
Thermoset polymers form the matrix in filled plastics and fiberreinforced composites used in a diversity of products. These range from consumer items and auto body panels to advanced composites for printed circuit boards (PCBs), aerspace structural components such as the Space Shuttle payload bay door and jet engine cowls and ducts, and expensive, high-performance sports equipment. Also, thermosets are used extensively as adhesives, molding compounds, and surface coatings, including protective solder masks for PCBs.
方案详情

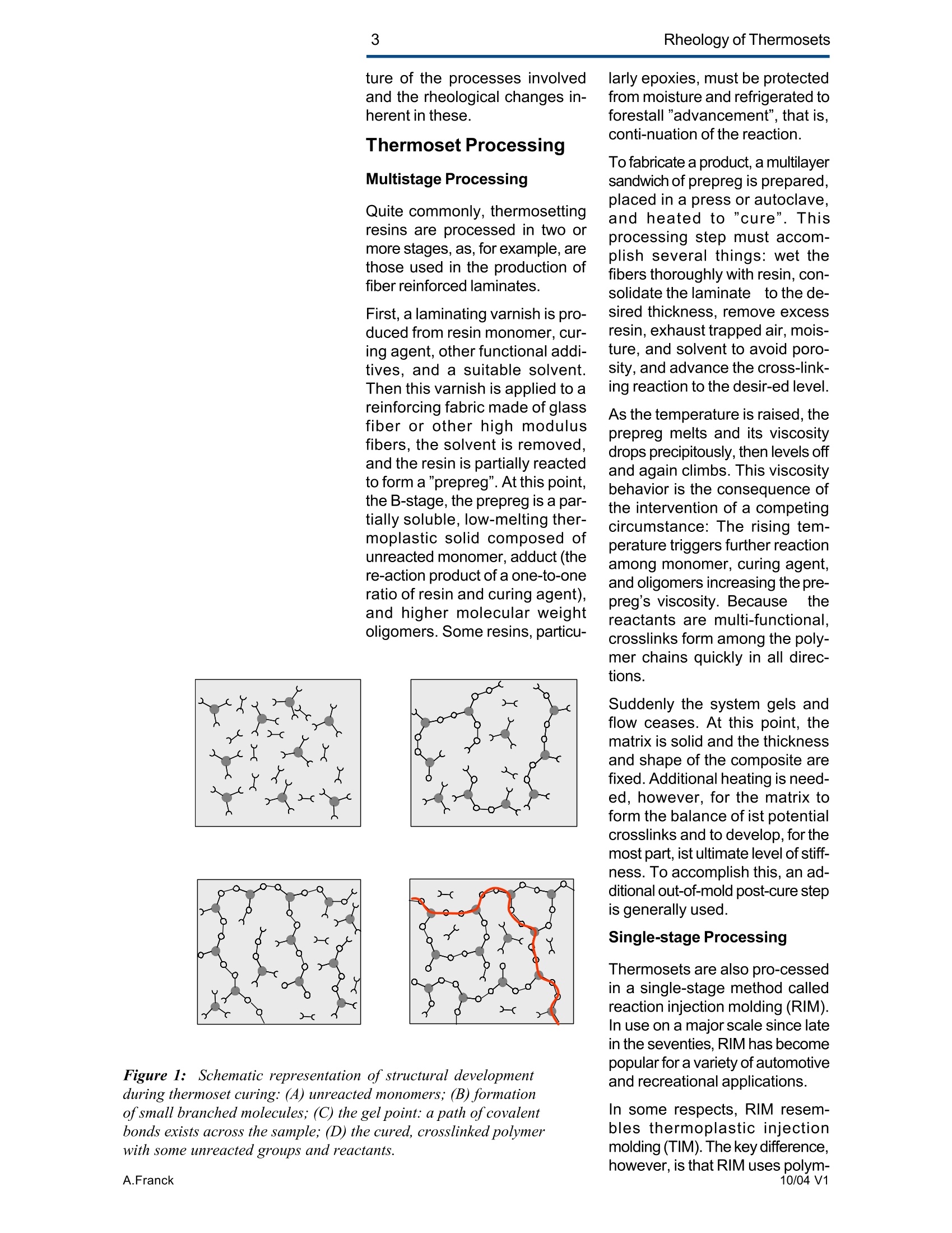
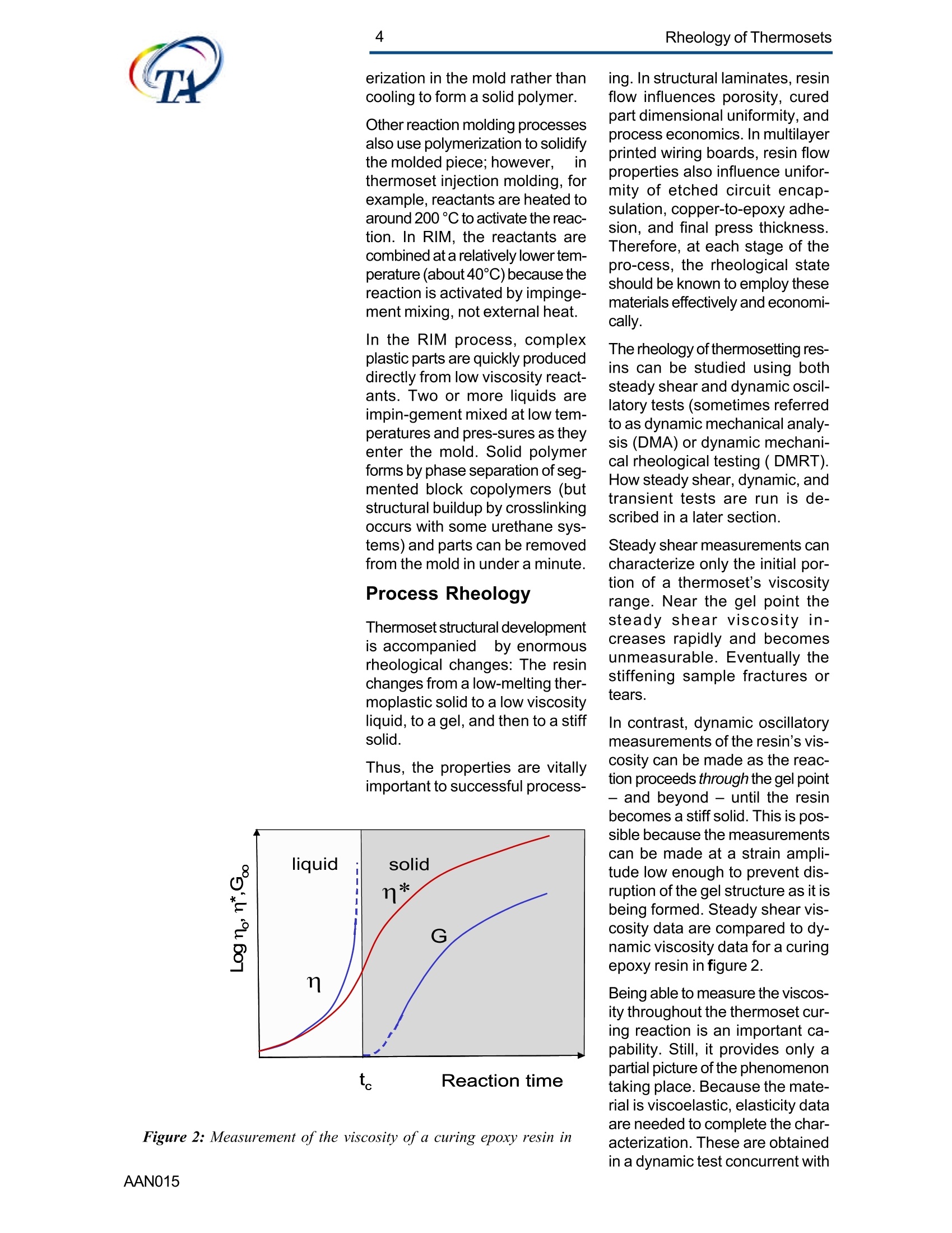
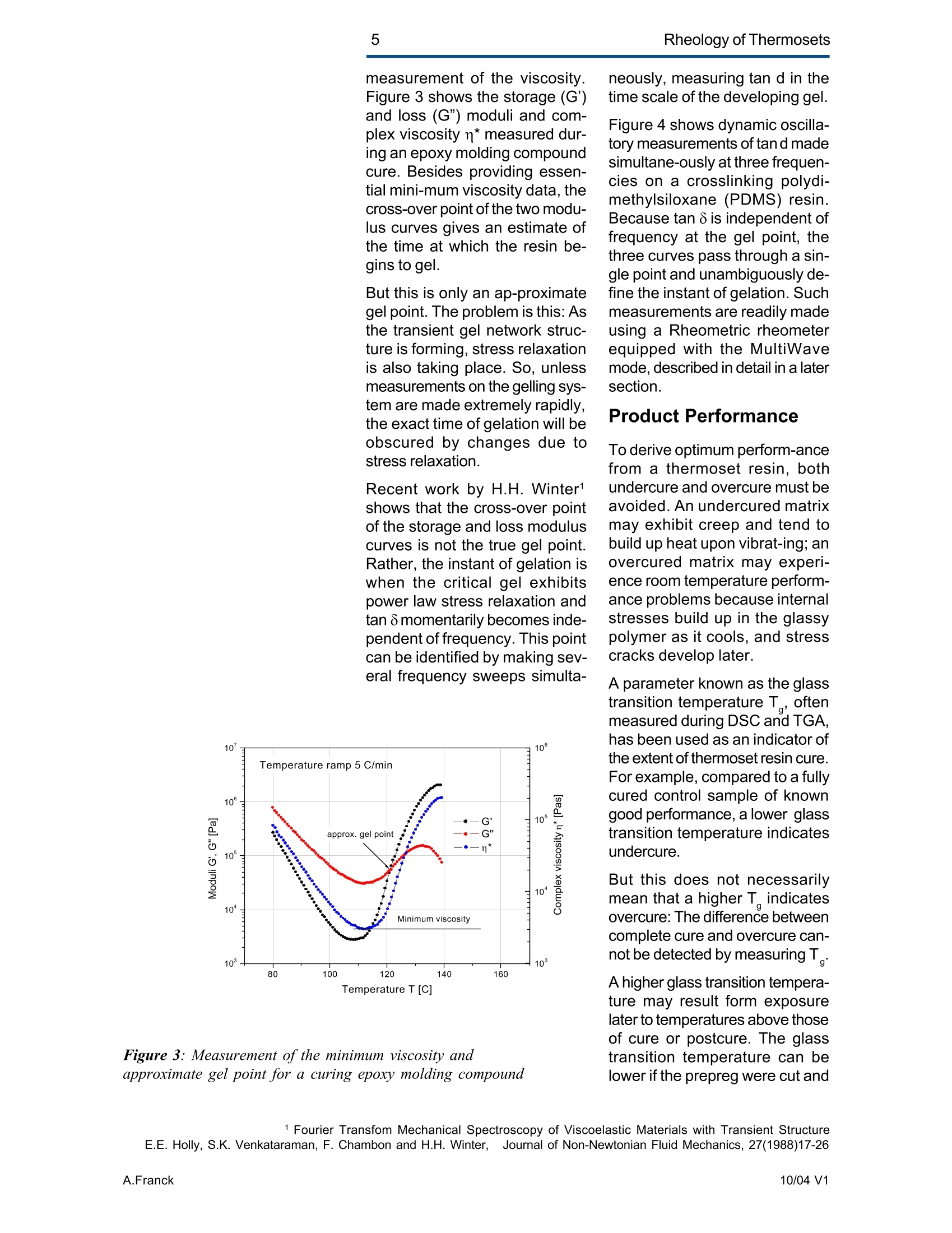
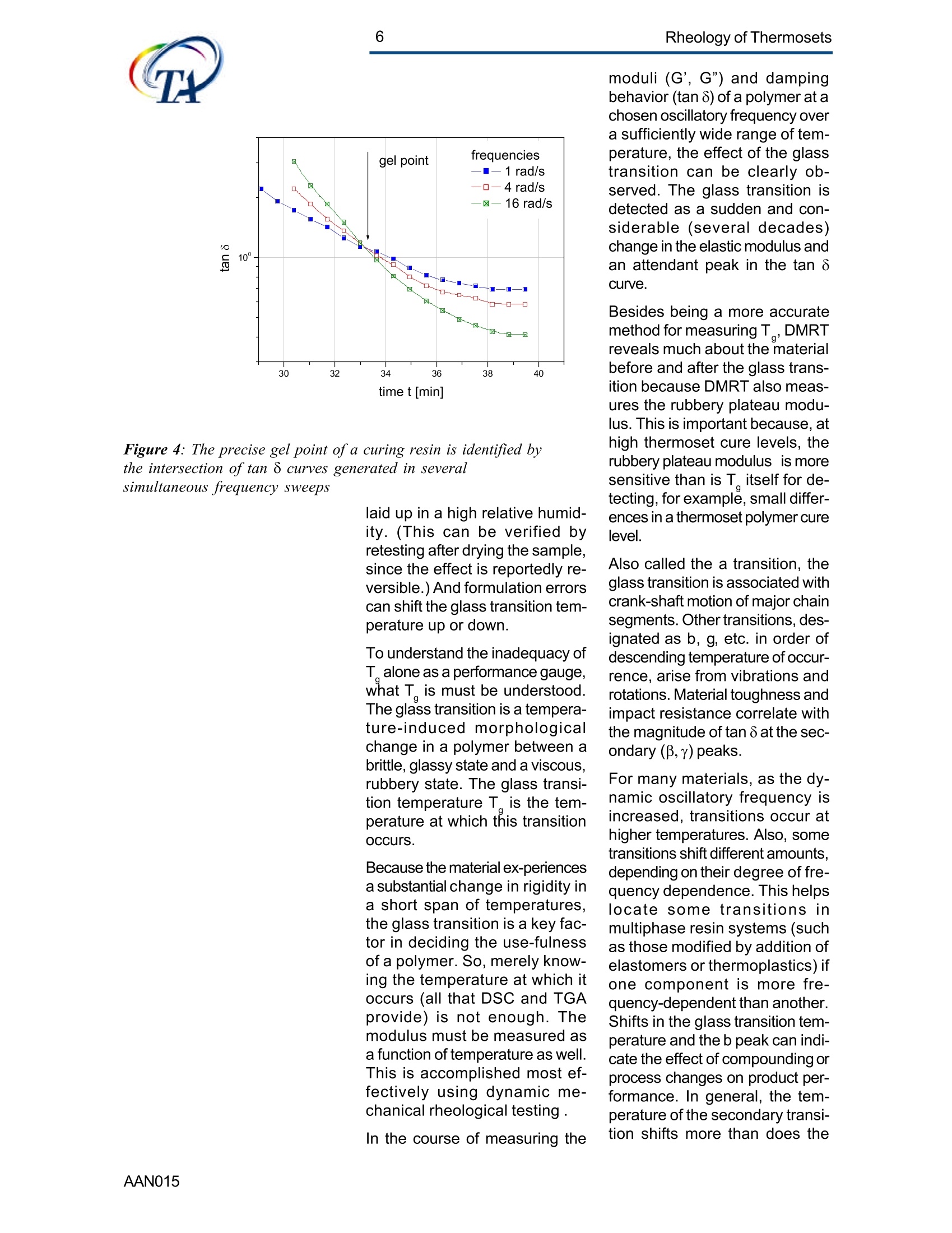
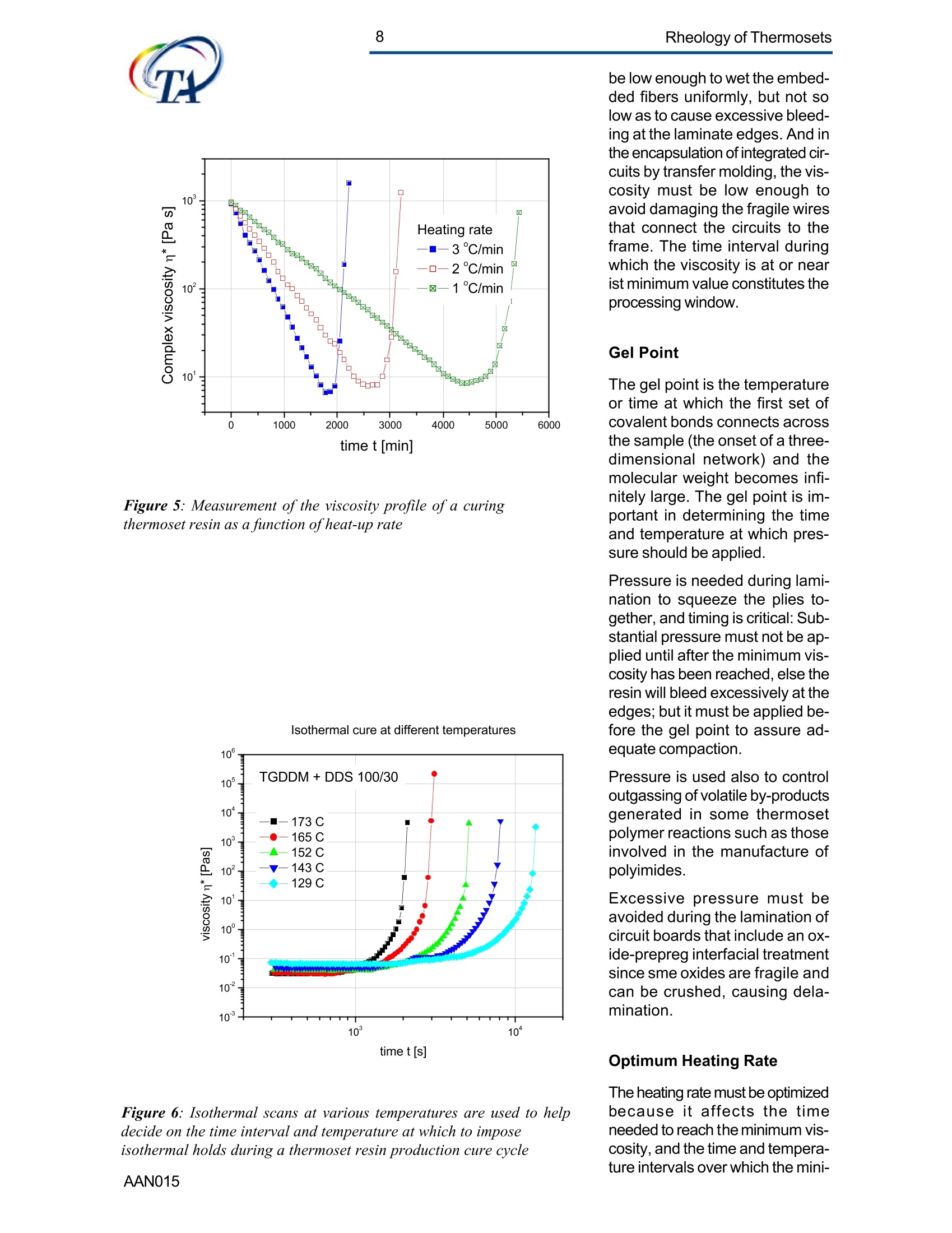
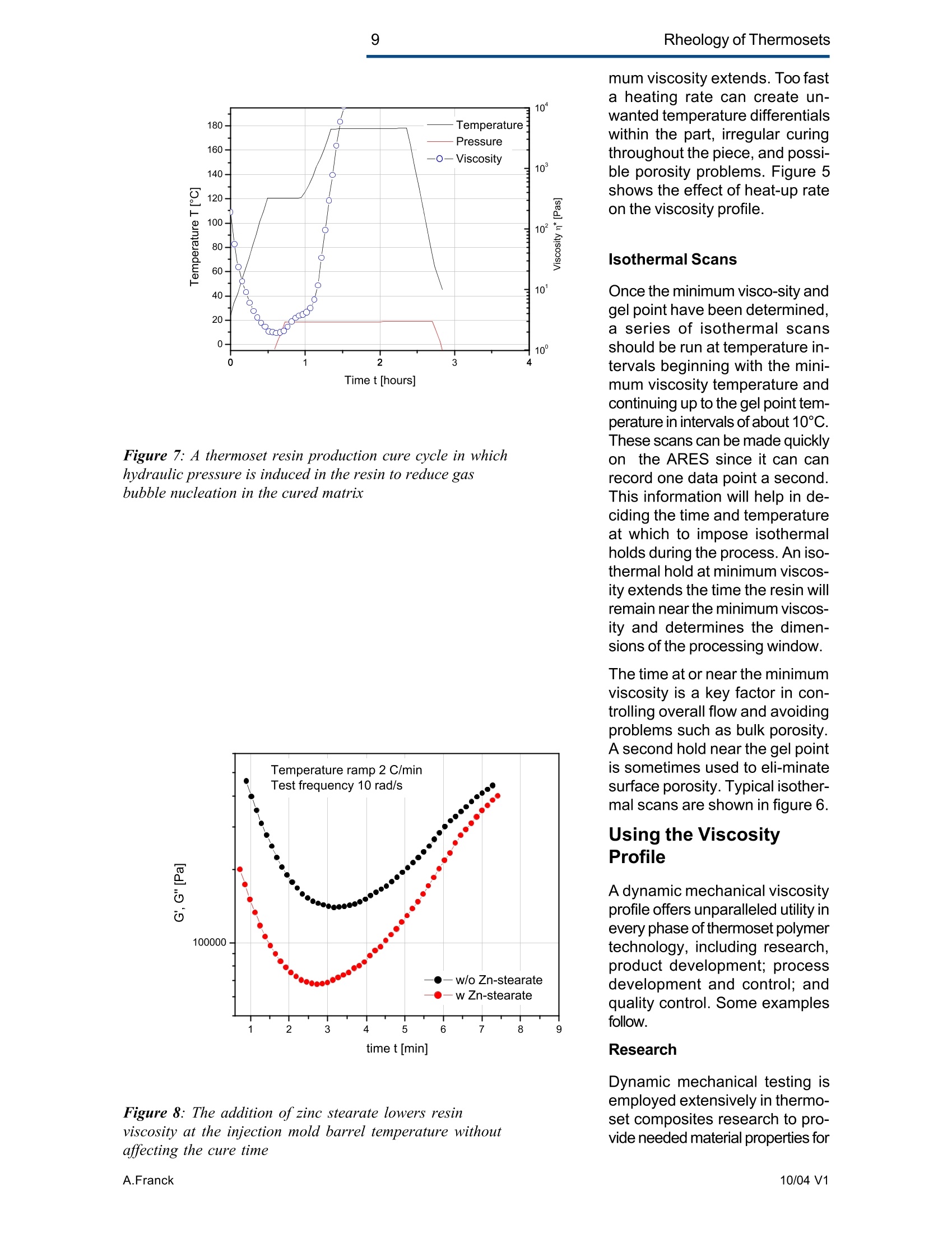
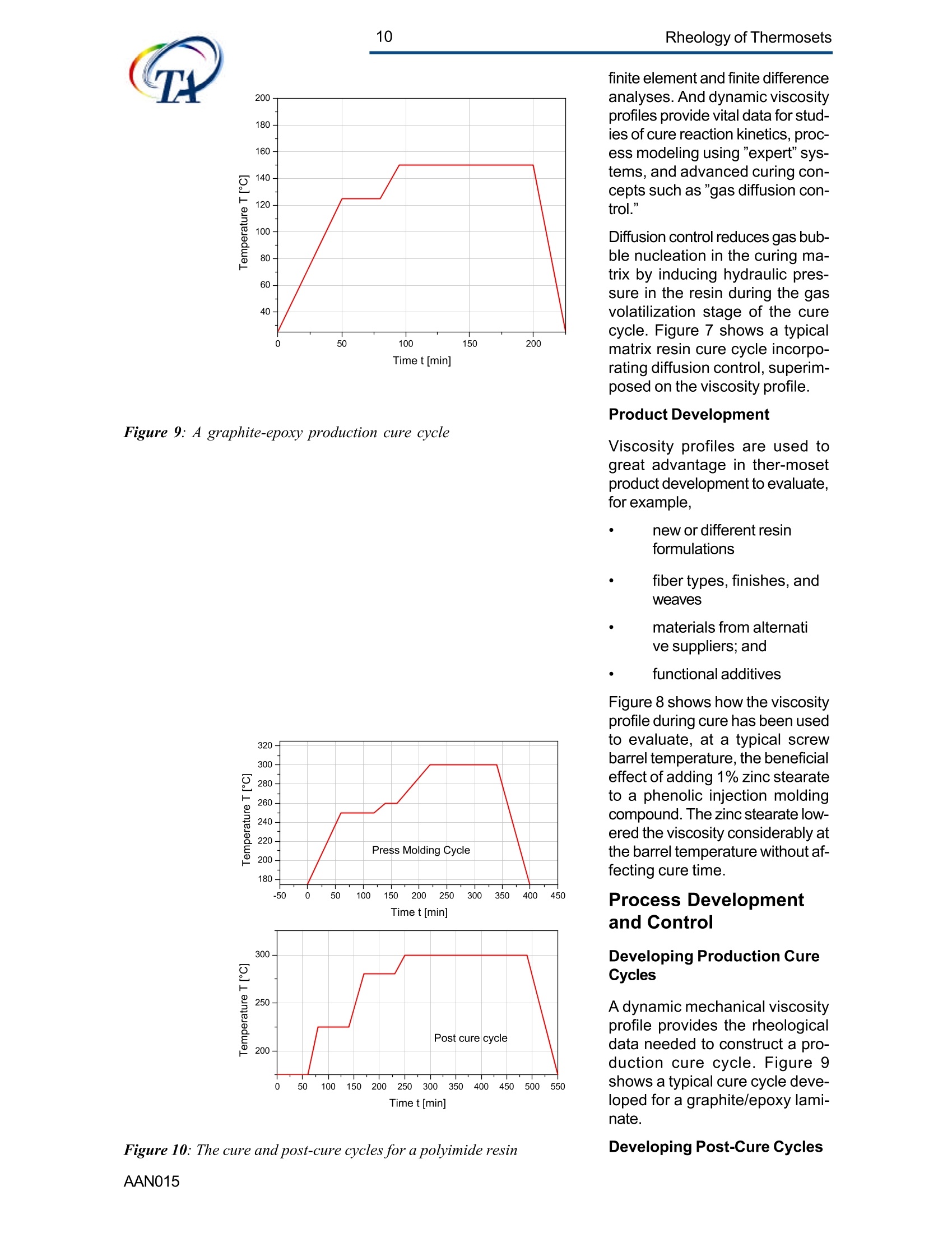
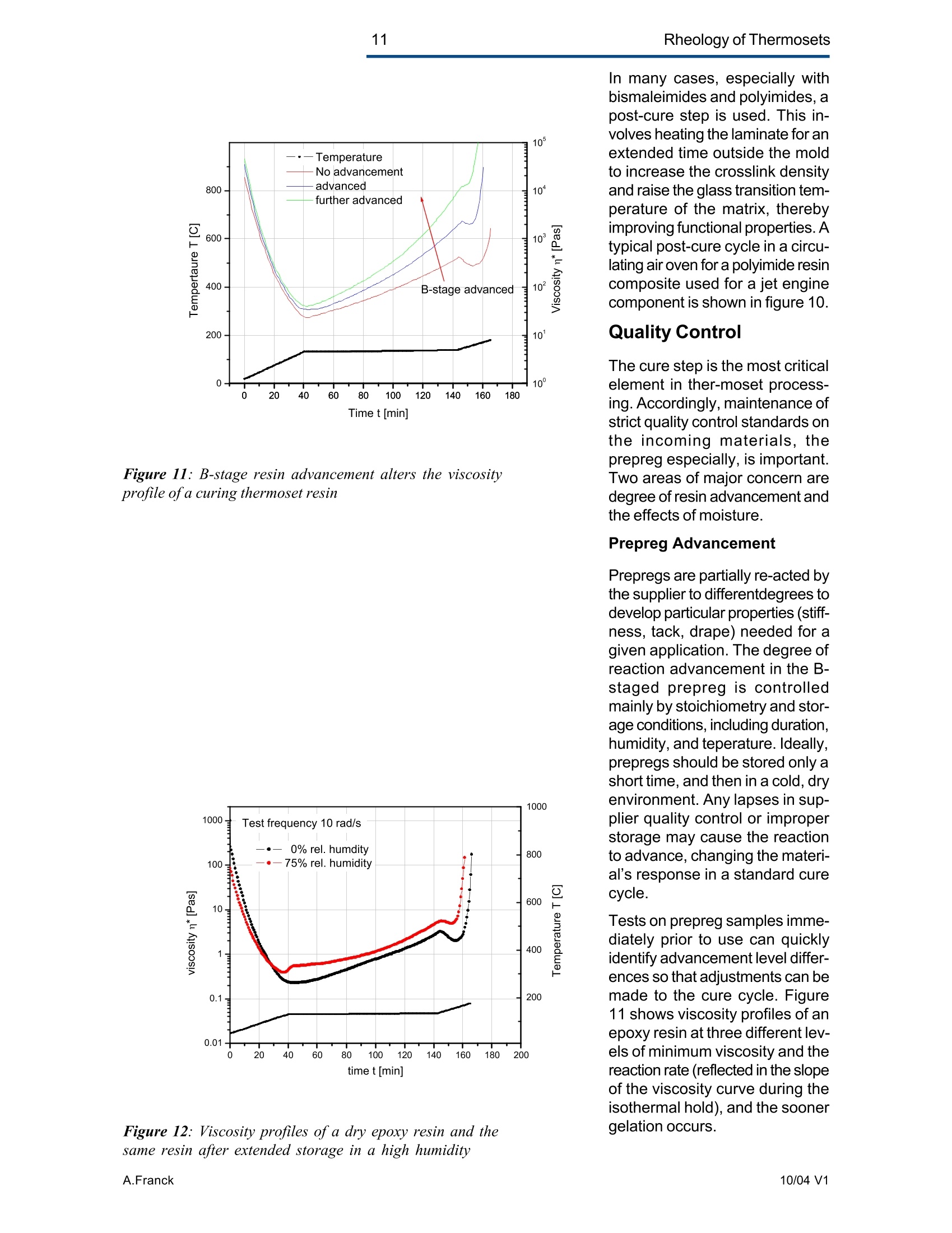
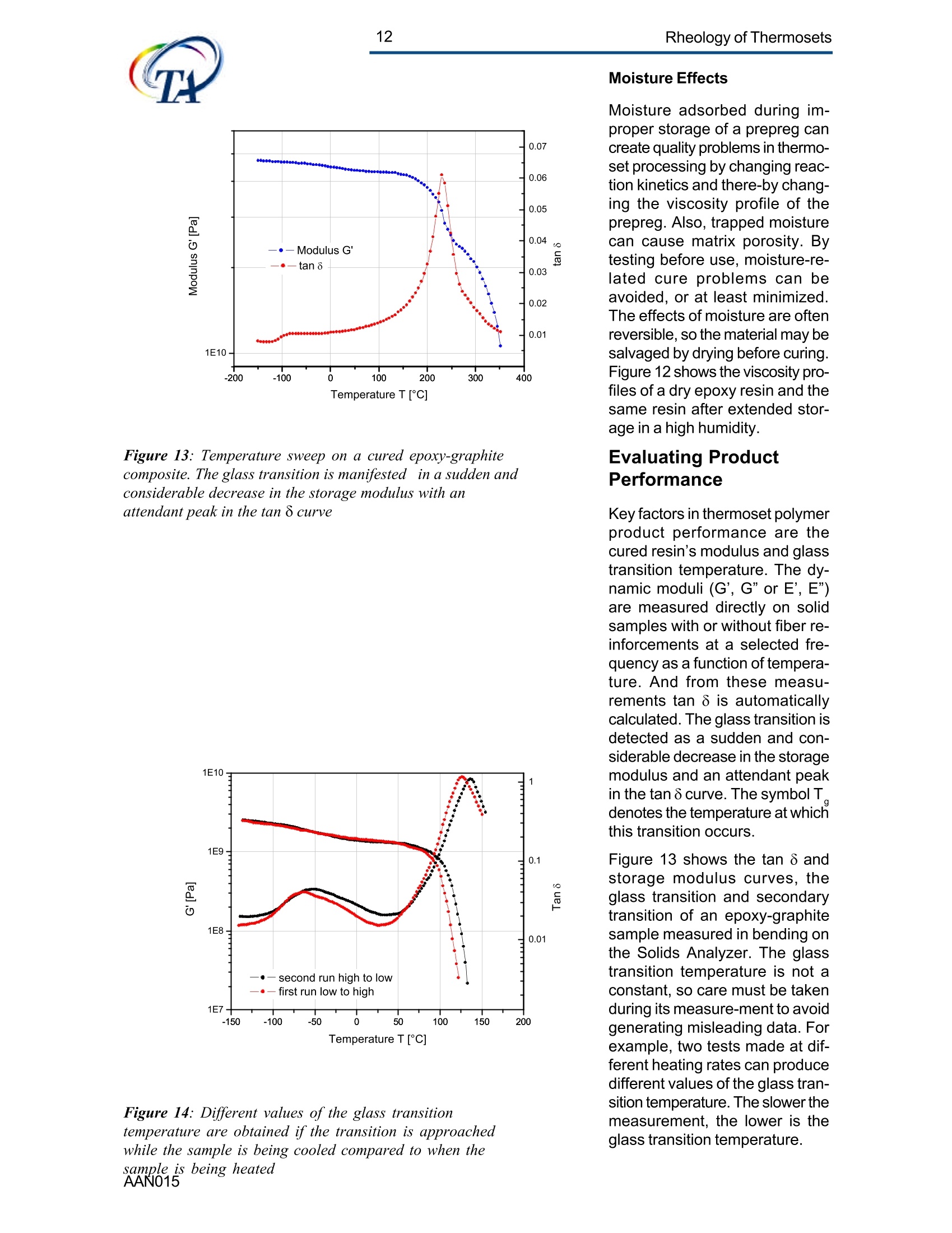
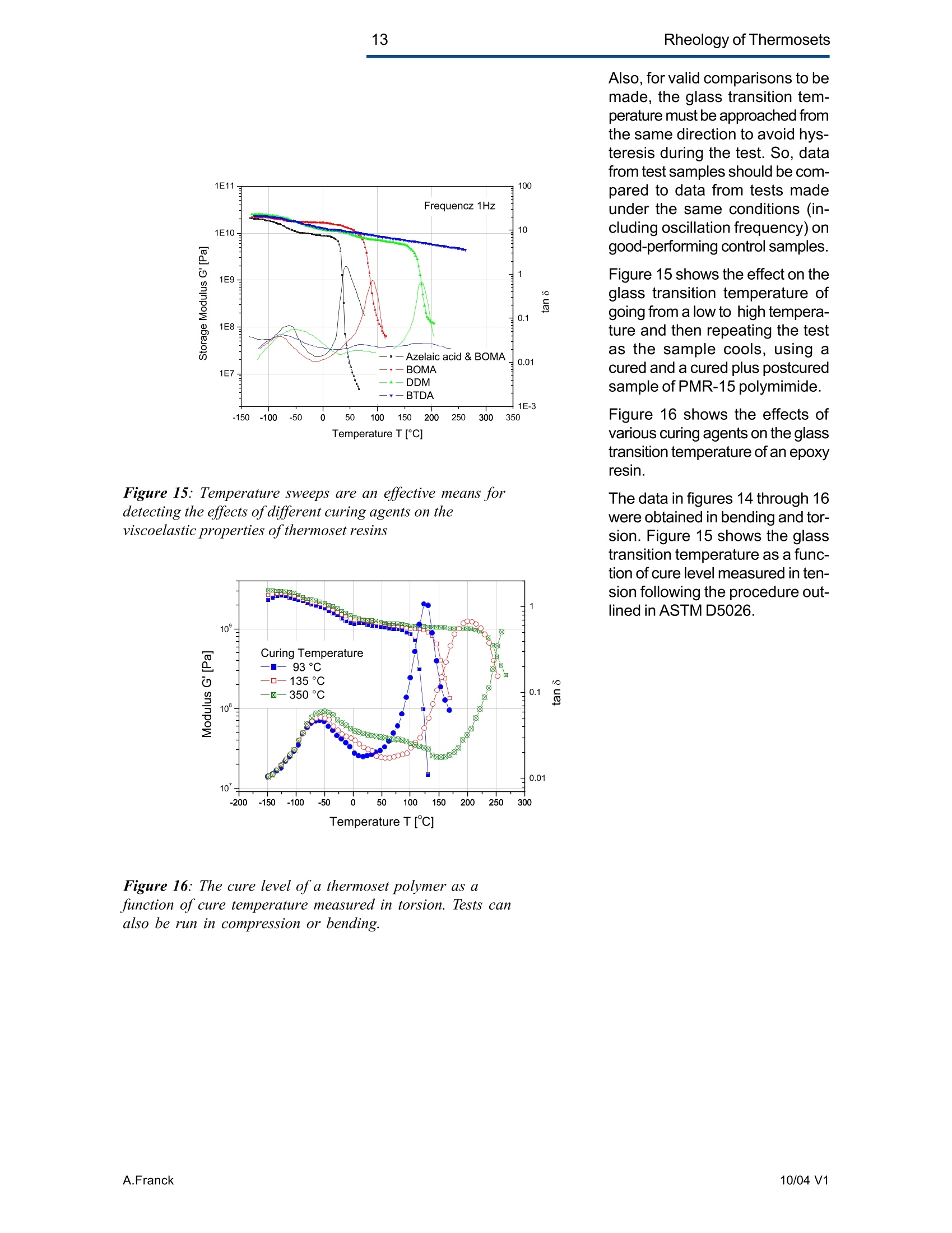
2Rheology of Thermosets UnderstandingRheology of Thermosets General ConsiderationsThermoset Polymer Uses Thermoset polymers form thematrix in filled plastics and fiber-reinforced composites used in adiversity of products. These rangefrom consumer items and autobody panels to advanced compos-ites for printed circuit boards(PCBs), aerspace structuralcompo-nents such as the SpaceShuttle payload bay door and jetengine cowls and ducts, and ex-pensive,high-performance sportsequipment. Also, thermosets areused extensively as adhe-sives,molding compounds, and surfacecoatings, including protective sol-der masks for PCBs. Thermosets versus Ther-moplastics and Elastomers Thermoset polymers are distin-quished from elastomers andthermoplastic polymers in severalways. Thermoplastics are processed inthe molten state, their final shapeand internal structure establishedby cooling, and they can be sof-tened and reshaped by reapplica-tion of heat and pressure. Also,their polymer chains,whether lin-ear or branched, remain di-screteafter molding. Thermoset poly-mers usually go through threestages. In the A-stage, some-times called a resole, the resin isstill soluble and fusible. In the B-stage, thermosets are nearly in-soluble, but are still thermoplas-tic. They can, however,spend onlya rela-tively short time in the mol-ten state because the tempera-tures that promote flow also causethe material to crosslink. Thecrosslinking reaction is accom-plished in the final stages of po-lymerization - the C-stage-dur-ing molding of the product under the controlled influence of heatand pressure over time. Thus,thermosets build their final struc-ture during processing, forming athree-dimensional internal struc-tural network of highly crosslinkedpolymer chains. And the finalproduct is insoluble and not ther-mally reformable. Elastomers share characteris-tics of thermoplastics andthermosets. Elastomers beginas thermoplastic polymers withdiscretechains that later developa network of covalent crosslinks.However, elastomers are distin-guished from thermosets by thefact that the crosslink network isformed in a separate post-polyme-rization step called vulcanization. Another factor distinguish-ing thethree classes of polymers is theglass transition temperature T-the temperature at which poly-mers reversibly transmute be-tween rubberyand glassy states.For elastomers, the glass transi-tion tempe-ature is below ambi-ent temperatures; for thermoplas-tics and thermosets, it is substan-tiallyabove ambiant. Studying the CrosslinkingReaction The formation of a thermosetcrosslinked network is shownschematically in figure 1. Understanding of this pro-cesshas been advanced substantiallyby use of rheological analysis-measurements of resin viscosity,shear modulus, and damping.More sensitive than even Fouriertransform infrared spectroscopyfor measuring extent of cure,rheological testing has becomea vital supplement to DSC, chro-matography, and wet chemicalanalysis in thermoset polymerre-search and develop-ment. Thereasons for this reside in the na- ture of the processes involvedand the rheological changes in-herent in these. Thermoset Processing Multistage Processing Quite commonly, thermosettingresins are processed in two ormore stages,as, for example, arethose used in the production offiber reinforced laminates. First, a laminating varnish is pro-duced from resin monomer, cur-ing agent, other functional addi-tives, and a suitable solvent.Then this varnish is applied to areinforcing fabric made of glassfiber or other high modulusfibers, the solvent is removed,and the resin is partially reactedto form a"prepreg”. At this point,the B-stage, the prepreg is a par-tially soluble, low-melting ther-moplastic solid composed ofunreacted monomer, adduct (there-action product of a one-to-oneratio of resin and curing agent),and higher molecular weightoligomers. Some resins, particu- Figure 1:: Schematic representation of structural developmentduring thermoset curing: (4) unreacted monomers; (B) formationof small branched molecules; (C) the gel point: a path ofcovalentbonds exists across the sample; (D) the cured, crosslinked polymerwith some unreacted groups and reactants. larly epoxies, must be protectedfrom moisture and refrigerated toforestall "advancement", that is,conti-nuation of the reaction. To fabricate a product, a multilayersandwich of prepreg is prepared,placed in a press or autoclave,and heated to "cure". Thisprocessing step must accom-plish several things: wet thefibers thoroughly with resin, con-solidate the laminate to the de-sired thickness, remove excessresin, exhaust trapped air, mois-ture, and solvent to avoid poro-sity, and advance the cross-link-ing reaction to the desir-ed level. As the temperature is raised, theprepreg melts and its viscositydrops precipitously, then levels offand again climbs. This viscositybehavior is the consequence ofthe intervention of a competingcircumstance: The rising tem-perature triggers further reactionamong monomer, curing agent,and oligomers increasing the pre-preg’s viscosity. Because thereactants are multi-functional,crosslinks form among the poly-mer chains quickly in all direc-tions. Suddenly the system gels andflow ceases.At this point, thematrix is solid and the thicknessand shape of the composite arefixed. Additional heating is need-ed, however, for the matrix toform the balance of ist potentialcrosslinks and to develop, for themost part, ist ultimate level of stiff-ness. To accomplish this, an ad-ditional out-of-mold post-cure stepis generally used. Single-stage Processing Thermosets are also pro-cessedin a single-stage method calledreaction injection molding (RIM).In use on a major scale since latein the seventies, RIM has becomepopular for a variety of automotiveand recreational applications. In some respects, RIM resem-bles thermoplastic injectionmolding(TIM). The key difference,however, is that RIM uses polym- erization in the mold rather thancooling to form a solid polymer. Other reaction molding processesalso use polymerization to solidifythe molded piece; however,inthermoset injection molding, forexample, reactants are heated toaround 200℃ to activate the reac-tion. In RIM, the reactants arecombined at a relatively lower tem-perature (about 40℃) because thereaction is activated by impinge-ment mixing, not external heat. In the RIM process, complexplastic parts are quickly produceddirectly from low viscosity react-ants. Two or more liquids areimpin-gement mixed at low tem-peratures and pres-sures as theyenter the mold. Solid polymerforms by phase separation of seg-mented block copolymers (butstructural buildup by crosslinkingoccurs with some urethane sys-tems) and parts can be removedfrom the mold in under a minute. Process Rheology Thermoset structural developmentis accompanied Iby enormousrheological changes: The resinchanges from a low-melting ther-moplastic solid to a low viscosityliquid, to a gel, and then to a stiffsolid. Thus, the properties are vitallyimportant to successful process- t. Reaction time Figure 2: Measurement of the viscosity ofa curing epoxy resin in ing. In structural laminates, resinflow influences porosity, curedpart dimensional uniformity, andprocess economics. In multilayerprinted wiring boards, resin flowproperties also influence unifor-mity of etched circuit encap-sulation, copper-to-epoxy adhe-sion, and final press thickness.Therefore, at each stage of thepro-cess, the rheological stateshould be known to employ thesematerials effectively and economi-cally. The rheology of thermosetting res-ins can be studied using bothsteady shear and dynamic oscil-latory tests (sometimes referredto as dynamic mechanical analy-sis (DMA) or dynamic mechani-cal rheological testing (DMRT).How steady shear,dynamic, andtransient tests are run is de-scribed in a later section. Steady shear measurements cancharacterize only the initial por-tion of a thermoset's viscosityrange. Near the gel point thesteady shear viscosity in-creases rapidly and becomesunmeasurable. Eventually thestiffening sample fractures ortears. In contrast, dynamic oscillatorymeasurements of the resin's vis-cosity can be made as the reac-tion proceeds through the gel point-and beyond- until the resinbecomes a stiff solid. This is pos-sible because the measurementscan be made at a strain ampli-tude low enough to prevent dis-ruption of the gel structure as it isbeing formed. Steady shear vis-cosity data are compared to dy-namic viscosity data for a curingepoxy resin in figure 2. Being able to measure the viscos-ity throughout the thermoset cur-ing reaction is an important ca-pability. Still, it provides only apartial picture of the phenomenontaking place. Because the mate-rial is viscoelastic, elasticity dataare needed to complete the char-acterization. These are obtainedin a dynamic test concurrent with measurement of the viscosity.Figure 3 shows the storage (G)and loss (G") moduli and com-plex viscosity n* measured dur-ing an epoxy molding compoundcure. Besides providing essen-tial mini-mum viscosity data, thecross-over point of the two modu-lus curves gives an estimate ofthe time at which the resin be-gins to gel. But this is only an ap-proximategel point. The problem is this: Asthe transient gel network struc-ture is forming, stress relaxationis also taking place. So, unlessmeasurements on the gelling sys-tem are made extremely rapidly,theexact time of gelation will beobscured by changes due tostress relaxation. Recent work by H.H. Winter1shows that the cross-over pointof the storage and loss moduluscurves is not the true gel point.Rather, the instant of gelation iswhen the critical gel exhibitspower law stress relaxation andtan 8 momentarily becomes inde-pendent of frequency. This pointcan be identified by making sev-eral frequency sweeps simulta- Figure 3: Measurement of the minimum viscosity andapproximate gel point for a curing epoxy molding compound neously, measuring tan d in thetime scale of the developing gel. Figure 4 shows dynamic oscilla-tory measurements of tan d madesimultane-ously at three frequen-cies on a crosslinking polydi-methylsiloxane (PDMS) resin.Because tan 8 is independent offrequency at the gel point, thethree curves pass through a sin-gle point and unambiguously de-fine the instant of gelation. Suchmeasurements are readily madeusing a Rheometric rheometerequipped with the MultiWavemode, described in detail in a latersection. Product Performance To derive optimum perform-ancefrom a thermoset resin, bothundercure and overcure must beavoided. An undercured matrixmay exhibit creep and tend tobuild up heat upon vibrat-ing; anovercured matrix may experi-ence room temperature perform-ance problems because internalstresses build up in the glassypolymer as it cools, and stresscracks develop later. A parameter known as the glasstransition temperatureT , oftenmeasured during DSC and TGA,has been used as an indicator ofthe extent of thermoset resin cure.For example, compared to a fullycured control sample of knowngood performance,a lower glasstransition temperature indicatesundercure. But this does not necessarilymean that a higher T indicatesovercure: The difference betweencomplete cure and overcure can-not be detected by measuring T. A higher glass transition tempera-ture may result form exposurelater to temperatures above thoseof cure or postcure. The glasstransition temperature can belower if the prepreg were cut and Figure 4: The precise gel point ofa curing resin is identified bythe intersection of tan 8 curves generated in severalsimultaneous frequency sweeps laid up in a high relative humid-ity. (This can be verified byretesting after drying the sample,since the effect is reportedly re-versible.)And formulation errorscan shift the glass transition tem-perature up or down. To understand the inadequacy ofTalone as a performance gauge,what T. is must be understood.The glass transition is a tempera-tdunfure-induced morphologicalchange in a polymer between abrittle, glassy state and a viscous,rubbery state. The glass transi-tion temperature T is the tem-perature at which this transitionOccurs. Because the material ex-periencesa substantial change in rigidity ina short span of temperatures,the glass transition is a key fac-tor in deciding the use-fulnessof a polymer. So, merely know-ing the temperature at which itoccurs (all that DSC and TGAprovide) is not enough. Themodulus must be measured asa function of temperature as well.This is accomplished most ef-fectively using dynamic me-chanical rheological testing. In the course of measuring the moduli (G', G") and dampingbehavior (tan 8) of a polymer at achosen oscillatory frequency overa sufficiently wide range of tem-perature, the effect of the glasstransition can be clearly ob-served. The glass transition isdetected as a sudden and con-siderable (several decades)change in the elastic modulus andan attendant peak in the tan 8curve. Besides being a more accuratemethod for measuring T, DMRTreveals much about the materialbefore and after the glass trans-ition because DMRT also meas-ures the rubbery plateau modu-lus. This is important because, athigh thermoset cure levels,therubbery plateau modulus is moresensitive than is T. itself for de-tecting, for example, small differ-ences in a thermoset polymer curelevel. Also called the a transition, theglass transition is associated withcrank-shaft motion of major chainsegments.Other transitions, des-ignated as b, g, etc. in order ofdescending temperature of occur-rence, arise from vibrations androtations. Material toughness andimpact resistance correlate withthe magnitude of tan 8 at the sec-ondary (B,y)peaks. For many materials, as the dy-namic oscillatory frequency isincreased, transitions occur athigher temperatures. Also, sometransitions shift different amounts.depending on their degree of fre-quency dependence. This helpslocate some transitions inmultiphase resin systems (suchas those modified by addition ofelastomers or thermoplastics) ifone component is more fre-quency-dependent than another.Shifts in the glass transition tem-perature and the b peak can indi-cate the effect of compoundingorprocess changes on product per-formance. In general, the tem-perature of the secondary transi-tion shifts more than does the glass transition temperature asfrequency is changed. How dynamic mechanical testingcan be employed to study the criti-cal aspects of thermoset rheol-ogy during resin curing and solidstate finished product performan-ce is described in the followingsections. Sample Selection andPreparation If the immediate application in-volves use of the neat resin, thentests should be run on material inthat form. If, however, prepregs areused, then tests should be madeon the staged resin mechanicallyextracted form the prepreg, or di-rectly on die-cut samples of lami-nates (for example, a three-plylaminate of prepreg stacked withthe middle and top ply oriented at45° and 90°with respect to thefiber orientation of the bottom ply). The die size is dictated by thediameter of the parallel plates tobe used for the dynamic mechani-cal tests. Disposable plates,should be used for tests thatcause the resin to cure since theresin can adhere strongly to theplates. The sample should bepressed (under vacuum, if possi-ble) to reduce ist bulk and holdthe plies together. To avoid slip-page, the test should be begunat a temperature at which theresin is tacky. Powdered resin samples shouldbe pulverized, dried, and pressedin a mold, forming a wafer of asize matching the test plate’s di-ameter. Generating ViscosityProfiles Developing the proper cure levelis perhaps the most critical stepfor a thermoset polymer. And bothunder-curing and overcuring mustbe avoided. The most effective and economi-cal way to do this is to design athermoset cure cycle derived from measurements of the cur-ing resin’s dynamic viscosity pro-file as a function of temperatureand time. Rheometers are designed to fa-cilitate making these measure-ments: A time/cure mode allowstest temperatures to be advanced(ramped) at rates of 1° to 5°℃ perminute in eight separately control-led heat zones. Each zone canbe programmed for a ramp rate,final temperature, total time,measure time, and percent strain.Thus, almost any cure cycle canbe conveniently simulated. Andrheometers are equipped with aMultiWave mode that provides themost precise measurement of thegel point available by measur-ngtan d in several simultaneous fre- quency sweeps. From a series of measure-mentsat different temperature rampingrates (generally 1°to 5C perminute), the storage (G’)and loss(G") moduli and the complex vis-cosity n*are derived, and the fol-lowing key information is ob-tained: Initial viscosity Minimum viscosity Gel point Optimum heating rate Initial Viscosity The initial viscosity, the first pointof the viscosity profile, indicatesthe onset of matrix melting, andis not influenced by heating rate.lt is a useful indicator of degree ofresin advancement for quality con-trol. Minimum Viscosity The minimum viscosity representsthe time period and temperaturerange over which resistance toflow is lowest. Knowing this andthe time needed to reach the mini-mum viscosity is critical in vari-ous processes: In laminate pro-duction, the resin viscosity must Figure 5: Measurement of the viscosity profile ofa curingthermoset resin as a function of heat-up rate Isothermal cure at different temperatures time t [s] Figure 6: Isothermal scans at various temperatures are used to helpdecide on the time interval and temperature at which to imposeisothermal holds during a thermoset resin production cure cycle be low enough to wet the embed-ded fibers uniformly, but not solow as to cause excessive bleed-ing at the laminate edges. And inthe encapsulation of integrated cir-cuits by transfer molding, the vis-cosity must be low enough toavoid damaging the fragile wiresthat connect the circuits to theframe. The time interval duringwhich the viscosity is at or nearist minimum value constitutes theprocessingwindow. Gel Point The gel point is the temperatureor time at which the first set ofcovalent bonds connects acrossthe sample (the onset of a three-dimensional network) and themolecular weight becomes infi-nitely large. The gel point is im-portant in determining the timeand temperature at which pres-sure should be applied. Pressure is needed during lami-nation to squeeze the plies to-gether, and timing is critical: Sub-stantial pressure must not be ap-plied until after the minimum vis-cosity has been reached,else theresin will bleed excessively at theedges; but it must be applied be-fore the gel point to assure ad-equate compaction. Pressure is used also to controloutgassing of volatile by-productsgenerated in some thermosetpolymer reactions such as thoseinvolved in the manufacture ofpolyimides. Excessive pressure must beavoided during the lamination ofcircuit boards that include an ox-ide-prepreg interfacial treatmentsince sme oxides are fragile andcan be crushed, causing dela-mination. Optimum Heating Rate The heating rate must be optimizedbecause it affects the timeneeded to reach the minimum vis-cosity,and the time and tempera-ture intervals over which the mini- Time t [hours] Figure 7: A thermoset resin production cure cycle in whichhydraulic pressure is induced in the resin to reduce gasbubble nucleation in the cured matrix Figure 8: The addition of zinc stearate lowers resinviscosity at the injection mold barrel temperature withoutaffecting the cure time mum viscosity extends. Too fasta heating rate can create un-wanted temperature differentialswithin the part, irregular curingthroughout the piece, and possi-ble porosity problems. Figure 5shows the effect of heat-up rateon the viscosityprofile. Isothermal Scans Once the minimum visco-sity andgel point have been determined,a series of isothermal scansshould be run at temperature in-tervals beginning with the mini-mum viscosity temperature andcontinuing up to the gel point tem-perature in intervals of about 10°C.These scans can be made quicklyon the ARES since it can canrecord one data point a second.This information will help in de-ciding the time and temperatureat which to impose isothermalholds during the process. An iso-thermal hold at minimum viscos-ity extends the time the resin willremain near the minimum viscos-ity and determines the dimen-sions of the processing window. The time at or near the minimumviscosity is a key factor in con-trolling overall flow and avoidingproblems such as bulk porosity.A second hold near the gel pointis sometimes used to eli-minatesurface porosity. Typical isother-mal scans are shown in figure 6. Using the ViscosityProfile A dynamic mechanical viscosityprofile offers unparalleled utility inevery phase of thermoset polymertechnology, including research,product development; processdevelopment and control; andquality control. Some examplesfollow. Research Dynamic mechanical testing isemployed extensively in thermo-set composites research to pro-vide needed material properties for Figure 9: graphite-epoxy production cure cycle finite element and finite differenceanalyses. And dynamic viscosityprofiles provide vital data for stud-ies of cure reaction kinetics, proc-ess modeling using "expert"sys-tems,and advanced curing con-cepts such as "gas diffusion con-trol." Diffusion control reduces gas bub-ble nucleation in the curing ma-trix by inducing hydraulic pres-sure in the resin during the gasvolatilization stage of the curecycle. Figure 7 shows a typicalmatrix resin cure cycle incorpo-rating diffusion control, superim-posed on the viscosity profile. Product Development Viscosity profiles are used togreat advantage in ther-mosetproduct development to evaluate,for example, new or different resinformulations fiber types,finishes,andweaves materials from alternative suppliers; and functional additives Figure 8 shows how the viscosityprofile during cure has been usedto evaluate, at a typical screwbarrel temperature, the beneficialeffect of adding 1% zinc stearateto a phenolic injection moldingcompound. The zinc stearate low-ered the viscosity considerably atthe barrel temperature without af-fecting cure time. Process Developmentand Control Developing Production Curecycles A dynamic mechanical viscosityprofile provides the rheologicaldata needed to construct a pro-ire eveleduction cure cycle. Figure 9shows a typical cure cycle deve-loped for a graphite/epoxy lami-nate. Figure 11: B-stage resin advancement alters the viscosityprofile ofa curing thermoset resin Figure 12: Viscosity profiles ofa dry epoxy resin and thesame resin after extended storage in a high humidity In many cases, especially withbismaleimides and polyimides, apost-cure step is used. This in-volves heating the laminate for anextended time outside the moldto increase the crosslink densityand raise the glass transition tem-perature of the matrix, therebyimproving functional properties.Atypical post-cure cycle in a circu-lating air oven for a polyimide resincomposite used for a jet enginecomponent is shown in figure 10. Quality Control The cure step is the most criticalelement in ther-moset process-ing. Accordingly,maintenance ofstrict quality control standards onthe incoming materials, theprepreg especially, is important.Two areas of major concern aredegree of resin advancement andthe effects of moisture. Prepreg Advancement Prepregs are partially re-acted bythe supplier to differentdegrees todevelop particular properties (stiff-ness, tack, drape) needed for agiven application. The degree ofreaction advancement in the B-staged prepreg is controlledmainly by stoichiometry and stor-age conditions, including duration,humidity, and teperature. Ideally,prepregs should be stored only ashort time, and then in a cold, dryenvironment. Any lapses in sup-plier quality control or improperstorage may cause the reactionto advance, changing the materi-al’s response in a standard curecycle. Tests on prepreg samples imme-diately prior to use can quicklyidentify advancement level differ-ences so that adjustments can bemade to the cure cycle. Figure11 shows viscosity profiles of anepoxy resin at three different lev-els of minimum viscosity and thereaction rate (reflected in the slopeof the viscosity curve during theisothermal hold), and the soonergelation occurs. c Figure 13: Temperature sweep on a cured epoxy-graphitecomposite. The glass transition is manifested in a sudden andconsiderable decrease in the storage modulus with anattendant peak in the tan 8 curve c Figure 14: Different values of the glass transitiontemperature are obtained if the transition is approachedwhile the sample is being cooled compared to when thesample is being heatedAAN015 Moisture adsorbed during im-proper storage of a prepreg cancreate quality problems in thermo-set processing by changing reac-tion kinetics and there-by chang-ing the viscosity profile of theprepreg. Also, trapped moisturecan cause matrix porosity. Bytesting before use, moisture-re-lated cure problems can beavoided, or at least minimized.The effects of moisture are oftenreversible, so the material may besalvaged by drying before curing.Figure 12 shows the viscosity pro-files of a dry epoxy resin and thesame resin after extended stor-age in a high humidity. Evaluating ProductPerformance Key factors in thermoset polymerproduct performance are thecured resin's modulus and glasstransition temperature. The dy-namic moduli (G, G”or E', E")are measured directly on solidsamples with or without fiber re-inforcements at a selected fre-quency as a function of tempera-ture. And from these measu-rements tan 8 is automaticallycalculated. The glass transition isdetected as a sudden and con-siderable decrease in the storagemodulus and an attendant peakin the tan 8 curve. The symbol Tdenotes the temperature at whichthis transition occurs. Figure 13 shows the tan 8 andstorage modulus curves, theglass transition and secondarytransition of an epoxy-graphitesample measured in bending onthe Solids Analyzer. The glasstransition temperature is not aconstant, so care must be takenduring its measure-ment to avoidgenerating misleading data. Forexample, two tests made at dif-ferent heating rates can producedifferent values of the glass tran-sition temperature. The slower themeasurement, the lower is theglass transition temperature. Figure 15: Temperature sweeps are an effective means fordetecting the effects of different curing agents on theviscoelastic properties of thermosetresins Figure 16: The cure level ofa thermoset polymer as afunction of cure temperature measured in torsion. Tests canalso be run in compression or bending. Also, for valid comparisons to bemade, the glass transition tem-perature must be approached fromthe same direction to avoid hys-teresis during the test. So, datafrom test samples should be com-pared to data from tests madeunder the same conditions (in-cluding oscillation frequency) ongood-performing control samples. Figure 15 shows the effect on theglass transition temperature ofgoing from a low to high tempera-ture and then repeating the testas the sample cools, using acured and a cured plus postcuredsample of PMR-15 polymimide. Figure 16 shows the effects ofvarious curing agents on the glasstransition temperature of an epoxyresin. The data in figures 14 through 16were obtained in bending and tor-sion. Figure 15 shows the glasstransition temperature as a func-tion of cure level measured in ten-sion following the procedure out-lined in ASTM D5026. Keywords: thermopsets, processing, elasticity, cure, viscosity, performance, gel point, vitrification, glass transition revised by A.J.Franck, TA Instruments AAN Thermoset polymers form the matrix in filled plastics and fiberreinforced composites used in a diversity of products. These range from consumer items and auto body panels to advanced composites for printed circuit boards (PCBs), aerspace structural components such as the Space Shuttle payload bay door and jet engine cowls and ducts, and expensive, high-performance sports equipment. Also, thermosets are used extensively as adhesives, molding compounds, and surface coatings, including protective solder masks for PCBs.
确定




还剩12页未读,是否继续阅读?
TA仪器为您提供《材料中热固性材料检测方案(流变仪)》,该方案主要用于其它中热固性材料检测,参考标准--,《材料中热固性材料检测方案(流变仪)》用到的仪器有TA仪器Discovery 流变仪
推荐专场
相关方案
更多
该厂商其他方案
更多















