磁铁矿球团的硬化是一个伴随着氧化、烧结和传热过程的物理化学综合复杂过程。在硬化过程中,随着球团性能和环境的变化,氧化和烧结过程会发生变化及机理会相互产生影响。为能够预测硬化过程并予以控制,需要对这些复杂过程的动力学进行研究。研究的一种方法是独立的确定各种现象的动力学性能。本文研究的目的是预测和研究不同加热速率下氧化后磁铁矿球团的烧结现象,试验中采用了三种不同的加热速率,并采用光学投影法热膨胀仪来俘获硬化过程中的烧结行为,并同时进行验证。
方案详情

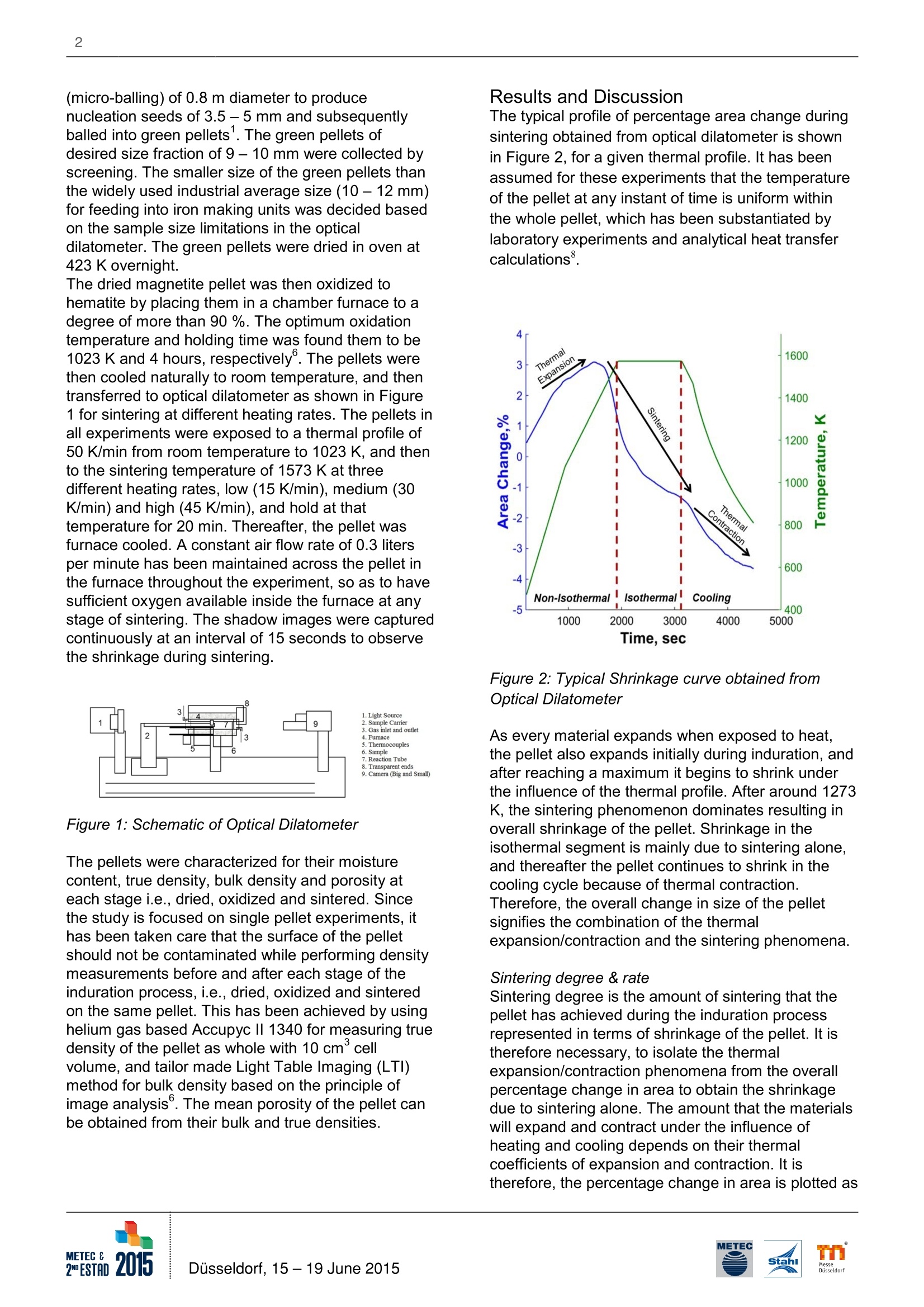
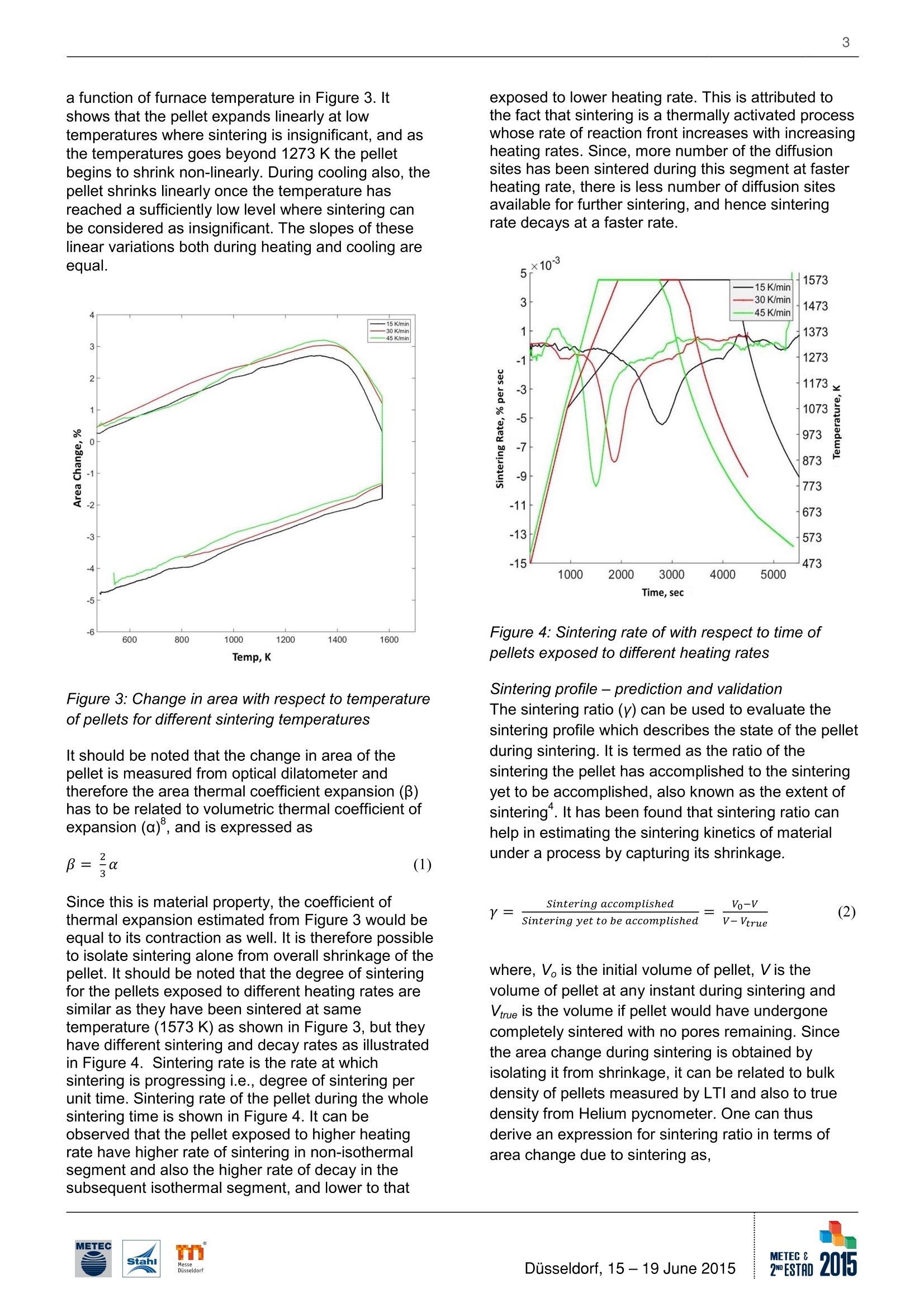
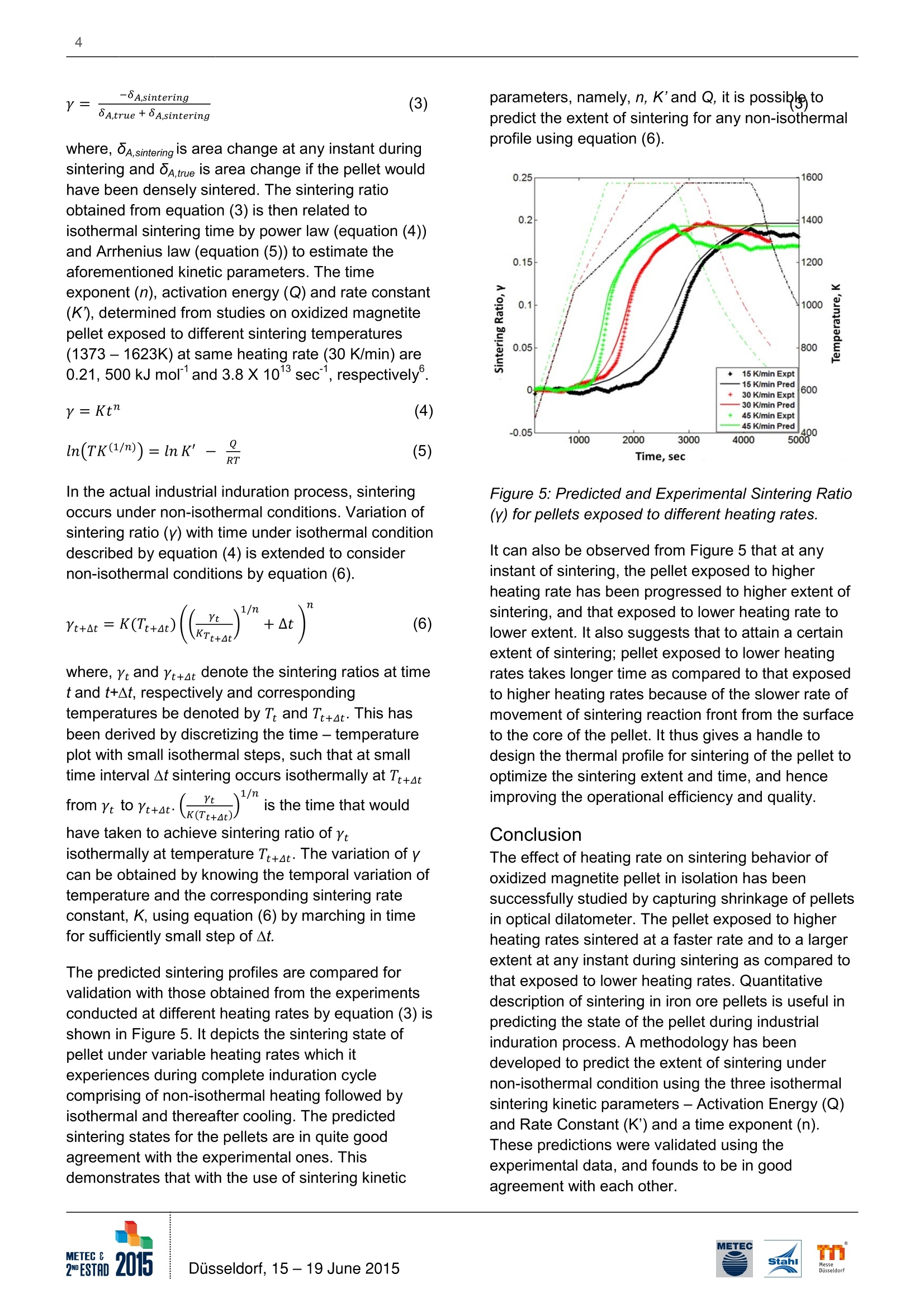
2 Effect of Heating Rates on the Sintering of Oxidized Magnetite Pellets duringInduration T. K. Sandeep Kumar, H. Ahmed, B. Bjorkman/ Lulea University of Technology (LTU)/ Sweden; N.N. Viswanathan/Indian Institute of Technology Bombay (IITB)/ India; C. Andersson, G. Magnusson/ Luossavaara-KiirunavaraAktiebolag (LKAB)/ Sweden. T. K. Sandeep Kumar, Lulea University of Technology (LTU), (+46) 9204910000, (+46) 920491399,kamesh.sandeep@ltu.se Summarv Magnetite pellet induration is a combination of complex physicochemical phenomena -oxidation, sintering and theheat transfer associated with them. Depending on the pellet properties and the environment it encounters duringthe induration, the oxidation and sintering course may vary and the mechanisms will interact. To be able to predicttheir course and control it,the kinetics of these phenomena needs to be understood. One approach is to determinethe kinetics of the phenomena in isolation. The present investigation is aimed to predict and studying the sinteringbehavior of oxidized magnetite (hematite) pellets exposed to different heating rates. Experiments have beencarefully performed at three different heating rates to capture the sintering behavior during induration using anoptical dilatometer, and also used for validation. Key Words IntroductionPelletization is the leading agglomeration techniquepracticed in across the world, especially for magnetiteore fines like in Sweden. Pelletization of magnetiteaids an added benefit in terms of energy generatedfrom exothermic nature of magnetite oxidation.Swedish steel industries pioneered in operating theirblast furnaces with cent percent pellets. This makes itnecessary to understand the entire process ofpelletization, where green pellets are strengthenedthrough heat hardening process for subsequent usein iron making units such as blast furnace and directreduced iron processes.Induration is carried out in a straight grate furnace orin straight grate furnace followed by a rotary Kilnfurnace. The hot gases are allowed to flow upward aswell as downward through the packed bed and incounter-current direction across the furnace forefficient heat transfer. During induration, magnetitepellet undergoes drying (273-523 K), oxidation (573- 1073 K) at lower temperatures and sintering (>1273 K) at higher temperatures. As the reaction frontmoves from the surface towards the center of thepellet by the diffusion of hot gases through pores, theoxidation should preferably precede the sintering. Inreality sintering and oxidation may proceedsimultaneously. This might for example causes theformation of a duplex structure in the pellet, with amagnetite core and hematite shell because ofmagnetite sintering which starts at a lower and thusinducing structural stress1. The formation of theduplex structure is one of the reasons why it isnecessary to understand the kinetics of themechanisms involved in these processes thoroughly. The methodology adapted was to study theseprocesses in isolation, beginning with that of sinteringof oxidized magnetite (hematite) pellet.To understand the kinetics, the process has to bequantified and can be estimated by three parametersnamely- Activation Energy (Q), Rate Constant (K)and time exponent (n). The sintering kinetics ofoxidized magnetite was studied by capturing theshrinkage of pellet during its sintering underisothermal conditions. These kinetics parametershave been estimated and discussed elsewhere°. Theshrinkage during sintering of single oxidizedmagnetite pellet is being captured by opticaldilatometer, which measures the dimensionalvariations solely on their optical images withouthaving any contact with the sample, and hence doesnot interfere in the process..The activation energyand rate constant estimated for the sintering ofoxidized magnetite pellet were 500 kJ moland 3.8 x101sec, respectively. In this paper, theseparameters have been used to predict and study theeffect of different heating rates on sintering ofoxidized magnetite pellet by conducting experiments. Experimental Method The source of magnetite chosen here is pelletconcentrate from Luossavaara-Kiirunavaara AB's(LKAB) mine in northern part of Sweden. Theconcentrate was collected carefullyby coning andquartering, and contains Fe304 >95% with Al203and SiO2 <0.6 % and 7% moisture by weight. Theconcentrate was mixed with 0.5 % dosage ofbentonite as binder in a laboratory mixer (Eirich R02).This green mix was then fed to drum pelletizer (micro-balling) of 0.8 m diameter to producenucleation seeds of 3.5-5 mm and subsequentlyballed into green pellets. The green pellets ofdesired size fraction of 9 - 10 mm were collected byscreening. The smaller size of the green pellets thanthe widely used industrial average size (10 -12 mm)for feeding into iron making units was decided basedon the sample size limitations in the opticaldilatometer. The green pellets were dried in oven at 423 K overnight.The dried magnetite pellet was then oxidized tohematite by placing them in a chamber furnace to adegree of more than 90 %. The optimum oxidationtemperature and holding time was found them to be1023 K and 4 hours,respectively. The pellets werethen cooled naturally to room temperature, and thentransferred to optical dilatometer as shown in Figure1 for sintering at different heating rates. The pellets inall experiments were exposed to a thermal profile of50 K/min from room temperature to 1023 K, and thento the sintering temperature of 1573 K at threedifferent heating rates, low (15 K/min), medium (30K/min) and high (45 K/min), and hold at thattemperature for 20 min. Thereafter, the pellet wasfurnace cooled. A constant air flow rate of 0.3 litersper minute has been maintained across the pellet inthe furnace throughout the experiment, so as to havesufficient oxygen available inside the furnace at anystage of sintering. The shadow images were capturedcontinuously at an interval of 15 seconds to observethe shrinkage during sintering. Figure 1: Schematic of Optical Dilatometer The pellets were characterized for their moisturecontent, true density, bulk density and porosity ateach stage i.e.,dried, oxidized and sintered. Sincethe study is focused on single pellet experiments, ithas been taken care that the surface of the pelletshould not be contaminated while performing densitymeasurements before and after each stage of theinduration process,i.e., dried, oxidized and sinteredon the same pellet. This has been achieved by usinghelium gas based Accupyc lI 1340 for measuring truedensity of the pellet as whole with 10 cmcellvolume, and tailor made Light Table Imaging (LTI)method for bulk density based on the principle ofimage analysis. The mean porosity of the pellet canbe obtained from their bulk and true densities. Results and Discussion The typical profile of percentage area change duringsintering obtained from optical dilatometer is shownin Figure 2, for a given thermal profile. It has beenassumed for these experiments that the temperatureof the pellet at any instant of time is uniform withinthe whole pellet, which has been substantiated bylaboratory experiments and analytical heat transfercalculations°. Figure 2: Typical Shrinkage curve obtained fromOptical Dilatometer As every material expands when exposed to heat,the pellet also expands initially during induration, andafter reaching a maximum it begins to shrink underthe influence of the thermal profile. After around 1273K, the sintering phenomenon dominates resulting inoverall shrinkage of the pellet. Shrinkage in theisothermal segment is mainly due to sintering alone,and thereafter the pellet continues to shrink in thecoolingcycle because of thermal contraction.Therefore, the overall change in size of the pelletsignifies the combination of the thermalexpansion/contraction and the sintering phenomena. Sintering degree & rate Sintering degree is the amount of sintering that thepellet has achieved during the induration processrepresented in terms of shrinkage of the pellet. It istherefore necessary, to isolate the thermalexpansion/contraction phenomena from the overallpercentage change in area to obtain the shrinkagedue to sintering alone. The amount that the materialswill expand and contract under the influence ofheating and cooling depends on their thermalcoefficients of expansion and contraction. It istherefore, the percentage change in area is plotted as a function of furnace temperature in Figure 3. Itshows that the pellet expands linearly at lowtemperatures where sintering is insignificant, and asthe temperatures goes beyond 1273 K the pelletbegins to shrink non-linearly. During cooling also, thepellet shrinks linearly once the temperature hasreached a sufficiently low level where sintering canbe considered as insignificant. The slopes of theselinear variations both during heating and cooling areequal. Figure 3: Change in area with respect to temperatureof pellets for different sintering temperatures It should be noted that the change in area of thepellet is measured from optical dilatometer andtherefore the area thermal coefficient expansion (B)has to be related to volumetric thermal coefficient ofexpansion (a), and is expressed as Since this is material property, the coefficient ofthermal expansion estimated from Figure 3 would beequal to its contraction as well. It is therefore possibleto isolate sintering alone from overall shrinkage of thepellet. It should be noted that the degree of sinteringfor the pellets exposed to different heating rates aresimilar as they have been sintered at sametemperature (1573 K) as shown in Figure 3, but theyhave different sintering and decay rates as illustratedin Figure 4. Sintering rate is the rate at whichsintering is progressing i.e., degree of sintering perunit time. Sintering rate of the pellet during the wholesintering time is shown in Figure 4. It can beobserved that the pellet exposed to higher heatingrate have higher rate of sintering in non-isothermalsegment and also the higher rate of decay in thesubsequent isothermal segment, and lower to that exposed to lower heating rate. This is attributed tothe fact that sintering is a thermally activated processwhose rate of reaction front increases with increasingheating rates. Since, more number of the diffusionsites has been sintered during this segment at fasterheating rate, there is less number of diffusion sitesavailable for further sintering, and hence sinteringrate decays at a faster rate. Figure 4: Sintering rate of with respect to time ofpellets exposed to different heating rates Sintering profile- prediction and validationThe sintering ratio (Y) can be used to evaluate thesintering profile which describes the state of the pelletduring sintering. It is termed as the ratio of thesintering the pellet has accomplished to the sinteringyet to be accomplished,also known as the extent ofsinteringt. It has been found that sintering ratio canhelp in estimating the sintering kinetics of materialunder a process by capturing its shrinkage. sintering accomplished Vo-VY= (2)Sintering yet to be accomplished V-Vtrue where, V is the initial volume of pellet, V is thevolume of pellet at any instant during sintering andVrue is the volume if pellet would have undergonecompletely sintered with no pores remaining. Sincethe area change during sintering is obtained byisolating it from shrinkage, it can be related to bulkdensity of pellets measured by LTl and also to truedensity from Helium pycnometer. One can thusderive an expression for sintering ratio in terms ofarea change due to sintering as, where, 8A,sintering is area change at any instant duringsintering and 8A,true is area change if the pellet wouldhave been densely sintered. The sintering ratioobtained from equation (3) is then related toisothermal sintering time by power law (equation (4))and Arrhenius law (equation (5)) to estimate theaforementioned kinetic parameters. The timeexponent (n),activation energy (Q) and rate constant(K), determined from studies on oxidized magnetitepellet exposed to different sintering temperatures(1373-1623K) at same heating rate(30 K/min) are0.21,500 kJ mor and 3.8 X1013sec, respectively. In the actual industrial induration process, sinteringoccurs under non-isothermal conditions. Variation ofsintering ratio (y) with time under isothermal conditiondescribed by equation (4) is extended to considernon-isothermal conditions by equation (6). where, Yt and Yt+at denote the sintering ratios at timet and t+At, respectively and correspondingtemperatures be denoted by Tt and Tt+4t. This hasbeen derived by discretizing the time - temperatureplot with small isothermal steps, such that at smalltime interval At sintering occurs isothermally at Tt+atfrolreto ye+at (r)/ is itsh teh et itmieme that wouldhave taken to achieve sintering ratio of Ytisothermally at temperature Tt+At. The variation of ycan be obtained by knowing the temporal variation oftemperature and the corresponding sintering rateconstant, K, using equation (6) by marching in timefor sufficiently small step of At. The predicted sintering profiles are compared forvalidation with those obtained from the experimentsconducted at different heating rates by equation (3) isshown in Figure 5. It depicts the sintering state ofpellet under variable heating rates which itexperiences during complete induration cyclecomprising of non-isothermal heating followed byisothermal and thereafter cooling. The predictedsintering states for the pellets are in quite goodagreement with the experimental ones. Thisdemonstrates that with the use of sintering kinetic parameters, namely, n, K’and Q, it is possible topredict the extent of sintering for any non-isothermalprofile using equation (6). Figure 5: Predicted and Experimental Sintering Ratio(Y) for pellets exposed to different heating rates. It can also be observed from Figure 5 that at anyinstant of sintering, the pellet exposed to higherheating rate has been progressed to higher extent ofsintering, and that exposed to lower heating rate tolower extent. It also suggests that to attain a certainextent of sintering; pellet exposed to lower heatingrates takes longer time as compared to that exposedto higher heating rates because of the slower rate ofmovement of sintering reaction front from the surfaceto the core of the pellet. It thus gives a handle todesign the thermal profile for sintering of the pellet tooptimize the sintering extent and time, and henceimproving the operational efficiency and quality. ConclusionThe effect of heating rate on sintering behavior ofoxidized magnetite pellet in isolation has beensuccessfully studied by capturing shrinkage of pelletsin optical dilatometer. The pellet exposed to higherheating rates sintered at a faster rate and to a largerextent at any instant during sintering as compared tothat exposed to lower heating rates. Quantitativedescription of sintering in iron ore pellets is useful inpredicting the state of the pellet during industrialinduration process. A methodology has beendeveloped to predict the extent of sintering undernon-isothermal condition using the three isothermalsintering kinetic parameters- Activation Energy (Q)and Rate Constant (K') and a time exponent (n).These predictions were validated using theexperimental data, and founds to be in goodagreement with each other. Acknowledgement We would like to thank Hjalmar Lundbohm ResearchCentre (HLRC) for their financial support. We wouldalso extend our gratitude for technical and laboratorysupport at LKAB as well as LTU, Sweden as andwhen required. References ( 1. Forsmo, S.P.E; F orsmo, S. E.; Samskog, P.O.;Bjorkman, B . M. T.: Mechanisms in Oxidation andSintering of Magnetite I ron Ore G reen Pellets;Powder Technology, vol. 1 8 3, No.2, 2 0 08, P. 247- 259. ) 2. Semberg, P.; Rutqvist, A.; Andersson, C.;Bjorkman, B.: Interaction between iron oxides andolivine in magnetite based pellets during reduction attemperatures below 1000℃; Ironmaking andSteelmaking, 2011, 38, No.5, P. 321-328. ( 3 . Tang, M.; Cho, H . J.; Pistorius, P .C.: E arly gaseousoxygen enrichment to enhance magnetite pelletoxidation; Metallurgical and Materials Transactions B,2014, vol. 45 B, P. 1-11. ) ( 4. Wynnyckyj, J.R.; McCurdy, W.A.: Causes of theShell and Core Structure and Layered Inhomogeneityin Iron Ore Pellets; Metallurgical Transactions, 1974,vol. 5,No. 10, P. 2207-2215. ) ( 5. Wynnyckyj, J.R.; Fahidy,T.Z.: Solid St a te Sinteringin the Induration o f Iron Ore Pellets; MetallurgicalTransactions, 1974, vol. 5 , No. 5, P. 991-1000. ) ( 6. Sandeep Kumar, T . K.; Viswanathan, N.N.;Ahmed, H.M.; Andersson, C.; Bjorkman, B.:Estimation of Sintering Kinetics of Oxidized MagnetitePellet using Optical D ilatometer; Metallurgical andMaterials Transactions B, 2014, vol. 4 5 B, P . 1 -9. ) ( 7 .Karamanov, A.; Dzhantov, B.; P aganelli, M.;Sighinolfi , D.: Glass Transition Temperature andActivation Energy of Sintering by Optical Dilatometry;Thermochimica Acta. 2013, vol. 553, No. 32, P.1-7. ) ( 8. Gaskell, D.R.: Introduction to the thermodynamicsof materials; vol. 2 , 5th e d ., New Yor k , USA: CRC Press, 2008. ) METEC EDESTADusseldorf, June 磁铁矿球团的硬化是一个伴随着氧化、烧结和传热过程的物理化学综合复杂过程。在硬化过程中,随着球团性能和环境的变化,氧化和烧结过程会发生变化及机理会相互产生影响。为能够预测硬化过程并予以控制,需要对这些复杂过程的动力学进行研究。研究的一种方法是独立的确定各种现象的动力学性能。本文研究的目的是预测和研究不同加热速率下氧化后磁铁矿球团的烧结现象,试验中采用了三种不同的加热速率,并采用光学投影法热膨胀仪来俘获硬化过程中的烧结行为,并同时进行验证。
确定


还剩3页未读,是否继续阅读?
上海依阳实业有限公司为您提供《磁铁矿颗粒中热膨胀,线膨胀检测方案(热膨胀仪)》,该方案主要用于金属矿产中热膨胀,线膨胀检测,参考标准--,《磁铁矿颗粒中热膨胀,线膨胀检测方案(热膨胀仪)》用到的仪器有光学投影法高温热膨胀仪
推荐专场
相关方案
更多
















