溶液响应高分子系统主要着眼于对两亲性可转变高分子(AIP)的研究,这种两亲性高分子可以形成溶液响应的胶束或者反转胶束结构。在这篇文章中,作者报告了一种新型制备溶液诱导双亲空壳微粒的方法。双亲性PEI-g-PMMA空壳微粒首先由PEI/PMMA核-壳微粒,经由去除壳内部的PMMA分子得到。这种空壳微粒可以稳定的分散在非极性溶液和水中。通过透射电镜、接触角测量技术和X射线光电子能谱技术,作者对这种微粒进行了详细的表征。通过重复多达6次循环的在二氯甲烷和水中的溶解,这种结构微粒仍旧可以保持结构的稳定。通过增加表面吸水量,DCM处理的表面的接触角也由94°降低至51°。结果显示这种两亲性的空壳微粒作为一种新型智能材料可以被运用在多方面,如在疏水药物运输,水溶性高分子包覆材料,多肽选择性吸附,催化剂等。
方案详情

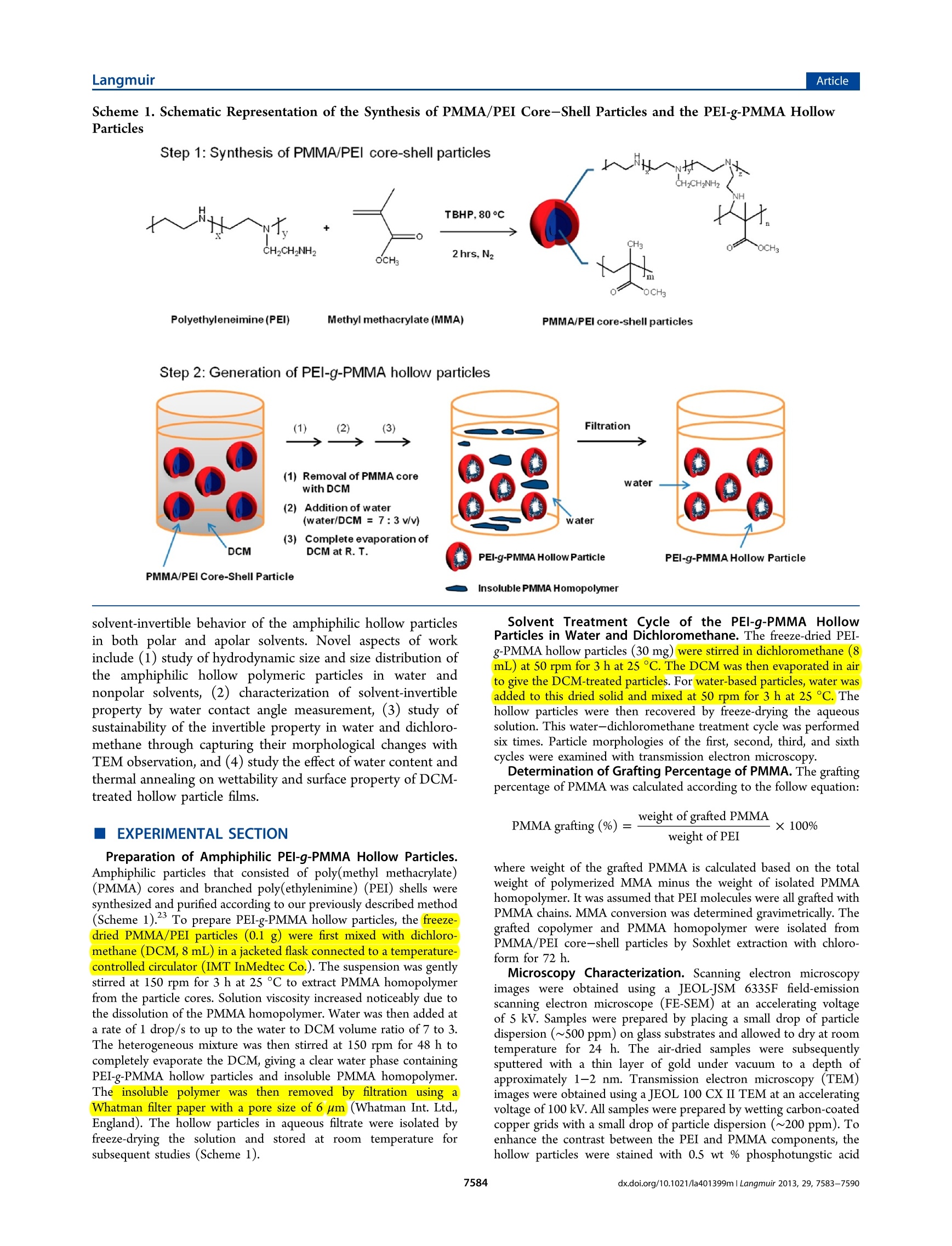
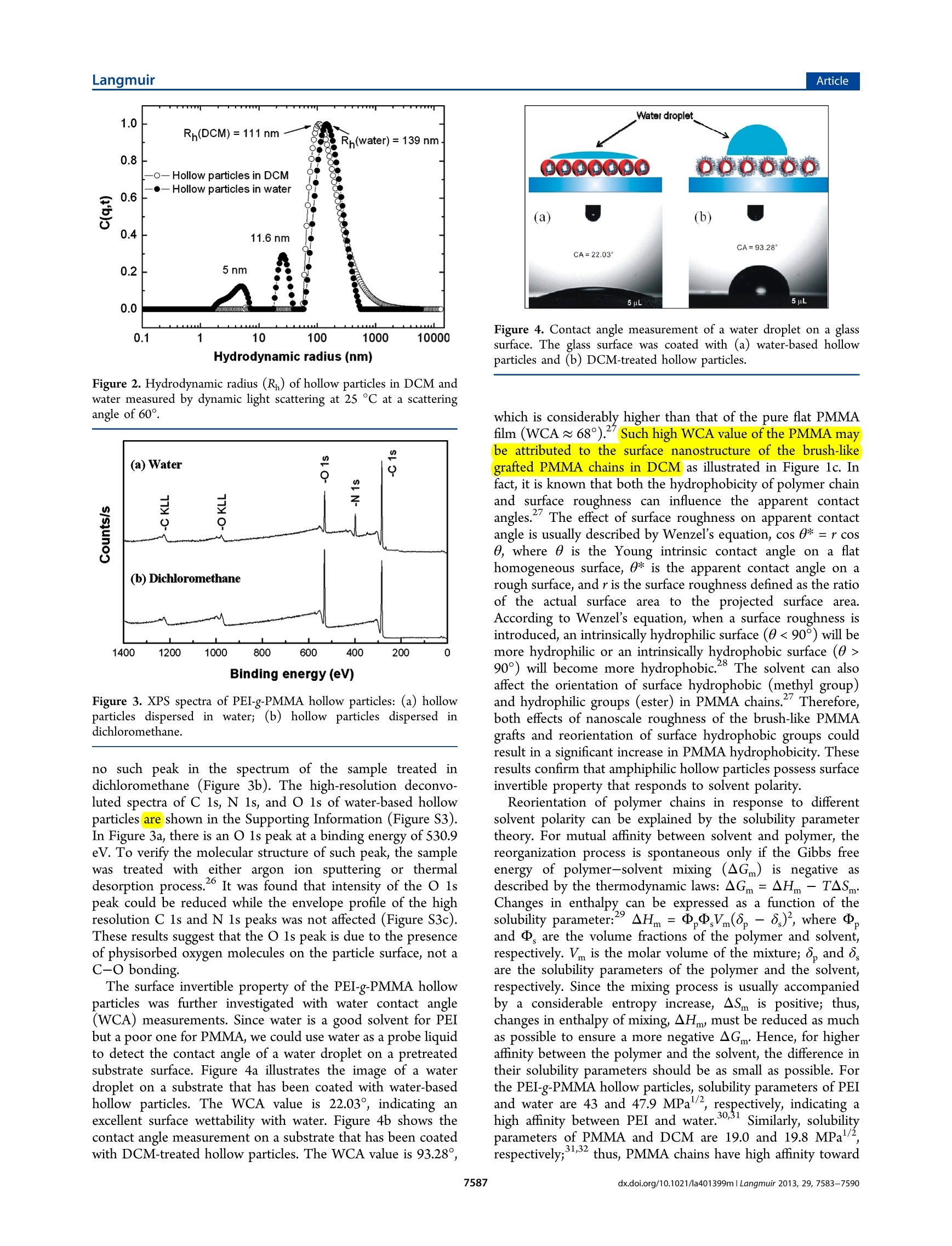
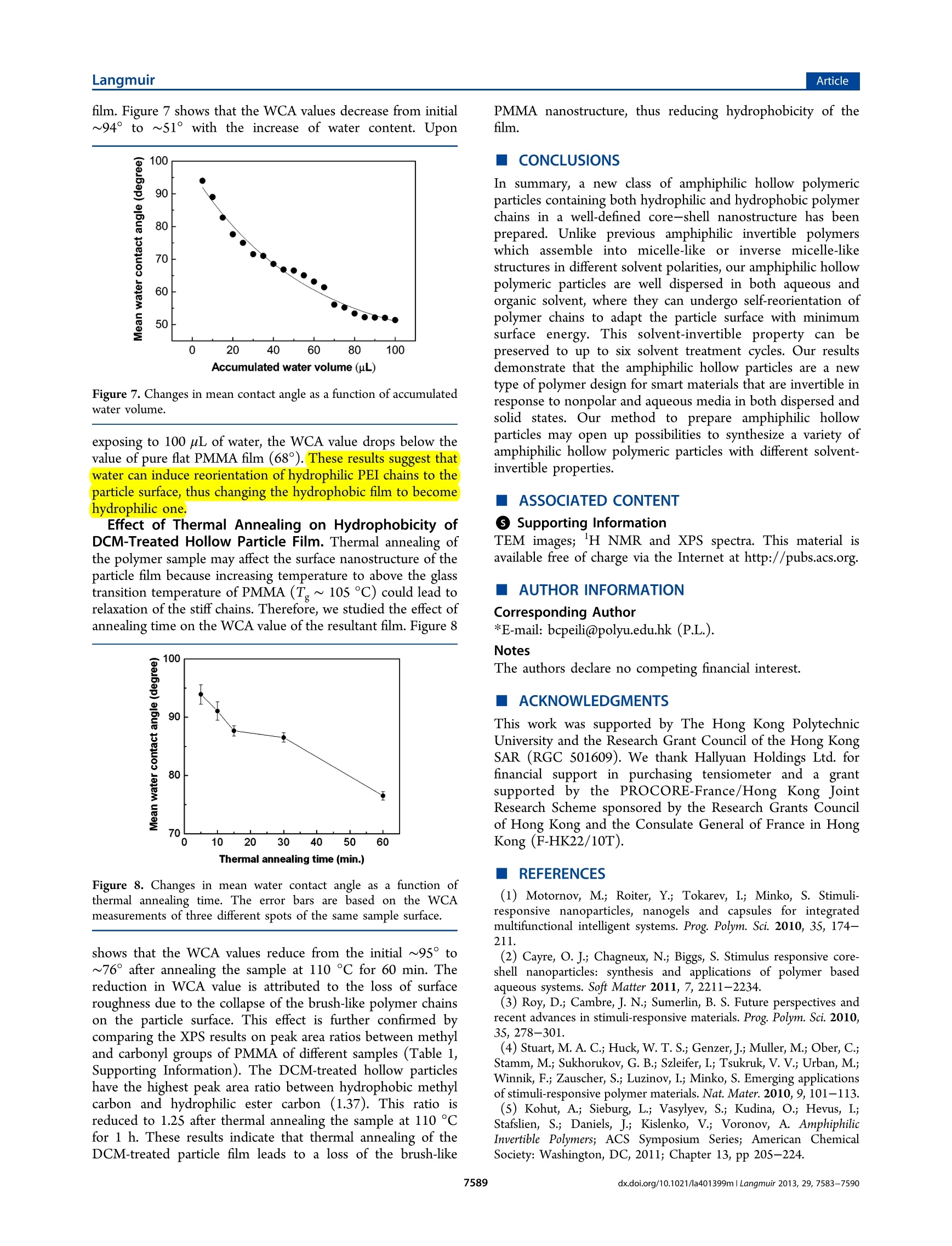
Langmuir-Articlepubs.acs.org/Langmuir LangmuirArticle Synthesis and Characterization of Solvent-Invertible AmphiphilicHollow Particles Cheng Hao Lee, Chun Him Wong, Djamila Ouhab, Redouane Borsali,and Pei Li* Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Hung Hom, Kowloon, HongKong SAR, P. R. China *Centre de Recherches sur les Macromolecules Vegetales (CERMAV-CNRS) and Joseph Fourier University, BP 53, F-38041Grenoble Cedex 9, France Supporting Information ABSTRACT: Previous researches on solvent-dependentpolymer systems mainly focus on amphiphilic invertiblepolymers (AIPs), whichare capable of forming solvent-dependent micellar or inverse micellar assemblies. However,poollyymer particles that are invertible in response to solventpolarity are almost unexplored. In this paper, we report a newtype of invertible hollow polymer (IHP))particle that iscomprised of polyethylenimine-g-poly(methyl methacrylate)(PEI-g-PMMA) copolymer. The amphiphilic PEI-g-PMMA PEI-g-PMMA hollow particles in water PEI-g-PMMA hollow particles in DCM hollow particles were first prepared through synthesis of well-defined PEI/PMMA core-shell particles, followed by removal ofPMMA homopolymer from the core. The resulting IHP particles can be stably dispersed in both nonpolar solvent and water. Wehave investigated the morphology and surface property of the particles in both dichloromethane (DCM) and water usingtransmission electron microscopy, water contact angle measurement, and X-ray photoelectron spectroscopy analysis to gaininsight into this unique particle dispersibility. Sustainability of the solvent-invertible property was carefully studied throughrepeated treatment ofthe IHP particles in DCM or water for up to six cycles. Solvent-dependent property of the dry films formedby IHP particles was also investigated through water contact angle measurement. Increasing water content on the DCM-treatedIHP particle film could reduce the water contact angle from 94° to 51°. Our results demonstrate that the amphiphilic hollowparticles are a new type of polymer design for smart materials that are invertible in response to nonpolar and aqueous media inboth dispersed and solid states. INTRODUCTION Smart polymer systems have received much attention inresearch because they are capable of undergoing conforma-tional andchemical changes upon receiving externalstimulus. -3 This kind of material plays an increasinglyimportant role in diverse applications such as drug delivery,diagnostics, biosensors, coatings, and textiles. Compared withthe widely studied pH-, temperature-, and electrolyte-sensitivepolymeric systems, much less attention has been paid to thesolvent-responsive polymeric system. Current studies mainlyfocus on amphiphilic invertible polymers (AIPs), which arecapable of assembling into polymer micelles in different solventenvironments. Various types of AIPs have been designed andsynthesized, such as amphiphilic homopolymers,6-8 amphi-philic copolymers,,10and amphiphilic dendrimers.1112 Themost distinctive advantage of AIPs is their rapid switching ofthe polymer conformation in response to different solventpolarities and the ability to subsequently self-assemble intowell-defined polymeric micelles under appropriate concen-trations. Other approaches to prepare solvent-responsivepolymeric systems are based on core-shell particles thatconsist of a solvent-responsive block copolymer shell or mixedblock copolymer brushes covalently linked to the particle cores.13-155These solvent-responsiveparticles have shownapplications in theedelivery off poorly water-soluble drug,16encapsulation of water-soluble molecules,selective peptidebinding18and catalysis.19 We herein report a new type of solvent-invertible polymericsystem and its properties. We have synthesized amphiphilichollow polymeric particles, which are capable of undergoingself-reorientation of polymer chains in response to differentsolvent polarities while their particle structure and stabilityremain intact.. To the best of our knowledge, this is the firstexample to demonstrate that amphiphilic hollow polymericparticles could possess solvent-invertible property in responseto a polar and an apolar solvent in both dispersed and solidstates.s.TThis work is a continuation of our previous research onthe use of amphiphilic hollow particles as a building block tocreate various supramolecular assemblies via the controlledaggregation and coalescence of the PEI-g-PMMA hollowparticles in a dichloromethane (DCM)-water mixture.20-22Objectives of this work is to carry out in-depth investigation of ( Received: A ugust 2 8, 2012 ) ( Revised: May 14, 2013 ) ( P ublished: May 30, 2013 ) Scheme 1. Schematic Representation of the Synthesis of PMMA/PEI Core-Shell Particles and the PEI-g-PMMA HollowParticles Step 1: Synthesis of PMMA/PEI core-shell particles Step 2: Generation of PEI-g-PMMA hollow particles solvent-invertible behavior of the amphiphilic hollow particlesin both polar and apolar solvents. Novel aspects of workinclude (1) study of hydrodynamic size and size distribution ofthe amphiphilic hollow polymeric particles in water andnonpolar solvents, (2) characterization of solvent-invertibleproperty by water contact angle measurement, (3) study ofsustainability of the invertible property in water and dichloro-methane through capturing their morphological changes withTEM observation, and (4) study the effect of water content andthermal annealing on wettability and surface property of DCM-treated hollow particle films. EXPERIMENTAL SECTION Preparation of Amphiphilic PEI-g-PMMA Hollow Particles.Amphiphilic particles that consisted of poly(methyl methacrylate)(PMMA) cores and branched poly (ethylenimine) (PEI) shells weresynthesized and purified according to our previously described method(Scheme 1).23 To prepare PEI-g-PMMA hollow particles, the freeze-dried PMMA/PEI particles (0.1 g) were first mixed with dichloro-methane (DCM, 8 mL) in a jacketed flask connected to a temperature-controlled circulator (IMT InMedtec Co.). The suspension was gentlystirred at 150 rpm for 3 h at 25°C to extract PMMA homopolymerfrom the particle cores. Solution viscosity increased noticeably due tothe dissolution of the PMMA homopolymer. Water was then added ata rate of 1 drop/s to up to the water to DCM volume ratio of 7 to 3.The heterogeneous mixture was then stirred at 150 rpm for 48 h tocompletely evaporate the DCM, giving a clear water phase containingPEI-g-PMMA hollow particles and insoluble PMMA homopolymer.The insoluble polymer was then removed by filtration using aWhatman filter paper with a pore size of 6 um(Whatman Int. Ltd.,England). The hollow particles in aqueous filtrate were isolated byfreeze-drying the solution and stored at room temperature forsubsequent studies (Scheme 1). Solvent Treatment Cycle of the PEI-9-PMMA HollowParticles in Water and Dichloromethane. The freeze-dried PEI-g-PMMA hollow particles (30 mg)) were stirred in dichloromethane (8mL) at 50 rpm for 3 h at 25 °C. The DCM was then evaporated in airto give the DCM-treated particles. For water-based particles, water wasadded to this dried solid and mixed at 50 rpm for 3 h at 25 °C. Thehollow particles were then recovered by freeze-drying the aqueoussolution. This water-dichloromethane treatment cycle was performedsix times. Particle morphologies of the first, second, third, and sixthcycles were examined with transmission electron microscopy. Determination of Grafting Percentage of PMMA. The graftingpercentage of PMMA was calculated according to the follow equation: where weight of the grafted PMMA is calculated based on the totalweight of polymerized MMA minus the weight of isolated PMMAhomopolymer. It was assumed that PEI molecules were all grafted withPMMA chains. MMA conversion was determined gravimetrically. Thegrafted copolymer and PMMA homopolymer were isolated fromPMMA/PEI core-shell particles by Soxhlet extraction with chloro-form for 72 h. Microscopy Characterization. Scanning electron microscopyimages were obtained using a JEOL-JSM 6335F field-emissionscanning electron microscope (FE-SEM) at an accelerating voltageof 5 kV. Samples were prepared by placing a small drop of particledispersion (~500 ppm) on glass substrates and allowed to dry at roomtemperature for 24 h. The air-dried samples were subsequentlysputtered with a thin layer of gold under vacuum to a depth ofapproximately 1-2 nm. Transmission electron microscopy (TEM)images were obtained using a JEOL 100 CX II TEM at an acceleratingvoltage of 100 kV. All samples were prepared by wetting carbon-coatedcopper grids with a small drop of particle dispersion (~200 ppm). Toenhance the contrast between the PEI and PMMA components, thehollow particles were stained with 0.5 wt % phosphotungstic acid solution for an appropriate time (30 s to 5 min.). The stained sampleswere then air-dried at room temperature before analysis. Dynamic Light Scattering Measurements. Light scatteringmeasurements were performed using an ALV laser goniometer, whichconsisted of a 22 mW red He-Ne linearly polarized laser (1= 632.8nm), an ALV/LSE-5004 multiple r digital correlator with a 125 nsinitial sampling time, and a temperature controller.4 The accessiblescattering angle ranged from 20° to 155°. Concentrations of hollowparticles in both water and DCM were ~1 mg/mL with a minimumsample volume of 0.8 mL. Sample solutions were added directly into10 mm diameter glass cells without prior filtration. The sample inwater was studied at scattering angles of 30°, 45°, 55°, and 60°, whilethe sample in DCM was studied at scattering angles of 60°, 75°, 90°,and 120°.All samples were measured at 25°C, and data were collectedusing digital ALV correlator control software. The counting time formeasuring the elastic or the quasi-elastic scattering intensities for eachsample varied from 60 to 300 s. The relaxation time distributions,A(t),obtained using CONTIN analysis were applied to the autocorrelationfunction, C(q,t).2 The reported particle sizes were calculated based onthe R, values measured at four different angles. The Rvalue at eachscattering angle was an average of at least three measurements. Water Contact Angle Measurements. Water contact angle(WCA) measurements were carried out using a Theta Lite OpticalTensiometer (Model TL100, Attension, Biolin Scientific, Sweden) anda Hamilton GASTIGHT microsyringe with a 22-gauge needle andsessile drop mode. Milli-Q water was used as the probe fluid.Hydrophilic glass plates with a thickness of 0.2 mm were thoroughlycleaned with detergent, followed by Milli-Q water and then ethanol.The cleaned glass plates were vacuum-dried at 60 °C before use. Allsamples were prepared via a drop-coating technique on cleaned glassplates, followed by air-drying the sample at room temperature for 24 h.A 5 uL water drop was added to the surface of the tested sample. Animage of the water droplet was recorded right at the instant when thedrop touched the sample surface. Data were also recorded after 30 s inorder to obtain a stable contact angle value. Thus, the advancing,receding, and mean contact angles were obtained at the 30th second.The reported WCA value was an average of six repeated measurementsof the same water droplet. The error uncertainties of all measurementswere within ±5%. Contact angle measurements of DCM-treated hollow particle filmsobtained from each DCM-treatment cycle were as follows:The freeze-dried hollow particles (5 mg) obtained from aqueous solution werestirred in 4 mL of DCM at 50 rpm for 3 h at 25 °C. This sample with afinal concentration of 5 mg/mL was used to perform six cycles ofwater-dichloromethane treatment according to the proceduredescribed before. All six cycles of dried DCM-treated particle filmsformed on glass substrates were stored in a desiccator prior to theirwater contact angle measurements. The reported WCA value of eachcycle of the DCM-treated particle film was calculated from an averageof six repeated measurements. Contact angle measurements on DCM-treated particle film withincreasing water content were as follows:; The freeze-dried hollowparticles (5 mg) obtained from aqueous solution were stirred in 4 mLof DCM at 50 rpm for 3 h at 25 °C. A final concentration of 5 mg/mLparticles in DCM was used to form a DCM-treated film on a glasssubstrate via a drop-coating technique. Water contact angles weremeasured on the sample surface 30 s after the addition of waterdroplet. After the water droplet on the sample dried completely,another drop of water was added to the same sample spot, followed byanother WCA measurement. This wetting-drying cycle was repeated20 times at the same sample location with a final accumulated watervolume of 100 uL. Contact angle measurements of the DCM-treated hollow particlefilm after thermal annealing were as follows: The freeze-dried hollowparticles (5 mg) obtained from aqueous solution were stirred in 4 mLof DCM at 50 rpm for 3 h at 25C. A DCM solution containinghollow particles (5 mg/mL) was used to form a DCM-treated particlefilm on a glass substrate via a drop-coating technique. The driedsamples were then heated at 110 C at 1 atm pressure for differentannealing durations (5, 10, 15, 30, and 60 min) in an oven (UM 100 Memmert Co., Germany). The annealed samples were cooled down toroom temperature (at least 30 min) prior to water contact anglemeasurement. The reported WCA values of all thermally annealedparticle films were the average of three measurements at three differentsample locations. X-ray Photoelectron Spectroscopy. X-ray photoelectron spec-troscopy (XPS) was used to examine the surface composition of foursamples which were coated on flat mica substrates, followed by dryingthem overnight in a vacuum oven at room temperature. These foursamples are (1) DCM-treated hollow particles at room temperature,(2) water-based hollow particles at room temperature, (3) DCM-treated hollowparticles which were thermally annealed at 110°C for lh, and (4) PMMA homopolymer prepared by bulk polymerization.XPS measurement was performed using a PHI 5600 (PhysicalElectronics) multidetection system equipped with a monochromatic AlKa X-ray source (1486.6 eV). The X-ray spot size was 400 um indiameter. The pass energy of exciting radiations was set at 187 and 45eV for survey scans. Charge compensation was achieved using acombination of electron and argon ion sputtering guns. The energyand emission currents of the electrons were 4 eV and 0.35 mA,respectively. The partial pressure for the argon ion flood gun wasmaintained at a vacuum level of 2× 10-8 mbar. Argon-ion sputteringwas made by a PHI VersaProbe ion gun at an incidence angle of 45°for removal of physiosorbed adsorbates. The beam energy and ioncurrent were set at 600 eV and 4 uA, respectively. The measuredsample size was confined to 600 ×600 um or 800×800 um. The ionsputtering was performed at the substrate temperature of 300 K for 10min in each cycle. All data acquisition and processing were achievedwith the PC-based Avantage software (version 1.85). Spectralcalibration was determined by setting the main C 1s component at285.0 eV. The surface composition was determined using themanufacturer’s sensitivity factors. Curve fitting of the spectra wasaccomplished using a nonlinear least-squares method, and a Gaussianfunction was assumed for the curve-fitting. RESULTS AND DISCUSSION Synthesis and Characterization of PEI-g-PMMAHollow Particles. We first prepared PEI-g-PMMA hollowparticles via a two-step synthesis as shown in Scheme 1: (1)Synthesis of PEI/PMMA core-shell particles according to ourpreviously described method.3 (2) Removal of PMMAhomopolymer from the particle cores with dichloromethane,followed by isolation of PEI-g-PMMA hollow particles in water.The amphiphilic PMMA/PEI core-shelll particles weresynthesized by dissolving the branched PEI ((M)~ 60 000g mol) in water, followed by addition of an appropriateamount of tert-butyl hydroperoxide (TBHP) at 80 C togenerate both PEI macroradicals and tert-butoxy radicalsthrough amine-peroxide redox reaction. The resultant PEImacroradicals could polymerize MMA to give a graftedcopolymer, while the tert-butoxy radicals could either initiatethe homopolymerization of MMA or abstract hydrogen atomsfrom the polymer backbone. The amphiphilic graft macro-radicals generated in situ could subsequently self-assemble toform micelle-like microdomains, followed by further emulsionpolymerization of the MMA to generate core-shell particles.Monomer conversions were usually above 90%. The resultingcore-shell particles were purified by repeated cycles ofcentrifugation and decantation, followed by freeze-drying togive white solids for subsequent studies. The purified core-shell particles were characterized withrespect to their particle size and morphology. Statisticallynumber- and volume-average hydrodynamic diameters of theparticles were 193 and 212 nm in water, respectively. Thus,particle polydispersity (D/D,) was equal to 1.10, indicating avery narrow particle size distribution. This property was confirmed by the scanning electron microscopy (SEM) imageas shown in Figure la. To identify the nanostructure of the Figure 1. (a) SEM image of PMMA/PEI core-shell particles. (b)TEM image of PMMA/PEI core-shell particles.(c) PEI-g-PMMAhollow particles in DCM. (d) PEI-g-PMMA hollow particles in water.Inset: HRTEM image of PEI-g-PMMA hollow particles in water. Theoverlapped region displays darker contrast than the nonoverlappedpart, indicating the hollow nature of the particles. Particles shown inTEM images were all stained with 0.5 wt % phosphotungstic acidsolution for 5 min. particles, we utilized heavy atoms as a probe to generate adarker contrast in TEM images. Since the amine-containing PEImolecules have much stronger binding affinity for heavy atomsthan the PMMA, staining the particles with a dilutephosphotungstic acid (PTA) solution could provide a bettercontrast to display the placement of the hydrophilic PEIsegments within the particles. Figure 1b shows a TEMmicrograph of PMMA/PEI core-shell particles in water. Theparticles display three observable and well-defined regions: (1)The white center part is the region of PMMA homopolymer.(2) The gray area is comprised of a mixture of PEI and PMMAsegments. (3) The darker outer region is the PEI-rich area (seeFigure S1 in Supporting Information). Since the particlescontain PMMA homopolymer in the cores, the homopolymercan be easily removed through dissolving them in DCM. Thus,homo- and grafted-PMMA could be isolated by a solventextraction method. Percentage of PMMA grafting on the PEIcan be estimated based on monomer conversion and weight ofthe isolated PMMA homopolymer. It was found that PMMAgrafting percentage (weight of PMMA grafts divided by weightof PEI) was ~195%. This result means that the weight fractionof the grafted PMMA was almost 2 times higher than that ofthe PEI. The grafting density of the PEI was also estimated tobe around 5% based on the molar ratio between TBHP initiatorand primary amine of the PEI. Unfortunately, the averagemolecular weight of the grafted PMMA was difficult todetermine since no solvent could completely dissolve thePEI-g-PMMA copolymer. To prepare hollow particles, freeze-dried PMMA/PEI core-shell particles were mixed with DCM under gentle stirring (below 150 rpm) at 25 ℃ for 3-4h, followed by addition ofwater to up to the water to DCM volume ratio of 7 to 3. TheDCM was slowly evaporated at room temperature to give aclear water phase containing PEI-g-PMMA hollow particles andinsoluble PMMA homopolymer. The insoluble polymer wasthen removed by filtration. The hollow particles in aqueouswere isolated by freeze-drying the solution (Scheme11).Chemical structures of the isolated PMMA homopolymer andthe PEI-g-PMMA copolymer were confirmed with H NMRspectroscopy (Figure S2, Supporting Information). Figure 1cshows the morphology of the PEI-g-PMMA hollow particlesinitially generated in DCM. The lighter region of the particleshell indicates the presence of the PMMA graft chains whichare highly extended in DCM with a rough surface. This particlemorphology explains why the PEI-g-PMMA hollow particleswere able to disperse in DCM. This is due to the fact thatPMMA grafted chains are localized at the outer shell, thusproviding steric stabilization for the particles. On the otherhand, the dark core region indicates the location of the PEImolecule. A small amount of staining agent might have beentrapped inside the particle core; thus, the core appeared to bequite dark. Figure 1c also shows that the PEI-g-PMMA hollowparticles in DCM have high uniformity. Figure 1d illustrates themorphology of the PEI-g-PMMA hollow particles in water. Thedarker PEI region is now localized on the outer layer of theparticle with PMMA grafted chains in the interior (lighterregion). The inversion of the shell layer from PMMA grafts toPEI in water results in stabilizing PEI-g-PMMA hollow particlesin water. The hollow nature of the PEI-g-PMMA particles wasconfirmed with HRTEM image (Figure 1d, inset). The bright-field HRTEM image shows a darker contrast in the overlappedregion that is attributed to higher electron scattering withincreased thickness. In addition, particle boundaries betweentwo overlapped particles are clearly observable. These resultsindicate that the PEI-g-PMMA particles indeed possess hollowcavity. Characterization of Solvent-Invertible Property of thePEI-9-PMMA Hollow Particles. The above TEM imagessuggest that hydrophilic PEI and hydrophobic grafted PMMAchains could undergo internal switching in order to minimizetheir surface energies in solution. To gain a better under-standing of the switching mechanism of the hollow particles, wehave determined whose hydrodynamic sizes in both water andDCM with dynamic light scattering (DLS) measurements. Itwas found that the average hydrodynamic radius (Rh) of thehollow particles in DCM (measured at scattering angles of 60°,75°, 90° and 120°) was 110 nm, while the average R, of hollowparticles in water (measured at scattering angles of 30°, 45°,55°, and 60°) was 150 nm. Figure 2 compares the Rh of thehollow particles dispersed in bothi water and DCM at ascattering angle of 60°. The two small peaks at 5 and 11.6 nmdetected in water are due to the presence of trace amounts offree PEI molecules. The DLS results suggest that the hollowparticles in both solvents have small differences in their size andsize distribution, indicating that the particles have remainedstable and intact during the switching process The surface invertible property of the PEI-g-PMMA hollowparticles was investigated with X-ray photoelectron spectros-copy analysis. The chemical composition of the outer layers ofthe hollow particles in both water and DCM could be identifiedsince the XPS detects region close to the sample surface to adepth of 10 nm or below. Figure 3a shows that hollow particlesin water contain an N 1s peak at about 400.5 eV, while there is Figure 2. Hydrodynamic radius (R,) of hollow particles in DCM andwater measured by dynamic light scattering at 25C at a scatteringangle of 60°. Figure 3. XPS spectra of PEI-g-PMMA hollow particles:(a) hollowparticles dispersed in water; (b) hollow particles dispersed indichloromethane. no such peak in the spectrum of the sample treated indichloromethane (Figure 3b). The high-resolution deconvo-luted spectra of C 1s, N 1s, and O 1s of water-based hollowparticles are shown in the Supporting Information (Figure S3).In Figure 3a, there is an O 1s peak at a binding energy of 530.9eV. To verify the molecular structure of such peak, the samplewas treated with either argon ion sputtering or thermaldesorption process.26 It was found that intensity of the O 1speak could be reduced while the envelope profile of the highresolution C 1s and N 1s peaks was not affected (Figure S3c).These results suggest that the O 1s peak is due to the presenceof physisorbed oxygen molecules on the particle surface, not aC-O bonding. The surface invertible property of the PEI-g-PMMA hollowparticles was further investigated with water contact angle(WCA) measurements. Since water is a good solvent for PEIbut a poor one for PMMA, we could use water as a probe liquidto detect the contact angle of a water droplet on a pretreatedsubstrate surface. Figure 4a illustrates the image of a waterdroplet on a substrate that has been coated with water-basedhollow particles. The WCA value is 22.03°, indicating anexcellent surface wettability with water. Figure 4b shows thecontact angle measurement on a substrate that has been coatedwith DCM-treated hollow particles. The WCA value is 93.28° Figure 4. Contact angle measurement of a water droplet on a glasssurface. The glass surface was coated with (a) water-based hollowparticles and (b) DCM-treated hollow particles. which is considerably higher than that of the pure flat PMMAfilm (WCA~68°).'sSuch high WCA value of the PMMA maybe attributed to the surface nanostructure of the brush-likegrafted PMMA chains in DCM as illustrated in Figure 1c. Infact, it is known that both the hydrophobicity of polymer chainand surface roughness can influence the apparent contactangles. The effect of surface roughness on apparent contactangle is usually described by Wenzel’s equation, cos 0* = r cosO, where 0 is the Young intrinsic contact angle on a flathomogeneous surface, 0* is the apparent contact angle on arough surface, and r is the surface roughness defined as the ratioof the actual surface area to the projected surface area.According to Wenzel’s equation, when a surface roughness isintroduced, an intrinsically hydrophilic surface (0<90°) will bemore hydrophilic or an intrinsically hydrophobic surface (0>90°) will become more hydrophobic.28 The solvent can alsoaffect the orientation of surface hydrophobic (methyl group)and hydrophilic groups (ester) in PMMA chains. Therefore,both effects of nanoscale roughness of the brush-like PMMAgrafts and reorientation of surface hydrophobic groups couldresult in a significant increase in PMMA hydrophobicity. Theseresults confirm that amphiphilic hollow particles possess surfaceinvertible property that responds to solvent polarity. Reorientation of polymer chains in response to differentsolvent polarity can be explained by the solubility parametertheory. For mutual affinity between solvent and polymer, thereorganization process is spontaneous only if the Gibbs freeenergy of polymer-solvent mixing (AG ) is negative asdescribed by the thermodynamic laws: AGm=AHm-TASm.Changes in enthalpycan be expressed as a function of thesolubility parameter:AHm=D,D,Vm(8,-8,), where ,and , are the volume fractions of the polymer and solvent,respectively. Vm is the molar volume of the mixture; 8, and8,are the solubility parameters of the polymer and the solvent,respectively. Since the mixing process is usually accompaniedby a considerable entropy increase, ASm is positive; thus,changes in enthalpy of mixing, AHm, must be reduced as muchas possible to ensure a more negative AGm. Hence, for higheraffinity between the polymer and the solvent, the difference intheir solubility parameters should be as small as possible. Forthe PEI-g-PMMA hollow particles, solubility parameters of PEIand water are 43 and 47.9 MPal/2, respectively, indicating ahigh affinity between PEI and water.30,31 Similarly, solubilityparameters of PMMA and DCM are 19.0 and 19.8 MPa1/2respectively;31,32 thus, PMMA chains have high affinity toward Figure 5. TEM images of hollow particles in both water and DCM. (a) Initial hollow particles in water. (b) The first treatment cycle of hollowparticles in DCM. (c) The second treatment cycle of hollow particles in water. (d) The second treatment cycle of the hollow particles in DCM. (e)The third treatment cycle of hollow particles in water. (f) The third treatment cycle of the hollow particles in DCM. (g) The sixth treatment cycle ofthe hollow particles in water. (h) The sixth treatment cycle of the hollow particles in DCM. dichloromethane, resulting in spontaneous chain stretching inDCM. Sustainability of Invertible Property of the HollowParticles in Solution. Upon confirming the solvent-invertibleproperty of the amphiphilic PEI-g-PMMA hollow particles, itwas of interest to elucidate if such property could be sustainedwith repeated polar and nonpolar solvent treatment cycles andthe resulting morphological changes after each cycle. Thus, weredispersed the freeze-dried hollow particles initially obtainedfrom water phase into DCM (around 3 h at room temperature)and examined the morphology of the resultant hollow particles.The TEM image shown in Figure Sb clearly displays well-defined core-shell particle morphology with PMMA grafts(lighter region) as the shell and PEI (darker region) as the core.The hollow particles in DCM were then recovered throughcomplete DCM evaporation. The dried solids were treated withwater again for 3 h at room temperature, and the particlemorphology in water was examined by TEM (Figure 5c). Thistreatment cycle was repeated six times, and morphologicalchanges were observed by TEM. Figure 5 shows that the solvent-invertible nature of theamphiphilic hollow particles could still be maintained even aftersix water/DCM treatment cycles. The particles remained intactduring these switching cycles. When the amphiphilic hollowparticles were dispersed in water, grafted PMMA chains tendedto localize in the core in order to reduce surface energy throughminimizing surface contact with water. At the same time, water-soluble PEI molecules tended to form the corona of the particleto maximize their conformation entropy. In the case ofdispersing the amphiphilic hollow particles in DCM, hydro-philic PEI segments were tucked into the core in order tominimize surface energy in nonpolar DCM. It was also notedthat originally well-defined PMMA and PEI regions becamemore and more ill-defined after six cycles of solvent treatment.This phenomenon might be due to the fact that the longhydrophilic PEI branches and the hydrophobic PMMA graftchains might become entangled or interpenetrated with eachother through repeated switching process. Thus, contrastbetween the hydrophilic PEI and hydrophobic PMMAsegments became ill-defined. On the other hand, short PMMA graft chains were more labile. They were still able tomove to the particle surface to form a thin PMMA shell asshown in Figure Sh. To confirm the above-mentioned morphological changesbased on TEM observation, we also determined the watercontact angles of the DCM-treated particle films obtained fromeach cycle. Figure 6 shows that WCA value in each consecutive Figure 6. Changes in mean water contact angle of films prepared fromeach cycle of DCM-treated hollow particles. cycle gradually decreases and drop to 71°at the sixth cycle.These results suggest that the surface roughness of the DCM-treated hollow particles reduces after each switching cycle dueDXto chain entanglement. Eventually only those short PMMAgraft chains can moveto thee particle surface, givingacomparable WCA value with the bulk PMMA (WCA= 68°).Results from this water contact angle study are consistent withthe TEM observation in Figure 5. Effect of Water Content on Wettability of DCM-Treated Hollow Particle Film. The above results showed thatPEI-g-PMMA hollow particles were responsive to solutionpolarity. We would also like to know if this switching propertycould occur in solid film or not. Therefore, we studied the effectof water content on wettability of DCM-treated hollow particle film. Figure 7 shows that the WCA values decrease from initial~94° to ~51° with the increase of water content. Upon Figure 7. Changes in mean contact angle as a function ofaccumulatedwater volume. exposing to 100 uL of water, the WCA value drops below thevalue of pure flat PMMA film (68°). These results suggest thatwater can induce reorientation of hydrophilic PEI chains to theparticle surface, thus changing the hydrophobic film to becomehydrophilic one. Effect of Thermal Annealing on Hydrophobicity ofDCM-Treated Hollow Particle Film. Thermal annealing ofthe polymer sample may affect the surface nanostructure of theparticle film because increasing temperature to above the glasstransition temperature of PMMA (T~105°C) could lead torelaxation of the stiff chains. Therefore, we studied the effect ofannealing time on the WCA value of the resultant film. Figure 8 Figure 8. Changes in mean water contact angle as a function ofthermal annealing time. The error bars are based on the WCAmeasurements of three different spots of the same sample surface. shows that the WCA values reduce from the initial ~95°to~76°after annealing the sample at 110℃ for 60 min. Thereduction in WCA value is attributed to the loss of surfaceroughness due to the collapse of the brush-like polymer chainson the particle surface. This effect is further confirmed bycomparing the XPS results on peak area ratios between methyand carbonyl groups of PMMA of different samples (Table 1,Supporting Information). The DCM-treated hollow particleshave the highest peak area ratio between hydrophobic methylcarbon and hydrophilic ester carbon (1.37). This ratio isreduced to 1.25 after thermal annealing the sample at 110 ℃for 1 h. These results indicate that thermal annealing of theDCM-treated particle film leads to a loss of the brush-like PMMA nanostructure, thus reducing hydrophobicity of thefilm. CONCLUSIONS In summary, a new class of amphiphilic hollow polymericparticles containing both hydrophilic and hydrophobic polymerchains in a well-defined core-shell nanostructure has beenprepared. Unlike previous amphiphilic invertible polymerswhich assemble into micelle-likete or inverse micelle-likestructures in different solvent polarities, our amphiphilic hollowpolymeric particles are well dispersed in both aqueous andorganic solvent, where they can undergo self-reorientation ofpolymer chains to adapt the particle surface with minimumsurface energy. This solvent-invertible property can bepreserved to up to six solvent treatment cycles. Our resultsdemonstrate that the amphiphilic hollow particles are a newtype of polymer design for smart materials that are invertible inresponse to nonpolar and aqueous media in both dispersed andsolid states. Our method to prepare amphiphilic hollowparticles may open up possibilities to synthesize a variety ofamphiphilic hollow polymeric particles with different solvent-invertible properties. ASSOCIATED CONTENT ⑤ Supporting Information TEM images; H NMR and XPS spectra. This material isavailable free of charge via the Internet at http://pubs.acs.org. AUTHOR INFORMATION Corresponding Author *E-mail: bcpeili@polyu.edu.hk (P.L.). Notes The authors declare no competing financial interest. ACKNOWLEDGMENTS This work was supported by The Hong Kong PolytechnicUniversity and the Research Grant Council of the Hong KongSAR (RGC 501609). We thank Hallyuan Holdings Ltd. forfinancial support in purchasing tensiometer anda grantsupported by the PROCORE-France/Hong Kong JointResearch Scheme sponsored by the Research Grants Councilof Hong Kong and the Consulate General of France in HongKong(F-HK22/10T). REFERENCES (1) Motornov, M.; Roiter, Y.; Tokarev, I; Minko, S. Stimuli-responsive nanoparticles, nanogels and capsules for integratedmultifunctional intelligent systems. Prog. Polym. Sci. 2010, 35, 174-211. (2) Cayre, O. J.; Chagneux, N.; Biggs, S. Stimulus responsive core-shell nanoparticles: synthesis and applications of polymer basedaqueous systems. Soft Matter 2011,7,2211-2234. (3) Roy, D.; Cambre, J. N.; Sumerlin, B. S. Future perspectives andrecent advances in stimuli-responsive materials. Prog. Polym. Sci. 2010,35, 278-301. (4) Stuart, M. A. C.; Huck, W. T. S.; Genzer,J.; Muller, M.; Ober, C.;Stamm, M.; Sukhorukov, G. B.; Szleifer, I.; Tsukruk, V. V.; Urban, M.;Winnik, F.; Zauscher, S.; Luzinov, I; Minko, S. Emerging applicationsof stimuli-responsive polymer materials. Nat. Mater. 2010, 9,101-113. (5) Kohut, A.; Sieburg, L.; Vasylyev, S.; Kudina, O.; Hevus, I.;Stafslien, S.; Daniels, J.; Kislenko, V.; Voronov, A. AmphiphilicInvertible Polymers; ACS Symposium Series; American ChemicalSociety: Washington, DC,2011; Chapter 13, pp 205-224. ( (6) E lamprakash, N.; S ivakumar, S .; Aathimanikandan, V.; Thayumanavan, S . Su p ramolecular as s emblies fr o m amp h iphilichomopolymers: T e sting t h e s c ope. J. A m. Chem. So c . 2 0 06, 12 8 , 16224-16230. ) ( (7) K ale, T. S.; K laikherd, A.; P opere, B .; Thayumanavan, S . Supramolecular a ssemblies o f amphiphilic homopolymers. L a ngmuir 2009,25, 9660-9670. ) ( (8) L a urent, B . A . ; G r ayson, S. M. Syn t hesis of c y clic amphiphilichomopolymers an d their potential application as polymeric m i celles. Polym. Chem. 2012, 3, 1846-1855. ) ( (9) Hevus, I ; K o hut, A. ; Vo r onov, A. A mp h iphilic invertiblepolyurethanes: s ynthesis and properties. Macromolecules 2010, 4 3, 7488-7494. ) ( (10) Voronov, A.; K ohut, A.; Peukert, W.; Voronov, S .; Gevus, O.;Takarev, V . I nvertible a rchitectures from a mphiphilic p o lyesters. Langmuir 2006, 2 2, 1946-1 9 48. ) ( (11) V utukuri, D . R.; B asu, S.; Thayumanavan, S . Dendrimers wi t h b oth polar and apolar nanocontainer characteristics. J. Am. Chem. Soc. 2004, 126,15636-15637. ) ( (12) Ramireddy, R . R . ; Raghupathi, K . R.; Torres, D . A. ; Thayumanavan, S. St i muli se n sitive a m phiphilic d e ndrimers. New J. Chem. 2 012, 36, 340-349. ) ( (13) Y in, M.; B auer, R.; Klapper, M.; Mullen, K. Amphiphilicmulticore-shell particles based on polyphenylene dendrimers. Ma c ro- mol. Chem. Phys. 2007, 208, 1646-1656. ) ( (14) Motornov, M. ; Sheparovych, R.; Lu p itskyy, R.; M a cWilliams, E. ; Hoy, O.; Luzinov, I.; Minko, S. Stimuli-responsive c olloidal systems f rom m i xed br u sh-coated nanoparticles. Ad v . Funct. Ma t er. 2007, 17, 2307-2314. ) ( (15) Z hu, L.; Z hao, B. Transmission electron microscopy study ofsolvent-induced p hase m o rphologies of en v ironmentally res p onsivemixed homopolymer brushes on silica particles. J. Phys. Chem. B 2008, 112, 1 1529-11536. ) ( (16) H e vus, I.; Modgil, A.; Dan i els, J.; Koh u t, A.; S un, C.; S t afs l ien,S.; Voronov, A. I n vertible micellar polymer assemblies for delivery ofpoorly w ater-soluble drugs. Biomacromolecules 2012, 13, 2 537-2545. ) ( (17) Q iu, L.; Z hang, J.; Y an, M .; Jin, Y .; Z hu, K . Reverse self- assemblies based on a m phiphilic polyphosphazenes for encapsulation of water-soluble molecules. Nanotechnology 2007, 18,475602-475610. ) ( (18) G omez-Escudero, A. ; Azagarsamy, M. A.; Theddu, N.; Vachet, R . W .; T hayumanavan, S. Se l ective peptide binding us i ng fa c iallyamphiphilic dendrimers. J . Am. Chem. Soc . 2008 , 130 , 11156-11163. ) ( (19) R yu, E . ; Cho, H . ; Z h ao, Y. Ca t alyzing me t hanolysis of alkylhalides i n t he i nterior o f an amphiphilic molecular bas k et. Org . Lett. 2007, 9 ,5147-5150. ) ( (20) Sunintaboon, P.; H o , K. M.; Li , P.; Cheng, S. Z. D.; Harris, F . W. Formation of nanostructured materials via c oalescence o famphiphilic hollow particles. J. Am. Chem. Soc. 2006, 128, 2168-2169. ) (21) Lee, C. H.; Ho, K. M.;Harris, F. W.; Cheng, S. Z. D.; Li, P.Formation of nanostructured materials using inexpensive hollowparticles of amphiphilic graft copolymers as building blocks: 1. Insightinto the mechanism of nanotube formation. Soft Matter 2009, S,4914-4921. ( (22) Lee, C. H.; Li, P. pH-induced formation of various hierarchical structures from amphiphilic core-shell nanotubes. RSC Adv. 2012, 2,1303-1306. ) ( (23) L i, P .; Zhu, J.; Sunintaboon, P .; Harris, F. W. N ew route toamphiphilic core-shell polymer nanospheres: g r aft copolymerization o f methyl m ethacrylate f rom water-soluble p olymer c h ains c o ntaining amino groups. L angmuir 2002, 1 8 , 8641-8646. ) ( (24) Berne, B .; Pecora, R. Dynamic Light Scattering: With Applications to Chemistry, Biology , and Physics; Dover Publications : Mineola, NY, 2000. ) (25) Dal B6, A. G.; Soldi, V.; Giacomelli, F. C.; Travelet, C.; Jean, B.;Pignot-Paintrand, I.;Borsali, R.; Fort, S. Self-assembly of amphiphilicglycoconjugates into lectin-adhesive nanoparticles. Langmuir 2012, 28,1418-1426. ( ( 26) Polzonetti, G.; R u sso,M. V.; Fur l ani, A.; Iucci,G. Interaction ofHO, O2an d CO, with the surface of polyphenylacetylene fi l ms: a n X PS i n vestigation. Chem. Phys. Lett . 1991 , 185, 105-110. ) ( ( 27) Ma, Y . ; Cao, X.; Feng, X. J.; Ma, Y . M .; Z ou, H. F a brication ofsuperhydrophobic film f rom PMMA with intrinsic water contact a n gle below 90°. P olymer 2007, 4 8, 7 455-7460. ) ( (28) A damson. A. W. ; Gast, A. P. Ph y sical Chemistry of Surfaces, 6thed.; Wiley: New York, 1 9 97; p 358. ) ( (29) M ark, J . E . , E d .; P h ysical P r operties o f Polymers H a ndbook;Springer: New York, 2007, p 289. ) ( (30) Madkour, T. M . A combined statistical mechanics a n d moleculardynamics approach for the evaluation of the miscibility of polymers i ngood, poor and non-solvents. Chem. Phys.2001,274, 1 87-198. ) ( ( 31) B arton, A. F . M. C RC Handbook of Solubility Parameters a nd Other Cohesion Parameters; CRC Press: Boston, MA, 1983; pp 147- 149. ) ( (32) B arton, A . F . M . CRC Ha n dbook of Polymer-Liquid Interaction Parameters and Solubility Parameters; CRC Press: Bo s ton, MA, 1990; p 265. ) CS Publications @ American Chemical Societydx.doi.org/laI Langmuir x.doi.org/laI Langmuir
确定





还剩6页未读,是否继续阅读?
瑞典百欧林科技有限公司为您提供《合成与表征溶液诱导双亲空壳微粒》,该方案主要用于其他中--检测,参考标准--,《合成与表征溶液诱导双亲空壳微粒》用到的仪器有Theta Lite 光学接触角仪
推荐专场
相关方案
更多
该厂商其他方案
更多