方案详情
文
Key Words
Angiotensin II Receptor Blocker, API, Automated Method
Scouting, Autosampler, Diuretics, Generic Method, UHPLC
Instrumentation, Vanquish UHPLC System
Goal
To develop an easy to use UHPLC method for the fast
separation of combinations of possible antihypertension agents
方案详情

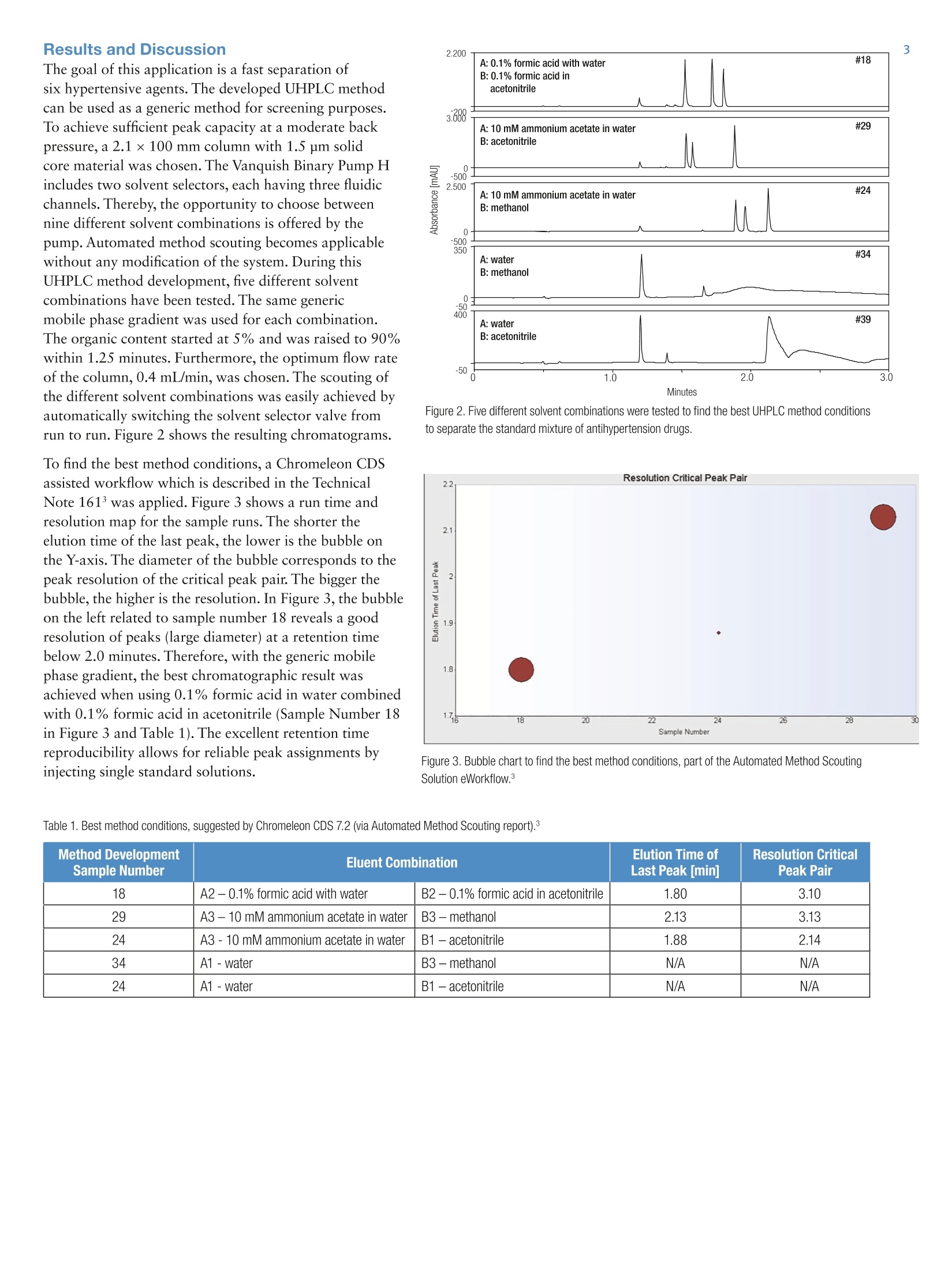
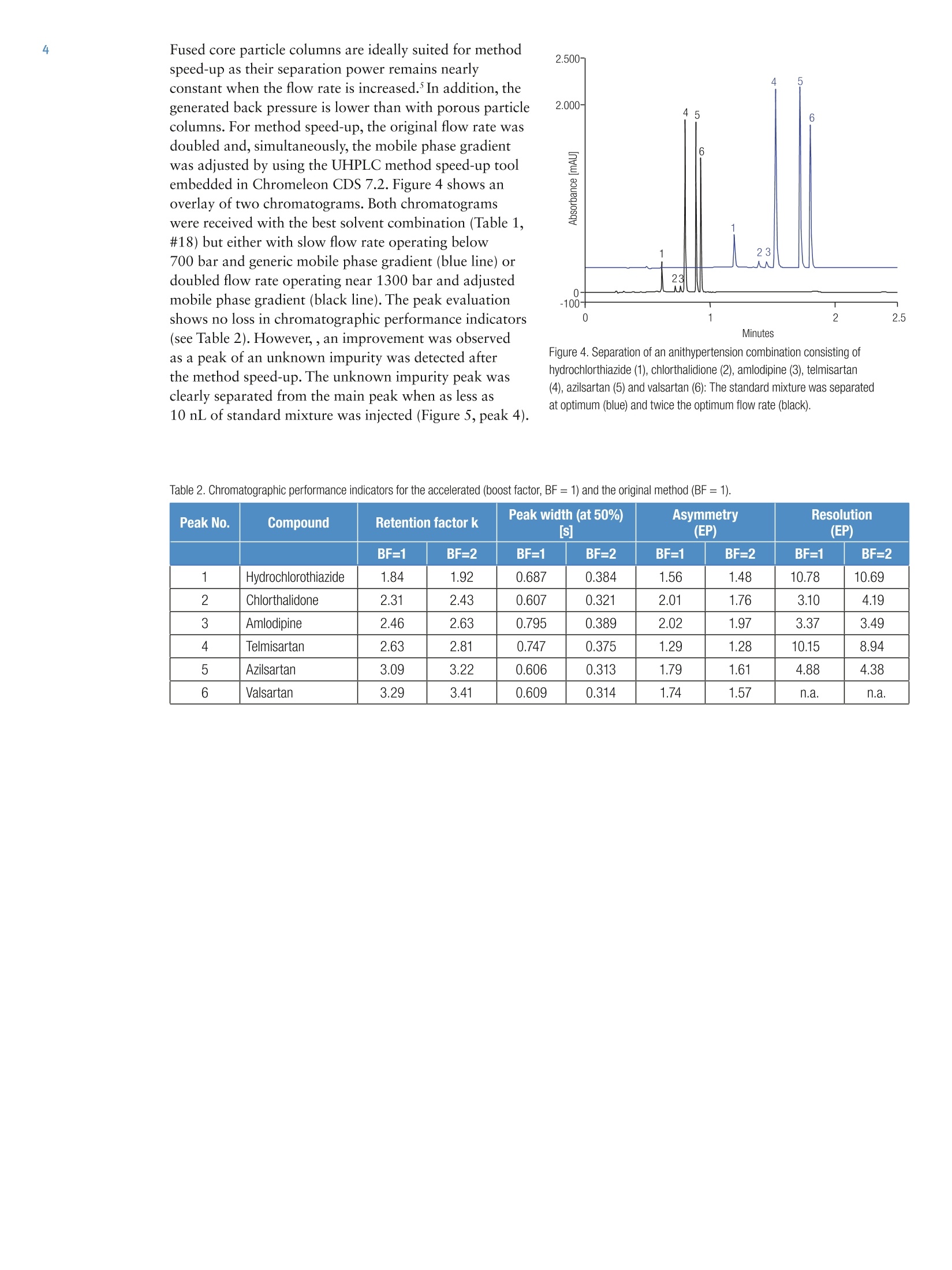
2 3 UHPLC Method Development forSimultaneous Determination ofAntihypertensive Combination Agents Susanne Fabel Thermo Fisher Scientific, Germering, Germany Key Words Angiotensin II Receptor Blocker, APl, Automated MethodScouting, Autosampler, Diuretics, Generic Method, UHPLCInstrumentation, Vanquish UHPLC System Goal To develop an easy to use UHPLC method for the fastseparation of combinations of possible antihypertension agents Introduction For antihypertension therapy, drug combinations areconsidered to be a better method to manage and reduceblood pressure than a single drug medication. Therefore,a current trend is the combination of several antihyperten-sion agents in a single pill. Additionally, a single doseapplication improves the compliance due to the moreconvenient option of once daily dosing. Typical combina-tions include agents from different pharmacologic classes,e.g. angiotensin II inhibitors, calcium channel blockers,or thiazides. The goal of this UHPLC method development was a fastseparation of potential combinations of antihypertensionagents. The latest generation of UHPLC instrumentationwas applied to separate a mixture of one calcium channelblocker (amlodipine besylate), two thiazide diuretics(hydrochlorothiazide and chlorthalidone), and threeangiotensin II receptor antagonists (azilsartan, telmisartan,valsartan). Scouting the effect of different mobile phasecompositions on the chromatographic result was sup-ported by tools embedded within Thermo Scientific""Dionex" Chromeleon" Chromatography Data System(CDS) software version 7.2. For subsequent evaluation ofbest UHPLC method conditions, the report template ofthe Automated Method Scouting solution was applied(see the related Thermo Scientific Technical Note 161).3Finally, the method was accelerated by applying themethod transfer tool of Chromeleon CDS 7.2.4 Due to thecharacteristics of the 1.5 um solid core particle material,the separation acceleration did not compromise peakresolution. With this developed UHPLC method, sixantihypertension agents are separated within one minute,at a maximum system pressure of 1300 bar (19000 psi). Reagents and Chemicals Compound Supplier P/N Valsartan Sigma-Aldrich SML0142-10MG Azilsartan Sigma-Aldrich SML0432-10MG Telmisartan Sigma-Aldrich T8949-10MG Amlodipine besylate Sigma-Aldrich A5605-10MG Hydrochlorothiazide Sigma-Aldrich H4759-5G Chlorthalidone Molekula 38140528 Formic acid Fisher Scientific A117-50 Ammonium acetate Fisher Scientific A114-50 Acetic acid Fisher Scientific A113-50 Methanol OPTIMAM LC/MS Fisher Scientific A456-212 Acetonitrile OPTIMA LC/MS Fisher Scientific A955-212 Ultra-pure lab water,18.2 MQcm at 25°C n.a. n.a. Standard Preparation Single stock solutions of all active pharmaceuticalingredients (APIs) were prepared by solving appropriateamounts in methanol. The stock concentration ofamlodipine besylate,valsartan, and azilsartan had aconcentration of 2 mg/mL. The stock solution ofhydrochlorothiazide had a concentration of 2.60 mg/mL,the stock solution of chlorthalidone had a concentrationof 1.81 mg/mL, and the stock solution of telmisartan hada concentration of 1 mg/mL. The single standard solutionswere mixed and diluted with water to yield standardmixture containing 320 ug/mL of valsartan, azilsartan,and telmisartan, 40 pg/mLamlodipine besylate, 50 pg/mLchlorthalidone and 50 pg/mL hydrochlorothiazide. Theconcentrations of the components refer to their typicalratio in hypertension drug formulations. Results and Discussion The goal of this application is a fast separation ofsix hypertensive agents. The developed UHPLC methodcan be used as a generic method for screening purposes.To achieve sufficient peak capacity at a moderate backpressure, a 2.1×100 mm column with 1.5 um solidcore material was chosen. The Vanquish Binary Pump Hincludes two solvent selectors, each having three fluidicchannels.Thereby, the opportunity to choose betweennine different solvent combinations is offered by thepump. Automated method scouting becomes applicablewithout any modification of the system. During thisUHPLC method development, five different solventcombinations have been tested. The same genericmobile phase gradient was used for each combination.The organic content started at 5%and was raised to 90%within 1.25 minutes. Furthermore, the optimum flow rateof the column, 0.4 mL/min, was chosen. The scouting ofthe different solvent combinations was easily achieved byautomatically switching the solvent selector valve from run to run. Figure 2 shows the resulting chromatograms. To find the best method conditions, a Chromeleon CDSassisted workflow which is described in the TechnicalNote 161 was applied. Figure 3 shows a run time andresolution map for the sample runs. The shorter theelution time of the last peak, the lower is the bubble onthe Y-axis. The diameter of the bubble corresponds to thepeak resolution of the critical peak pair. The bigger thebubble, the higher is the resolution. In Figure 3, the bubbleon the left related to sample number 18 reveals a goodresolution of peaks (large diameter) at a retention timebelow 2.0 minutes. Therefore, with the generic mobilephase gradient, the best chromatographic result wasachieved when using 0.1% formic acid in water combinedwith 0.1% formic acid in acetonitrile (Sample Number 18in Figure 3 and Table 1). The excellent retention timereproducibility allows for reliable peak assignments byinjecting single standard solutions. Figure 2. Five different solvent combinations were tested to find the best UHPLC method conditionsto separate the standard mixture of antihypertension drugs. Figure 3. Bubble chart to find the best method conditions, part of the Automated Method ScoutingSolution eWorkflow.3 Table 1. Best method conditions, suggested by Chromeleon CDS 7.2 (via Automated Method Scouting report).3 Method DevelopmentSample Number Eluent Combination Elution Time ofLast Peak min] Resolution CriticalPeak Pair 18 A2- 0.1% formic acid with water B2 -0.1% formic acid in acetonitrile 1.80 3.10 29 A3-10mM ammonium acetate in water B3-methanol 2.13 3.13 24 A3 - 10 mM ammonium acetate in water B1 -acetonitrile 1.88 2.14 34 A1-water B3-methanol N/A N/A 24 A1 -water B1 -acetonitrile N/A N/A Fused core particle columns are ideally suited for methodspeed-up as their separation power remains nearlyconstant when the flow rate is increased.In addition, thegenerated back pressure is lower than with porous particlecolumns. For method speed-up, the original flow rate wasdoubled and, simultaneously, the mobile phase gradientwas adjusted by using the UHPLC method speed-up toolembedded in Chromeleon CDS 7.2. Figure 4 shows anoverlay of two chromatograms.Both chromatogramswere received with the best solvent combination (Table 1,#18) but either with slow flow rate operating below700 bar and generic mobile phase gradient (blue line) ordoubled flow rate operating near 1300 bar and adjustedmobile phase gradient (black line). The peak evaluationshows no loss in chromatographic performance indicators(see Table 2). However,, an improvement was observedas a peak of an unknown impurity was detected afterthe method speed-up. The unknown impurity peak wasclearly separated from the main peak when as less as10 nL of standard mixture was injected (Figure 5, peak 4). Figure 4. Separation of an anithypertension combination consisting ofhydrochlorthiazide (1), chlorthalidione (2), amlodipine (3), telmisartan(4), azilsartan (5) and valsartan (6): The standard mixture was separatedat optimum (blue) and twice the optimum flow rate (black). Table 2. Chromatographic performance indicators for the accelerated (boost factor, BF=1)and the original method (BF=1). Peak No. Compound Retention factor k Peak width (at 50%) [s] Asymmetry (EP) Resolution (EP) BF=1 BF=2 BF=1 BF=2 BF=1 BF=2 BF=1 BF=2 1 Hydrochlorothiazide 1.84 1.92 0.687 0.384 1.56 1.48 10.78 10.69 2 Chlorthalidone 2.31 2.43 0.607 0.321 2.01 1.76 3.10 4.19 3 Amlodipine 2.46 2.63 0.795 0.389 2.02 1.97 3.37 3.49 4 Telmisartan 2.63 2.81 0.747 0.375 1.29 1.28 10.15 8.94 5 Azilsartan 3.09 3.22 0.606 0.313 1.79 1.61 4.88 4.38 6 Valsartan 3.29 3.41 0.609 0.314 1.74 1.57 n.a. n.a. A calibration curve was recorded for each API by utilizingthe wide injection volume range of the Vanquish UHPLCsystem. In Figure 5, the impressing area reproducibilitywith 10 nL injection is shown. From six consecutive0.01 pL injections, the relative standard deviations in peakarea and retention time are listed in Table 3. The achievedrelative standard deviation of the peak area is below fivepercent, an excellent value giving the fact that this resultwas achieved without changing any settings on theinstrument hardware or software. Furthermore, injectionvolumes from 0.01 pLup to 1 pL of a standard mixturediluted tenfold with water were injected to generate thecalibration curve. For the smaller peaks chlorthalidone,amlodipine and the unknown, the calibration curvewas calculated from 0.05 pL up to 1 pL. The excellentinjection linearity of the Vanquish autosampler resultedin superior calibration coefficients as shown in Table 3and Figure 6. Figure 5. Overlay of six consecutive 0.01 pL injections of standard mixture(after method speed-up). Table 3. Reproducibility of peak area and retention time at 0.01 pL. Coefficients for calibration curvefrom 0.01 pL up to 1 pL. Reproducibility at lowestinjection volume 0.01 pL Autosampler linearityby injection volumes0.01 pL-1 pL PeakNo. Compound Retention time Peak AreaRSD [%]n=6 Calibration Coefficient(calibration points) RSD [%](n=6) 1 Hydrochlorothiazide 0.07 4.98 0.99992 (6) 2 Chlorthalidone 0.07 3.53 0.99991 (5) 3 Amlodipine 0.04 3.32 0.99967(5) 4 Unknown 0.05 2.22 0.99671 (5) 5 Telmisartan 0.03 2.53 0.99963 (6) 6 Azilsartan 0.04 1.41 0.99774 (6) 7 Valsartan 0.04 3.05 0.99999 (6) Figure 6: Calibration curves generated for the hypertensive agent mixture (standardmixture diluted tenfold in water) by applying injection volumes from 0.01 pL up to 1 pL. Conclusion The fast and easy method development with latestgeneration UHPLC equipment has been demonstrated.Scouting of five different mobile phase combinations tofind the best method condition was done without userinteraction. Therefore, the eluent switching capabilitiesof the Vanquish Binary Pump Hand Chromeleon 7.2software support was applied for data evaluation. Thehighly efficient Accucore Vanquish C18+ column with1.5 um particles allowed method speed-up by a factorof two without compromising resolution. It was demon-strated that the Vanquish Split Sampler HT couldreproducibly inject within the nanoliter range and withoutthe need for hardware or software changes. Superiorcalibration curves were generated by applying the wideinjection volume range of the Vanquish Split Sampler HT. ( References ) ( 1. Jones, J. D. ; Jackson, S. H . ; Agboton, C.; Martin,T.S.; Pharmacy and Therapeutics,2011 , 36 (10) p. 634-640 ) 2. Thermo Fisher Scientific. Vanquish UHPLC System,2014.[Online] http://www.thermoscientific.com/vanquish (accessed March 3,2015). 3.Thermo Scientific Technical Note 161: LC MethodDevelopment by an Automated Method ScoutingSystem, 2015.[Online] http://www.thermoscientific.com/content/dam/tfs/ATG/CMD/cmd-documents/sci-res/app/chrom/lc/sys/TN-161-HPLC-Method-Scouting-TN71514-EN.pdf(accessed May 22, 2015). SCIENTIFIC AThermo Fisher Scientific BrandANEN
确定




还剩4页未读,是否继续阅读?
赛默飞色谱与质谱为您提供《化学药中理化常数检测方案 》,该方案主要用于化药制剂中含量测定检测,参考标准--,《化学药中理化常数检测方案 》用到的仪器有赛默飞优谱佳UHPLC+高效液相色谱系统、赛默飞 Vanquish™ UHPLC超高效液相色谱系统、赛默飞 Vanquish Flex UHPLC系统
推荐专场
相关方案
更多
该厂商其他方案
更多