方案详情
文
■ 单内标校正,极大的简化了定量分析,无基体影响;
■ 对于任何基体的样品可单独进行校准和定量分析;
■ 多元素实时分析,可进行痕量和超痕量分析;
■ 不受样品的类型和不同应用需求影响;
■ 独特的液体或固体样品的微量分析,分析所需样品量小;
■ 优秀的检出限水平;
■ 出色的动态线性范围;
■ 无需任何化学前处理,无记忆效应;
■ 非破坏性分析,运行成本低廉。
方案详情

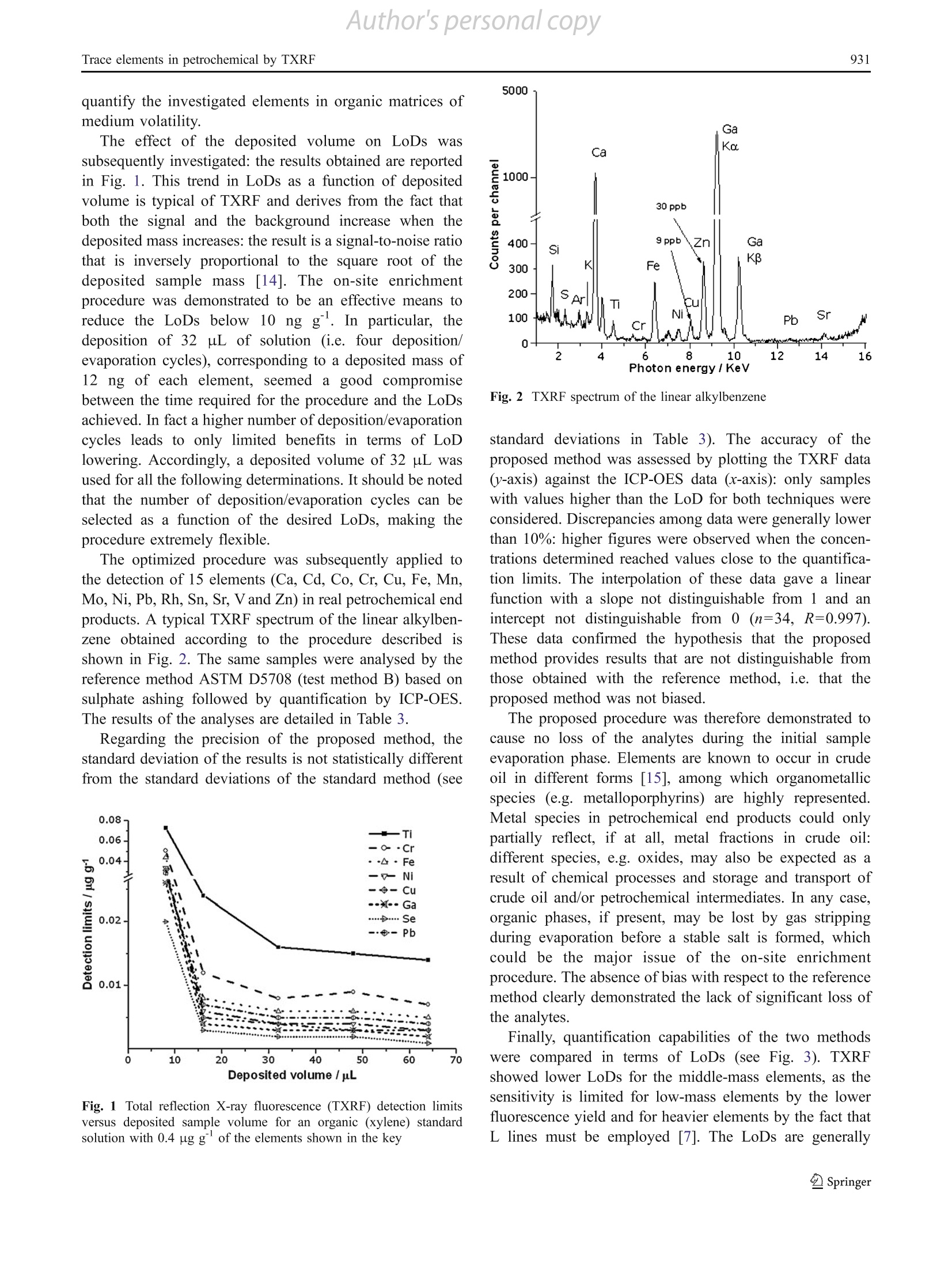
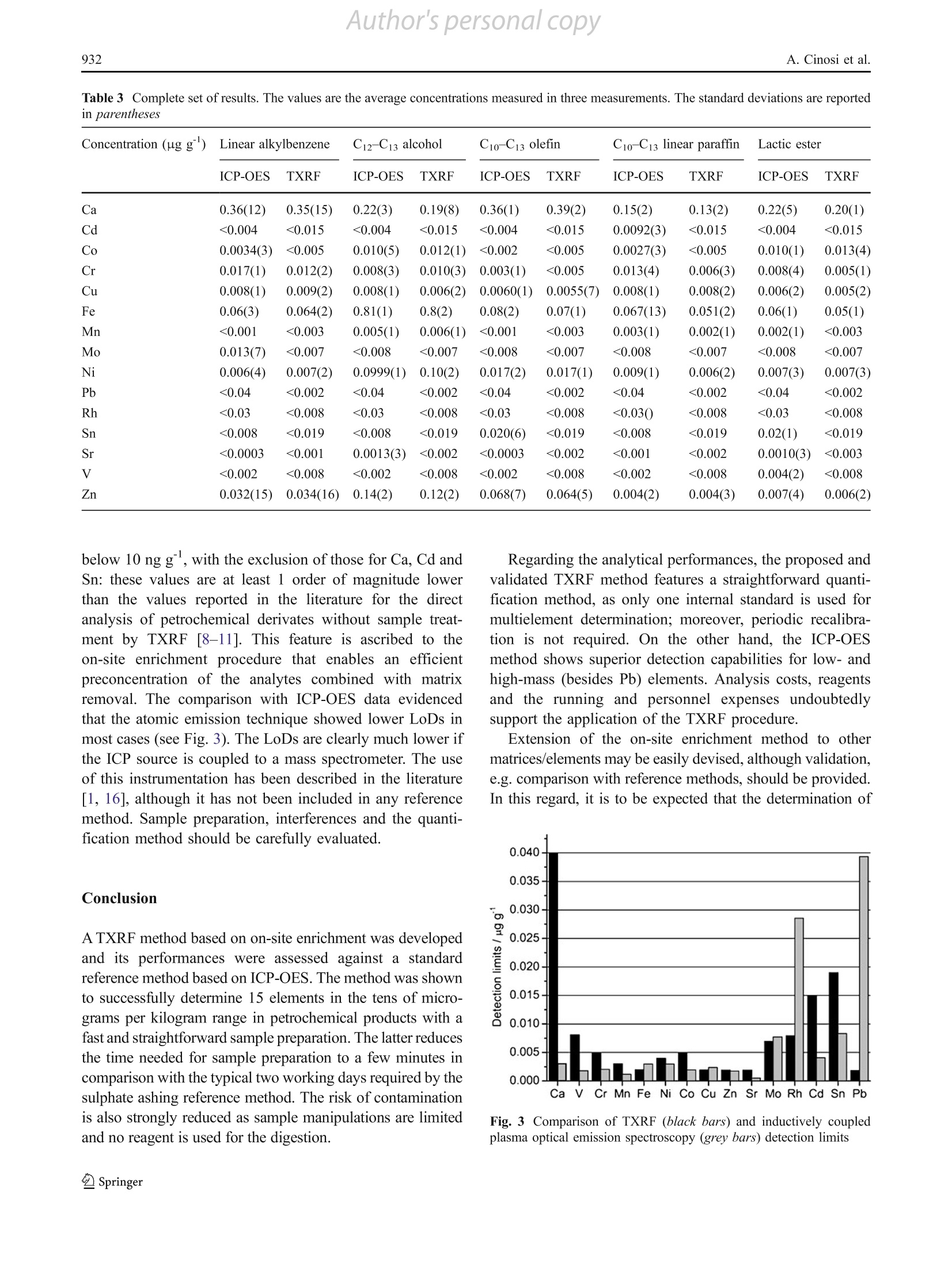
Author's personal copyAnal Bioanal Chem (2011) 399:927-933DOI 10.1007/s00216-010-4352-xORIGINAL PAPER Author's personal copyA. Cinosi et al.928 A novel total reflection X-ray fluorescence procedurefor the direct determination of trace elementsin petrochemical products Amedeo Cinosi·Nunzio Andriollo · Giancarlo Pepponi·Damiano Monticelli Received: 28 September 2010/Revised: 15 October 2010/Accepted: 17 October 2010 /Published online:3 November 2010C Springer-Verlag 2010 Abstract A total reflection X-ray fluorescence (TXRF)procedure was developed for the determination of metaltraces in petrochemical end products or intermediates forsurfactant synthesis.. The method combines a ffast andstraightforward sample preparation, i.e. deposition on thesample holder and evaporation of the sample matrix, withan efficient quantification method based on internalstandardization (organic gallium standard). The methoddeveloped showed detection limits below 0.05 ugg and inmost cases below 0.005 ug g. Fifteen elements (Ca, Cd,Co, Cr, Cu, Fe, Mn, Mo, Ni, Pb, Rh, Sn, Sr, V and Zn) weredetermined in matrices such as paraffins, n-olefins, linearalkylbenzenes, long-chain alkyl alcohols and esters: typicalmetal contents were below 1 ug g. The results werecompared with the reference method ASTM D5708 (test A. Cinosi·N. AndriolloSasol Italy SpA,via Reali 4.20037 Paderno Dugnano, Italy G. Pepponi Fondazione Bruno Kesslervia Sommarive 18. 38050 Povo, Trento, Italy D. Monticelli() Dipartimento di Scienze Chimiche e Ambientali, Universita degli Studi dell'Insubria -sede di Como, via Valleggio 11, 22100 Como, CO, Italy e-mail: damiano.monticelli@uninsubria.it Present Address.A. CinosiGNR srl.via Torino 7-28010,Agrate Conturbia, Novara, Italy method B) based on inductively coupled plasma opticalemission spectroscopy: advantages and drawbacks of thetwo procedures were critically evaluated. The TXRFmethod developed showed comparable precision andabsence of bias with respect to the reference method. Acomparison of the performances of the two methods ispresented. Keywords Total reflection X-ray fluorescence·Inductivelycoupled plasma optical emission spectroscopy.Traceelements· Petrochemical products· Direct analysis Introduction Elemental impurities in petrochemical products are a keyfactor for monitoring the quality of a whole productionchain. Information on trace element content and the relativeratio of the trace elements greatly helps in solving issuesrelated to the production processes and the logistic mediaquality (storage and transport). In particular, it is possible todetect corrosion phenomena (e.g. the presence of Fe, Cr,Ni, etc.) or to reveal contamination of the products withelements from catalyst residues (e.g. Co, Ni, Pt, Rh, Cr, Cuand Ba). Several standard methods for the quantitative analysis ofelements dispersed in organic phases have been developed,aiming at assuring the repeatability and reproducibility ofthe measurements. The first group of standard methods(Table 1, methods 1-6) involves a treatment with concen-trated sulphuric acid (sulphate ashing) at high temperature(about 550°℃); the main advantage of these methods is thata low limit of detection (LoD) can be obtained. Thisprocedure is nevertheless time-consuming, requiring fromseveral hours up to some days, hence causing a significant Table 1 Standard methods for the analysis of trace elements in petrochemical derivates (see [17] for ASTM methods, http://www.iso.ch for ISOstandards and http://www.uni.com for the UNI EN method) Method Standard method Analytes Matrix Technique 1 ASTM D5863 (test method A) Ni, V, Fe Crude oils and residual AAS fuels 2 ISO 8691 V, low levels (0.4-4 mg/kg) Liquid fuels AAS 3 ASTM D5708 (test method B) Ni, V, Fe Crude oils and residual ICP-OES fuels 4 ASTM D3340 Li, Na Lubricant greases AAS 5 ASTM D5184 Al, Si Fuel oils ICP-OES and AAS 6 ISO 10478 Al, Si Fuel oils ICP-OES and AAS 7 ASTM D3237 Pb Fuel AAS 8 ASTM D3605 Ca, Na, Pb, V Gas fuel AAS 9 ASTM D3831 Mn MMT in gasoline AAS 10 ASTM D4268 Ba, Ca, Mg, Zn Unused lubricant oil AAS 11 ASTM D4951 B, Ba, Ca, Cu, Mg, P, S, Zn Lubricant oil ICP-OES 12 ASTM D5185 Ag, Al, B, Ba, Ca, Cr, Cu, Fe, K, Mg, Mn, Mo, Used lubricant oil ICP-OES 13 ASTM D5708 (test method A) Na, Ni, P, Pb,S, Si, Sn, Ti, V, Zn Ni, Fe, V Crude oils and residual ICP-OES fuels 14 ASTM D5863 (test method B) Fe, Na, Ni, V Crude oils and residual AAS fuels 15 UNI EN 13131 Ni, V Petroleum products AAS 16 ASTM D7111 Ag, Al, Ba, Ca, Cr, Cu, Fe, K, Li, Mg, Mn, Mo, Middle distillate fuels ICP-OES 17 ASTM D4927 Na, Ni, Pb, Si, Ti, V, Zn Ba, Ca, P, S, Zn Lubricant and additive EDXRF component 18 ASTM D5059 Pb Gasoline EDXRF 19 ASTM D6443 Ca, Cl, Cu, Mg, P, S, Zn Unused lubricating oil WDXRF and additives 20 ASTM D6481 Ca, P,S, Zn Lubricant oil EDXRF 21 ASTM D6842 Ca, P,S, Zn Unused lubricant oil EDXRF 22 ISO 14597 Ni, V and additive Petroleum products EDXRF MMT methylcyclopentadienyl manganese tricarbonyl, AAS atomic absorption spectroscopy, ICP-OES inductively coupled plasma opticalemission spectroscopy, EDXRF energy-dispersive X-ray fluorescence, WDXRF wavelength-dispersive X-ray fluorescence probability of contamination. Moreover, “volatile” ele-ments, such as Hg, As and Se, could be partially lost andthus underestimated. A second group of analytical methods(Table 1, methods 7-15) involves sample dilution with asuitable solvent (e.g. toluene) and subsequent determinationby an atomic technique such as inductively coupled plasma(ICP) optical emission spectroscopy (OES) or atomicabsorption spectroscopy. However, this uncomplicated andfast sample preparation causes sample dilution (and thushigher LoDs) and possible loss of elements contained in thenonsoluble phase of the sample (e.g. oxides). Direct analysis ofmiddle-distillate fuels is described inreference method ASTM D7111 (method 16 in Table 1) for19 trace elements, with LoDs in the range 0.1-2 mgkg. Microwave-assisted wet digestion is an efficient alterna-tive method to dissolve organic samples [1]. The closed-vessel microwave systems permit the digestion of the sample in a short time, but, on the other hand, only a fewhundred milligrams of sample can be treated. Openfocussed microwave systems allow digestion of largersamples [2-6]. Besides issues related to sample preparation, the cali-bration step should be carefully evaluated when “heavy”matrices are analysed. Spectral interferences as well asmatrix effects are to be expected: different strategies, suchas reconstructing a synthetic matrix, internal standardiza-tion or standard addition, should be adopted to overcomepossible error sources. In addition, a multielement calibra-tion should be performed. All of these methods requiremuch work and are time-consuming. X-ray fluorescence has also been investigated for traceelement determination in petrochemical products: sixstandard methods based on this technique are currentlyavailable, enabling the analysis of four to seven elements in different matrices (Table 1, methods 17-22). These proce-dures are fast and independent of the species distribution ofthe metal in the samples. The LoDs are neverthelessgenerally in the milligram per kilogram range. These valuesare often too high to meet product specifications, especiallyfor applications in pharmaceuticals, cosmetics and electron-ics, where a sub-milligram per kilogram quantification ismandatory (see, e.g., ISO standards 14706 and 17331 fortrace element analysis on silicon surfaces by total reflectionX-ray fluorescence, TXRF). TXRF is a special configuration of energy-dispersiveX-ray fluorescence in which the sample is illuminated ingrazing incidence conditions (below the critical angle fortotal reflection) and the fluorescence is observed with adetector positioned very close to the sample and at a rightangle with respect to the primary beam. Thanks to theelimination of ideally all matrix effects and to the increasedsignal-to-noise ratio, due to the very low spectral back-ground, TXRF leads to drastically reduced LoDs (see theauthoritative review by von Bohlen [7]). Applications of TXRF to fractions of petrochemicalproducts or petroleum crude oil have already beendescribed by different authors [8-12]. Several sampletreatments were used: direct analysis [8], dilution with orwithout evaporation [10, 12], and acidic mineralization [11,12]. ReuSs [L9] has shown that better performances areattained with O2 plasma ashing over digestion in nitric acidor extraction into an aqueous solution for vegetable andmineral oils. All of these methods generally report LoDshigher than 1 mgkg for the direct analysis of petroleumfractions. Ojeda et al.[8] succeeded in lowering the LoDsto 0.6, 0.1 and 0.4 for V, Fe and Ni respectively,with W Laas the excitation source and Compton scattering as aninternal reference for quantification. The aim of the present work is the definition of a TXRFmethod for the direct, preparation-free and sub-milligramper kilogram determination of trace elements in petrochem-ical products. The procedure involves the evaporation ofthe matrix and the use of a single internal standard adequatefor organic samples. The proposed method is comparedwith the reference method ASTM D5708 (test method B)based on ICP-OES determination: advantages anddrawbacks of the two procedures are critically evaluated. Experimental Apparatus A simultaneous ICP-OES Jarrell Ash IRIS instrument fromThermo was employed for all measurements. The instru-ment is equipped with a cyclonic spray chamber and astandard Meinhard nebulizer. The principal instrumentalparameters are shown in Table 2. The TXRF measurements were performed using an ItalStructures TX2000 total reflection X-ray spectrometer. Theinstrument is equipped with a 12-position carousel forautomatic sample exchange. Monochromatic (obtained by aW/C multilayer reflector) Mo Ko excitation or part of thewhite continuous spectrum at 33 keV from a long fine-focus tube with a Mo/W anode was used as the X-raysource. The fluorescent X-rays derived from the samplewere detected using a solid-state Li-drifted Si detector of20-mm’active area with an 8-um-thick Be window(Rontec, Germany). The measured energy resolution (fullwidth at half maximum) of the detector was 137 eV for MnKo. Data elaboration (intensity measurement and quantifi-cation) was carried out by the software packages providedby the manufacturer. The TXRF operating parameters areshown in Table 2. Reagents, standard and solutions Ultrapure quality reagents, H2SO4 (Fluka, 98% m/m),HNO3 (Fluka, 65% m/m) and ultrapure water produced bya Millipore Milli-Q system (18 MQcm) were used for thesulphate ashing procedure, the preparation of aqueouscalibration standards and sample dilution for ICP-OESanalysis. Multielement standard solutions of Ag, Al, Cr, Cu,Fe, Mg, Na, Ni, Pb, Si, Sn, Ti and Se were obtained bydiluting by mass with electronic grade xylene (Acros) thestandard solutions CONOSTANQ S-12 blended standard(Conoco, alkylaryl sulphonate elements, 100 ug g) andcoNOSTAN@ selenium standard (100 ug gfronConoco), respectively. The Ga standard was obtained by dissolving theappropriate mass of gallium(III) B-diketone (Alfa Aesar)in N-methyl pyrrolidone (Fluka) to obtain a 300 ug gGa Table 2 ICP-OES and total reflection X-ray fluorescence (TXRF) instrumental set-up ICP-OES TXRF RF view Radial Mo K 33 keV RF power 1,200 W Voltage (kV) 40 45 Nebulizer flow 0.65 L min ' Current (mA) 40 40 Auxiliary gas 0.65 L min Live time (s) 1,000 1,000 Sample uptake 1.85 mL min Internal standard Ga Ga stock solution. Gallium was chosen as the internal standardbecause of its very low abundance in petrochemicalproducts and good sensitivity in TXRF determination. Thismetallorganic standard shows high solubility and could beeasily employed as an internal standard in other organicmatrices. The Ga concentration was checked by TXRFusing a CONOSTAN@ cobalt 5,000 ug gbase oil as theinternal standard: in addition, no significant contaminationby other elements was evidenced. The Ga stock solutionwas diluted by mass with xylene up to a concentration of10 ugg and used as an internal standard solution forTXRF quantitative analysis. The stock solution was stablefor at least 1 month when it was stored in a low-densitypolyethylene closed bottle and kept in the dark. Sample analysis Five samples3 \were analysed for thisSstudy: linearalkylbenzene (HybleneTM, boiling point about 290 C),C12-C13 linear alcohol (LialTM, boiling point about 260°C), C1o-C13 olefin (boiling point about 210 °C), C1o-C13linear paraffin (LinparTM, boiling range 185-230 ℃) andlactic esters (Cosmacol ELITM, boiling point above 250 C asreported by the producer). Almost all the samples were eitherend products or intermediates of surfactant production;Cosmacol ELITM is a base product for cosmetic preparations. The analytical procedure for TXRF determinations wasas follows. Polished quartz diskss were used as sampleholders after siliconizing. Typically, a 10-uL aliquot ofsilicone solution in 2-propanol (Serva, catalogue no. 35130)was deposited onto the surface to be exposed to the X-raybeam and evaporated to dryness at room temperature. Thisstep helps to stabilize the drop of the deposited sample andto obtain a thin layer in the centre of the disk. A 10-g portion of the sample was spiked with the Gainternal standard solution (typically 0.4 g) in 15-mLdisposable polyethylene test tubes (Kartell) equipped witha screw-cap closure and accurately mixed with an orbitalshaker. Eight microlitres of this solution was transferred tothe centre of the quartz reflector disk and heated to drynesson a ceramic hot plate at 90-100 C. The effect of on-site enrichment, i.e. deposition ofincreasing sample masses, was investigated by performingthe above-mentioned procedure up to eight times (volumes8-64 pL, total mass 0.06-0.48 pg). This procedure, i.e.repeated deposition and evaporation of a small volume ofsolution, aimed at limiting the dispersion of the depositeddrop on the disk surface. LoDs were calculated as theconcentration of the element giving a signal equivalent to 3times the standard deviation of the background signal, inaccordance with IUPAC recommendations [13].l. Regarding the excitation sources, the Mo Ko line wasused for elements with Z<399 and Z>55, whereas the 33 keV from the continuous spectrum was employed forMo, Rh, Sn, Ag and Pd, since it gave a better sensitivity.All samples were analysed in triplicate: average valueswere reported. The same samples analyzed by TXRF were also treatedaccording to the reference standard test method ASTMD5708 (test method B) employing sulphate ashing andanalysed by ICP-OES. This treatment warrants that allspecies of the elements, such as organometallic andinorganic forms, are transformed into oxides or sulphatessoluble in acidic solutions with minimal dilution of thesample. Briefly, 10-g aliquots of the samples were weighedinto a 250-mL quartz crucible, mineralized with 10 mL ofsuprapure H2SO4 (98%) and carefully heated on a ceramichot plate to dryness. The residue was burned in a mufflefurnace at 550 C: the residual ashes were subsequentlydigested with suprapure HNO3 (65%). After evaporation toincipient dryness, the residue salts were dissolved withnitric acid and made up to volume in a 25-mL volumetric flaskwith ultrapure water. Several hours is required for both thesulphuric digestion and the muffle furnace ashing. Quantifica-tion was performed by external calibration: three multielementstandard solutions prepared from 1.000 ug g single-elementICP standard solution were used. The LoD in ICP-OES analysis was determined accordingto IUPAC recommendations as 33times the standarddeviation of ten blank measurements divided by thesensitivity [13]. The average values and the precision,expressed as standard deviation, were obtained from threeindependent sample preparations. Results and discussion The initial part of the research was devoted to thedevelopment of the TXRF procedure and the determinationof its analytical figures of merit. The procedure wasinitiallyassessseedd through arecovery test to evaluate the accuracy and precision ofthe proposed method, in particular, the reliability of theon-site enrichment procedure and of the use of gallium(III) B-diketone as an internal standard required to betested. Accordingly, a recovery test using a multielementstandard solution diluted in fatty alcohols to which theGa standard solution had been added as the internalstandard was performed. Concentrations in the sub-milligram per kilogram range (element concentration of0.4 ugg) were examined. As a result, Ti, Cr, Fe, Ni Cu,Se and Pb recoveries were demonstrated not to bestatistically different from 100%, ranging from 9191to121% with a median of 106% (the standard deviations ofthe three replicates spanned the range10-18%). Theproposed method was tthus demonstrated to correctly quantify the investigated elements in organic matrices ofmedium volatility. The effect of the deposited volume on LoDss wassubsequently investigated: the results obtained are reportedin Fig. 1. This trend in LoDs as a function of depositedvolume is typical of TXRF and derives from the fact thatboth the signal and the background increase when thedeposited mass increases: the result is a signal-to-noise ratiothat is inversely proportional to the square root of thedeposited sample mass[[14]. The on-site enrichmentprocedure was demonstrated to be an effective means toreduce the LoDs below 10 ng g. In particular, thedeposition of 32 pL of solution (i.e. four deposition/evaporation cycles), corresponding to a deposited mass of12 ng of each element, seemed a good compromisebetween the time required for the procedure and the LoDsachieved. In fact a higher number of deposition/evaporationcycles leads to only limited benefits in terms of LoDlowering. Accordingly, a deposited volume of 32 pL wasused for all the following determinations. It should be notedthat the number of deposition/evaporation cycles can beselected as a function of the desired LoDs, making theprocedure extremely flexible. The optimized procedure was subsequently applied tothe detection of 15 elements (Ca, Cd, Co, Cr, Cu, Fe, Mn,Mo, Ni, Pb, Rh, Sn, Sr, V and Zn) in real petrochemical endproducts. A typical TXRF spectrum of the linear alkylben-zene obtained according to the procedure described isshown in Fig. 2. The same samples were analysed by thereference method ASTM D5708 (test method B) based onsulphate ashing followed by quantification by ICP-OES.The results of the analyses are detailed in Table 3. Regarding the precision of the proposed method, thestandard deviation of the results is not statistically differentfrom the standard deviations of the standard method (see Fig. 1 Total reflection X-ray fluorescence (TXRF) detection limitsversus deposited sample volume for an organic (xylene) standardsolution with 0.4 ug gof the elements shown in the key Fig. 2 TXRF spectrum of the linear alkylbenzene standard deviations in Table3). The accuracy of theproposed method was assessed by plotting the TXRF data(y-axis) against the ICP-OES data (x-axis): only sampleswith values higher than the LoD for both techniques wereconsidered. Discrepancies among data were generally lowerthan 10%: higher figures were observed when the concen-trations determined reached values close to the quantifica-tion limits. The interpolation of these data gave a linearfunction with a slope not distinguishable from 1 and anintercept not distinguishable from 0 (n=34, R=0.997).These data confirmed the hypothesis that the proposedmethod provides results that are not distinguishable fromthose obtained with the reference method, i.e. that theproposed method was not biased. The proposed procedure was therefore demonstrated tocause no loss of the analytes during the initial sampleevaporation phase. Elements are known to occur in crudeoil in different forms [15], among which organometallicspecies (e.g. metalloporphyrins) are highly represented.Metal species in petrochemical end products could onlypartially reflect, if at all, metal fractions in crude oil:different species, e.g. oxides, may also be expected as aresult of chemical processes and storage and transport ofcrude oil and/or petrochemical intermediates. In any case,organic phases, if present, may be lost by gas strippingduring evaporation before a stable salt is formed, whichcould be the major issue of the on-site enrichmentprocedure.The absence of bias with respect to the referencemethod clearly demonstrated the lack of significant loss ofthe analytes. Finally, quantification capabilities of the two methodswere compared in terms of LoDs (see Fig. 3). TXRFshowed lower LoDs for the middle-mass elements, as thesensitivity is limited for low-mass elements by the lowerfluorescence yield and for heavier elements by the fact thatL lines must be employed [7]. The LoDs are generally Table 3 Complete set of results. The values are the average concentrations measured in three measurements. The standard deviations are reportedin parentheses Concentration (ug g) Linear alkylbenzene C12-C13 alcohol C1o-C13 olefin C10-C13 linear paraffin Lactic ester ICP-OES TXRF ICP-OES TXRF ICP-OES TXRF ICP-OES TXRF ICP-OES TXRF Ca 0.36(12) 0.35(15) 0.22(3) 0.19(8) 0.36(1) 0.39(2) 0.15(2) 0.13(2) 0.22(5) 0.20(1) Cd <0.004 <0.015 <0.004 <0.015 <0.004 <0.015 0.0092(3) <0.015 <0.004 <0.015 Co 0.0034(3) <0.005 0.010(5) 0.012(1) <0.002 <0.005 0.0027(3) <0.005 0.010(1) 0.013(4) Cr 0.017(1) 0.012(2) 0.008(3) 0.010(3) 0.003(1) <0.005 0.013(4) 0.006(3) 0.008(4) 0.005(1) Cu 0.008(1) 0.009(2) 0.008(1) 0.006(2) 0.0060(1) 0.0055(7) 0.008(1) 0.008(2) 0.006(2) 0.005(2) Fe 0.06(3) 0.064(2) 0.81(1) 0.8(2) 0.08(2) 0.07(1) 0.067(13) 0.051(2) 0.06(1) 0.05(1) Mn <0.001 <0.003 0.005(1) 0.006(1) <0.001 <0.003 0.003(1) 0.002(1) 0.002(1) <0.003 Mo 0.013(7) <0.007 <0.008 <0.007 <0.008 <0.007 <0.008 <0.007 <0.008 <0.007 Ni 0.006(4) 0.007(2) 0.0999(1) 0.10(2) 0.017(2) 0.017(1) 0.009(1) 0.006(2) 0.007(3) 0.007(3) Pb <0.04 <0.002 <0.04 <0.002 <0.04 <0.002 <0.04 <0.002 <0.04 <0.002 Rh <0.03 <0.008 <0.03 <0.008 <0.03 <0.008 <0.03() <0.008 <0.03 <0.008 Sn <0.008 <0.019 <0.008 <0.019 0.020(6) <0.019 <0.008 <0.019 0.02(1) <0.019 Sr <0.0003 <0.001 0.0013(3) <0.002 <0.0003 <0.002 <0.001 <0.002 0.0010(3) <0.003 V <0.002 <0.008 <0.002 <0.008 <0.002 <0.008 <0.002 <0.008 0.004(2) <0.008 Zn 0.032(15) 0.034(16) 0.14(2) 0.12(2) 0.068(7) 0.064(5) 0.004(2) 0.004(3) 0.007(4) 0.006(2) below 10 ng g, with the exclusion of those for Ca, Cd andSn: these values are at least 1 order of magnitude lowerthan the values reported in the literature for the directanalysis of petrochemical derivates without sample treat-ment by TXRF [8-11]. This feature is ascribed to theon-site enrichment procedure that enables an efficientpreconcentration of the analytes combined with matrixremoval. The comparison with ICP-OES data evidencedthat the atomic emission technique showed lower LoDs inmost cases (see Fig. 3). The LoDs are clearly much lower ifthe ICP source is coupled to a mass spectrometer. The useof this instrumentation has been described in the literature[1, 16], although it has not been included in any referencemethod. Sample preparation, interferences and the quanti-fication method should be carefully evaluated. Conclusion A TXRF method based on on-site enrichment was developedand its performances were assessed against a standardreference method based on ICP-OES. The method was shownto successfully determine 15 elements in the tens of micro-grams per kilogram range in petrochemical products with afast and straightforward sample preparation. The latter reducesthe time needed for sample preparation to a few minutes incomparison with the typical two working days required by thesulphate ashing reference method. The risk of contaminationis also strongly reduced as sample manipulations are limitedand no reagent is used for the digestion. Regarding the analytical performances, the proposed andvalidated TXRF method features a straightforward quanti-fication method, as only one internal standard is used formultielement determination; moreover, periodic recalibra-tion is not required. On the other hand, the ICP-OESmethod shows superior detection capabilities for low- andhigh-mass (besides Pb) elements. Analysis costs, reagentsand the running and personnel expenses undoubtedlysupport the application of the TXRF procedure. Extension of the on-site enrichment method to othermatrices/elements may be easily devised, although validation,e.g. comparison with reference methods, should be provided.In this regard, it is to be expected that the determination of Fig. 3 Comparison of TXRF (black bars) and inductively coupledplasma optical emission spectroscopy (grey bars) detection limits trace elements in different medium-volatility products (e.g.petroleum derivatives, biodiesel and pharmaceutical/cosmeticintermediates) could greatly benefit from the application oftheon-site enrichment procedure with TXRF detection. References ( 1. Bettinelli M , Spezi a S , Baron i U, Bizzarri G (1995) J Anal At Spectrom 10:555-560 ) ( 2. Kowalewska Z , R uszczynska A, Bulska E ( 2 005) Spectrochim A cta B 60:351 -3 59 ) ( 3. Lamble KJ, H ill SJ (1998) Analyst 1 23:103R- 13 3R ) ( 4. Liu J, Sturgeon R, Willie SN ( 1995) Analyst 120:1905-1909 ) ( 5. Sun YC, C hi P H, Shiue MY (2001) Anal Sci 1 7 :1395-1399 ) ( 6 . Wondimu T, Goessler W , I r golic KJ ( 2000) F resenius J Anal C h em 367:35-42 ) ( 7 . von B ohlen A (2009) Spectrochim A c ta B 64:821-832 ) ( 8. Ojeda N, Greaves ED, A lvarado J , Sajo-Bohus L (1993) Spectrochim Acta B 48:247-253 ) ( 9 . Reus U (1991) S pectrochim A cta B 46:1403-1411 ) ( 10. Schirmacher M, Freimann P , Schmidt D , D ahlmann G (1 9 93) Spectrochim Acta B 48:199-205 ) ( 11. Vilhune n JK, Bohle n AV, Schmeling M, Klockenkamper R,Klockow D (1997) Spectrochim Acta B 52:953- 9 59 ) ( 1 2 . Past i F , Torbol i A , Zanetti C, March i M (1996) J Phys I V 6 ( C4):627-633 ) 13. IUPAC (1995) Pure Appl Chem 67:1699 14. Bilbrey DB, Leland DJ, Leyden DE, Wobrauschek P, Aiginger H(1987) XRay Spectrom 16:161-165 15. Caumette G, Lienemann CP, Merdrignac I, Bouyssierea B,Lobinski R (2009) J Anal At Spectrom 24:263-276 16. Maryutina TA, Soin AV (2009) Anal Chem 81:5896-5901 ( 17. Nadkarni RA (2007) Guide to ASTM test methods for the an a lysis o f petroleum products and lubricants , 2n d edn . ASTM I nterna- tional , West Conshohocken ) Springer 本文使用全反射X射线荧光(TXRF)方法测定石化中间体或终产品中的痕量金属元素。该方法结合了基于内标(有机镓标样)的快速检测和简便的样品制备技术,即在载样片上沉积蒸发后的样品基质,以及有效的定量方法,检测限低于0.05μg/g,大多数情况下低于0.005μg/g。十五种元素(Ca, Cd, Co, Cr, Cu, Fe, Mn, Mo, Ni, Pb, Rh, Sn, Sr, V, Zn)在基质如石蜡,线型烷基苯,长链烷基醇和酯中测定出,典型金属元素含量低于1μg/g。将结果与基于ICP光谱测试的参考方法ASTM D5708(B)进行比较评估,TXRF方法表现出较优异的精确性和低偏差。
确定





还剩5页未读,是否继续阅读?
利曼中国为您提供《石化中间体或终产品中Ca, Cd, Co, Cr, Cu, Fe, Mn等元素含量检测方案(能散型XRF)》,该方案主要用于柴油中含量分析检测,参考标准--,《石化中间体或终产品中Ca, Cd, Co, Cr, Cu, Fe, Mn等元素含量检测方案(能散型XRF)》用到的仪器有TX 2000 全反射X荧光光谱仪
推荐专场
X荧光光谱、XRF(能量色散型X荧光光谱仪)
相关方案
更多
该厂商其他方案
更多
















