方案详情
文
Titer standardisation of the titration solution
The titer of the solution is determined in use on a daily basis. To do so, 10.0 ml of the potassium dichromate solution 0.02 mol/l are diluted with water to approx. 100 ml. 30 ml of sulphuric acid are added carefully to this solution. After cooling down the solution is placed on the magnetic stirrer and titrated using 3 times the method ?Titer COD“. A triple determination is carried out. The average value is automatically calculated in mol/l and stored into the exchange unit.
方案详情

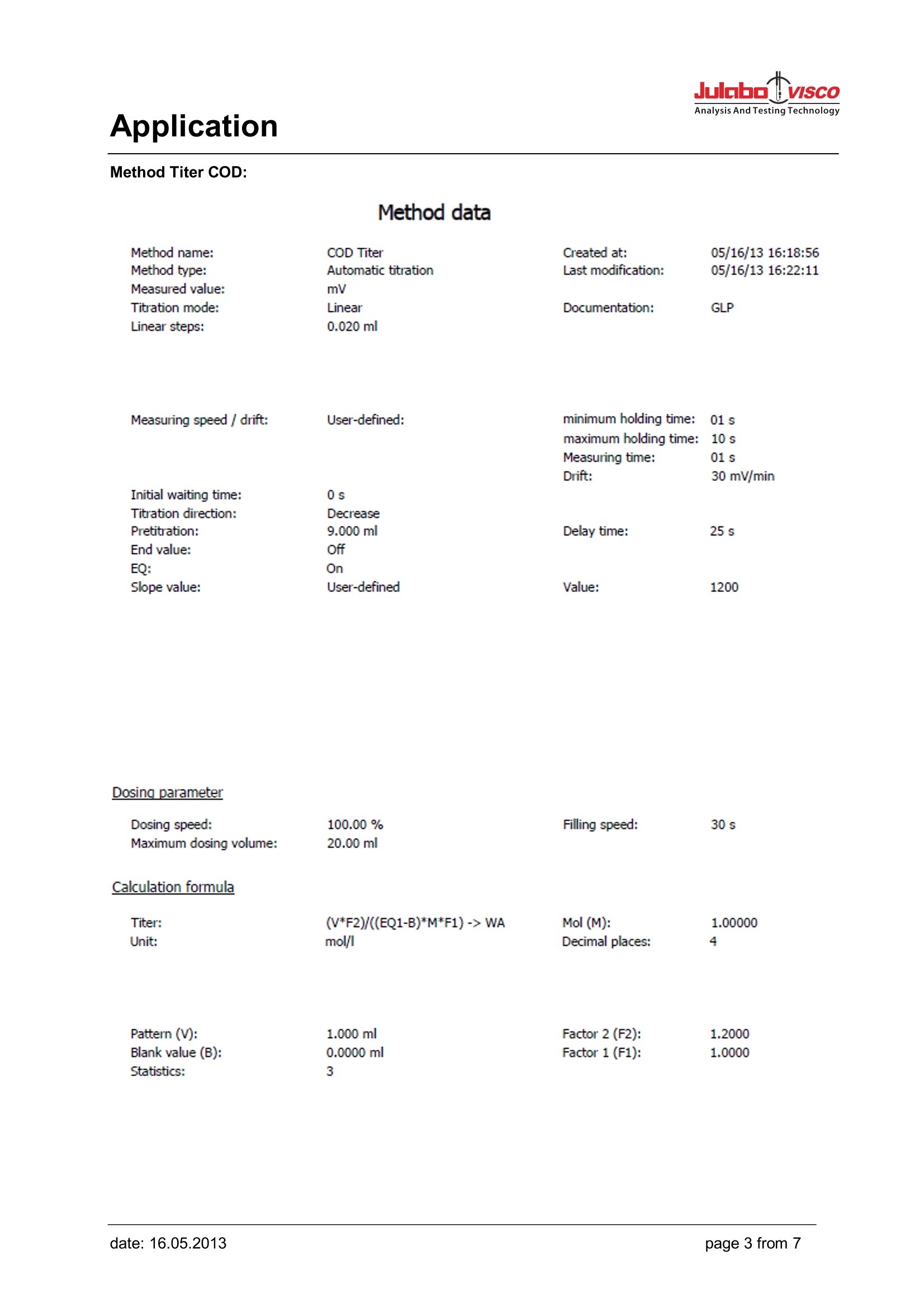
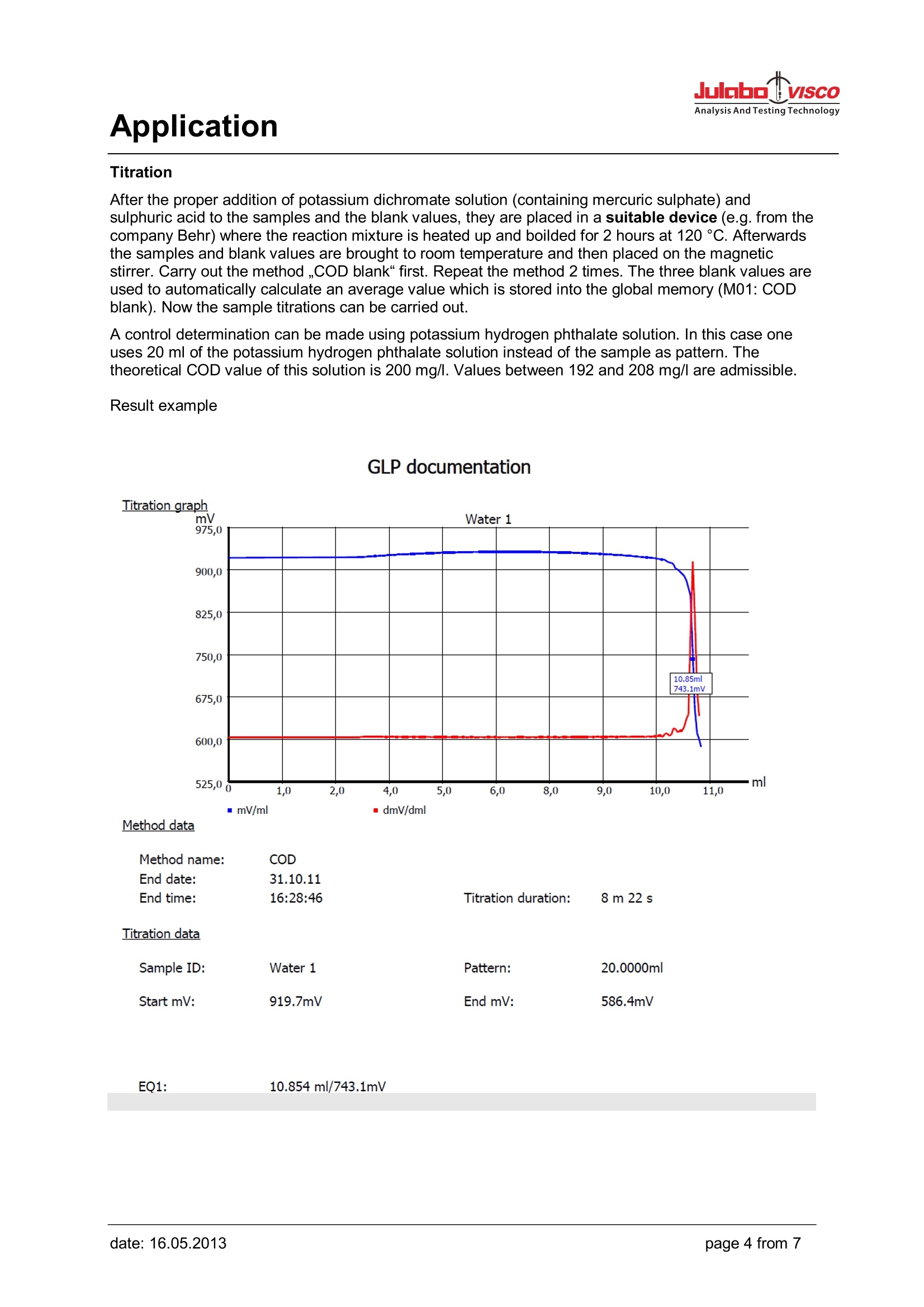
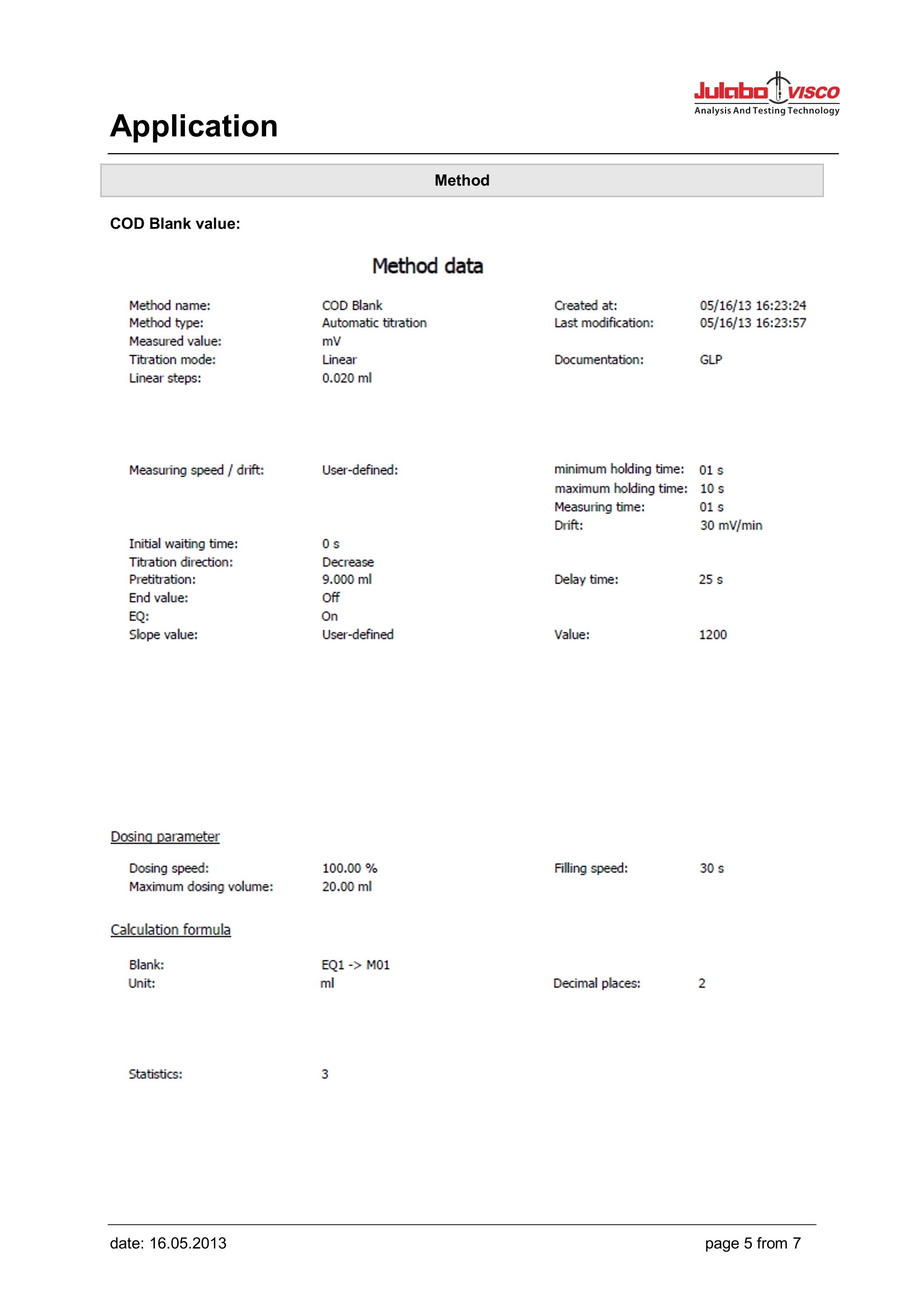
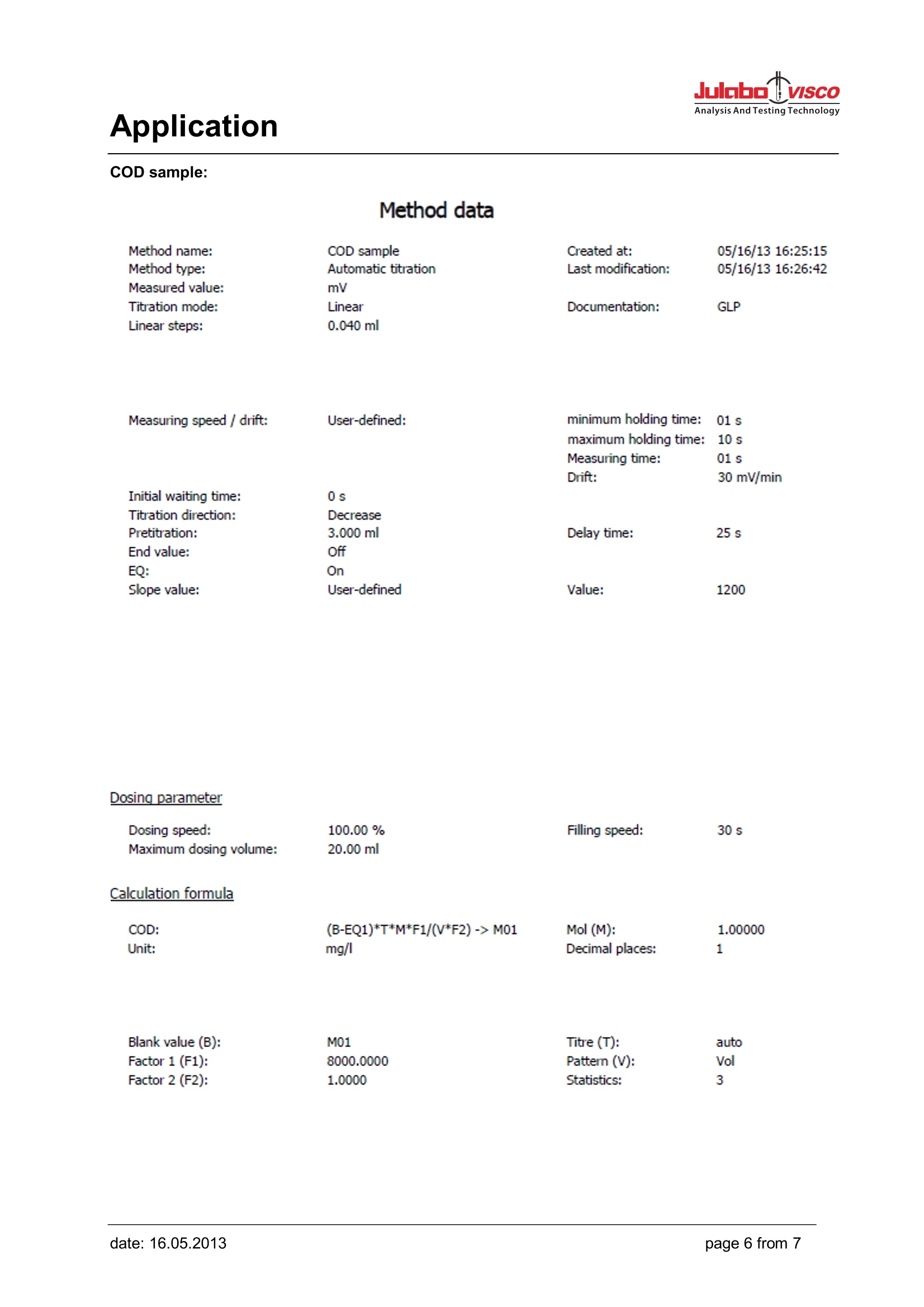
Analysis And Testing Technology Application Application Use Determination of the chemical oxygen demand above concentration of 15 mg/l. In the case ofundiluted samples the procedure can be used up to 300 mg/l. The chloride contents of the undilutedsample must not exceed 1 g/l. Apart from that, the work should be performed according to theprocedure laid down in Part 41-2. Appliances Titrator: TL 6000/7000 (TL 6000/7000 M1/20 or TL 6000/7000 M1/50) consists of Basic device Magnetic stirrer TM 235 20 mL exchange unit WA 20 or 50 mL exchange unit WA 50, with amber glass bottle for thetitrant, complete Electrodes Electrode: Pt 61 Titration tip TZ 1623 Option: longer stand rod with (450 mm) Z 601 Reagents Titrant: Ferrous (II) ammonium sulphate solution 0.120 mol/l Sulphuric acid, potassium hydrogen phthalate solution as standard, potassium dichromatesolution (containing mercuric sulphate) 0.02 mol/l Description Preparation of the titration solution 47.1 g of ferrous (II) ammonium sulphate are dissolved in water in a 11 volumetric flask. 20 ml ofsulphuric acid ¥=1.84g/lare added, solution is topped up with water to 1000 ml. Titer standardisation of the titration solution The titer of the solution is determined in use on a daily basis. To do so, 10.0 ml of the potassiumdichromate solution 0.02 mol/l are diluted with water to approx. 100 ml. 30 ml of sulphuric acid areadded carefully to this solution. After cooling down the solution is placed on the magnetic stirrer andtitrated using 3 times the method ,,Titer COD". A triple determination is carried out. The average valueis automatically calculated in mol/l and stored into the exchange unit. Application Method Titer COD: Method data Method name: COD Titer Created at: GLP Method type: Automatic titration Last modification: Measured value: mV Titration mode: Linear Documentation: Linear steps: 0.020 ml Measuring speed / drift: User-defined: minimum holding time: 01 s maximum holding time: : 10 s Measuring time: 01 s Drift: 30 mV/min Initial waiting time: 0s Titration direction: Decrease Pretitration: 9.000 ml Delay time: 25 s End value: EQ: Slope value: User-defined Value: 1200 Dosing parameter Dosing speed: 100.00 % Filling speed: 30 s Maximum dosing volume: 20.00 ml Calculation formula Titer: (V*F2)/((EQ1-B)*M*F1)-> WA Mol (M): 1.00000 Unit: mol/l Decimal places: 4 Pattern (V): 1.000 ml Factor 2 (F2): 1.2000 Blank value (B): 0.0000 ml Factor 1 (F1): 1.0000 Statistics: 3 Application Titration After the proper addition of potassium dichromate solution (containing mercuric sulphate) andsulphuric acid to the samples and the blank values, they are placed in a suitable device (e.g. from thecompany Behr) where the reaction mixture is heated up and boilded for 2 hours at 120°C. Afterwardsthe samples and blank values are brought to room temperature and then placed on the magneticstirrer. Carry out the method ,,COD blank" first. Repeat the method 2 times. The three blank values areused to automatically calculate an average value which is stored into the global memory (M01: CODblank). Now the sample titrations can be carried out. A control determination can be made using potassium hydrogen phthalate solution. In this case oneuses 20 ml of the potassium hydrogen phthalate solution instead of the sample as pattern. Thetheoretical COD value of this solution is 200 mg/l. Values between 192 and 208 mg/l are admissible. Result example GLP documentation mV/ml Method data Method name: COD End date: 31.10.11 End time: 16:28:46 Titration duration: 8 m 22 s Titration data Sample ID:Water 1Pattern:20.0000mlStart mV:919.7mVEnd mV:586.4mV EQ1: 10.854 ml/743.1mV Application Method COD Blank value: Method data Method name: COD Blank Created at: GLP Method type: Automatic titration Last modification: Measured value: mV Titration mode: Linear Documentation: Linear steps: 0.020 ml Measuring speed / drift: User-defined: minimum holding time: :01 s maximum holding time: 10 s Measuring time: 01 s Drift: 30 mV/min Initial waiting time: 0s Titration direction: Decrease Pretitration: 9.000 ml Delay time: 25 s End value: EQ: Slope value: User-defined Value: 1200 Dosing parameter Dosing speed: 100.00% Filling speed: 30 s Maximum dosing volume: 20.00 ml Calculation formula Blank: EQ1 -> M01 Unit: ml Decimal places: 2 Statistics: 3 Application COD sample: Method data Method data Method name: COD sample Created at: Method type: Automatic titration Last modification: Measured value: mV Titration mode: Linear Documentation: GLP Linear steps: 0.040 ml Measuring speed/ drift: User-defined: minimum holding time: 01 s maximum holding time: 10 s Measuring time: 01 s Drift: 30 mV/min Initial waiting time: 0s Titration direction: Decrease Pretitration: 3.000 ml Delay time: 25 s End value: Off EQ: On Slope value: User-defined Value: 1200 Dosing parameter Dosing speed: 100.00 % Filling speed: 30 s Maximum dosing volume: 20.00 ml Calculation formula COD: (B-EQ1)*T*M*F1/(V*F2)-> M01 Mol(M): 1.00000 Unit: mg/I Decimal places: 1 Blank value (B): M01 Titre (T): auto Factor 1 (F1): 8000.0000 Patter (V): Vol Factor 2 (F2): 1.0000 Statistics: 3 Application After use, the Pt 61 electrode should be placed in KCI 3 mol/l immediately. It has to be made sure thatthere is always sufficient electrolyte solution in the electrode. For further details, please refer to theelectrode's operating instructions. Electrode handling Notes If you have any questions on the application, you can feel free to contact us.. JULABO TECHNOLOGY(Beijing)Co.,LTD 地址:北京市朝阳区酒仙桥东路1号院M8号楼C厅3层301室 Add : Room 301,C hall,M8 office building,No.1 jiuxianqiao east road ,Chaoyang District,Beijing,China Tel.: 4008092068昌Fax: +86 (0)10 64390585 ③Web: www.julabo.cn, www.julabo-visco.com 优莱博技术(北京)有限公司 page from date: date: age from
确定



还剩5页未读,是否继续阅读?
优莱博技术(北京)有限公司为您提供《检测化学需氧量》,该方案主要用于其他中--检测,参考标准--,《检测化学需氧量》用到的仪器有ChemTron VTR-80高低温磁力搅拌反应装置、Chemtron CAT 7500容量法卡式水份滴定仪、Chemtron CAT2-16全自动电位滴定仪
推荐专场
卡氏水分测定仪/卡氏水份测定仪
更多
相关方案
更多
























