
方案详情
文
The elution profiles of several essential oils of the Myrtaceae family were acquired using a single
enantioselective stationary phase column.
方案详情

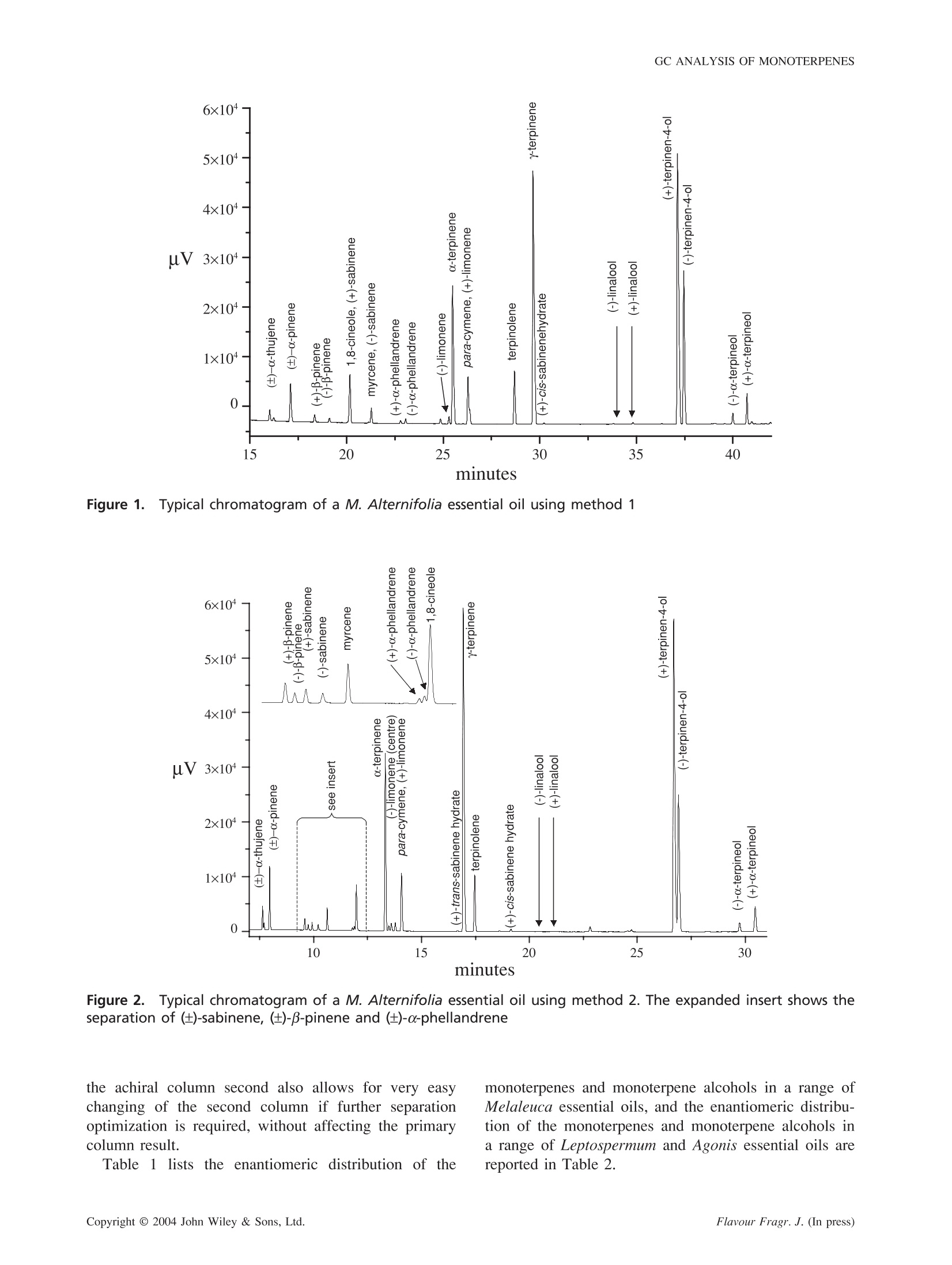
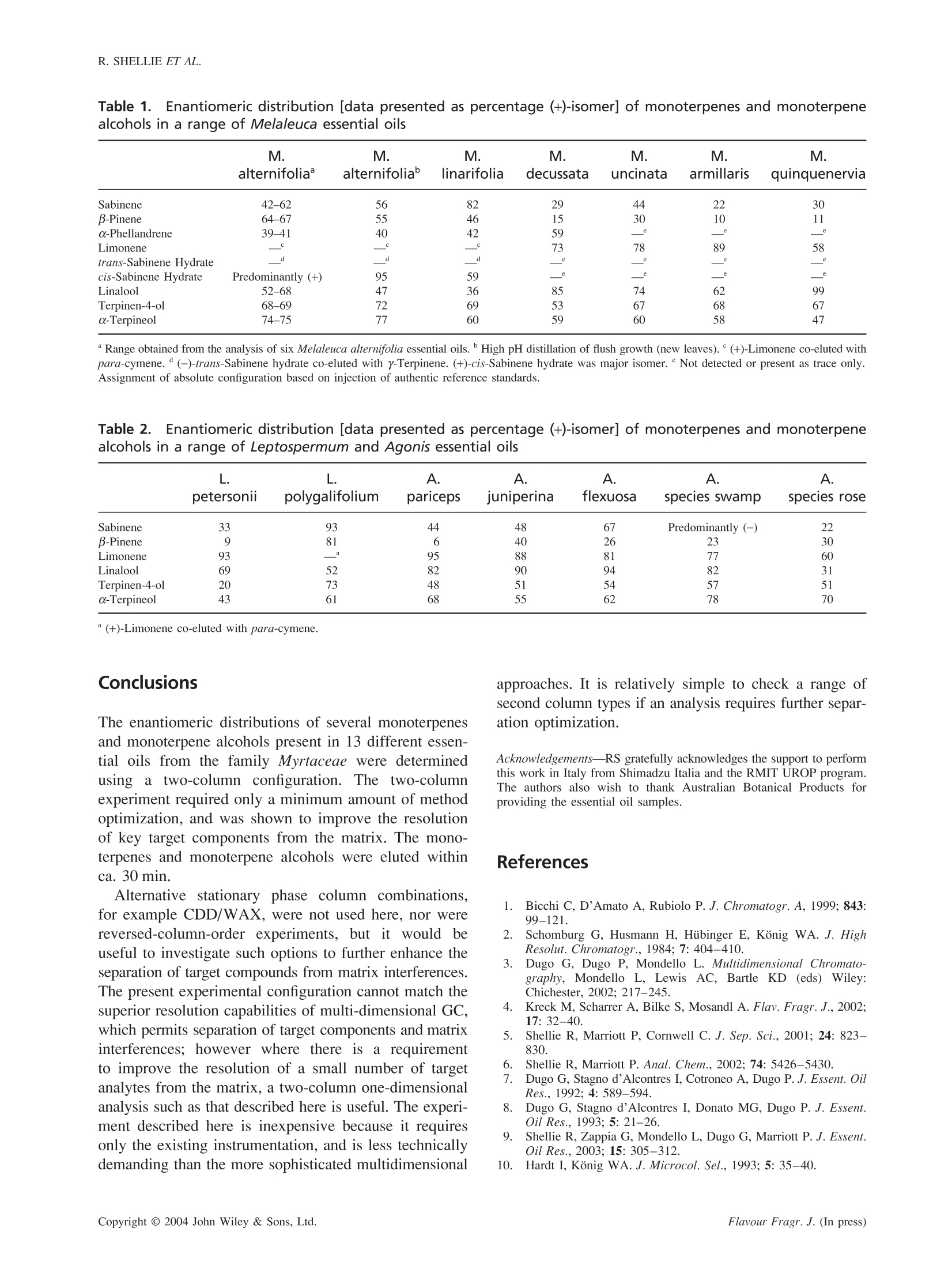
FLAVOUR AND FRAGRANCE JOURNALFlavour Fragr. J. (In press)Published online in Wiley InterScience (www.interscience.wiley.com). DOI: 10.1002/ffj.1368 R. SHELLIE ETAL. Enantioselective gas chromatographic analysis ofmonoterpenes in essential oils of the family Myrtaceae Robert Shellie, Luigi Mondello,2* Giovanni Dugo’ and Philip Marriott Australian Centre for Research on Separation Science, Department of Applied Chemistry, RMIT University, GPO Box 2476VMelbourne, Victoria 3001, Australia ’ Dipartimento Farmaco-chimico, Facoltá di Farmacia, Universitá di Messina, Viale Annunziata, 98168 Messina, Italy Received 22 February 2003 Revised 5 July 2003 Accepted 22 July 2003 ABSTRACT:The elution profiles of several essential oils of the Myrtaceae family were acquired using a singleenantioselective stationary phase column. These chromatograms were compared with those obtained by using a two-column coupled system, which comprised a low-polarity stationary phase column connected to the terminal end ofthe enantioselective column. The enantiomeric distributions of B-pinene, sabinene, a-phellandrene, limonene, trans-and cis-sabinene hydrate, linalool, terpinen-4-ol and o-terpineol were determined. The use of a two-column coupledsystem is demonstrated to be useful for improving the resolution of target (chiral) compounds from otherwiseinterfering matrix components. Copyright @ 2004 John Wiley & Sons, Ltd. KEY WORDS:enantioselectivegaschromatography;;cyclodextrin;essential0oil,, ttea tree;Melaleuca:Leptospermum; Agonis Introduction In the present investigation, the enantiomeric distribu-tions of the monoterpenes and monoterpene alcoholsin commercial-type tea tree essential oils, and otheressential oils from the family Myrtaceae were determinedusing enantioselective gas chromatography. Melaleucaalternifolia and M. linarifolia are widely recognizedbecause they are a source of commercial tea tree essentialoil. Here the lesser known essential oils of M. decussata,M. uncinata, M. armillaris, M. quinquinervia were alsoanalysed, as well as five Agonis essential oils, and twoessential oils from the genus Leptospermum. Agonis is asmall genus of 11 species, all of which occur naturallyonly in southwestern Australia. Leptospermum is a genusof about 86 species, distributed throughout Australia andextending to Malaysia and New Zealand. Enantioselective GC analysis of terpenoid compoundsis commonly performed using capillary columns coatedwith cyclodextrin derivatives (CDD) diluted in a suitablepolymeric stationary phase. Whilst the use of CDDcoated columns often provides adequate resolution of theindividual enantiomers of chiral compounds easily,theanalysis of complex matrices such as essential oils canbe problematic. There is an increased demand on theavailable resolving power of the column to ensure thatthe target (chiral) compounds are well resolved from ( * C o rrespondence to: L . Mondello, Dipartimento Farmaco-chimico, F a coltadi F a rmacia, Un i versita di M essina, Viale A n nunziata, 9 8 168 Messina, Ita l y.E- m ail: lmondello@pharma.unime.it ) matrix interferences. To circumvent this problem manyanalysts have adopted multi-dimensional enantioselectiveGC (enantio-MDGC) approaches for the analysis ofessential oils. The enantio-MDGC approach, which was first describedby Schomburg and co-workers’ requires unresolved targetcomponents to be heart-cut from the primary (achiral)column and delivered to the (chiral) analytical column,which provides resolution and quantitative measurementof the individual enantiomers. There have been numerousapplications of enantio-MDGC for food, flavour andfragrance analysis, including its recent use for the ana-lysis of M. alternifolia. A variant of the multidimensionalGC technique is comprehensive gas chromatography(GC × GC). Enantioselective essential oils analysis hasbeen reported using comprehensive enantio-GC × GC(where the first column contained the enantioselectivestationary phase), and comprehensive GC × enantio-GC(where the enantioselective stationary phase columnwas applied in the more conventional second dimensionposition). The former of these two examples was appliedto the analysis of M. alternifolia essential oils. In the present study a less technically demandingapproach to achieving adequate resolution of the target(chiral) compounds in these essential oils was invest-igated. Dugo and co-workers’8 previously showed that atwo-column combination (which essentially provides acapillary column of mixed stationary phase) was usefulin enhancing the resolution of target compounds frommatrix interferences. A two-column approach, similar tothe work of Dugo et al. but improved with the use of a chiral-non chiral stationary phases combination, will betaken here. Experimental Materials Samples were provided by Australian Botanical Products(Hallam, Australia). Prior to injection all samples werediluted 1:100 (v/v) in n-hexane. Instrumental All analyses were performed on a Shimadzu GC-17A gaschromatograph (Shimadzu Italia, Milan, Italy) equippedwith FID detector operated at 280℃. Method 1 used a diethyl-tert-butylsilyl-B-cyclodextrinenantioselective stationary phase column (Mega, Milan,Italy). This column is prepared in a base polymer ofPS086. The column dimensions were 25 m × 0.25 mm(0.25 um film thickness). The oven was temperatureprogrammed from an initial oven temperature of 45 °C(constant for 6 min) to 200°C at 2°C/min. The carriergas was hydrogen, which was supplied at a head pressureof 43 kPa. An injection volume of 0.1 ul was used for allanalyses and the split ratio was 100:1. Method 2 used two capillary columns which wereserially coupled using a zero dead-volume glass press-fitcapillary column joiner. The first column was a diethyl-tert-butylsilyl-B-cyclodextrin enantioselective stationaryphase column. The column dimensions were 25 m x0.25 mm (0.25 um film thickness). Column 2 was anRtx5-MS low-polarity stationary phase column. Thecolumn dimensions were 30 m × 0.25 mm (0.25 umfilm thickness). The oven was temperature programmedfrom an initial oven temperature of 80 °C (constant for6 min) to 200°℃ at 1.2C/min. The carrier gas washydrogen, which was supplied at a head pressure of104 kPa. An injection volume of 0.1 ul was used for allanalyses and the split ratio was 100:1. Results and Discussion The two-column approach produced more satisfactoryresults than analysis using only the enantioselectivestationary phase column. Typical results from the twoapproaches are contrasted below. Method 1 The diethyl-tert-butylsilyl-B-cyclodextrin enantioselectivestationary phase was able to separate the individual pairs of enantiomers of numerous components of teatree essential oil.(±)-Sabinene,(±)-B-pinene,(±)-0-phellandrene., (±)-limonene,(±)-trans- andd ((±)-cis-sabinene hydrate, (±)-linalool, (±)-terpinen-4-ol and(±)-a-terpineol were all adequately sepatated (baseline-resolution-or-better). However the determination of the. enantiomeric distribution of each of these compounds(using Method 1) was problematic because there wereseveral interfering matrix components. A typical chro-matogram of Melaleuca alternifolia essential oil usingthe diethyl-tert-butylsilyl-B-cyclodextrin stationary phasecolumn is shown in Fig. 1. (+)-Sabinene co-eluted with1,8-cineole, and (-)-sabinene was poorly resolved frommyrcene. Likewise, (+)-limonene was poorly resolvedfrom para-cymene, and (+)-trans-sabinene hydrate couldnot be resolved from terpinolene. In each case thepossibility of correctly determining the enantiomericdistribution of the target compound was hindered. Method 2 The chromatogram of the essential oil shown in Fig. 1 ispresented in Fig. 2 using the two-column combinationUsing the new experimental setup, (-)-trans-sabinenehydrate co-eluted withhyterpinene. Althoughtrans-sabinene hydrate is abundant in the flush growth samplethere appears to be only small amounts present in theother oils analysed, and hence this component wasconsidered to be less important for the characterization ofthe mature-type essential oils. Whilst problematic in theformer experiment, both isomers of sabinene eluted with-out matrix interference from the two-column combina-tion. In the high terpinen-4-ol/low 1,8-cineole type oils,(+)-limonene was not quantifiable, because it could notbe resolved from para-cymene. Both isomers of limonenecould be satisfactorily quantified in the high 1,8-cineole/low terpinen-4-ol oils, which typically contained lower.amounts of para-cymene. For example, L. petersoniiwhich is commonly called the lemon-scented tea-tree,produces an essential oil with a high limonene content.Thus the para-cymene interference was smallwithrespect to the (+)-limonene content. It may be possible tohave resolution of both (+)- and (-)-limonene from para-cymene by reversing the order of the capillary columns,as this was achieved previously using a similar set-up.However, for the present study the enantioselectivestationary phase column was purposely installed at theinjector side of the two-column combination. Thisensured that components had a lower effective elutiontemperature from the column, and hence better resolutionof enantiomers was expected. Also in this manner,the second column should not affect the enantiomericresolution achieved (provided that the same temperatureprogram is used), but the second column will affectthe resolution of enantiomers from the matrix.Having Figure 1.Typical chromatogram of a M. Alternifolia essential oil using method 1 minutes Figure 2.Typical chromatogram of a M. Alternifolia essential oil using method 2. The expanded insert shows theseparation of (±)-sabinene, (±)-B-pinene and (±)-o-phellandrene monoterpenes and monoterpene alcohols in a range ofMelaleuca essential oils, and the enantiomeric distribu-tion of the monoterpenes and monoterpene alcohols ina range of Leptospermum and Agonis essential oils arereported in Table 2. the achiral column second also allows for very easychanging of the second column if further separationoptimization is required, without affecting the primarycolumn result. Table 1 lists the enantiomeric distribution of the Table 11.. Enantiomeric distribution [data presented as percentage (+)-isomer] of monoterpenes and monoterpenealcohols in a range of Melaleuca essential oils M. M. M. M. M. M. M. alternifolia alternifolia° linarifolia decussata uncinata armillaris quinquenervia Sabinene 42-62 56 82 29 44 22 30 B-Pinene 64-67 55 46 15 30 10 11 a-Phellandrene 39-41 40 42 59 e e Limonene c c 73 78 89 58 trans-Sabinene Hydrate d d d e e e e cis-Sabinene Hydrate Predominantly (+) 95 59 e e e e Linalool 52-68 47 36 85 74 62 99 Terpinen-4-ol 68-69 72 69 53 67 68 67 o-Terpineol 74-75 77 60 59 60 58 47 " Range obtained from the analysis of six Melaleuca alternifolia essential oils. High pH distillation of flush growth (new leaves).(+)-Limonene co-eluted withpara-cymene.(-)-trans-Sabinene hydrate co-eluted with y-Terpinene. (+)-cis-Sabinene hydrate was major isomer. Not detected or present as trace only.Assignment of absolute configuration based on injection of authentic reference standards. Table 2. Enantiomeric distribution [data presented as percentage (+)-isomer] of monoterpenes and monoterpenealcohols in a range of Leptospermum and Agonis essential oils L. L. A. A. A. A. A. petersonii polygalifolium pariceps juniperina flexuosa species swamp species rose Sabinene 33 93 44 48 67 Predominantly (-) 22 B-Pinene 9 81 6 40 26 23 30 Limonene 93 _a 95 88 81 77 60 Linalool 69 52 82 90 94 82 31 Terpinen-4-ol 20 73 48 51 54 57 51 o-Terpineol 43 61 68 55 62 78 70 (+)-Limonene co-eluted with para-cymene. Conclusions The enantiomeric distributions of several monoterpenesand monoterpene alcohols present in 13 different essen-tial oils from the family Myrtaceae were determinedusing aatwo-column configuration. The two-columnexperiment required only a minimum amount of methodoptimization, and was shown to improve the resolutionof key target components from the matrix. The mono-terpenes and monoterpene alcohols were eluted withinca. 30 min. Alternative stationary phase column combinations,for example CDD/WAX, were not used here, nor werereversed-column-order experiments, but it would beuseful to investigate such options to further enhance theseparation of target compounds from matrix interferences.The present experimental configuration cannot match thesuperior resolution capabilities of multi-dimensional GC,which permits separation of target components and matrixinterferences; however where there is a requirementto improve the resolution of a small number of targetanalytes from the matrix, a two-column one-dimensionalanalysis such as that described here is useful. The experi-ment described here is inexpensive because it requiresonly the existing instrumentation, and is less technicallydemanding than the more sophisticated multidimensional approaches. It is relatively simple to check a range ofsecond column types if an analysis requires further separ-ation optimization. Acknowledgements-RS gratefully acknowledges the support to performthis work in Italy from Shimadzu Italia and the RMIT UROP program.The authors also wish to thank Australian Botanical Products forproviding the essential oil samples. References 1. Bicchi C, D'Amato A, Rubiolo P. J. Chromatogr. A, 1999; 843:99-121. 2. Schomburg G, Husmann H, Hubinger E, Konig WA. J. HighResolut.Chromatogr., 1984;7:404-410. 3. Dugo G, Dugo P, Mondello L. Multidimensional Chromato-graphy, Mondello L, Lewis AC, Bartle KD (eds)Wiley:Chichester, 2002;217-245. ( 4. K reck M, Scharrer A, Bilke S , M o sandl A. Flav. F r a gr. J. , 2002; 17: 32 - 40. ) ( 5. S hellie R, M arriott P , C ornwell C . J . S e p. S c i., 2 0 01;24 : 82 3 - 830. ) ( 6 S hellie R, Marriott P. Anal. Chem., 2 002; 7 4:5 4 26-54 3 0. 7 ) ( Dugo G , S t agno d'Alcontres I, Cotroneo A , Dugo P. J. Essent. O i l Res., 1992; 4 : 589-594. ) ( 8. D ugo G, S t agno d ' Alcontres I , D onato M G , Du g o P. J . Es s ent.Oil Res., 1993; 5: 21-26. ) 9. Shellie R, Zappia G, Mondello L, Dugo G, Marriott P. J. Essent.Oil Res., 2003;15:305-312. ( 10. H ardt I , Konig WA. J. Microcol. Se l ., 1993; 5: 3 5 -40 . ) Copyright C John Wiley & Sons, Ltd. Flavour Fragr. J. (In press)Copyright C John Wiley & Sons, Ltd.
确定


还剩2页未读,是否继续阅读?
扬州华明仪器设备有限公司为您提供《食用油、油脂中营养成分检测方案 》,该方案主要用于食用植物油中营养成分检测,参考标准--,《食用油、油脂中营养成分检测方案 》用到的仪器有
相关方案
更多







