
方案详情
文
This application report shows that the ICP-AES is an appropriate technique for the analysis of glass samples due to the very large dynamic range. The high reproducibility of the ULTIMA was shown and enables a high throughput with less time needed for re-slope of the calibration curves. The accuracy for major elements was obtained using the internal standard.
方案详情

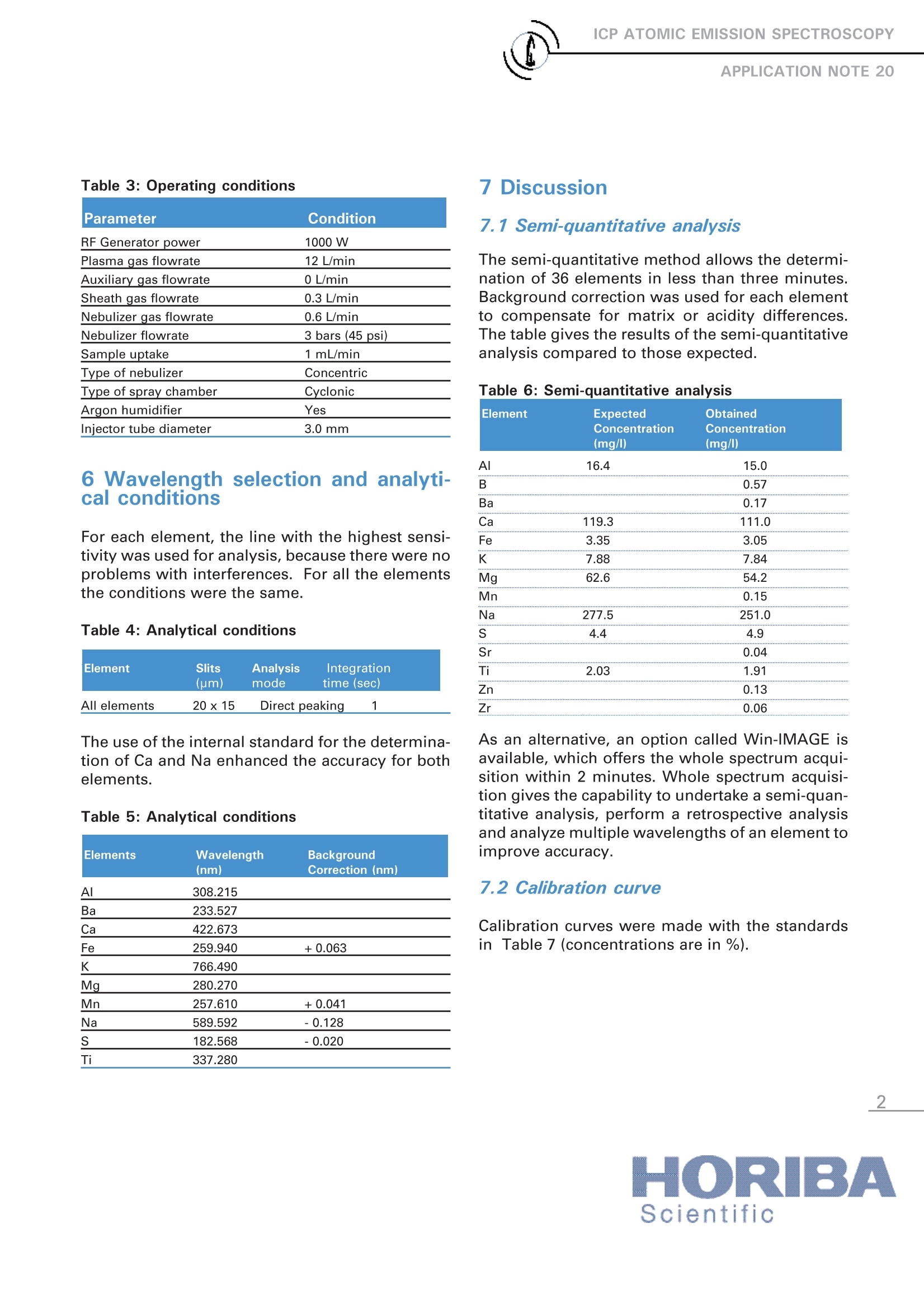
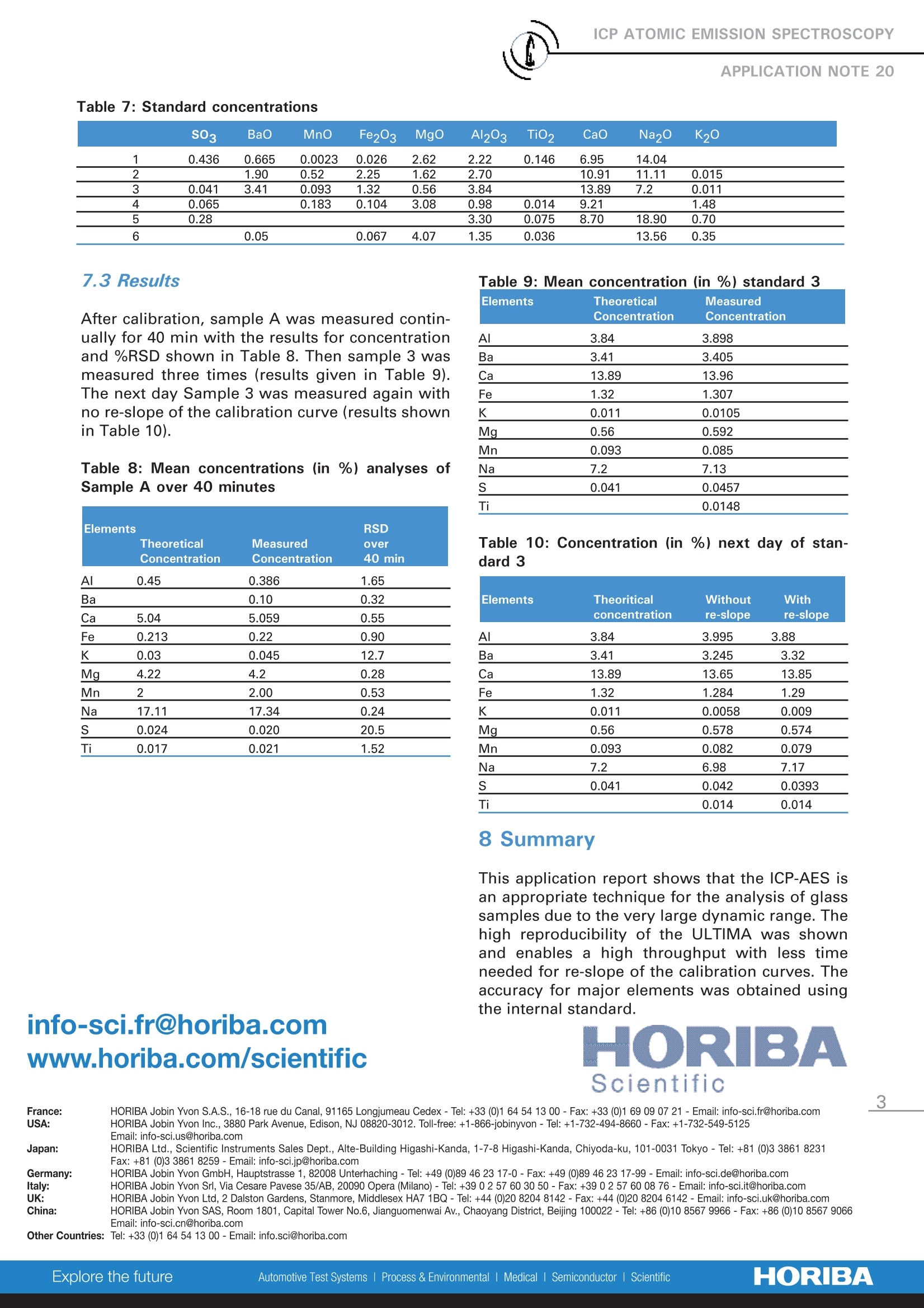
ICP ATOMIC EMISSION SPECTROSCOPY Analysis of Glass Samples Nathalie Le Corre HORIBA Scientific Longjumeau, France Keywords: glass 1 Introduction ICP-AESs iss a multi-element technique thatallows the analysis of nearly all the elements ofthe periodic table. It is very suitable for thedetermination of trace and major elements inthe same sample or matrix. This ApplicationNote presents data on the analysis of 9 ele-ments in glass samples. 2 Principle 2.1 Technique used The elemental analysis of these samples wasundertaken by Inductively Coupled PlasmaAtomic Emission Spectrometry (ICP-AES).).Thesample is nebulized then transferred to anargon plasma. It is decomposed, atomized andionized whereby the atoms and ions are excited.We measure the intensity of the light emittedwhen the atoms or ions return to lower levels ofenergy. Each element emits light at characteris-tic wavelengths and these lines can be used forquantitative analysis after a calibration. 2.2 Wavelength choice The choice of the wavelength in a given matrixcan be made using the "profile"function, or byusing Win-IMAGE, which is rapid semi-quantita-tive analysis mode using multiple wavelengths.The principle is the same in either case: recordthe scans of analytes at low concentration, andof the matrix. By superimposing the spectra, wesee possible interferences. 3 Sample preparation 1 g of glass was dissolved in 20 mL of 20% HF.Heat was applied to dissolve the glass and evap-orate to a lower volume. The sample was dilut-ed with 10 mL of 50 % HCl and then with deion-ized water up to a volume of 100 mL. 0.1 g ofHBO3 was added to improve the solubility offluoride forming elements. In this sample preparation process, the Si matrix is lost as volatile SiF6, which reduces thematrix and dissolved solids. 4. Instrument specification The work was done on a ULTIMA.The specifica-tions of this instrument are listed Table 1 and 2. Table 1: Specification of spectrometer Parameters Specifications Mounting Czerny Turner Focal length 1m Nitrogen purge Yes Variable resolution Yes Grating number of grooves 2400 gr/mm Order 2nd order Table 2: Specification of RF Generator Parameters Specifications Type of generator Solid state Observation Radial Frequency 40.68 MHz Control of gas flowrate by computer Control of pump flow by computer Cooling air An internal standard (Y ll at 371 nm) was usedto improve the Ca and Na results. A separatemonochromator was employed to allow forsimultaneous measurement of the internalstandard with the measurement of the analyte. 5 0perating conditions The operating conditions are listed in Table 3below. Table 3:Operating conditions Parameter Condition RF Generator power 1000 W Plasma gas flowrate 12 L/min Auxiliary gas flowrate 0 L/min Sheath gas flowrate 0.3 L/min Nebulizer gas flowrate 0.6 L/min Nebulizer flowrate 3 bars (45 psi) Sample uptake 1 mL/min Type of nebulizer Concentric Type of spray chamber Cyclonic Argon humidifier Yes Injector tube diameter 3.0 mm 6 Wavelength selection and analyti-cal conditions For each element, the line with the highest sensi-tivity was used for analysis, because there were noproblems with interferences. For all the elementsthe conditions were the same. Table 4: Analytical conditions Element Slits Analysis Integration (pm) mode time (sec) All elements 20x15 Direct peaking 1 The use of the internal standard for the determina-tion of Ca and Na enhanced the accuracy for bothelements. Table 5: Analytical conditions Elements Wavelength Background (nm) Correction (nm) Al 308.215 Ba 233.527 Ca 422.673 Fe 259.940 +0.063 766.490 Mg 280.270 Mn 257.610 +0.041 Na 589.592 -0.128 182.568 -0.020 Ti 337.280 7 Discussion 7.1 Semi-quantitative analysis The semi-quantitative method allows the determi-nation of 36 elements in less than three minutes.Background correction was used for each elementto compensate for matrix or acidity differences.The table gives the results of the semi-quantitativeanalysis compared to those expected. Table 6: Semi-quantitative analysis Element Expected Obtained Concentration Concentration (mg/l) (mg/l) Al 16.4 15.0 B 0.57 Ba 0.17 Ca 119.3 111.0 Fe 3.35 3.05 K 7.88 7.84 Mg 62.6 54.2 Mn 0.15 Na 277.5 251.0 S 4.4 4.9 Sr 0.04 Ti 2.03 1.91 Zn 0.13 Zr 0.06 As an alternative, an option called Win-IMAGE isavailable,which offers the whole spectrum acqui-sition within 2 minutes. Whole spectrum acquisi-tion gives the capability to undertake a semi-quan-titative analysis, perform a retrospective analysisand analyze multiple wavelengths of an element toimprove accuracy. 7.2 Calibration curve Calibration curves were made with the standardsin Table 7 (concentrations are in %). SO3 Bao MnO Fe203 MgO Al203 TiO2 CaO Na2O K20 1 0.436 0.665 0.0023 0.026 2.62 2.22 0.146 6.95 14.04 2 1.90 0.52 2.25 1.62 2.70 10.91 11.11 0.015 3 0.041 3.41 0.093 1.32 0.56 3.84 13.89 7.2 0.011 4 0.065 0.183 0.104 3.08 0.98 0.014 9.21 1.48 5 0.28 3.30 0.075 8.70 18.90 0.70 6 0.05 0.067 4.07 1.35 0.036 13.56 0.35 7.3 Results After calibration, sample A was measured contin-ually for 40 min with the results for concentrationand %RSD shown in Table 8. Then sample 3 wasmeasured three times (results given in Table 9).The next day Sample 3 was measured again withno re-slope of the calibration curve (results shownin Table 10). Table 8: Mean concentrations (in %) analyses ofSample A over 40 minutes RSD Elements Theoretical Measured over Concentration Concentration 40 min Al 0.45 0.386 1.65 Ba 0.10 0.32 Ca 5.04 5.059 0.55 Fe 0.213 0.22 0.90 K 0.03 0.045 12.7 Mg_ 4.22 4.2 0.28 Mn 2 2.00 0.53 Na 17.11 17.34 0.24 0.024 0.020 20.5 Ti 0.017 0.021 1.52 info-sci.fr@horiba.comwww.horiba.com/scientific Table 9: Mean concentration (in %) standard 3 Elements Theoretical Measured Concentration Concentration Al 3.84 3.898 Ba 3.41 3.405 Ca 13.89 13.96 Fe 1.32 1.307 K 0.011 0.0105 Mg 0.56 0.592 Mn 0.093 0.085 Na 7.2 7.13 0.041 0.0457 Ti 0.0148 Table 10: Concentration (in %) next day of stan-dard 3 Elements Theoritical Without With concentration re-slope re-slope Al 3.84 3.995 3.88 Ba 3.41 3.245 3.32 Ca 13.89 13.65 13.85 Fe 1.32 1.284 1.29 K 0.011 0.0058 0.009 Mg 0.56 0.578 0.574 Mn 0.093 0.082 0.079 Na 7.2 6.98 7.17 0.041 0.042 0.0393 Ti 0.014 0.014 8 Summary This application report shows that the ICP-AES isan appropriate technique for the analysis of glasssamples due to the very large dynamic range.Thehigh reproducibility of the ULTIMA was shownand enables a high throughput with less timeneeded for re-slope of the calibration curves. Theaccuracy for major elements was obtained usingthe internal standard. France: HORIBA Jobin Yvon S.A.S., 16-18 rue du Canal, 91165 Longjumeau Cedex - Tel: +33 (0)1 64 54 13 00-Fax: +33 (0)1 69 09 07 21-Email: info-sci.fr@horiba.com ORIBAScientific ORIBAExplore the futureAutomotive Test Systems Process & EnvironmentalMedicalSemiconductorl Scientific This application report shows that the ICP-AES is an appropriate technique for the analysis of glass samples due to the very large dynamic range. The high reproducibility of the ULTIMA was shown and enables a high throughput with less time needed for re-slope of the calibration curves. The accuracy for major elements was obtained using the internal standard.
确定

还剩1页未读,是否继续阅读?
HORIBA(中国)为您提供《玻璃样品中铅检测方案(ICP-AES)》,该方案主要用于建筑玻璃中铅检测,参考标准--,《玻璃样品中铅检测方案(ICP-AES)》用到的仪器有HORIBA Ultima Expert高性能ICP光谱仪
推荐专场
相关方案
更多
该厂商其他方案
更多















