方案详情
文
■ 单内标校正,极大的简化了定量分析,无基体影响;
■ 对于任何基体的样品可单独进行校准和定量分析;
■ 多元素实时分析,可进行痕量和超痕量分析;
■ 不受样品的类型和不同应用需求影响;
■ 独特的液体或固体样品的微量分析,分析所需样品量小;
■ 优秀的检出限水平;
■ 出色的动态线性范围;
■ 无需任何化学前处理,无记忆效应;
■ 非破坏性分析,运行成本低廉。
方案详情

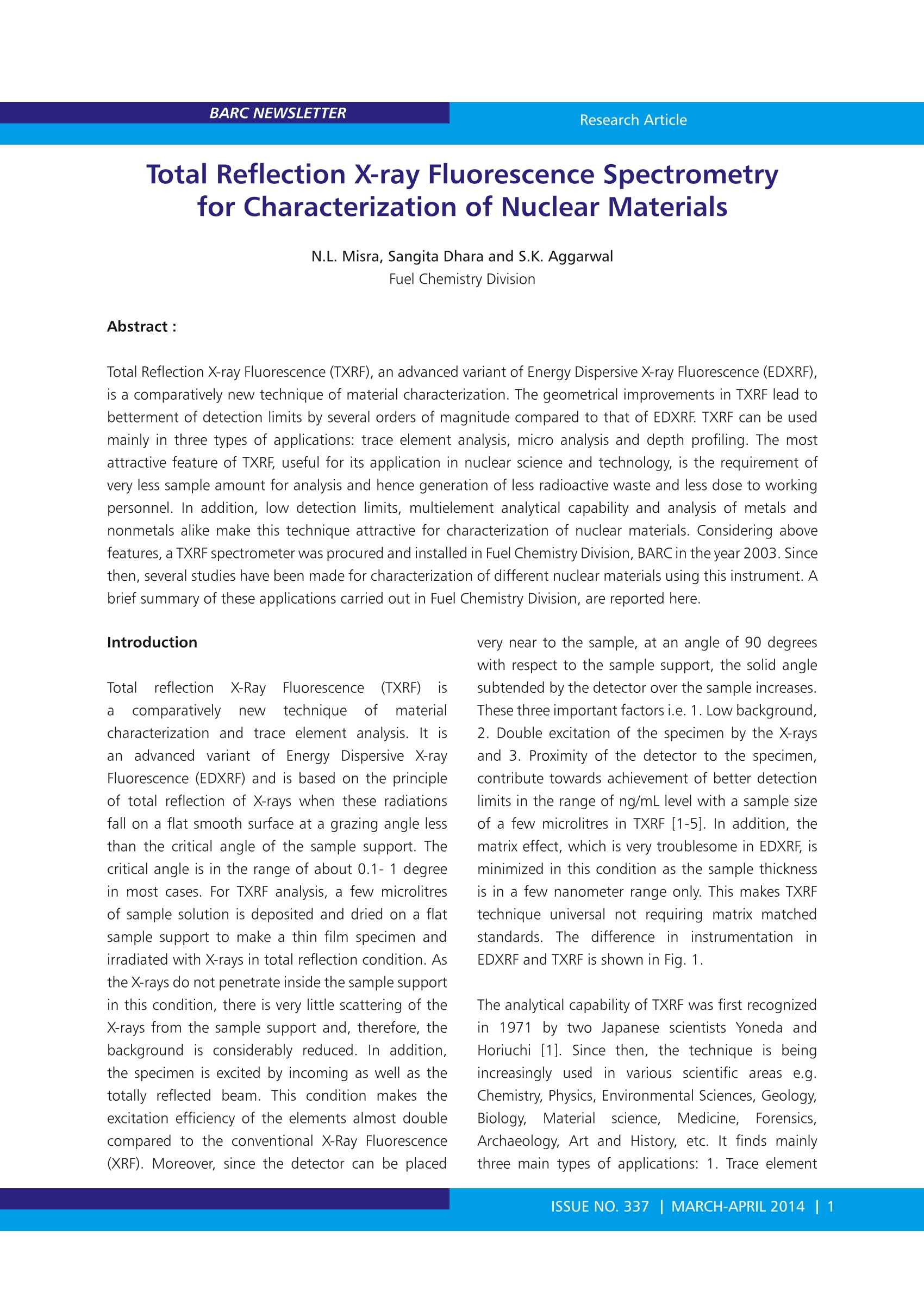
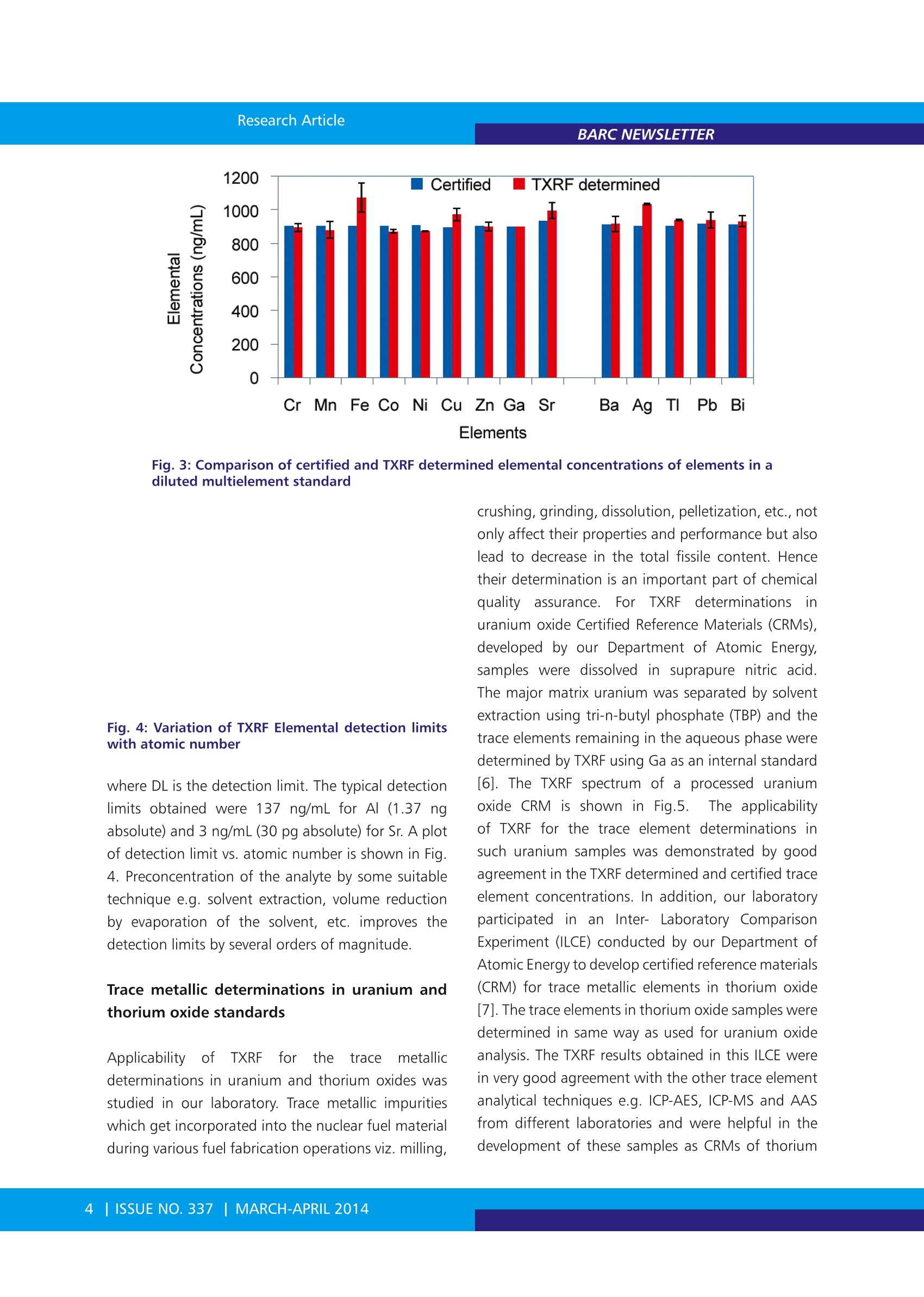
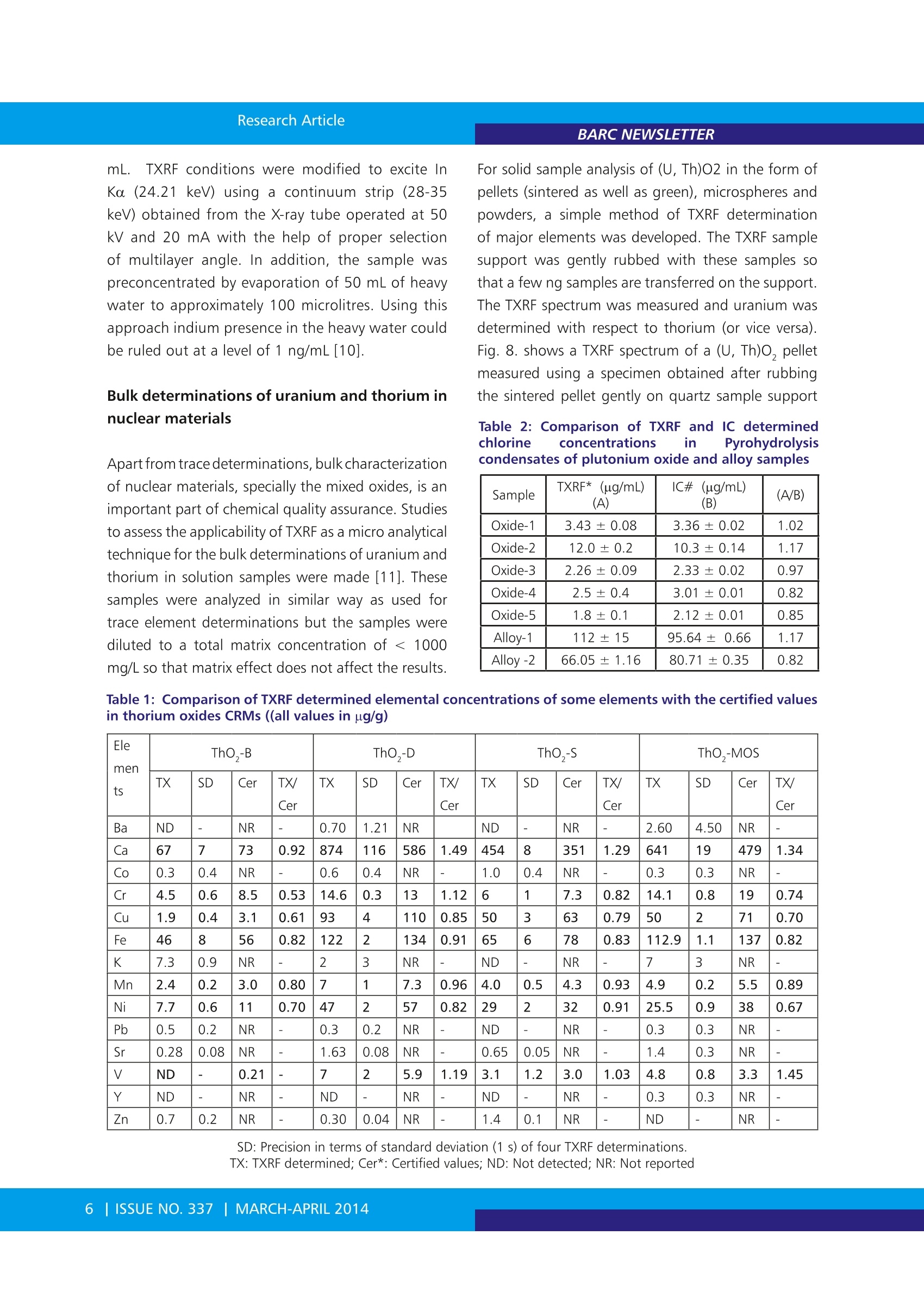
BARC NEWSLETTER Research Article Total Reflection X-ray Fluorescence Spectrometryfor Characterization of Nuclear Materials N.L. Misra, Sangita Dhara and S.K. AggarwalFuel Chemistry Division Abstract : Total Reflection X-ray Fluorescence (TXRF), an advanced variant of Energy Dispersive X-ray Fluorescence (EDXRF),is a comparatively new technique of material characterization. The geometrical improvements in TXRF lead tcbetterment of detection limits by several orders of magnitude compared to that of EDXRF. TXRF can be usedmainly in three types of applications: trace element analysis, micro analysis and depth profiling. The mostattractive feature of TXRF, useful for its application in nuclear science and technology, is the requirement ofvery less sample amount for analysis and hence generation of less radioactive waste and less dose to workingpersonnel. In addition, low detection limits, multielement analytical capability and analysis of metals andnonmetals alike make this technique attractive for characterization of nuclear materials. Considering abovefeatures, a TXRF spectrometer was procured and installed in Fuel Chemistry Division, BARC in the year 2003. Sincethen, several studies have been made for characterization of different nuclear materials using this instrument. Abrief summary of these applications carried out in Fuel Chemistry Division, are reported here. lotal reflection X-Ray IFluorescence(TXRF) isa comparatively new technique of materialcharacterization andtrace element analysis. It isanadvanceddvariant ofEnergy Dispersive X-rayFluorescence (EDXRF) and is based on the principleof total reflection of X-rays when these radiationsfall on a flat smooth surface at a grazing angle lessthan the critical angle of the sample support. Thecritical angle is in the range of about 0.1- 1 degreein most cases. For TXRF analysis, a few microlitresof sample solution is deposited and dried on a flatsample support to make a thin film specimen andirradiated with X-rays in total reflection condition. Asthe X-rays do not penetrate inside the sample supportin this condition, there is very little scattering of theX-rays from the sample support and, therefore, thebackground is considerably reduced. In addition,the specimen is excited by incoming as well as thetotally reflected beam. This condition makes theexcitation efficiency of the elements almost doublecompared to the conventional X-Ray Fluorescence(XRF). Moreover, since the detector can be placed very near to the sample, at an angle of 90 degreeswith respect to the sample support, the solid anglesubtended by the detector over the sample increases.These three important factors i.e. 1. Low background,2. Double excitation of the specimen by the X-raysand 3. Proximity of the detector to the specimen,contribute towards achievement of better detectionlimits in the range of ng/mL level with a sample sizeof a few microlitres in TXRF [1-5]. In addition, thematrix effect, which is very troublesome in EDXRF, isminimized in this condition as the sample thicknessis in a few nanometer range only. This makes TXRFtechnique universal not requiring matrix matchedstandardsS.TThe difference in1linstrumentationnl inEDXRF and TXRF is shown in Fig. 1. The analytical capability of TXRF was first recognizedin1971 by two Japanese scientists Yoneda andHoriuchi [1]. Since then, the technique is beingincreasingly used in various scientific areas e.g.. Chemistry, Physics, Environmental Sciences, Geology,Biology,Material science. .Medicine.. ForensicsArchaeology, Art and History, etc. It finds mainlythree main types of applications: 1. Trace element analysis 2. Micro analysis and 3. Depth profiling[1-2,5]. For trace and micro analysis, about 10 uLof the sample in solution form is deposited on aquartz or any other suitable material sample support,dried and TXRF spectrum is recorded. The elementalconcentrations are determined by comparison ofthe normalized intensities of each element afterconsideration of its characteristic X-ray line sensitivitywith that of an internal standard mixed previously inthe sample. Since this technique requires very smallamount of sample (picogram to nanogram level ofanalyte on the sample support), has multielementanalytical capability, can analyze metals and non-metals alike and is sample non-consumptive, it isvery much suited for the trace element determinationin nuclear materials which are often radioactive. Allthese features result in minimizing the generationof radioactive waste, saving of precious nuclearmaterials and less dose to the analyst as well as theinstrument when this technique shall be used foranalysis of radioactive samples. Fig. 1: Difference in EDXRF and TXRF instrumental set-up A TXRF spectrometer was procured and installedin Fuel Chemistry Division, in November 2003 forcharacterization of nuclear materials. Since then,this instrument has been used to develop TXRFmethodology for application in different areas ofnuclear material characterization. This spectrometerhas been used for trace element analysis: metallicas well as non-metallic in uranium, thorium andplutonium oxides5 aCnd carbides, major elementdeterminations in mixeduranium-thorium oxidematrices, certification of presence of indium at level< 1 ng/mL in heavy water, determination of uraniumin sea water and other type of waters, forensic andenvironmental sample analysis. Some of the resultsobtained in these studies are reported in the presentarticle. Instrumentation An ITAL STRUCTURES TXRF spectrometer: TX-2000was used in these studies. The spectrometer isequipped to use single or dual target X-ray tubesfor sample excitation. For most of the experimentsa Mo-W dual target tube was used. Such dual targettubes can be tuned to allow either Mo Ka, K, W Loor W LB beams to fall on sample for excitation withoutchanging the X-ray tube. Mo Ko can efficiently excitemedium and high atomic number elements K andL lines respectively and W Lo/β can excite K lines oflow Z elements. Mo Kp has low intensity but canbe useful for elements e.g. Nb. A W-C multilayerwith 2d= 49.4 A was used to monochromatize theincident radiation emitted from the X-ray tube sothat a particular energye.g. Mo Ko /KB or W La/ Lcan be used for sample excitation. The spectrometergenerator is usually operated at voltage and current of40 kV and 30 mA, respectively. The sample chamberhas the capability to measure twelve samples in oneloading sequentially. Polished quartz sample supportsof 30 mm diameter and 3 mm thickness were usedas sample carriers. The characteristic X-rays emittedfrom the sample are detected with aSi (Li) detectorhaving a resolution of 139 eV (FVHM) at 5.9 keV (MnKa). The X-ray spectra were acquired and processed by computer programs TXRFACQ32 and EDXRF32,respectively, provided with the instrument. Calibration and validation In TXRF analysis, the elemental quantification is verysimple due to negligible matrix effects and can bedone by adding an internal standard to the samplesolution. After thorough mixing of these solutions,a few microlitre aliquots of the sample solutionsare deposited on the sample supports. The samplesupports are flat polished surface normally made ofQuartz, Plexiglas, Gold, etc. For the calibration ofthe spectrometer we used MERCK ICP-multielementstandard (MES) solution IV. This standard contains23 elements having elemental concentrations of1000 mg/L and was diluted in suprapure nitric acidto bring the concentration of the elements to a fewug/mL levels. A TXRF spectrum of such standard withelemental concentration of 5 ug/mL and a samplesize of 10 uL deposited on quartz sample support isshown in Fig.2. The net intensities of all the elementalX-ray lines in MES were calculated by finding thearea under the peak for each element using TXRFdata processing program EDXRF32. The absolutesensitivities were calculated by taking the ratio of netintensity to concentration. The relative sensitivitieswith respect to internal standards were calculatedby dividing the absolute sensitivity of each elementwith the absolute sensitivity of the internal standard.In TXRF analysis, relative sensitivity values are used absolute sensitivity value. The relative sensitivityvalues are constant for a particular spectrometer withfixed instrumental parameters. The quantification isdone using the equation: where, Cx is the concentration of the analyte presentin the sample, Nx and NIS are the net intensitiesof analyte X-ray lines being used for the analysisand that of internal standard respectively, Sx andSIS are the relative sensitivities of the analyte andinternal standard elemental X-ray lines and Cis is theconcentration of internal standard. Before applying this technique for trace elementdeterminations in nuclear materials, it was validatedby analyzing a diluted MERCK ICP- multielementstandard(MES))solution V having elementaconcentrations of 900 ng/mL. The analytical resultsobtained with Ga as internal standard are shown by abar graph in Fig. 3. Using the TXRF spectrum of abovestandard, the detection limits were also determinedfor sample volume of 10 uL and 1000s measurementtime employing the formula: DL=Goncentration3*Background A Peak Arena ((2) for quantification because it isvery difficult to make specimensof same area and thickness beingexposed to incoming X-rays inreproducible geometry in eachmeasurement. The benefit ofusingrelative sensitivity values is that thechange in X-ray intensities due todifferent area and sample amountbeing exposed do not cause errorsin quantification, as this type ofmeasurement shall nottresultin change in relative sensitivityvalue whereas it may change the Fig. 3: Comparison of certified and TXRF determined elemental concentrations of elements in adiluted multielement standard Fig. 4: Variation of TXRF Elemental detection limitswith atomic number where DL is the detection limit. The typical detectionlimits obtained were 137 ng/mL for Al (1.37 ngabsolute) and 3 ng/mL (30 pg absolute) for Sr. A plotof detection limit vs. atomic number is shown in Fia.4. Preconcentration of the analyte by some suitabletechnique e.g. solvent extraction, volume reductionby evaporation of the solvent, etc. improves thedetection limits by several orders of magnitude Trace metallic determinations in uranium andthorium oxide standards Applicabilityy 0of TXRF for)rthe trace metallicdeterminations in uranium and thorium oxides wasstudied in our laboratory. Trace metallic impuritieswhich get incorporated into the nuclear fuel materialduring various fuel fabrication operations viz. milling, crushing, grinding, dissolution, pelletization, etc.,notonly affect their properties and performance but alsolead to decrease in the total fissile content. Hencetheir determination is an important part of chemicalqualityyassurance. ForTXRFFdeterminationsinuranium oxide Certified Reference Materials (CRMs),developed by our Department of Atomic Energysamples were dissolved in suprapure nitric acidThe major matrix uranium was separated by solventextraction using tri-n-butyl phosphate (TBP) and thetrace elements remaining in the aqueous phase weredetermined by TXRF using Ga as an internal standard[6]. The TXRF spectrum of a processed uraniumoxide CRM is shown in Fig.5. The applicabilityofTXRF for the trace element determinations insuch uranium samples was demonstrated by goodagreement in the TXRF determined and certified traceelement concentrations. In addition, our laboratoryparticipated in an Inter- Laboratory ComparisonExperiment (ILCE) conducted by our Department ofAtomic Energy to develop certified reference materials(CRM) for trace metallic elements in thorium oxide[7]. The trace elements in thorium oxide samples were.determined in same way as used for uranium oxideanalysis. The TXRF results obtained in this ILCE werein very good agreement with the other trace elementanalytical techniques e.g. ICP-AES, ICP-MS and AASfrom different laboratories and were helpful in thedevelopment of these samples as CRMs of thorium oxide. A comparison of the certified concentrationsof trace elements in these CRMs and respective TXRFdetermined values are shown in Table 1. The goodagreement in these values gave us confidence aboutthe applicability and potential of TXRF for traceelement (above Z=13) determinations in uraniumand thorium using air as ambient atmosphere. Trace non-metallic determinations in nuclearmaterials Though TXRF can analyze metals and non-metalsalike, some elements require special treatment ofthe sample before thesecan be analyzed by TXRF.Chlorine is one such element. Normally chlorineis determined as chloride by lon Chromatography(IC). For such determinations of chlorine in nuclearmaterials, it is first separated from the main matrixusing pyrohydrolysis. The evolved chlorine, obtainedafter pyrohydrolysis, is collected in an acidic buffer.However when such solution containing Cl was mixedwith Co internal standard and a few microlitres ofthe solution were deposited on quartz supports andTXRF spectra were measured using W Lo as excitationsource, the TXRF spectra did not show any Cl Ka peak.This was due to the fact that when solution containingchlorine in acidic medium was dried on the samplesupports, the chlorine is driven out as HCl. In order toavoid such loss of chlorine in TXRF determination innuclear materials e.g. uranium, thorium, plutoniumoxides and carbides, the pyrohydrolysis methodologywas modified by collecting the evolved chlorine in5mM NaOH solution. This solution was mixed with Co internal standard in basic medium. The TXRFspectrum of the specimen prepared by such modifiedmethodology showed clear Cl Ko peak. The TXRFdetermined Cl concentrations were compared withIC determined Cl determinations. It was found thatthe TXRF results were comparatively less accurateand precise. This may be because Cl Ka, especiallywhen Cl is present in trace amounts, has interferencewith Ar Ko peak coming from the presence ofAr in atmosphere. In order to countercheck thisproblem, TXRF measurements were made usingHelium purge on the TXRF supports in such a waythat path between the sample and the detector isfilled with helium during TXRF measurements. Thisresulted in elimination of Ar Ka peak (as shown inFig. 6) and improved the precision and accuracy ofCl determinations by TXRF. Using this approach, theagreement in TXRF determined and IC determinedchlorine concentrations [8] could be improved. Thismethod of Cl determination has the advantage that itavoids the process of cumbersome sample dissolutionand requirement of putting the spectrometer insidethe glove box for radioactive samples. The TXRFspectrum of a pyrohydrolysed condensate obtainedfrom a PuO, sample and measured using heliumpurge is shown in Fig. 7. The agreement in TXRF andIC determined concentrations of Cl in some samplesis shown in Table 2. In addition, we have developedTXRF method for sulphur determination in uraniummatrix with good agreement in expected and TXRFdetermined sulphur concentrations [9]. Determination of indium in heavy water sample Heavy water is used as a coolant and moderator inpressurized heavy water reactors. This water shouldbe free from even ultra trace level of elementswhich produce highly radioactive isotopes afterneutron absorption as this will create radiationexposure risk to personnel working in reactorarea. Indium is one such element. It produces116nm isotope after neutron absorption. 116Inm hashalf-life of 54 minutes and emits y rays of energy> 1MeV. One of the heavy water samples was tobe certified to contain indium at a level < 1 ng/ mL.TXRF conditions were modified to excite InKo (24.21 keV) using a continuum strip (28-35keV) obtained from the X-ray tube operated at 50kV and 20 mA with the help of proper selectionof multilayer angle. In addition, the sample wasoreconcentrated by evaporation of 50 mL of heavywater to approximately 100 microlitres. Using thisapproach indium presence in the heavy water couldbe ruled out at a level of 1 ng/mL[10]. Bulk determinations of uranium and thorium innuclear materials Apart from trace determinations,bulk characterizationof nuclear materials, specially the mixed oxides, is animportant part of chemical quality assurance. Studiesto assess the applicability of TXRF as a micro analyticaltechnique for the bulk determinations of uranium andthorium in solution samples were made [11]. Thesesamples were analyzed in similar way as used fortrace element determinations but the samples werediluted to a total matrix concentration of < 1000mg/L so that matrix effect does not affect the results. For solid sample analysis of (U, Th)O2 in the form ofpellets (sintered as well as green), microspheres andpowders, a simple method of TXRF determinationof major elements was developed. The TXRF samplesupport was gently rubbed with these samples sothat a few ng samples are transferred on the support.The TXRF spectrum was measured and uranium wasdetermined with respect to thorium (or vice versa).Fig. 8. shows a TXRF spectrum of a (U, Th)O, pelletmeasured using a specimen obtained after rubbingthe sintered pellet gently on quartz sample support Table 2: Comparison of TXRFF aand1IC determinedchlorine concentrations in Pyrohydrolysiscondensates of plutonium oxide and alloy samples Sample TXRF* (ug/mL)(A) IC#(ug/mL)(B) (A/B) Oxide-1 3.43±0.08 3.36±0.02 1.02 Oxide-2 12.0±0.2 10.3±0.14 1.17 Oxide-3 2.26±0.09 2.33±0.02 0.97 Oxide-4 2.5±0.4 3.01±0.01 0.82 Oxide-5 1.8±0.1 2.12±0.01 0.85 Alloy-1 112±15 95.64±0.66 1.17 Alloy -2 66.05±1.16 80.71±0.35 0.82 Elements ThO,-B ThO,-D ThO,-S ThO,-MOS TX SD Cer TX/Cer TX SD Cer TX/Cer TX SD Cer TX/Cer TX SD Cer TX/Cer Ba ND - NR - 0.70 1.21 NR ND NR - 2.60 4.50 NR - Ca 67 7 73 0.92 874 116 586 1.49 454 8 351 1.29 641 19 479 1.34 Co 0.3 0.4 NR - 0.6 0.4 NR 1.0 0.4 NR - 0.3 0.3 NR - Cr 4.5 0.6 8.5 0.53 14.6 0.3 13 1.12 6 1 7.3 0.82 14.1 0.8 19 0.74 Cu 1.9 0.4 3.1 0.61 93 4 110 0.85 50 3 63 0.79 50 2 71 0.70 Fe 46 8 56 0.82 122 2 134 0.91 65 6 78 0.83 112.9 1.1 137 0.82 K 7.3 0.9 NR - 2 3 NR - ND - NR 7 3 NR - Mn 2.4 0.2 3.0 0.80 7 1 7.3 0.96 4.0 0.5 4.3 0.93 4.9 0.2 5.5 0.89 Ni 7.7 0.6 11 0.70 47 2 57 0.82 29 2 32 0.91 25.5 0.9 38 0.67 Pb 0.5 0.2 NR - 0.3 0.2 NR - ND - NR - 0.3 0.3 NR - Sr 0.28 0.08 NR - 1.63 0.08 NR 0.65 0.05 NR - 1.4 0.3 NR - V ND - 0.21 - 7 2 5.9 1.19 3.1 1.2 3.0 1.03 4.8 0.8 3.3 1.45 Y ND - NR - ND -一 NR - ND - NR - 0.3 0.3 NR - Zn 0.7 0.2 NR - 0.30 0.04 NR -一 1.4 0.1 NR - ND NR - SD: Precision in terms of standard deviation (1 s) of four TXRF determinations. Fig. 6. Effect of helium purge on the Ar Ko Intensity in TXRF spectrum Fig.7. A TXRF Spectrum of pyrohydrolysis condensate of PuO,sample in Helium atmosphere Fig. 8. A TXRF spectrum of (U,Th)O, sintered pellet obtainedfrom a specimen prepared by gently rubbing the pellet onquartz sample support and presenting it for TXRF measurementsThe advantages of this methodare. requirement of very small sample amount(in nanogram range) for analysis andnegligible sample preparation and almostnon-destructivee sampleeanalysis. Theprecision obtained was better than 2% inboth the solution and the solid samplesThe deviation of the TXRF determinedvalues from the expected values for mostof the cases was less than 2%. This studyinitiated a new kind of application ofTXRF as a micro analytical technique for. the bulk determination of constituents inradioactive samples. Conclusions Applicability of TXRF for trace as well asbulk determinations in nuclear materialswas successfully demonstrated Thestudies have established that TXREan upcoming technique for traceelementitcdetermination.1,is not onl\comparable but better in certain aspectse.g. non-consumption of the sample, lowoperating cost, multielement analyticalcapabilityincludingmetalsanddnon- metals, no memory effect, negligible matrix effect,analysis of almost all the elements from Z=11 (Na)using Ilab source, compared to most of ttheconventional trace determination techniques.For lowatomic number elements, Vacuum Chamber TXRFis advantageous. For radioactive materials, it has anadded advantage that it requires very less amount ofsample and produces negligible waste and this savesunnecessary radioactive exposures to the workingpersonnel, poses less problem of radioactive wastemanagement and saves precious nuclear materialsfrom wastage. The new developments in TXRF e.g.vacuum chamber based TXRF,TXRF-XANES/EXAFS,TotalRReflection XPS, whenaapplied to nuclearmaterials, shall not only cover almost whole periodictable for elemental determination but also be ableto give additional information e.g. speciation,atomicenvironment, etc. Acknowledgements: The authors express their sincere thanks to all co-authors of various publications included in this article. ( References: ) ( 1. R. k Klockenkaemper, Total Reflection X-rayFluorescence Analysis, Chemical A n alysis, vol. 140, John Wiley & S ons, N ew York, 1996. ) ( 2. F R.E. Van Grieken, A. Markowicz, Handbook of X-ray Spectrometry, Marcel Dekker I nc., New York, 1993. ) ( 3. N. L. M isra and K . D. S i ngh Mudher, To t al Reflection X-ray Fluorescence : A technique f or trace element a n alysis in m a terials, Progress i n Crystal Growth and Characterization of Materials45 (2002)65-74. ) ( 4. Thesis entitled "Analytical Characterization of T echnologically Important Materials using ) ( TXRF and E DXRF" s ubmitted by S angita DharaLenka to Homi B h abha In s titute of N a tional Institute, Mumbai for the award of PhD Degree i n the y ear 2 011.http://www.hbni.ac.in/ phdthesis/11phdthesis.htm ) ( 5. R. K lockenkaemper, A . von Bohlen, Total-reflection X-ray fluorescence moving towards nanoanalysis: a survey, Spectrochim. Acta Part B56(2001)2005-2018. ) 6.N.L. Misra, K.D. Singh Mudher, V.C. Adya, B.Rajeswari, V.Venugopal, Determination of traceelements in uranium oxide by Total ReflectionX-ray Fluorescence spectrometry, Spectrochim.Acta Part B 60 (2005)834-840. 7. N.L. Misra, Sangita Dhara, V.C. Adya, S.V.Godbole,K.D. Singh Mudher, S.K. Aggarwal, Traceelement determination in thorium oxide usingtotal reflection X-ray fluorescence spectrometry,Spectrochim. Acta Part B 63 (2008)81-85. 8.Sangita Dhara, Nand Lal Misra, Uday KumarThakur, DiptiShah,R. M. Sawant, K. L.Ramakumarand Suresh K. Aggarwal, A total reflection X-rayfluorescence methodfor the determinationof chlorine at trace levels in nuclear materialswithout sample dissolution, X-ray Spectrom. 41(2012)316-320. 9. Sangita Dhara, N.L. Misra and S.K. Aggarwal,Determination of sulphur in uranium matrix bytotal reflection X-ray fluorescence spectrometry,Spectrochim. Acta Part B 63 (2008) 1395-1398. 10.N.L.Misra, Characterizationof NuclearMaterials by Total Reflection X-ray FluorescenceSpectrometry, J. Radioanal. Nucl. Chem. 300(2014) 137-145. 11. Sangita Dhara, NandLal 1Misra, Khush DevSingh Mudher, Suresh Kumar Aggarwal, Bulkdetermination of uranium and thorium inpresence of each other by Total Reflection X-rayFluorescence spectrometry, Spectrochim. ActaPart B 62 (2007)82-85. ISSUE NO. MARCH-APRIL | 全反射X射线荧光技术(TXRF)是能量色散型X射线荧光(EDXRF)技术的高级变体,是一种相对较新的材料表征技术。与EDXRF相比,TXRF的几何改进导致检测极限提高了几个数量级。 TXRF主要用于三类应用:痕量元素分析,微观分析和深度剖析。 TXRF最有吸引力的特点是其在核科学与技术领域的应用,因为分析所需的样品量非常少,所以产生的放射性废物少,工作人员接触的剂量也小。此外,低检测限、多元素分析能力以及金属和非金属元素的分析使得这种技术对于核材料的表征具有很大优势。基于上述特点,印度巴巴原子研究中心(BARC) 燃料化学部门于2003年安装TXRF光谱仪,至今已经有多项研究使用此仪器对不同的核材料进行了表征。
确定





还剩6页未读,是否继续阅读?
利曼中国为您提供《核材料中Ba,Ga等元素检测方案(能散型XRF)》,该方案主要用于太阳能中Ba,Ga等元素检测,参考标准--,《核材料中Ba,Ga等元素检测方案(能散型XRF)》用到的仪器有TX 2000 全反射X荧光光谱仪
推荐专场
X荧光光谱、XRF(能量色散型X荧光光谱仪)
相关方案
更多
该厂商其他方案
更多
















