Raptor C18提供出色的分辨率,短总循环次数。更快的分析效率,2.7µ
颗粒。EPA方法537。全氟烷基酸是人造化合物用作制造各种表面活性剂产品,如消防泡沫,涂料添加剂,纺织品及清洁用品。他们已检测到世界各地的环境,并在世界各地使用非常大的数量。这些化合物是环境退化过程的主因.它们被广泛分布在土壤,空气,地下水,城市垃圾填埋场,和全氟辛烷磺酸富集电位酸(PFOS)和全氟辛酸(PFOA),特别是,对环境和人类健康构成潜在的不利影响。
方案详情

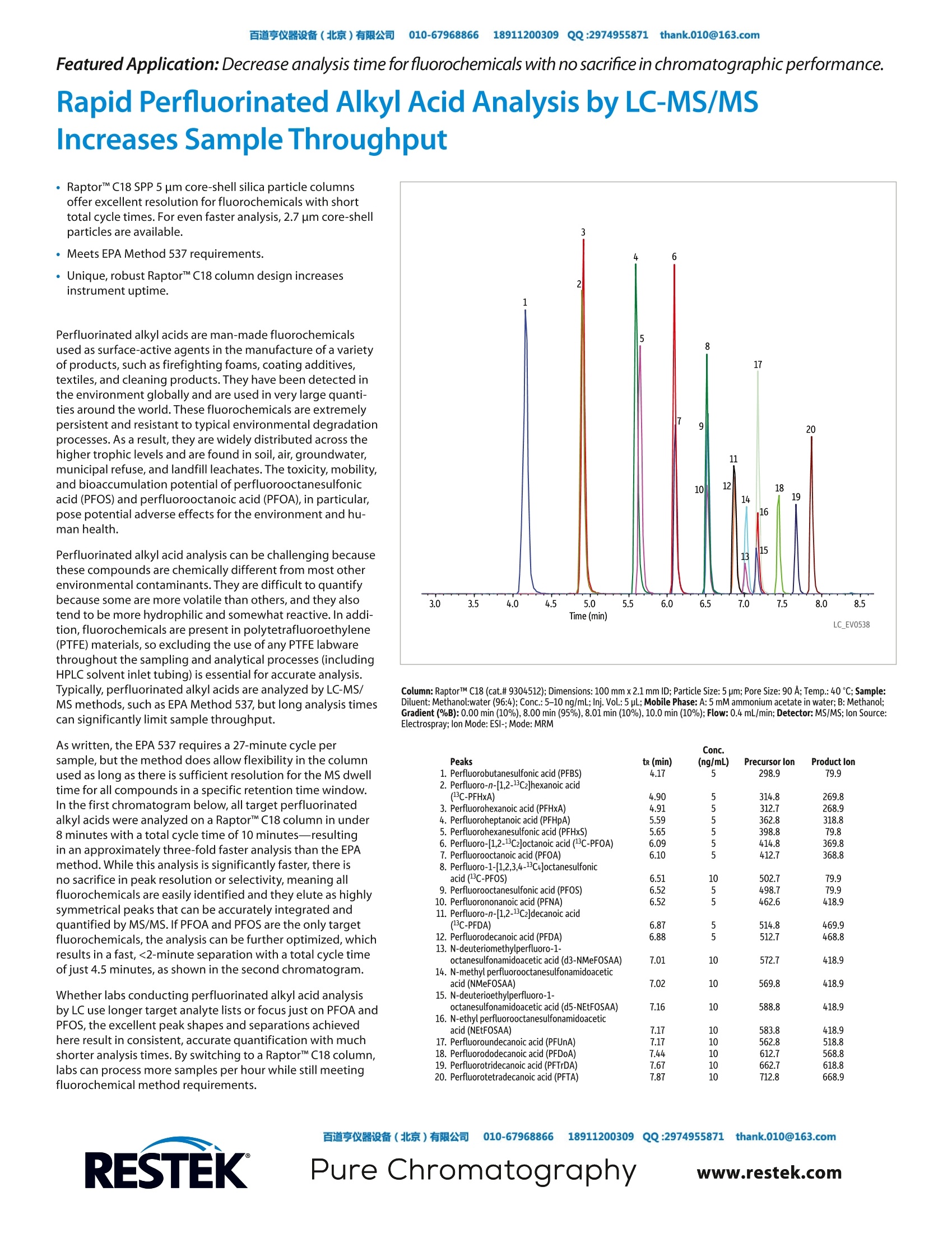
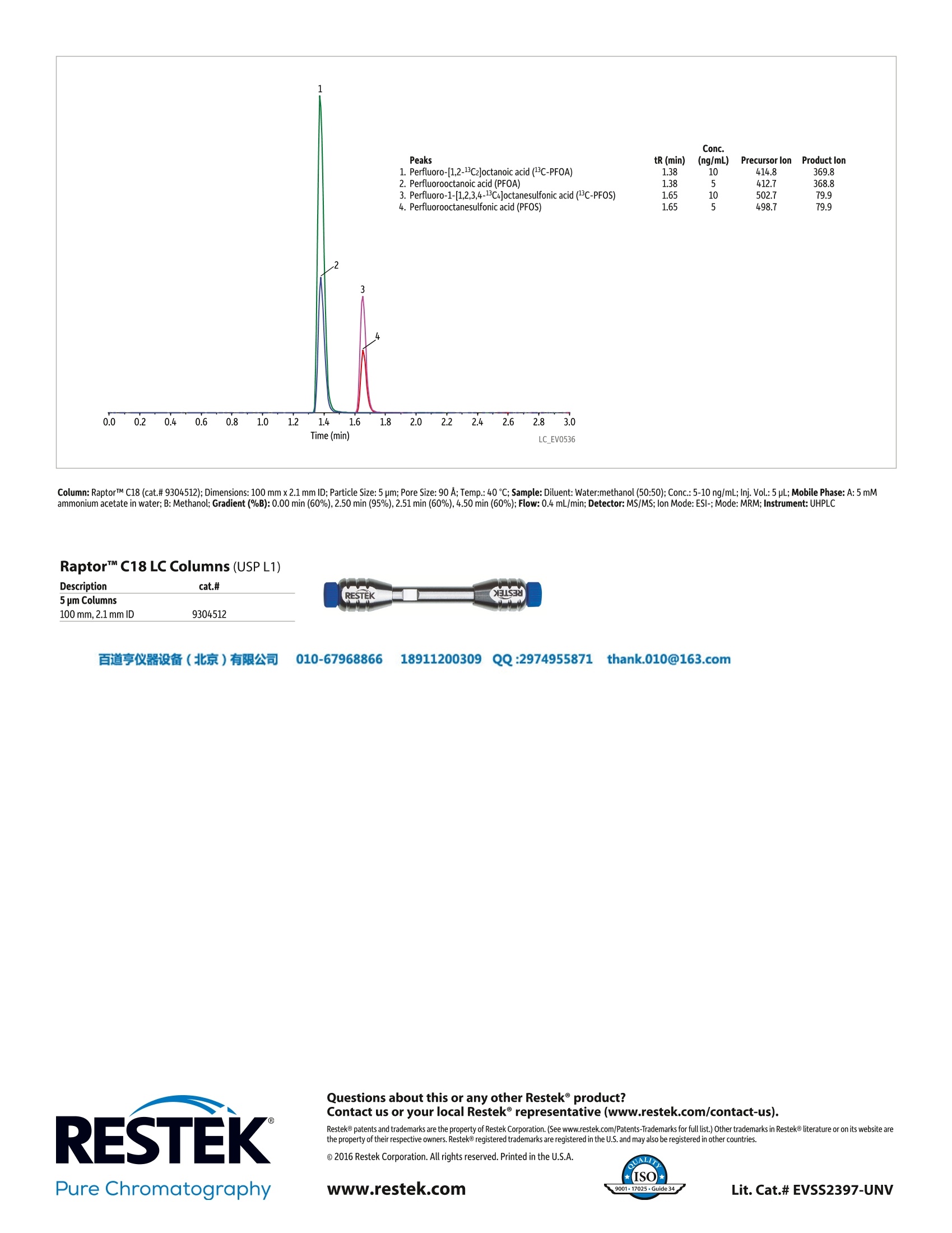
百道亨仪器设备(北京)有限公司 010-67968866 189112003099 QQ:29749558711thank.010@163.com ·Raptor"M C18 SPP 5 um core-shell silica particle columnsoffer excellent resolution for fluorochemicals with shorttotal cycle times. For even faster analysis, 2.7 um core-shellparticles are available. ·Meets EPA Method 537 requirements. ·Unique, robust Raptor C18 column design increasesinstrument uptime. Perfluorinated alkyl acids are man-made fluorochemicalsused as surface-active agents in the manufacture of a varietyof products, such as firefighting foams, coating additives,textiles, and cleaning products. They have been detected inthe environment globally and are used in very large quanti-ties around the world. These fluorochemicals are extremelypersistent and resistant to typical environmental degradationprocesses. As a result, they are widely distributed across thehigher trophic levels and are found in soil, air, groundwater,municipal refuse, and landfill leachates. The toxicity,mobility,and bioaccumulation potential of perfluorooctanesulfonicacid (PFOS) and perfluorooctanoic acid (PFOA), in particular,pose potential adverse effects for the environment and hu-man health. Perfluorinated alkyl acid analysis can be challenging becausethese compounds are chemically different from most otherenvironmental contaminants.They are difficult to quantifybecause some are more volatile than others, and they alsotend to be more hydrophilic and somewhat reactive. In addi-tion, fluorochemicals are present in polytetrafluoroethylene(PTFE) materials, so excluding the use of any PTFE labwarethroughout the sampling and analytical processes (includingHPLC solvent inlet tubing) is essential for accurate analysis.Typically, perfluorinated alkyl acids are analyzed by LC-MS/MS methods, such as EPA Method 537, but long analysis timescan significantly limit sample throughput. As written, the EPA 537 requires a 27-minute cycle persample, but the method does allow flexibility in the columnused as long as there is sufficient resolution for the MS dwelltime for all compounds in a specific retention time window.In the first chromatogram below, all target perfluorinatedalkyl acids were analyzed on a RaptorC18 column in under8 minutes with a total cycle time of 10 minutes-resultingin an approximately three-fold faster analysis than the EPAmethod. While this analysis is significantly faster, there isno sacrifice in peak resolution or selectivity,meaning allfluorochemicals are easily identified and they elute as highlysymmetrical peaks that can be accurately integrated andquantified by MS/MS. If PFOA and PFOS are the only targetfluorochemicals, the analysis can be further optimized, whichresults in a fast,<2-minute separation with a total cycle timeof just 4.5 minutes, as shown in the second chromatogram. Whether labs conducting perfluorinated alkyl acid analysisby LC uselonger target analyte lists or focus just on PFOA andPFOS, the excellent peak shapes and separations achievedhere result in consistent, accurate quantification with muchshorter analysis times. By switching to a RaptorC18 column,labs can process more samples per hour while still meetingfluorochemical method requirements. Rapid Perfluorinated Alkyl Acid Analysis by LC-MS/MSIncreases Sample Throughput Column: RaptorTM C18 (cat.# 9304512); Dimensions: 100 mmx 2.1 mm ID; Particle Size: 5 pm; Pore Size: 90 A;Temp.: 40℃; Sample:Diluent: Methanol:water (96:4); Conc.:5-10 ng/mL; Inj. Vol.:5 pL; Mobile Phase: A: 5 mM ammonium acetate in water; B: Methanol;Gradient (%B):0.00 min (10%),8.00 min (95%), 8.01 min (10%),10.0 min (10%); Flow: 0.4 mL/min; Detector: MS/MS; Ion Source:Electrospray;Ion Mode: ESI-;Mode: MRM Peaks tr (min) (ng/mL) Precursor lon Product lon 1. Perfluorobutanesulfonic acid (PFBS) 4.17 5 298.9 79.9 (C-PFHxA) 4.90 55555 314.8 269.8 2. Perfluoro-n-[1,2-1C2]hexanoic acid 3. Perfluorohexanoic acid (PFHxA) 4.91 312.7 268.9 4. Perfluoroheptanoic acid (PFHpA) 5.59 362.8 318.8 5. Perfluorohexanesulfonic acid (PFHxS) 5.65 398.8 79.8 6. Perfluoro-[1,2-13C2]octanoic acid (3C-PFOA) 6.09 414.8 369.8 7. Perfluorooctanoic acid (PFOA) 6.10 5 412.7 368.8 8. Perfluoro-1-[1,2,3,4-13C4]octanesulfonic acid("C-PFOS) 6.51 10 502.7 79.9 9. Perfluorooctanesulfonic acid (PFOS) 6.52 498.7 79.9 10. Perfluorononanoic acid (PFNA) 6.52 55 462.6 418.9 11. Perfluoro-n-[1,2-1C2]decanoic acid (C-PFDA) 6.87 5 514.8 469.9 12. Perfluorodecanoic acid (PFDA) 6.88 5 512.7 468.8 13. N-deuteriomethylperfluoro-1- octanesulfonamidoacetic acid (d3-NMeFOSAA) 7.01 10 572.7 418.9 14. N-methyl perfluorooctanesulfonamidoacetic acid (NMeFOSAA) 7.02 10 569.8 418.9 15. N-deuterioethylperfluoro-1- octanesulfonamidoacetic acid (d5-NEtFOSAA) 7.16 10 588.8 418.9 16. N-ethyl perfluorooctanesulfonamidoacetic acid (NEtFOSAA) 583.8 418.9 17. Perfluoroundecanoic acid (PFUnA) 562.8 518.8 18. Perfluorododecanoic acid (PFDoA) 7.44 612.7 568.8 19. Perfluorotridecanoic acid (PFTrDA) 662.7 618.8 20. Perfluorotetradecanoic acid (PFTA) 712.8 668.9 Column: RaptorTM C18 (cat.# 9304512); Dimensions: 100 mm x 2.1 mm ID; Particle Size: 5 pm; Pore Size: 90 A; Temp.: 40℃; Sample: Diluent: Water:methanol (50:50); Conc.: 5-10 ng/mL; Inj. Vol.: 5 pL; Mobile Phase: A: 5 mMammonium acetate in water; B: Methanol; Gradient (%B): 0.00 min (60%), 2.50 min (95%), 2.51min (60%), 4.50 min (60%); Flow: 0.4 mL/min; Detector: MS/MS; Ion Mode: ESI-; Mode:MRM; Instrument: UHPLC Raptor" C18 LC Columns (USPL1) Description cat.#5 pm Columns100mm , 2 . 1 mm I D 93 0 4 51 2 XIS3d Contact us or your local Restek° representative (www.restek.com/contact-us).RESTEK ( R estek@ p a tents and trademarks are t h e property of Restek Corporation. (See www.restek.com/Pa t ents-Trademarks for full list.) Other trademarks in Restek@ literat u re or on its we b site arethe property of their r e spective owners. Restek@registered trademarks are regi s t e r e d in the U.S.and m ay a l so be registered in oth e r countries. ) o 2016 Restek Corporation. All rights reserved. Printed in the U.S.A. 亨仪器设备(北京)有限公司 QQ:hank.comPure Chromatographywww.restek.com Raptor™ C18 SPP 5 µm core-shell silica particle columnsoffer excellent resolution for fluorochemicals with shorttotal cycle times. For even faster analysis, 2.7 µm core-shellparticles are available.• Meets EPA Method 537 requirements.• Unique, robust Raptor™ C18 column design increasesinstrument uptime.Perfluorinated alkyl acids are man-made fluorochemicalsused as surface-active agents in the manufacture of a varietyof products, such as firefighting foams, coating additives,textiles, and cleaning products. They have been detected inthe environment globally and are used in very large quantities around the world. These fluorochemicals are extremelypersistent and resistant to typical environmental degradationprocesses. As a result, they are widely distributed across thehigher trophic levels and are found in soil, air, groundwater,municipal refuse, and landfill leachates. The toxicity, mobility,and bioaccumulation potential of perfluorooctanesulfonicacid (PFOS) and perfluorooctanoic acid (PFOA), in particular,pose potential adverse effects for the environment and human health.Perfluorinated alkyl acid analysis can be challenging becausethese compounds are chemically different from most otherenvironmental contaminants. They are difficult to quantifybecause some are more volatile than others, and they alsotend to be more hydrophilic and somewhat reactive. In addition, fluorochemicals are present in polytetrafluoroethylene(PTFE) materials, so excluding the use of any PTFE labwarethroughout the sampling and analytical processes (includingHPLC solvent inlet tubing) is essential for accurate analysis.Typically, perfluorinated alkyl acids are analyzed by LC-MS/MS methods, such as EPA Method 537, but long analysis timescan significantly limit sample throughput.As written, the EPA 537 requires a 27-minute cycle persample, but the method does allow flexibility in the columnused as long as there is sufficient resolution for the MS dwelltime for all compounds in a specific retention time window.In the first chromatogram below, all target perfluorinatedalkyl acids were analyzed on a Raptor™ C18 column in under8 minutes with a total cycle time of 10 minutes—resultingin an approximately three-fold faster analysis than the EPAmethod. While this analysis is significantly faster, there isno sacrifice in peak resolution or selectivity, meaning allfluorochemicals are easily identified and they elute as highlysymmetrical peaks that can be accurately integrated andquantified by MS/MS. If PFOA and PFOS are the only targetfluorochemicals, the analysis can be further optimized, whichresults in a fast, <2-minute separation with a total cycle timeof just 4.5 minutes, as shown in the second chromatogram.Whether labs conducting perfluorinated alkyl acid analysisby LC use longer target analyte lists or focus just on PFOA andPFOS, the excellent peak shapes and separations achievedhere result in consistent, accurate quantification with muchshorter analysis times. By switching to a Raptor™ C18 column,labs can process more samples per hour while still meetingfluorochemical method requirements.
确定
还剩1页未读,是否继续阅读?
百道亨仪器设备(北京)有限公司为您提供《表面活性剂稀释液中全氟烷基酸检测方案(液相色谱柱)》,该方案主要用于环境水(除海水)中有机污染物检测,参考标准--,《表面活性剂稀释液中全氟烷基酸检测方案(液相色谱柱)》用到的仪器有
相关方案
更多









