方案详情
文
Preparation of the sample
The sample is weighed in accurately with four decimal places into a 100 mL beaker and filled up to 60 mL with distilled water. After that 5 mL of a sulfuric acid (25%) are added.
方案详情

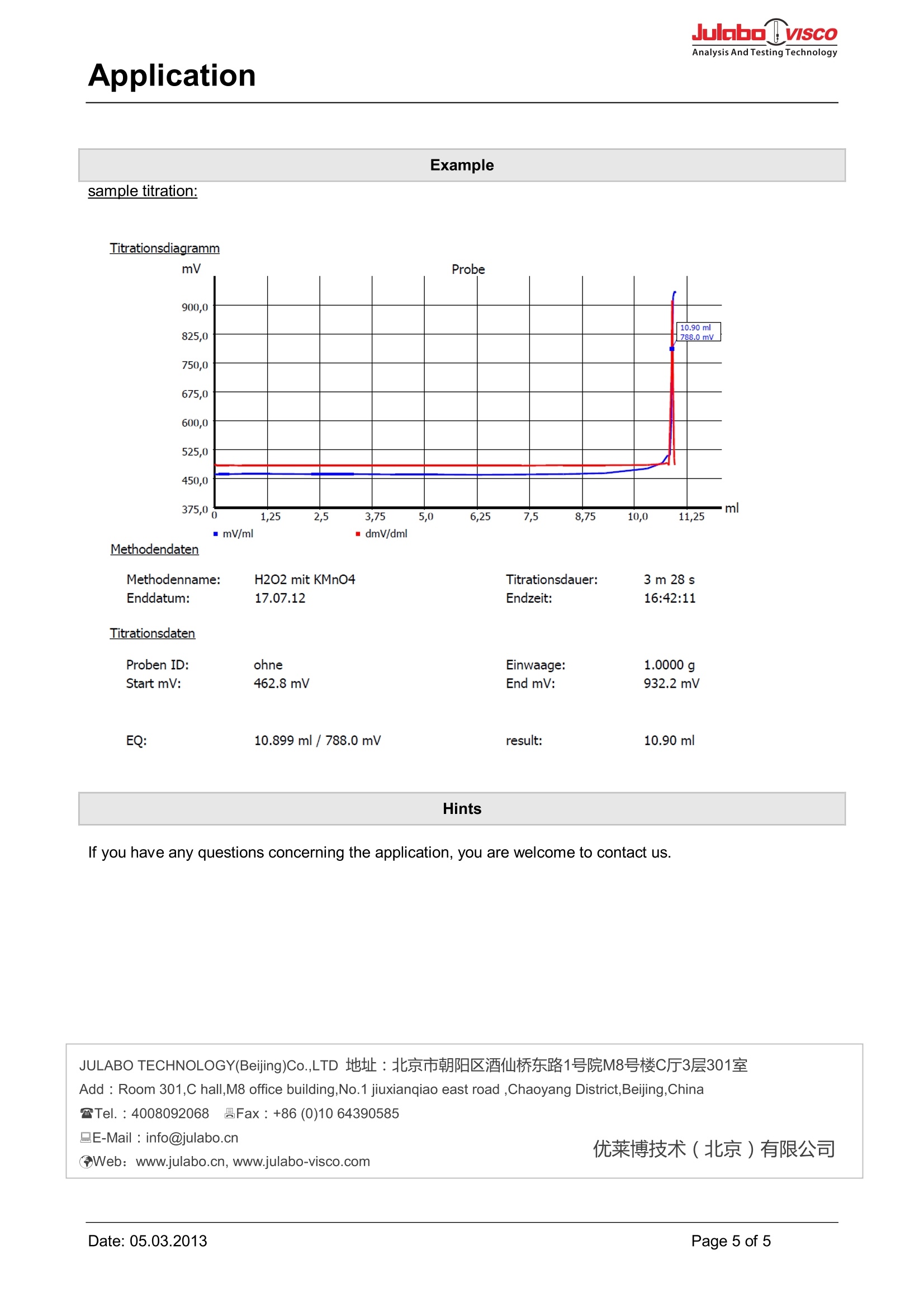
Analysis And Testing TechnologyApplication Julobo : lVISCOAnalysis And Testing Technology Quantitative analysis ofhydrogen peroxide Application Content Content.. 2 Use. 2 Appliances.2 Electrodes 2 Reagents.2 Descriptionl..m 2 Method .4 Example. 5 Hints 5 Use The concentration of hydrogen peroxide in a solution is determined by redox titration with potassiumpermanganate. Appliances Titrator: TitroLine 6000/7000/7750 Burette: WA 20 Electrodes Electrode : Pt 62, Pt 6280 or Pt 62 RG Electrolyte : KCI (3 mol/L) Reagents Titration agent: potassium permanganate (KMnO4) 0,02mol/L (0.1 N) Standardization: with ferrous sulphate (Fe(II)) other reagents: sulfuric acid (H2SO4) ca. 25% Description Preparation of the sample The sample is weighed in accurately with four decimal places into a 100 mL beaker and filled up to 60 mLwith distilled water. After that 5 mL of a sulfuric acid (25%) are added. Titration Hydrogen peroxide is determined by titration with potassium permanganate. The reaction runs in an acidsolution and is based on the following chemical equation: chemical equation: 2MnOA4 +5H202+6H*→>2 Mn²++50T+8H0 The chemical equation shows that the ratio of permanganate and hydrogen peroxide is 2:5,what is allowed for the calculation below. calculation: H,0,%]5.c(KMnOa)·V(KMnO)-(KMnO)·M(HzO2)1002·m(H202)·1000 c(KMnO4): concentration of the measure solution [mol/L] (here: 0,02 mol/L) V(KMnO4): value of the measure solution [mL] t(KMnO4): titre of the measure solution M(H202): molar mass of H2O2 (34,0146 g/mol) m(H2O2): amount of the sample That means that 1 ml 0.02 mol/l KMnO4= 1,701 mg H202 Please take the titration parameter out of the suitable method. To get proper results you have to make a titrefor the potassium permanganate with ferrous sulfate. Application Method Method data Method name: H202 Created at: Method type: Automatic titration Last modification: Measured value: mV Damping settings: None Titration mode: Dynamic Documentation: GLP Dynamic: Average Measuring speed / drift: User-defined: minimum holding time: 05 s maximum holding time: 12 s Measuring time: 03 s Drift: 50 mV/min Initial waiting time: 0s Titration direction: Increase Pretitration: 1.000 ml Delay time: 20 s End value: off EQ: On (1) Slope value: Steep Value: 700 Dosing parameter Dosingspeed:15.00%Filling speed:30sMaximum dosing volume:50.00 mlCalculation formulaH202:(EQ1-B)*T*M*F1/(W*F2)Mol (M):1.70100Unit:%Decimal places:2m-value:EQ2*T*M*F1/(W*F2)Mol (M):1.00000Unit:mmol/lDecimal places:2Blank value (B):0.0000 mlTitre (T):1.00000000Factor 1 (F1):0.1000Weight (W):manFactor 2 (F2):1.0000Statistics:Off Example sample titration: Titrationsdiagramm ml mV/ml m dmV/dml Methodendaten Hints lf you have any questions concerning the application, you are welcome to contact us. JULABO TECHNOLOGY(Beijing)Co.,LTD 地址:北京市朝阳区酒仙桥东路1号院M8号楼C厅3层301室 Add : Room 301,C hall,M8 office building,No.1 jiuxianqiao east road ,Chaoyang District,Beijing,China Tel.:4008092068 昌Fax: +86 (0)10 64390585 品E-Mail : info@julabo.cn Web: www.julabo.cn, www.julabo-visco.com 优莱博技术(北京)有限公司 Page of Date: Date:age of
确定



还剩3页未读,是否继续阅读?
优莱博技术(北京)有限公司为您提供《检测过氧化氢》,该方案主要用于其他中--检测,参考标准--,《检测过氧化氢》用到的仪器有ChemTron VTR-80高低温磁力搅拌反应装置、Chemtron CAT 7500容量法卡式水份滴定仪、Chemtron CAT2-16全自动电位滴定仪
推荐专场
卡氏水分测定仪/卡氏水份测定仪
更多
相关方案
更多
























