方案详情
文
Unique protomer collision cross section values (CCS) can be determined and used as an additional identification parameter
in a routine screening workflow. Individual protomer spectra are generated along with proposed fragmentation pathways. The impact of matrix upon protomer ratios can be observed routinely using new ion mobility processing functionality within the UNIFI Scientific Information System. The ACQUITY UPLC I-Class System can be used in conjunction with ion mobility as a development tool to generate more robust analytical methods. Ability to perform retrospective UPLC and ion mobility
data review.
方案详情

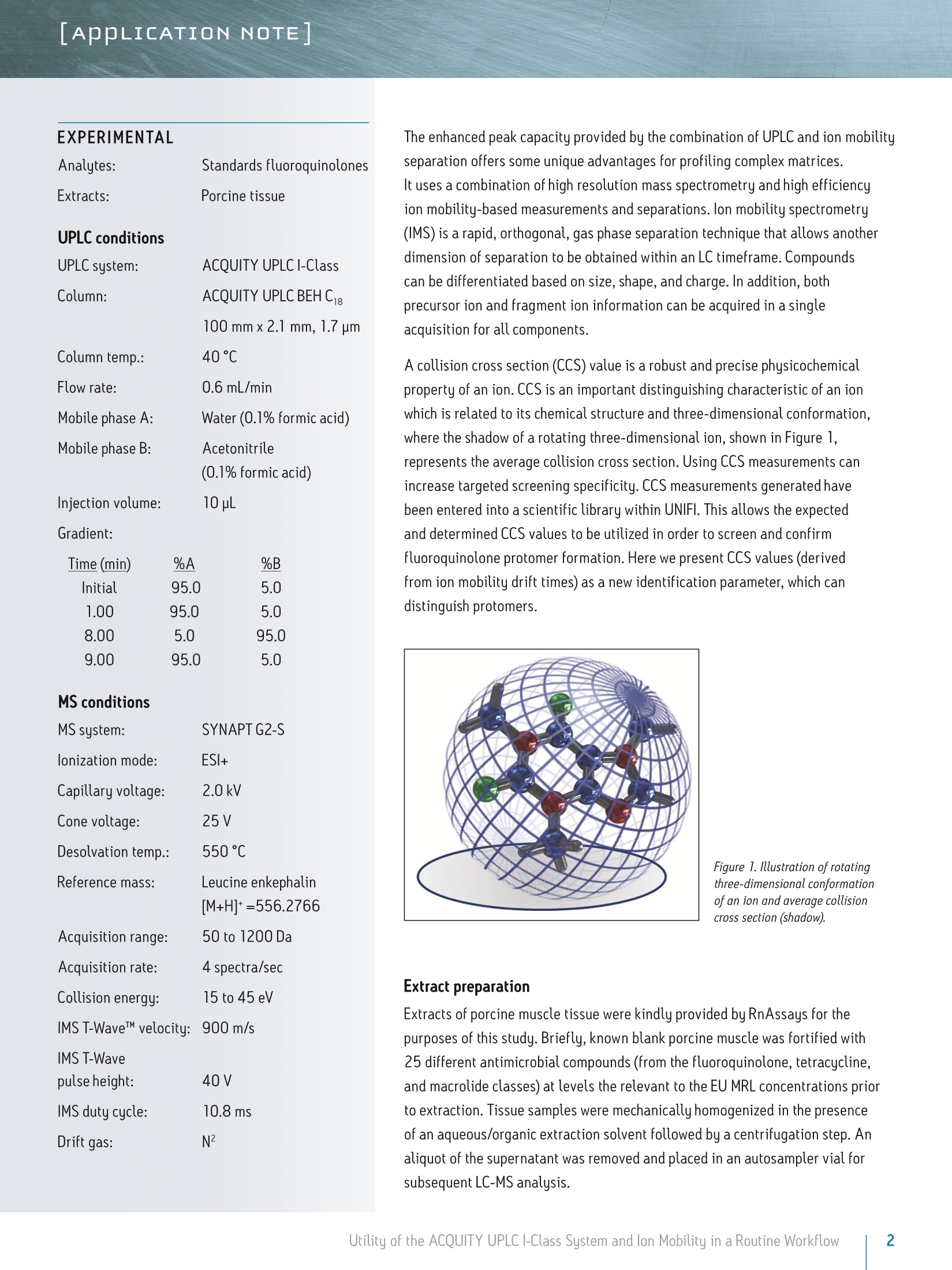
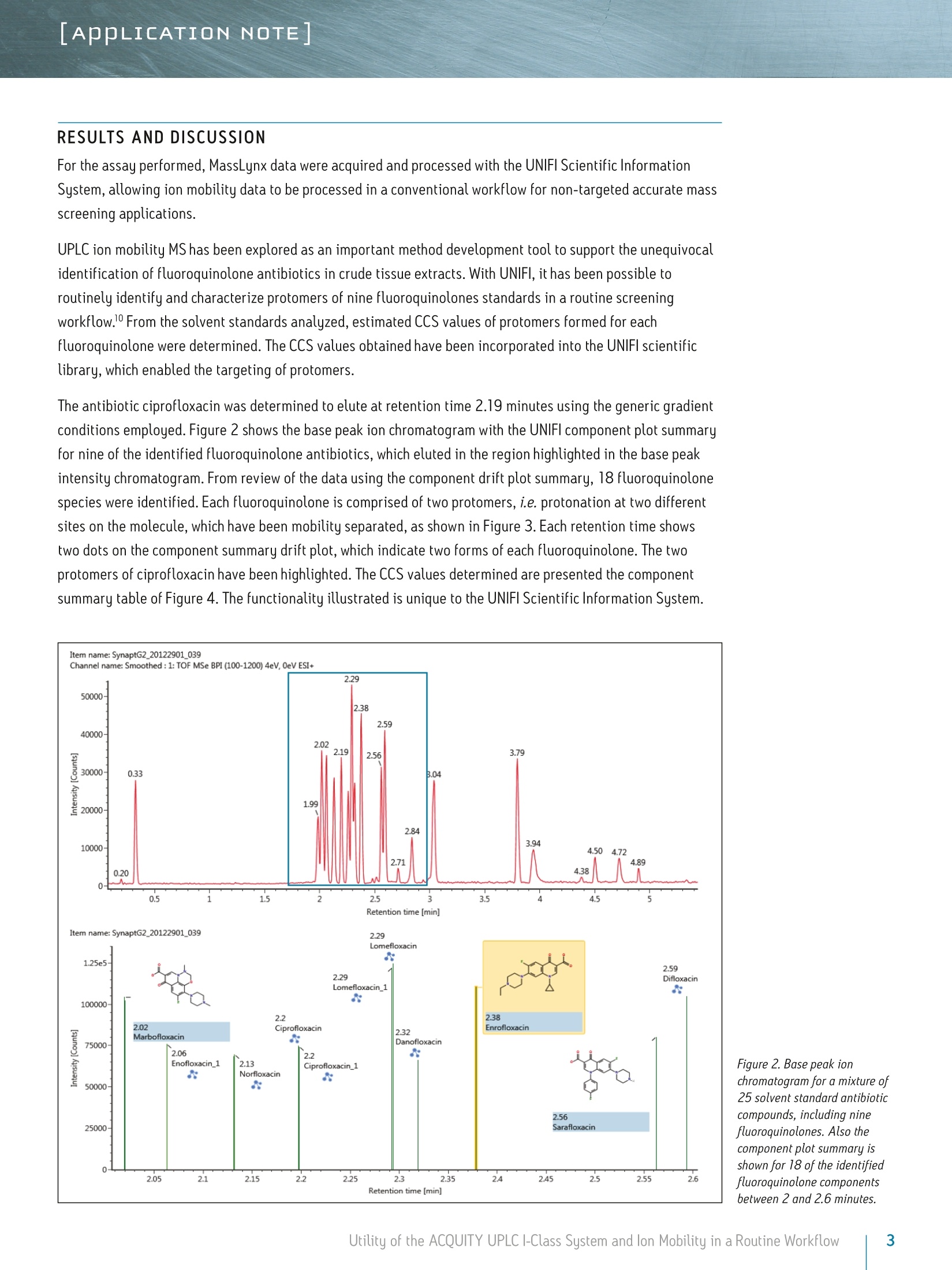
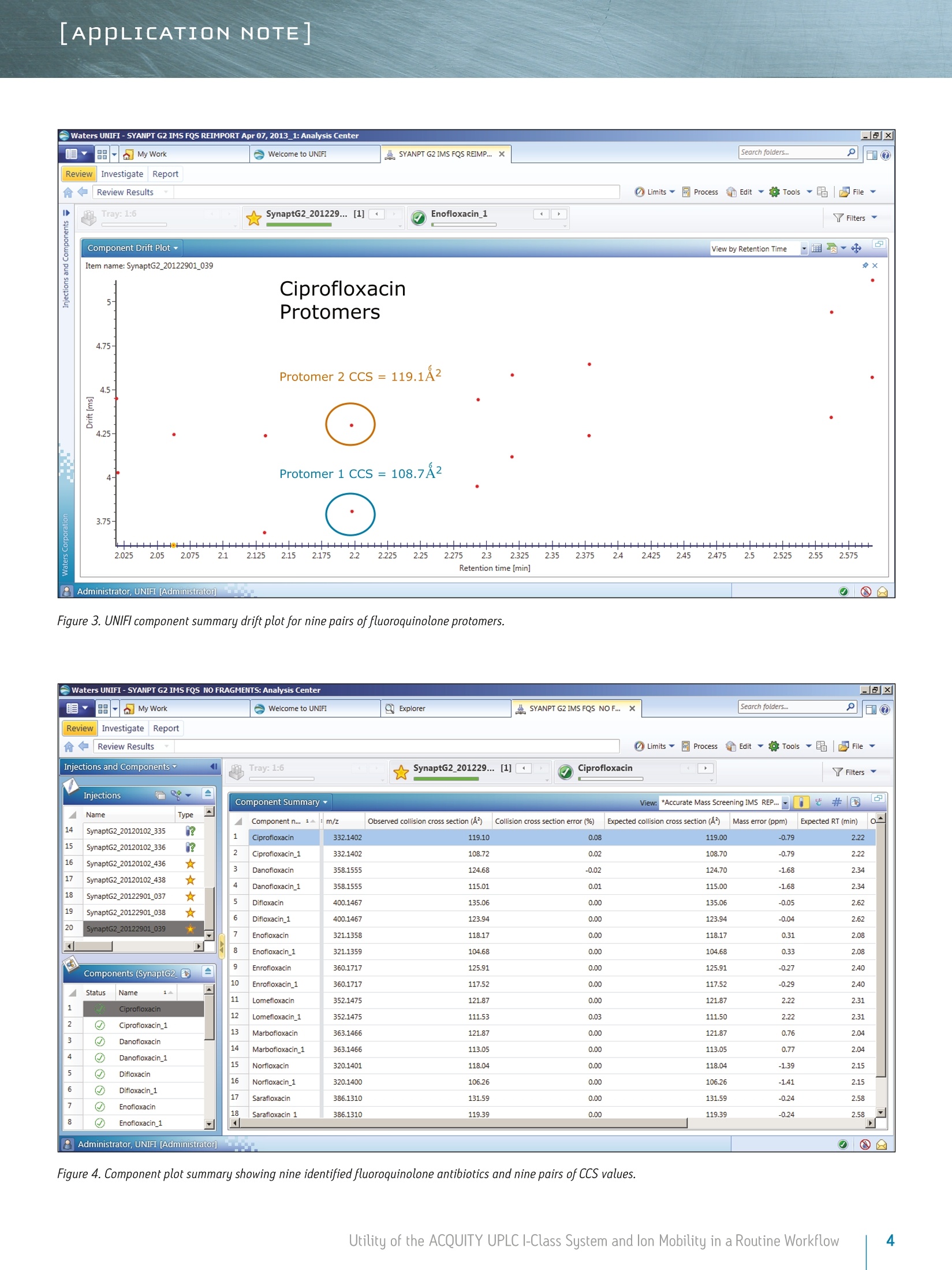
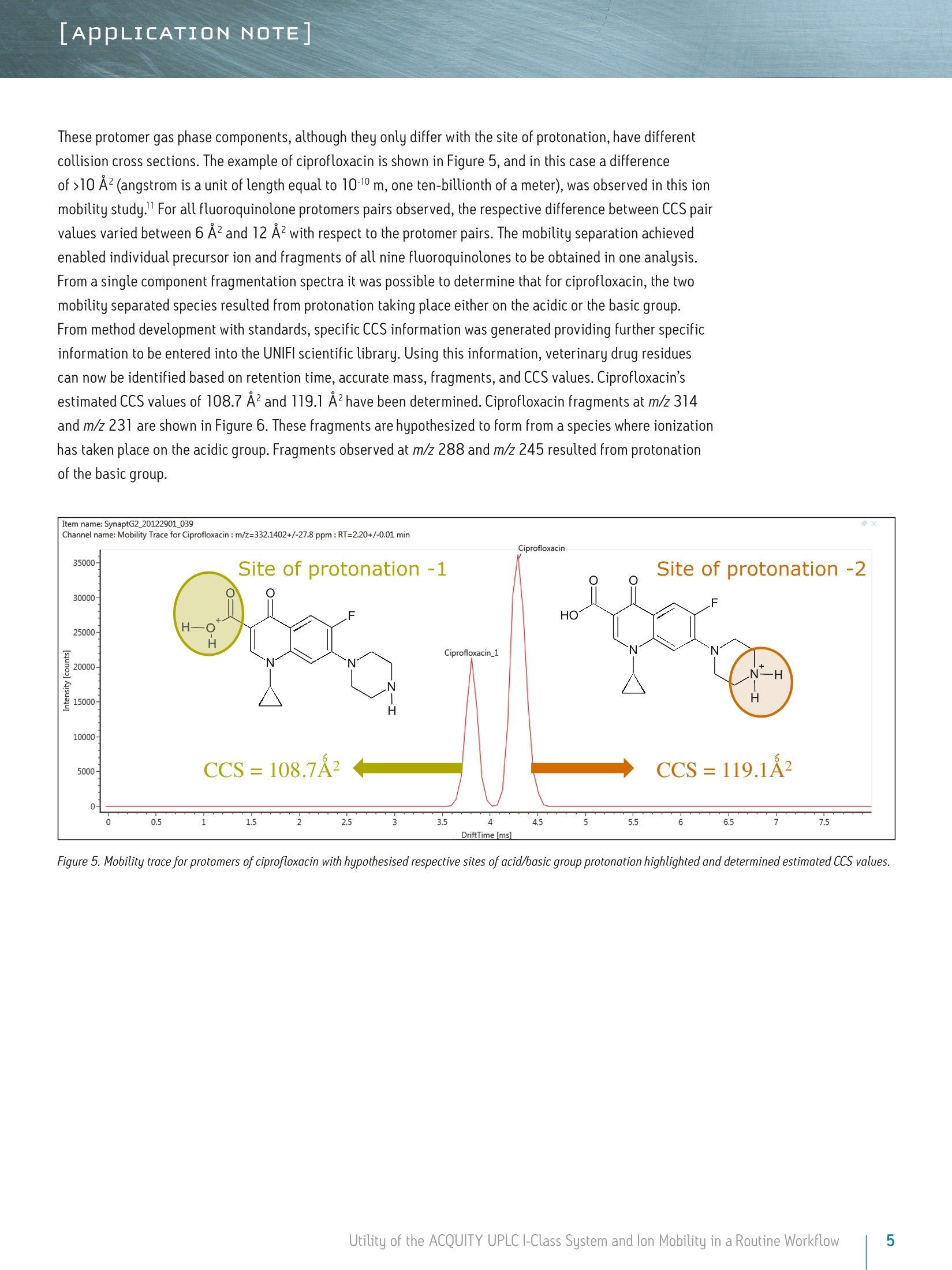
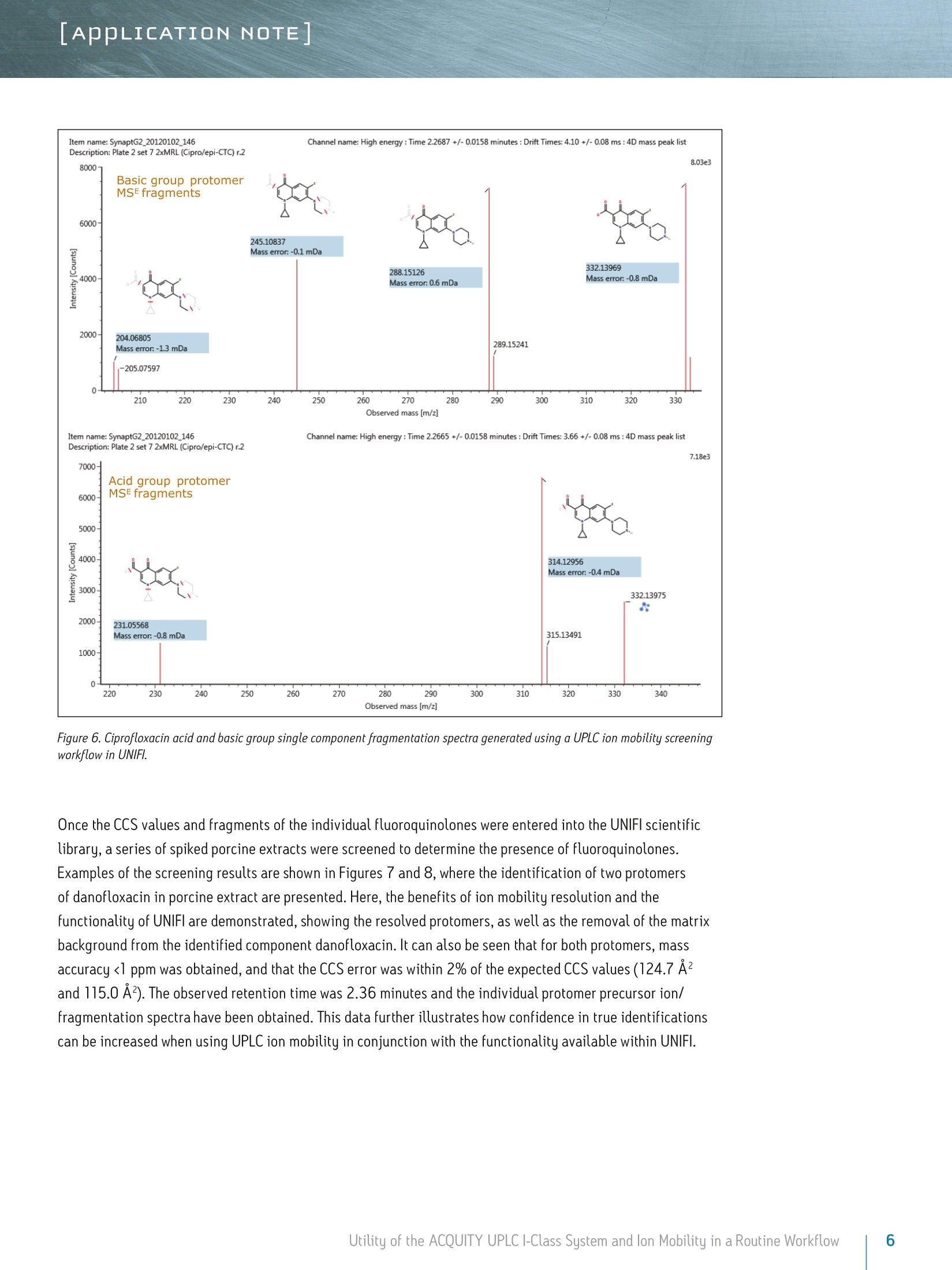
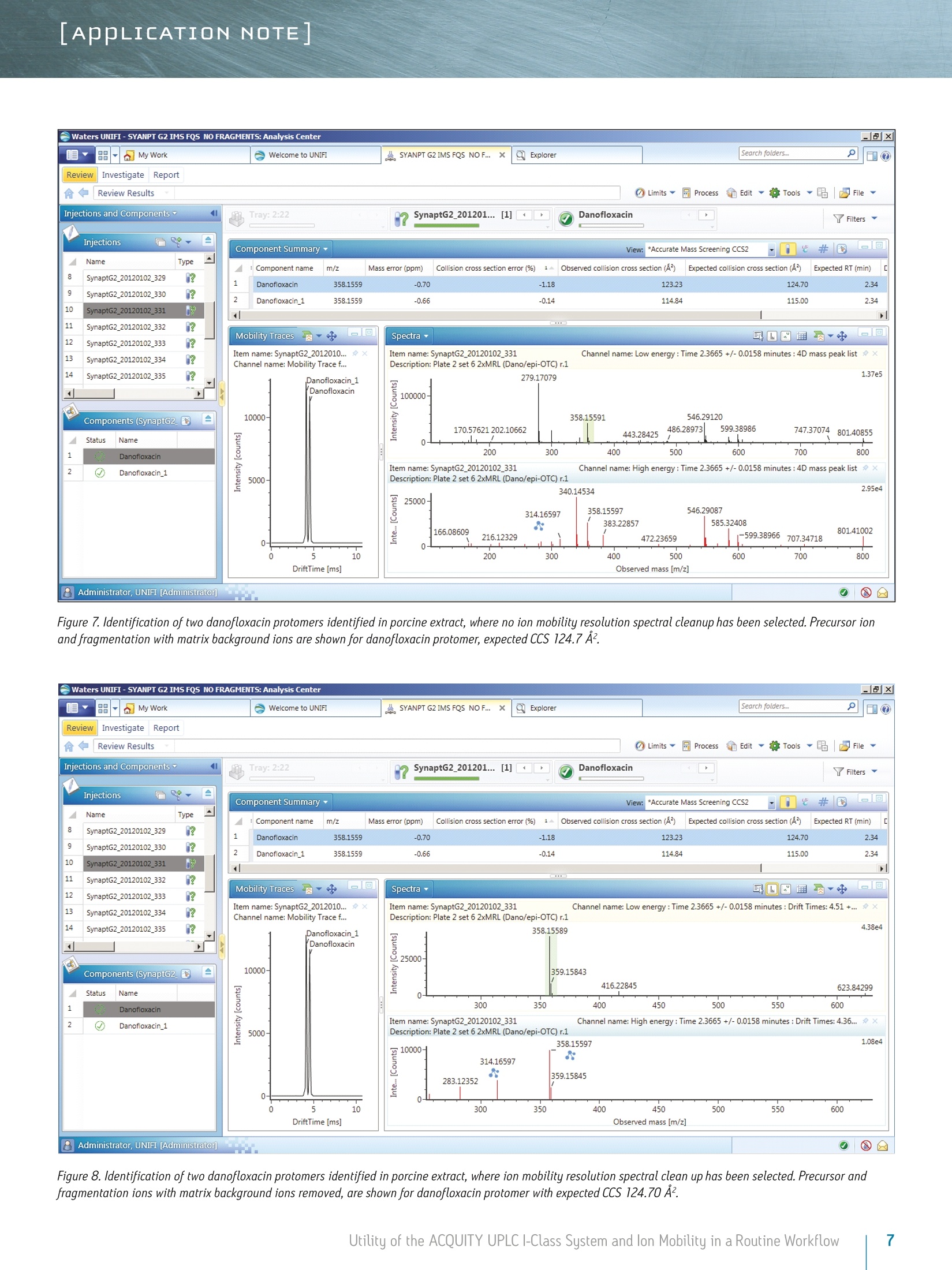
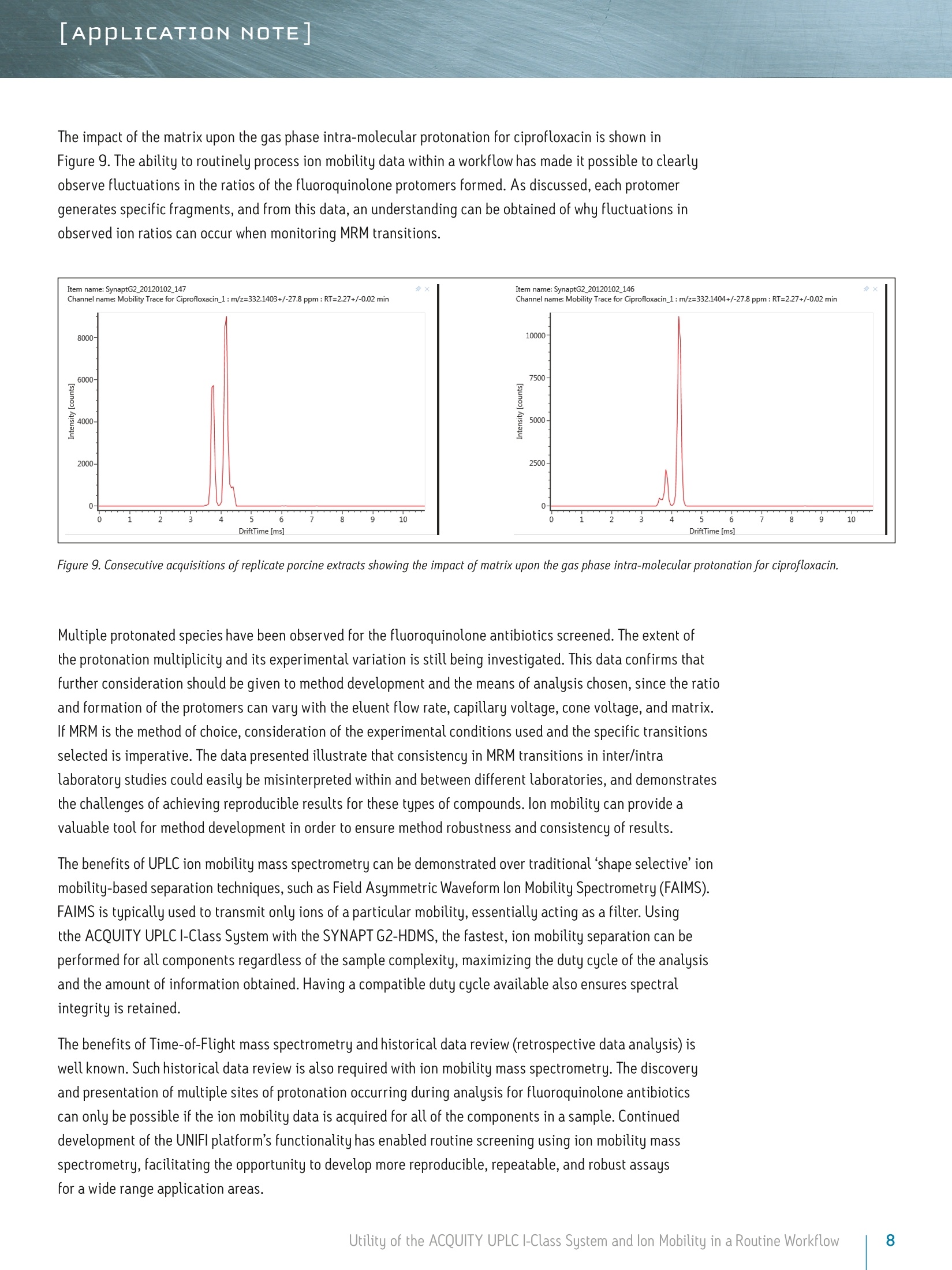
LAPPLICATION NOTE Utility of the ACQUITY UPLC I-Class System and lon Mobilityin a Routine Workflow to Understand the Challenge of AnalyzingFluoroquinolone Antibiotic Residues Michael McCullagh, Ramesh Rao, and Sara SteadWaters Corporation, Wilmslow, UK APPLICATION BENEFITS lULnique protomer collision cross sectionvalues (CCS) can be determined and usedas an additional identification parameterin a routine screening workflow. Individual protomer spectra aregenerated along with proposedfragmentation pathways. The impact of matrix upon protomer ratioscan be observed routinely using new ionmobility processing functionality withinthe UNIFI Scientific Information System. The ACQUITY UPLCB I-Class System canbe used in conjunction with ion mobilityas a development tool to generate morerobust analytical methods. Ability to perform retrospectiveUPLC andion mobilitudata review. WATERS SOLUTIONS ACQUITY UPLCI-Class SystemACQUITY UPLC BEH C Column SYNAPT G2-S High DefinitionMass Spectrometry@(HDMS@) System MassLynx@ and UNIFI Software for data processing KEY WORDS Protomer, collision cross section,CCS, ion mobility,spectral cleanup INTRODUCTION Across many application areas, the applicability of ion mobility to smallmolecule analysis continues to increase, along with the understanding of howthis technology can help address current analytical challenges. The reason,challenge, and methods of achieving successful fluoroquinolones analysis werebriefly discussed in a previous application note, where we described the use ofthe ACQUITY UPLCI-Class System combined with ion mobility mass spectrometryto show how fluoroquinolone class of compounds can form protomers.-5ldentification and characterization of the protomers of fluoroquinolones cannow be routinely screened for using the Waters@UNIFI Scientific InformationSystem. The software within UNIFI allows for the routine characterization of thefragmentation pathways of the respective protomers to be visualized. In addition,it is possible to see the direct impact of the matrix upon protomer formation, andhence obtain a greater insight of the challenges of using MRM to perform residueanalysis of fluoroquinolones. Fluoroquinolones are a family of synthetic broad-spectrum antimicrobial agentsthat have been administered to livestock for different purposes, including theprevention and control of infections and for growth promotion. Due to concernsregarding the spread of resistant microorganisms in the human population, theU.S. Food and Drug Administration (U.S. FDA) introduced a ban on the use ofenrofloxacin and ciprofloxacin in livestock production in September, 2005.9The use of antibiotic growth promoting agents (AGPs) in animalhusbandry hasbeen forbidden in the European Union (EU) since 2006. This application note explores the use of routine screening with UPLC and ionmobility to identify multiple protonation sites and different fragmentationpatterns within the fluoroquinolone class of antibiotics. It can be used as animportant method development tool to support the unequivocal identification offluoroquinolone antibiotics in crude tissue extracts. UPLC and ion mobility havebeen utilized to analyze crude extracts of porcine muscle tissue to determine thepresence of antibiotic residues including the fluoroquinolone class. EXPERIMENTAL Analytes:Standards fluoroquinolonesExtracts:Porcine tissue UPLC conditions ACQUITY UPLCI-Class UPLC system:Column: ACQUITY UPLC BEH C 100 mm x 2.1 mm, 1.7 um Column temp.: 40C Flow rate: 0.6 mL/min Mobile phase A: Water (0.1% formic acid) Mobile phase B: Acetonitrile (0.1% formic acid) Injection volume: 10 uL Gradient: Time (min) %A Initial 95.0 1.00 95.0 8.00 5.0 95.0 9.00 95.0 5.0 MS conditions MS system: SYNAPT G2-S lonization mode: ESI+ Capillary voltage: 2.0 kV Cone voltage: 25 V Desolvation temp.: 550℃ Reference mass: Leucine enkephalin [M+H]+=556.2766 Acquisition range: 50 to 1200 Da Acquisition rate: 4 spectra/sec Collision energy: 15 to 45 eV IMS T-Wave'M velocity: 900 m/s IMS T-Wave pulse height: 40V IMS duty cycle: 10.8 ms Drift gas: N2 The enhanced peak capacity provided by the combination of UPLC and ion mobilityseparation offers some unique advantages for profiling complex matrices.It uses a combination of high resolution mass spectrometry and high efficiencyion mobility-based measurements and separations. Ion mobility spectrometry(IMS) is a rapid, orthogonal, gas phase separation technique that allows anotherdimension of separation to be obtained within an LC timeframe. Compoundscan be differentiated based on size, shape, and charge. In addition, bothprecursor ion and fragment ion information can be acquired in a singleacquisition for all components. A collision cross section (CCS) value is a robust and precise physicochemicalproperty of an ion. CCS is an important distinguishing characteristic of an ionwhich is related to its chemical structure and three-dimensional conformation.where the shadow of a rotating three-dimensional ion, shown in Figure 1,represents the average collision cross section. Using CCS measurements canincrease targeted screening specificity. CCS measurements generated havebeen entered into a scientific library within UNIFI. This allows the expectedand determined CCS values to be utilized in order to screen and confirmfluoroquinolone protomer formation. Here we present CCS values (derivedfrom ion mobility drift times) as a new identification parameter, which candistinguish protomers. Figure 1. llustration of rotatingthree-dimensional conformationof an ion and average collisioncross section (shadow). Extract preparation Extracts of porcine muscle tissue were kindly provided by RnAssays for thepurposes of this study. Briefly, known blank porcine muscle was fortified with25 different antimicrobial compounds (from the fluoroquinolone, tetracycline,and macrolide classes) at levels the relevant to the EU MRL concentrations priorto extraction. Tissue samples were mechanically homogenized in the presenceof an aqueous/organic extraction solvent followed by a centrifugation step. Analiquot of the supernatant was removed and placed in an autosampler vial forsubsequent LC-MS analysis. RESULTS AND DISCUSSION For the assay performed, MassLynx data were acquired and processed with the UNIFI Scientific InformationSystem, allowing ion mobility data to be processed in a conventional workflow for non-targeted accurate massscreening applications. UPLC ion mobility MS has been explored as an important method development tool to support the unequivocalidentification of fluoroquinolone antibiotics in crude tissue extracts. With UNIFl, it has been possible toroutinely identify and characterize protomers of nine fluoroquinolones standards in a routine screeningworkflow. From the solvent standards analyzed, estimated CCS values of protomers formed for eachfluoroquinolone were determined. The CCS values obtained have been incorporated into the UNIFI scientificlibrary, which enabled the targeting of protomers. The antibiotic ciprofloxacin was determined to elute at retention time 2.19 minutes using the generic gradientconditions employed. Figure 2 shows the base peak ion chromatogram with the UNIFI component plot summaryfor nine of the identified fluoroquinolone antibiotics, which eluted in the region highlighted in the base peakintensity chromatogram. From review of the data using the component drift plot summary, 18 fluoroquinolonespecies were identified. Each fluoroquinolone is comprised of two protomers, i.e. protonation at two differentsites on the molecule, which have been mobility separated, as shown in Figure 3. Each retention time showstwo dots on the component summary drift plot, which indicate two forms of each fluoroquinolone. The twoprotomers of ciprofloxacin have been highlighted. The CCS values determined are presented the componentsummary table of Figure 4. The functionality illustrated is unique to the UNIFI Scientific Information System. Figure 2. Base peak ionchromatogram for a mixture of25 solvent standard antibioticcompounds, including ninefluoroquinolones. Also thecomponent plot summary isshown for 18 of the identifiedfluoroquinolone componentsbetween 2 and 2.6 minutes. Figure 3. UNIFI component summary drift plot for nine pairs of fluoroquinolone protomers. Component Summary Injections 厂 g Explorer View: *Accurate Mass Screening IMS REP...-0 #@ Component n... 1 m/z Observed collision cross section (A) Collision cross section error (%) Expected collision cross section (A) Mass error(ppm) Expected RT (min) Ciprofloxacin 332.1402 119.10 0.08 119.00 -0.79 2.22 Ciprofloxacin_1 332.1402 108.72 0.02 108.70 -0.79 2.22 Danofloxacin 358.1555 124.68 -0.02 124.70 -1.68 2.34 Danofloxacin1 358.1555 115.01 0.01 115.00 -1.68 2.34 Difloxacin 400.1467 135.06 0.00 135.06 -0.05 2.62 Difloxacin_1 400.1467 123.94 0.00 123.94 -0.04 2.62 Enofloxacin 321.1358 118.17 0.00 118.17 0.31 2.08 Enofloxacin_1 321.1359 104.68 0.00 104.68 0.33 2.08 Enrofloxacin 360.1717 125.91 0.00 125.91 -0.27 2.40 Enrofloxacin_1 360.1717 117.52 0.00 117.52 -0.29 2.40 Lomefloxacin 352.1475 121.87 0.00 121.87 2.22 2.31 Lomefloxacin_1 352.1475 111.53 0.03 111.50 2.22 2.31 Marbofloxacin 363.1466 121.87 0.00 121.87 0.76 2.04 Marbofloxacin1 363.1466 113.05 0.00 113.05 0.77 2.04 Norfloxacin 320.1401 118.04 0.00 118.04 -1.39 2.15 Norfloxacin_1 320.1400 106.26 0.00 106.26 -1.41 2.15 Sarafloxacin 386.1310 131.59 0.00 131.59 -0.24 2.58 Sarafloxacin 1 386.1310 119.39 0.00D 119.39 -0.24 2.58 8 Figure 4. Component plot summary showing nine identified fluoroquinolone antibiotics and nine pairs of CCS values. These protomer gas phase components, although they only differ with the site of protonation, have differentcollision cross sections. The example of ciprofloxacin is shown in Figure 5, and in this case a differenceof>10 A2(angstrom is a unit of length equal to 10-10 m, one ten-billionth of a meter), was observed in this ionmobility study."For all fluoroquinolone protomers pairs observed, the respective difference between CCS pairvalues varied between 6 A2 and 12 A? with respect to the protomer pairs. The mobility separation achievedenabled individual precursor ion and fragments of all nine fluoroquinolones to be obtained in one analysis.From a single component fragmentation spectra it was possible to determine that for ciprofloxacin, the twomobility separated species resulted from protonation taking place either on the acidic or the basic group.From method development with standards, specific CCS information was generated providing further specificinformation to be entered into the UNIFI scientific library. Using this information, veterinary drug residuescan now be identified based on retention time, accurate mass, fragments, and CCS values. Ciprofloxacin’sestimated CCS values of 108.7 A and 119.1 A?have been determined. Ciprofloxacin fragments at m/z 314and m/z 231 are shown in Figure 6. These fragments are hypothesized to form from a species where ionizationhas taken place on the acidic group. Fragments observed at m/z 288 and m/z 245 resulted from protonationof the basic group. Figure 5. Mobility trace for protomers of ciprofloxacin with hypothesised respective sites ofacid/basic group protonation highlighted and determined estimated CCS values. Observed mass [m/z] Figure 6. Ciprofloxacin acid and basic group single component fragmentation spectra generated using a UPLC ion mobility screeningworkflow in UNIFI. Once the CCS values and fragments of the individual fluoroquinolones were entered into the UNIFI scientificlibrary, a series of spiked porcine extracts were screened to determine the presence of fluoroquinolones.Examples of the screening results are shown in Figures 7 and 8, where the identification of two protomersof danofloxacin in porcine extract are presented. Here, the benefits of ion mobility resolution and thefunctionality of UNIFI are demonstrated, showing the resolved protomers, as well as the removal of the matrixbackground from the identified component danofloxacin. It can also be seen that for both protomers, massaccuracy <1 ppm was obtained, and that the CCS error was within 2% of the expected CCS values (124.7 A2and 115.0 A2). The observed retention time was 2.36 minutes and the individual protomer precursor ion/fragmentation spectra have been obtained. This data further illustrates how confidence in true identificationscan be increased when using UPLC ion mobility in conjunction with the functionality available within UNIFl. Figure 7. Identification of two danofloxacin protomers identified in porcine extract, where no ion mobility resolution spectral cleanup has been selected. Precursor ionand fragmentation with matrix background ions are shown for danofloxacin protomer, expected CCS 124.7 A2. Figure 8. Identification of two danofloxacin protomers identified in porcine extract, where ion mobility resolution spectral clean up has been selected. Precursor andfragmentation ions with matrix background ions removed, are shown for danofloxacin protomer with expected CCS 124.70 A’. The impact of the matrix upon the gas phase intra-molecular protonation for ciprofloxacin is shown inFigure 9. The ability to routinely process ion mobility data within a workflow has made it possible to clearlyobserve fluctuations in the ratios of the fluoroquinolone protomers formed. As discussed, each protomergenerates specific fragments, and from this data, an understanding can be obtained of why fluctuations inobserved ion ratios can occur when monitoring MRM transitions. Figure 9. Consecutive acquisitions of replicate porcine extracts showing the impact of matrix upon the gas phase intra-molecular protonation for ciprofloxacin. Multiple protonated species have been observed for the fluoroquinolone antibiotics screened. The extent ofthe protonation multiplicity and its experimental variation is still being investigated. This data confirms thatfurther consideration should be given to method development and the means of analysis chosen, since the ratioand formation of the protomers can vary with the eluent flow rate, capillary voltage, cone voltage, and matrix.If MRM is the method of choice, consideration of the experimental conditions used and the specific transitionsselected is imperative. The data presented illustrate that consistency in MRM transitions in inter/intralaboratory studies could easily be misinterpreted within and between different laboratories, and demonstratesthe challenges of achieving reproducible results for these types of compounds. lon mobility can provide avaluable tool for method development in order to ensure method robustness and consistency of results. The benefits of UPLC ion mobility mass spectrometry can be demonstrated over traditional shape selective'ionmobility-based separation techniques, such as Field Asymmetric Waveform lon Mobility Spectrometry (FAIMS).FAIMS is typically used to transmit only ions of a particular mobility, essentially acting as a filter. Usingtthe ACQUITY UPLCI-Class System with the SYNAPT G2-HDMS, the fastest, ion mobility separation can beperformed for all components regardless of the sample complexity, maximizing the duty cycle of the analysisand the amount of information obtained. Having a compatible duty cycle available also ensures spectralintegrity is retained. The benefits of Time-of-Flight mass spectrometry andhistorical data review (retrospective data analysis) iswell known. Such historical data review is also required with ion mobility mass spectrometry. The discoveryand presentation of multiple sites of protonation occurring during analysis for fluoroquinolone antibioticscan only be possible if the ion mobility data is acquired for all of the components in a sample. Continueddevelopment of the UNIFI platform’s functionality has enabled routine screening using ion mobility massspectrometry, facilitating the opportunity to develop more reproducible, repeatable, and robust assaysfor a wide range application areas. CONCLUSIONS iSSeparation of different intra-molecular protonated species hasbeen achieved uniquely using ion mobility. 1 SSingle component precursor ion and fragmentation spectra canbe generated for all components simultaneously. Multiple sites of protonation have been identified andconfirmed from the individual fragmentation spectraof each protomer species. CCS values can be used as an identification point in additionto retention time, precursor ion accurate mass, and accuratemass fragmentation spectra. Ion mobility separations can be effectively utilized to resolveanalyte peaks from matrix interferences and remove the needfor complex sample cleanup. 1LUPLC ion mobility mass spectrometry observations have thepotential to explain the differences sometimes observed ininter-laboratory studies, where participants report resultsobtained from monitoring specific MRM transitions. The UNIFI Scientific Information System enabled the routineinterrogation of UPLC ion mobility MS data acquired usingthe SYNAPT G2-S and SYNAPT G2-Si HDMS systems. Based on the observations of characteristic ionization forfluoroquinolone antibiotics included in this study, the useof UPLC ion mobility for method development purposesis warranted. THE SCIENCE OF WHAT'S POSSIBLE. Waters, UNIFI, ACQUITY UPLC, UPLC, SYNAPT, High Definition Mass Spectromety, HDMS, MassLynx, and The Science of What’s Possibleare registered trademarks of Waters Corporation. T-Wave is a trademark of Waters Corporation. All other trademarks are the propertyof their respective owners. Acknowledgements Waters would like to kindly acknowledge Aldert Bergwerffand Wouter de Keizer (RnAssays) for the provision of thesamples analyzed. References 1. Waters Applicaton Note: 720004720EN; Mike McCullagh, Sara Stead,Jonathan Williams, Wouter de Keizer, Aldert Bergwerff. 2.Verdon E, Couedor P, Roudaut B, Sanders PJ. Multiresidue method forsimultaneous determination of ten quinolone antibacterial residues inmultimatrix/multispecies animal tissues by liquid chromatography withfluorescence detection: single laboratory validation study. AOAC Inter. 2005;88:1179-1192. 3.A Kaufmann, P Butcher, K Maden, M Widmer, K Giles, D Uria. Are liquidchromatography/electrospray tandem quadrupole fragmentation ratiosunequivocal confirmation criteria? Rapid Commun. Mass Spectrom. 2009;23:985-998. 4.HG Mol,P Zomer, M de Koning. Qualitative aspects and validation of ascreening method for pesticides in vegetables and fruits based on liquidchromatography coupled to full scan high resolution (Orbitrap) massspectrometry. Anal Bioanal Chem. 2012;403:2891-2908. 5.TR Croley, K D White, J H Callahan, S M Musser. The chromatographic role inhigh resolution mass spectrometry for non-targeted analysis. J Am Soc MassSpectrom. 2012;23:1569. 6.CCommission Decision (2002/657EEC) Official Journal of the EuropeanCommunities 2002. 7.(.Final Decision of the Commissioner, Docket #2000N-157 1 0ctober 24,2000, Withdrawal of the approval of the new animal drug application forenrofloxacin in poultry. Department of Health and Human Services, U.S. Foodand Drug Administration. 8. FDA Center for Veterinary Medicine, June 2, 1997, fda order prohibits extralabel use of fluoroquinolones and glycopeptides. 9.Official Journal of the European Union,L268:29-43,Regulation (EC)No 1831/2003 of the European parliament and of the council of 22September 2003 on additives for use in animal nutrition. 10. Official Journal of the European Union, L24:1-8 Annexl, Council Regulation(EEC) No 2377/90 of 26 June 1990 laying down a community procedure forthe establishment of maximum residue limits of veterinary medicinal productsin foodstuffs of animal origin. 11. P M Lalli, B Alglesias, HE Toma, G F de Sa,R J Daroda, J C Silva Filho,JE Szulejko, K Araki, M N Eberlin. Protomers: formation,separation, andcharacterization via travelling wave ion mobility mass spectrometry.J Mass Spectrom. 2012;47(6):712-9. Waters Corporation 34 Maple Street Milford, MA 01757 U.S.A. Utility of the ACQUITY UPLC I-Class System and lon Mobility in a Routine Workflow
确定

还剩7页未读,是否继续阅读?
沃特世科技(上海)有限公司(Waters)为您提供《肉制品中氟喹诺酮类抗生素检测方案 》,该方案主要用于畜禽肉及副产品中兽药残留检测,参考标准--,《肉制品中氟喹诺酮类抗生素检测方案 》用到的仪器有Waters ACQUITY UPLC I-Class 超高效液相色谱
推荐专场
相关方案
更多
该厂商其他方案
更多