumentation TSQ Quantiva MS Polarity Positive Spray Voltage 3500V Vaporizer Temp. 317°℃ Sheath Gas Pressure z 40 Arb Aux Gas Pressure 12 Arb Capillary Temp. 333°C Collision Pressure 1.5 mTorr Dwell Time 75 ms Scan Time Auto Q1 (FWHM) 0.7 Q3 (FWHM) 0.7 Table 2. Compound transition details. Compound Desmopressin Octreotide Precursor (m/Z) 535.4 510.4 Products (m/Z) 328.2 159.2 Collision energy 18 34 Data Processing The Thermo Scientific"Xcalibur""v3.0, with SII 1.1 forXcalibur Data System was used for data acquisition andanalysis. Results and Discussion Extraction recovery was assessed at the LLOQ, Low, Mid,and High QC levels and was found to be greater than97% in all cases, as described in Table 3 and Figure 3.Matrix effects were assessed and found to be less than 4%in all cases, as described in Table 4 and Figure 4. Table 3. Compound transition details. Compound % Recoveryat LL00 % Recoveryat QCL % Recoveryat QCM %Recoveryat QCH Average%Recovery Desmopressin 97.7 98.3 97.8 98.6 98.2 Octreotide 98.6 98.5 98.5 100 99.0 Desmopressin %Recovery Octreotide (IS) Figure 3. SPE recovery data. Table 4. Matrix effects data. Compound % Matrix Effects at LLOQ % Matrix Effects at QCL % Matrix Effects at QCM % Matrix Effects at QCH Desmopressin -3.86 -0.0543 -1.72 -1.23 Octreotide -1.38 0.373 -2.89 -1.64 Figure 4. Matrix effects data. Excellent linearity was observed with a coefficient ofdetermination (r2) value of 0.9981 over the concentrationrange of 10 to 5000 pg/mL. Linear 1/x weighting wasemployed (Figure 5 and Table 5). The precision and accuracy was measured at LLOQ, Low,Mid, and High QC values (10,30,2500, and 4000 pg/mL,respectively) and are shown in Tables 5 and 6. Figure 5. Desmopressin calibration line (10 to 5000 pg/mL). Table 5. Desmopressin accuracy data. Compound Linearity Range (pg/mL) Coefficient ofDetermination (r) Mean Relative Error(%) at QCLL0Q (n=6) Mean Relative Error(%) at QCL (n=6) Mean Relative Error(%) at QCM (n=6) Mean Relative Error(%) at QCH (n=6) Desmopressin 10 to 5000 0.9981 0.347 3.74 3.24 3.82 Table 6. Desmopressin precision data. Compound % RSD at QCLLOQat 10 pg/mL (n=6) % RSD at QCL at 30pg/mL(n=6) % RSD at QCM at 2500pg/mL (n=6) % RSD at QCH at4000 pg/mL (n=6) Desmopressin 8.41 7.41 2.59 4.24 By exploiting the high pressure capabilities of theVanquish UHPLC system, in conjunction with theAccucore Vanquish C18+ UHPLC column and a simplebinary gradient, good separation of desmopressin, internalstandard, and matrix components was achieved within2.8 minutes with excellent peak shape. A typicalchromatogram for the internal standard is presented inFigure 6. Chromatograms of the ULOQ(5000 pg/mL)and LLOQ (10 pg/mL) are presented in Figures 7 and 8. Figure 6. Octreotide (IS) at 5000 pg/mL. Figure 7. Desmopressin ULOQ sample (5000 pg/mL). Figure 8. Desmopressin LLOQ sample (10 pg/mL). Following the extraction of blank human plasma, a smallbackground peak was observed at the same retention timeas desmopressin (2.5 minutes). This peak was also presentin the blank extracts from pooled plasma (Figure 9) andfrom six individual plasma sources (data not shown) toapproximately the same level. The peak is less than 10%by area than that of the LLOQ sample and so is notconsidered to interfere with the assay. For an assessment of carryover, blank reagent wassubjected to the extraction procedure and injected ontothe analytical system following a ULOQ sample extractedfrom human plasma. No peak was observed at or aroundthe retention time for desmopressin, which indicated acarryover-free assay (Figure 10). Figure 9. Desmopressin extracted blank +IS sample. Conclusion The results presented demonstrate the successfuldevelopment of an accurate, precise, and selectiveSPE-LC-MS/MS method for the analysis of desmopressinfrom human plasma. Use of micro-elution SPE allowedfor concentration of the sample prior to analysis withoutthe need for post-extraction processing. Utilizing SOLApmixed-mode SPE provided both a clean and repeatableextract across the concentration range. SOLAp WCX SPE provides a fast, robust, and high-throughput method of extraction of desmopressin fromhuman plasma samples. High levels of recovery and low levels of matrix effectswere observed, together with high levels of accuracyand precision across the concentration range. ·Use of the Vanquish UHPLC System with an AccucoreVanquish C18+ 1.5 pm analytical column allowed for afast separation from similar matrix components. Figure 10. Reagent carryover blank injected after the highestcalibration sample (5000 pg/mL). References 1. Neudert, L.; Zaugg, M.; Wood, S.; Struwe, P. A HighSensitivity Dual Solid Phase Extraction LC-MS/MSAssay for the Determination of the Therapeutic PeptideDesmopressin in Human Plasma (White Paper 2011),http://celerion.com/wordpress/wp-content/uploads/2011/09/A-High-Sensitivity-Dual-Solid-Phase-Extract-LC-MSMS-Assay-for-the-Determination-of-the-Therapeutic-Peptide-Desmopressin-in-Human-Plasma_2011.pdf Useful Links AppsLab Library The eWorkflow and the Chromeleon Backup (cmbx)file can be downloaded at AppsLab Library:https://appslab.thermoscientific.com/ S CIENTIFIC AThermo Fisher Scientific BrandANEN SOLAµ WCX, Accucore Vanquish, Vanquish, desmopressin, LC-MS/MS, UHPLC, mixed-mode SPE, solid-phase extraction, micro-elution, peptides, bioanalysis, biopharmaTo describe an accurate, precise, high-throughput workflow for the analysis of desmopressin from human plasma utilizing micro-elution solid-phase extraction (SPE), followed by liquid chromatography separation coupled to triple quadrupole mass spectrometry detection (LC-MS/MS).The results presented demonstrate the successful development of an accurate, precise, and selective SPE-LC-MS/MS method for the analysis of desmopressin from human plasma. Use of micro-elution SPE allowed for concentration of the sample prior to analysis without the need for post-extraction processing. Utilizing SOLAµ mixed-mode SPE provided both a clean and repeatable extract across the concentration range.
确定



还剩5页未读,是否继续阅读?
赛默飞色谱与质谱为您提供《人血浆中去氨加压素分析检测方案(液质联用仪)》,该方案主要用于全血/血清/血浆中去氨加压素分析检测,参考标准--,《人血浆中去氨加压素分析检测方案(液质联用仪)》用到的仪器有赛默飞 ISQ EC单四极杆质谱
推荐专场
相关方案
更多
该厂商其他方案
更多
















