
根据欧洲药典,许多药品的水份测定采用烘干箱或干燥器来进行.通常,这类物质是无法直接采用卡尔菲休滴定法来测定其水份含量, 因为样品多半会与样品发生副反应. 取代烘干箱的方法,我们采用自动干燥炉系统来进行微量水份测定.该系统包括774型Oven Sample Processor 和库仑法卡氏微量水份测定仪. 使用这套系统所获得的测定数据完全符合欧洲药典所规定的范围而且具有非常好的重现性.同时,大大提高分析工作者大工作效率和减轻工作强度,是非常值得药厂和药检部门考虑的方法. 本文以英文撰写,作者: Dr. Karl-Heinz Surborg / 德国 Bonn 大学 Ms. Regina Schlink, Ms. Claudia Dengler & Dr. Peter Kirschenbuhle r / Metrohm Ltd. 全文可于此下载. 中文翻译正在进行中.
方案详情

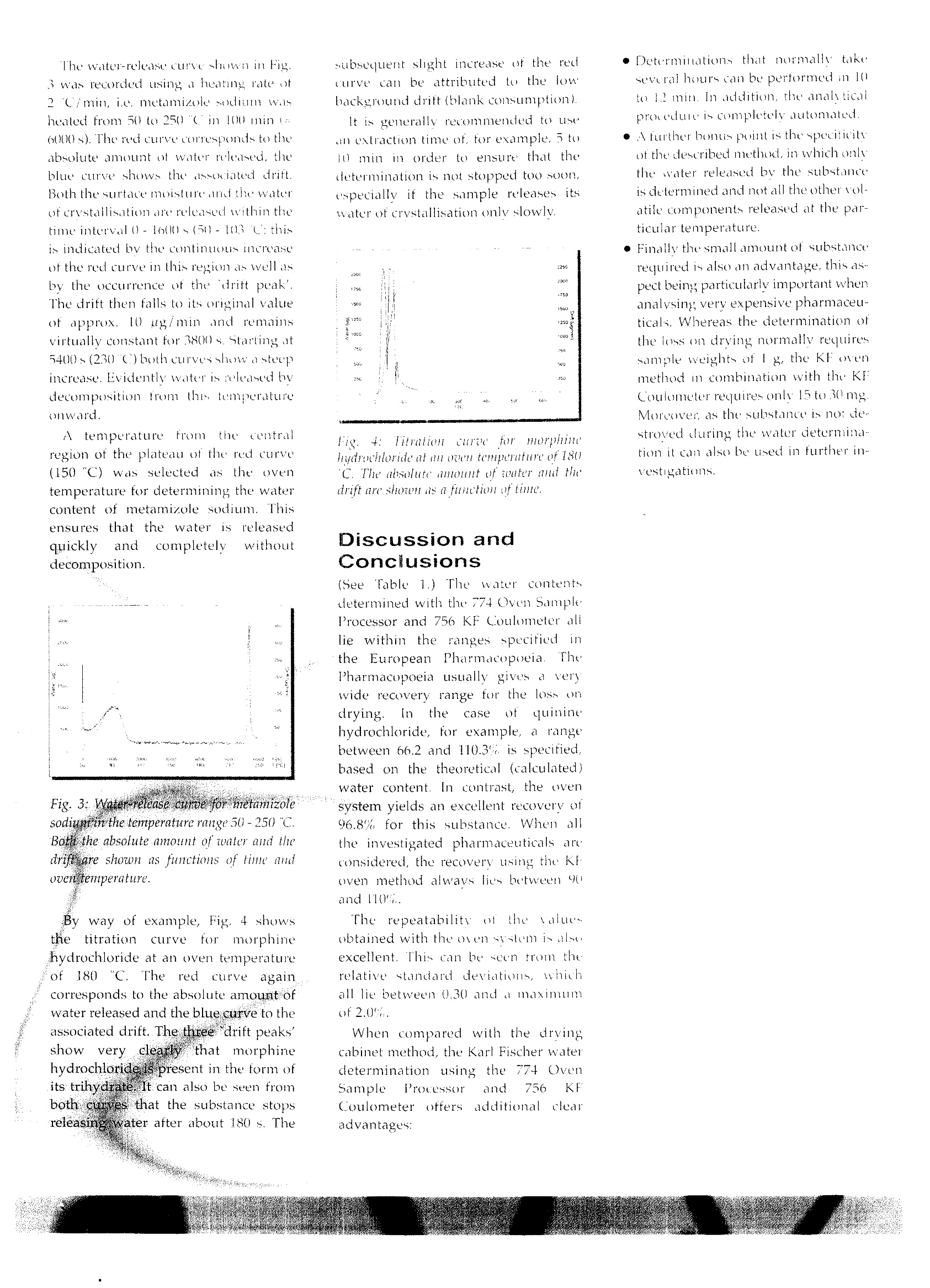
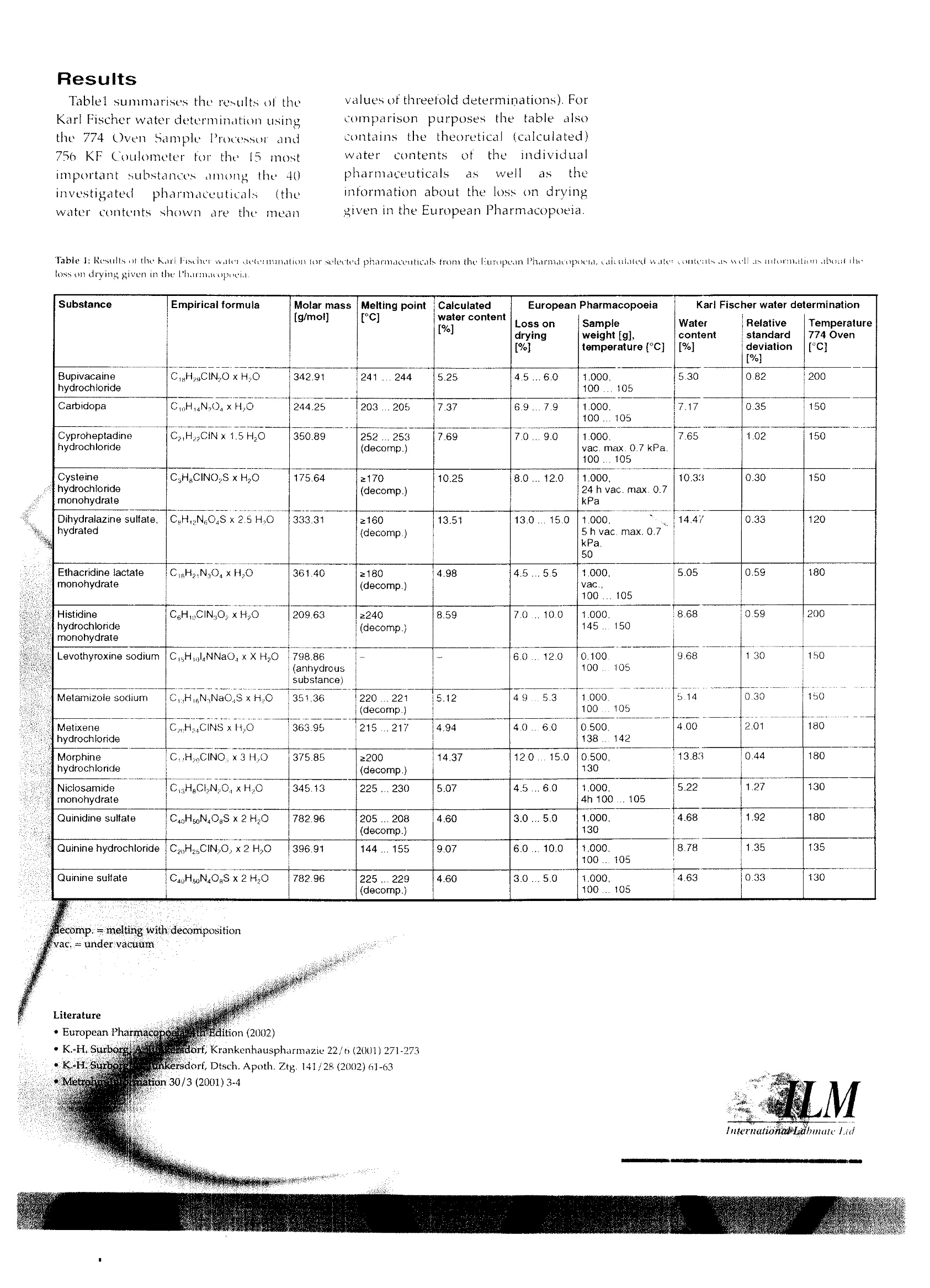
:六学 Automatic Karl FischeWater Determinationin Pharmaceuticals Authors Reginu Scllink, Claudia Dengler,Dr. Peter Kirschenbiih ler Metrohm Lidl. Herisau, SwitzerlundDr. Karl-Heinz Surborg University of Bonn, Germany According to the European Pharmacopoeiathe water content of many pharmac-euticals is determined by loss on dryingin a drying cabinet or desiccator. Usuallythese are substances that cannot beanalysed by means of direct Karl Fischontitration. Instead ofapplying the dryingcabinetmethod. wehgoe used alautomated Kart Excher oven sustem -consisting orthe 774 Oven SampleProcessoand a KF coulometer - for thewaleeterminations. The valuesaaed woith this system all lie withintha ranges specified in thePharmacopgeiaand show excellent repeatabthns Many active pharmaceutical ingredientsand adjuvants contain water in anadsorbed form (surface water)or boundas a hydrate (water of crystallisation).The water content of medicamentsstrongly influences their quality, shelflife and stability as well as the release ofthe active substances. The determinationOf watertherefore assumesgreatimportance in pharmaceutical analysis. The European Pharmacopoeia, 4thEdition(2002), describes variousmethods for determining the watercontent of pharmaceuticals. By far themost important method is the KarlFischer titration. Normally the titrationis carried out volumetrically (semi-micro determination). For substanceswith1ca very lowwater content acoulometric KF titration (micro-determination) is performed. KF Oven Method forDifficult Samples Many substances release their wateronly slowly or at high temperatures.They are therefore not suitable for adirect Karl Fischer titration..Anadditional problem is the low solubilityof certain samples in alcohols. In thesecases traditional methods recommendthe use of toxic solvents to promotedissolution or alternatively extensivesample preparation procedures. Othersubstances undergo side reactions withthe KF reagents, thus falsifying theresult. The European Pharmacopoeiaspecifies that these typespharmaceuticals are not to be analysedbyKarlFischer titration but bydetermining the loss on drying in adrying cabinet or desiccator (undervacuum if necessary). However, withthis method all volatile componentsreleased at the particular temperature(e.g. impurities) are determined andnot specifically the water content of thesubstance. By using the KF oven method theabove-mentionedproblems car beavoided. The substance underinvestigation is heated in a tube ovenand the released water is transferred by a carrier gas to the titration cell where itis determined bv Karl Fischer titration. As only the water enters the KF celland the sample itself does not comeinto contact with the KF reagent, thismeans that unwanted side reactionsand matrix effects are eliminated. Automation BringsClear Advantages The 774 Oven Sample Processor allowsto automate the KF oven method. Incontrast to the conventionalKarlFischer drying oven, the samples are nolonger introducedbyy means oftiasample boat, but the vial technique isapplied instead. The substances to beanalysed are weighed directly intosample vials, which are then sealedtightly and placed in the rack of theOvenSample Processor. For theanalysis the sample vessel is moved bythe turntable ttetlthe appropriate,ition above the ovenindthenlowered automatically into the heatingblock. At the sametime a doublehollow needle pierces the septum of thevial. Via the inlet needle a stream of drycarrier gas (air or inert gas) is passedthrough the heated sample. The carriergas, loaded with the released moisture,then flows through the outlet needleand a heated transfer tube directly intothe titration cell, where the Karl Fischerwater determination takespiace.Depending on the sample'swatercontent, the determination is carriedouteithervolumetrically or at the tracelevel, coulometrically. The automation of the Karl Fischerwater determination using the 774Oven Sample Processor brings decisiveadvantages: e Strictly reproducible analysis condi-tions for all samples as demonstratedby the significantly improved repeat.abilily of the results. ·Considerably increased Samplethroughput and therefore improvedetticiency, · Manual sanple preparation is reducedto a niinimun ● No contamination of the oven and ti-tration cell; consequently there are nocarryover and memory effects. ·Much lower reagent consumption asthe titration solution only requireschanging at infrequent intervals. ·lmproved water release from thesample as the carrier gas does notjust pass over the sample but directlythrough it in addition, thee OvenSampleProcessor allows temperature gradientsto be run. Using the recorded water-release curve, it is possible to determinethe optimum analysis temperature forthe particular sample. The curve alsoallows statements to be made about thekinetics of water release as a function oftemperature. Scope of theinvestigationsCarried Out Usingthe kOvenmethodanalysed about 40plarmaceuticalsfrom the European Pharmacopoeid.The analyses were carried out withthe 774 Oven Sample Processor incombination with a 756 KF CoulometerThe investigated pharmaceuticals weresubstances with a defined water content,some of which undergo side reactionswith the KF reagents and thereforecannot be analysed bydirectKarlFischer titration. As stated above, accordingto thePharmacopoeia the water content ofsuch substances must be determined byloss on drying in a drying cabinet ordesiccator (under vacuum if necessarv). Instruments andAccessories 774 Oven Sample Processor ·756 KF Coulometer, including KFcell without diaphragm · 728 Magnetic Stirrer · 6.5617.000 complementary equip-ment for automatic reagent exchange ●700 Dosino · PCwith VESUV 3.0 Metrodata sott-ware for data acquisition, storage andreprocessing Reagents Hvdranal Coulomat AGOven, Riedel-de Haen no. 34739 e Hvdranal Water Standard KF Oven(potassium citrate monohydrate),Riedel-de Haen no.34748 Nitrogen as inert carrier gas Fig. 1: The atomuted anulysis system usedfor the investigations, consisting of 774OacuSampleProcessor anil756KFCouloneter Analytical Procedure Between 15 and 30mgofthepharmaceuticals to be analysed isweighed into the sample vials, whichare then hermetically sealed withPTFE-coated septa. At least a threefolddetermination is carried out on eachsubstance. Prior to each determination thecomplete system is conditioned until aconstant low drift (approx. 10ugimin)is15aattained. During thisprocedure the needle is located in aspecial conditioning vessel on the rackof the Oven Sample Processor. In order to obtain correct results, theblank of the sample vials - i.e. themoisture adhering to the vessel walls,vialcap and septum--must bdetermined and taken into accountwhen calculating the water content ofthe samples. This is done by analysingthreeeempty vials at the oventemperature required for theparticular sample. The complete system is checked atregular intervals with a certified KFstandard (Hydranal Water StandardKFOven). Determinationof the AnalysisTemperatures When selecting the temperature to beusedtor driving off the water, thethermal stability(instability) of ththeparticular pharmaceuticalILISt betaken into account as well as the factthatwaitel is only released atsufficiently rapid cate att tteemmpperattireabovel00 Fig. 2: Structural formula ad enpiricalforuh of metanizole sodium, preseut asmonohudrate, This means that the oven temperatureshould be chosen as high as possibleto ensure short determination times,but still be 20 to 30 below thedecomposition temperature The analysis. temperatures aredetermined onthe basis of the water-release curves that were recorded for allthe investigated pharmaceuticals in thetemperature range 50- 250 C. Fig.3shows such a water-release curve formetamizole sodium. In addition, all thepharmaceuticals were examined bymeans of a Kofler microscope and theirmelting points were determined. Thisinstrument allows the substance to beclosely observed during the heatingrupand melting phases; any alterations suchas colour changes, sublimation ordecomposition reactions can be easilyrecognised. Metamizole sodium, whose structuraland empirical formulas are shown inFig. 2. melts at 220 to 221℃ withdecomposition. Water determination bydirect volumetric or coulometric KFtitration is not possible as the substanceis oxidised! completely or partiallyby iodine. The water-release curve shown in Fig.3 was recorded using a heating rate ot?C/min, i.e. metamizole sodium washeated from 50 to 250( in 100 mini=600(s). The red curve corresponds to theabsolute amount ot water released, theblue curve shows the associated drift.Both the surtace moisture and the waterof crystallisation are released within thetime interval0)-1600s(50-103℃; thisis indicated by the continuous increaseof the red curve in this region as well asby the occurrence of the drift peak'.The drift then falls to its original valueof approx. 10 ng/min and remainsvirtually constant for 3800 s. Starting at5400s(230C) both curves show asteepincrease. Evidently water is released bytecomposition from this temperatureonward. A temperature fromttihecentralregion of the plateau of the red curv(150"C) was selected as the oventemperature for determining the watercontent of metamizole sodium. Thisensures that the water isSIreleasedquickly and completely withoutdecomposition. Fig. 3: Watar rclense curee yor metamizole sodium in the temperature range 50-250℃. Boti the absolute amount of toater and thedrift are shown as functions of time audoven temperature. Byway of example, Fig. 4 showsthetitration Curve forImorphinehydrochloride at an oven temperatureot 180C. Thee red curveagaincorresponds to the absolute amount ofwater released and the blue curve to theassociated drift. The three drift peaks'showw very clearly thatmorphinehydrochloride is present in the form ofits trihydrate. It can also be seen fromboth curves that the substance stopsreleasingwater after about 180 s. The subsequent slight increase of the redcurve can be attributed to the i Abackground drift (blank consumption). lt is generally recommended to useain extraction time of, for example,5 toj0 min in order to ensure that thedetermination is not stopped too soon,especially if the sample releases itswater of crystallisation only slowly. Fig4: itration curee tom morphinthydrochloride at an ozen temperuture of 180C. The absolute anount of teater and the'drift are shornt as a furiction oftime. Discussion andConclusions (SeeTable 1.)The water contentsdetermined with the 774 Oven SampleProcessor and 756 KF Coulometer alllie within the ranges specifiedirthe European Pharmacopoeia..ThePharmacopoeiaaLusually gives a verywide recovery range for the loss ondrying In the case quininehydrochloride, for example, a rangebetween 66.2 and 110.3% is specified,based on the theoretical (calculated)water content. In contrast, the ovensystem yields an excellent recovery of96.8% for this substance. When allthe investigated pharmaceuticals areconsidered, the recovery using the KFoven method always lies between 90and 110%. The repeatability the valuesobtained with the oven systemis alseexcellent. This can be seen trom therelative standard deviations, whichall lie between 0.30 and a maximumof 2.0%, When comparedl with the drvingcabinet method, the Karl Fischer waterdetermination using the 774 OvenSample Processor and 756 KFCoulometer offersadditionalc leearadvantages: · Determinations that normally tetakeseveral hours can be performed in 10to 1 min. In addition, the analyticalprovedure is completely automated. A further bontis point is the speciticityof the described method, in which onlythe water released bv the substancejs determined and not all the other volatile components released at the par-ticular temperature. ● Finally the small amount of substancerequired is also an advantage, this aspect being particularly important whenanalysing very expensive pharmaceu-ticals. Whereas the determination ofthe loss on drying normally requiressample weights of I g, the KF ovenmethod in combination with the KFCoulometer requires only 15 to 30 mg.Moreover as the substance is no de-stroved during the water determination it can also be used in further investigations. Results Tablel summarises the results of theKarl Fischer water determination usingthe 774 Oven Sample Processor and756 KF Coulometer for the 15 mostimportant substances among the 40investigated pharmaceuticals (thewater contents shown are the mean values of threefold determinations). Forcomparison purposes the table alsocontains the theoretical (calculated)water contents Ot the individualpharmaceuticals as well as theinformation about the loss on dryinggiven in the European Pharmacopoeia. Table 1: Results ot the Karl Fischer water cielerination for selected pharmaceuticals from the European Pharmacopoeld,caleulated wale: conteats aswell as intormation about theloss on drying given in the Pharmacopoei. Substance Empirical formula Molar mass[g/mol] Melting point[C] Calculatedwater content[%] Loss ondrying[%] European Pharmacopoeia Sampleweight [g], temperature [C] Watercontent[%] Relativestandarddeviation[%] Temperature774 Oven [°C1 Bupivacainehydrochloride C8H2gCIN,OxH,O 342.91 241...244 .5.25 4.5...6.0 1.000,100....105 5.30 0.82 200 Carbidopa CH,N,O.xH,0 244.25 203...205 7.37 6.9...7.9 1.000.100...105 7.17 0.35 150 Cyproheptadinehydrochloride CHCINx1.5H,O 350.89 252...253(decomp.) 7.69 7.0...9.0 1.000.vac.max. 0.7 kPa.100...105 7.65 1.02 150 Cysteinehydrochloridemonohydrate CHgCINO,S xH,0 175.64 ≥170(decomp.) 10.25 8.0...12.0 1.000.24 h vac. max. 0.7kPa 10.33 0.30 150 Dihydralazine sulfate,hydrated CHN,O,Sx2.5 H,O 333.31 2160 (decomp.) 13.51 13.0 ... 15.0 1.000.5 h vac. max. 0.7kPa.50 14.47 0.33 120 Ethacridine lactatemonohydrate C8H2N0xH,O 361.40 ≥180(decomp.) 4.98 4.5...5.5 1.000,vac..100...105 5.05 0.59 180 Histidine hydrochloridemonohydrate CeH,CIN,0,xH0 209.63 2240(decomp.) 8.59 7.0...10.0 1.000145...150 8.68 0.59 200 Levothyroxine sodium CHLNNaO:xXH,O 798.86(anhydroussubstance) 6.0...12.0 0.100.100..105 9.68 1.30 150 Metamizole sodium CHNNaOSxH,O 351.36 220...221(decomp.) 5.12 49...5.3 1.000.100...105 5.14 0.30 150 Metixenehydrochloride CHCINSxH,O 363.95 215...217 4.94 4.0...6.0 0.500.138..142 4.00 2.01 180 Morphinehydrochloride C,HCINO, x3 H,O 375.85 2200(decomp.) 14.37 12.0...15.0 0.500,130 13.83 0.44 180 Niclosamidemonohydrate C3HgCLN,O.xH,O 345.13 225...230 5.07 4.5...6.0 1.000.4h 100..105 5.22 1.27 130 Quinidine sulfate C40H50NOgSx2HO 782.96 205...208(decomp.) 4.60 3.0...5.0 1.000,130 4.68 1.92 180 Quinine hydrochloride C20H2sCIN,O,x2 H,O 396.91 144...155 9.07 6.0...10.0 1.000.100...105 8.78 1.35 135 Quinine sulfate CagH5oN.OSx2HO 782.96 225...229(decomp.) 4.60 3.0...5.0 1.000.100...105 4.63 0.33 130 comp.= melting with decomposition g=undervacuum Literature · European Pharmac ltion (2002) ( ·K.H. Surbore sdorf, Krankenhauspharmazie 22/6 (2001)271-273 ) · K-H. Surbor rsdorf, Dtsch. Apoth.Ztg. 141/28(2002)61-63 Metrc lon30/3 (2001)3-4 fnternetionatibmale Lid
确定


还剩2页未读,是否继续阅读?
扬州华明仪器设备有限公司为您提供《化学药中理化常数检测方案 》,该方案主要用于化药制剂中限度检查检测,参考标准--,《化学药中理化常数检测方案 》用到的仪器有
相关方案
更多







