方案详情
文
采用安谱公司的MIP-PAH多环芳烃净化柱,可以有效去除油脂对于分析的干扰,快速,简便,RSD在8%以内
方案详情

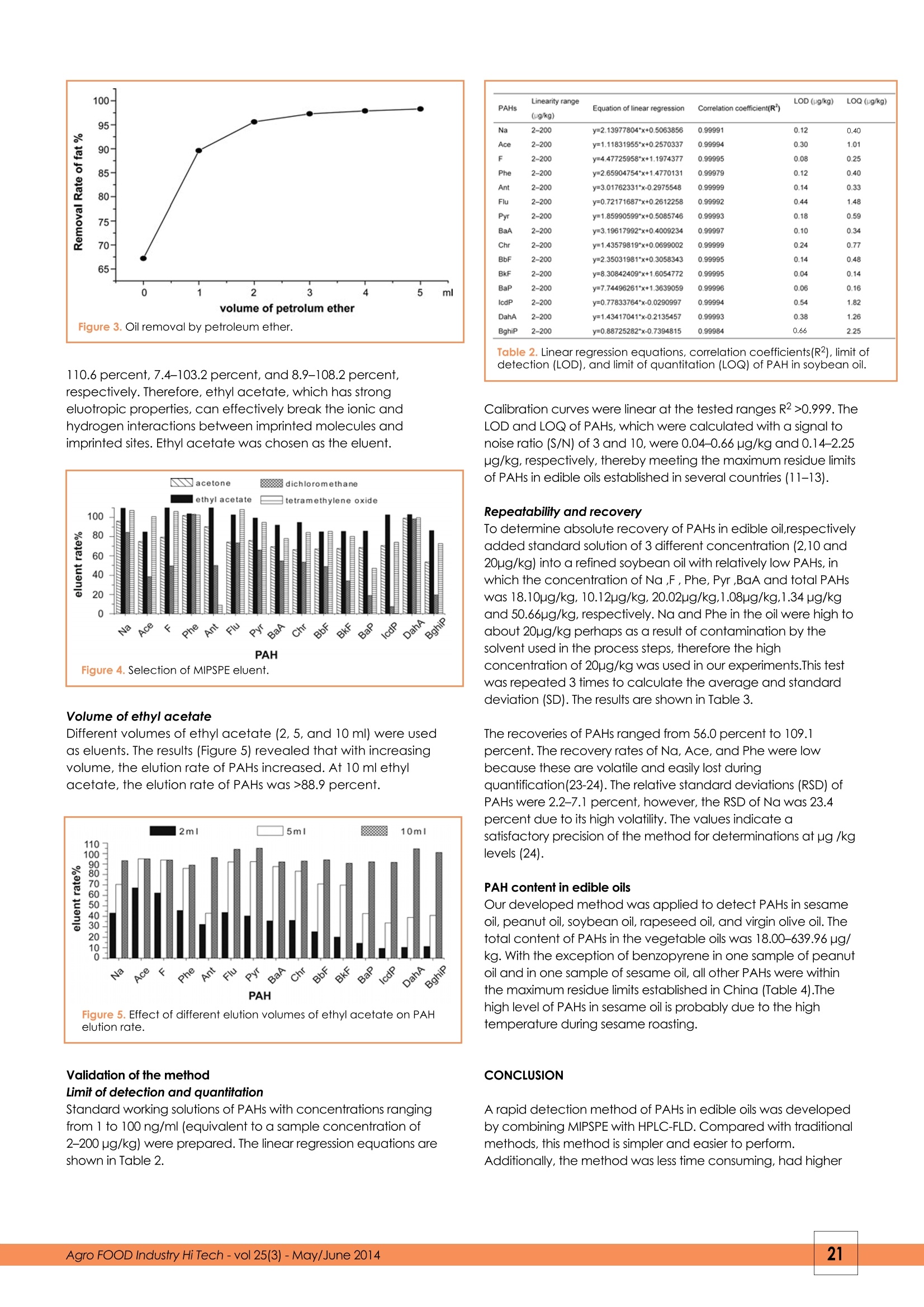
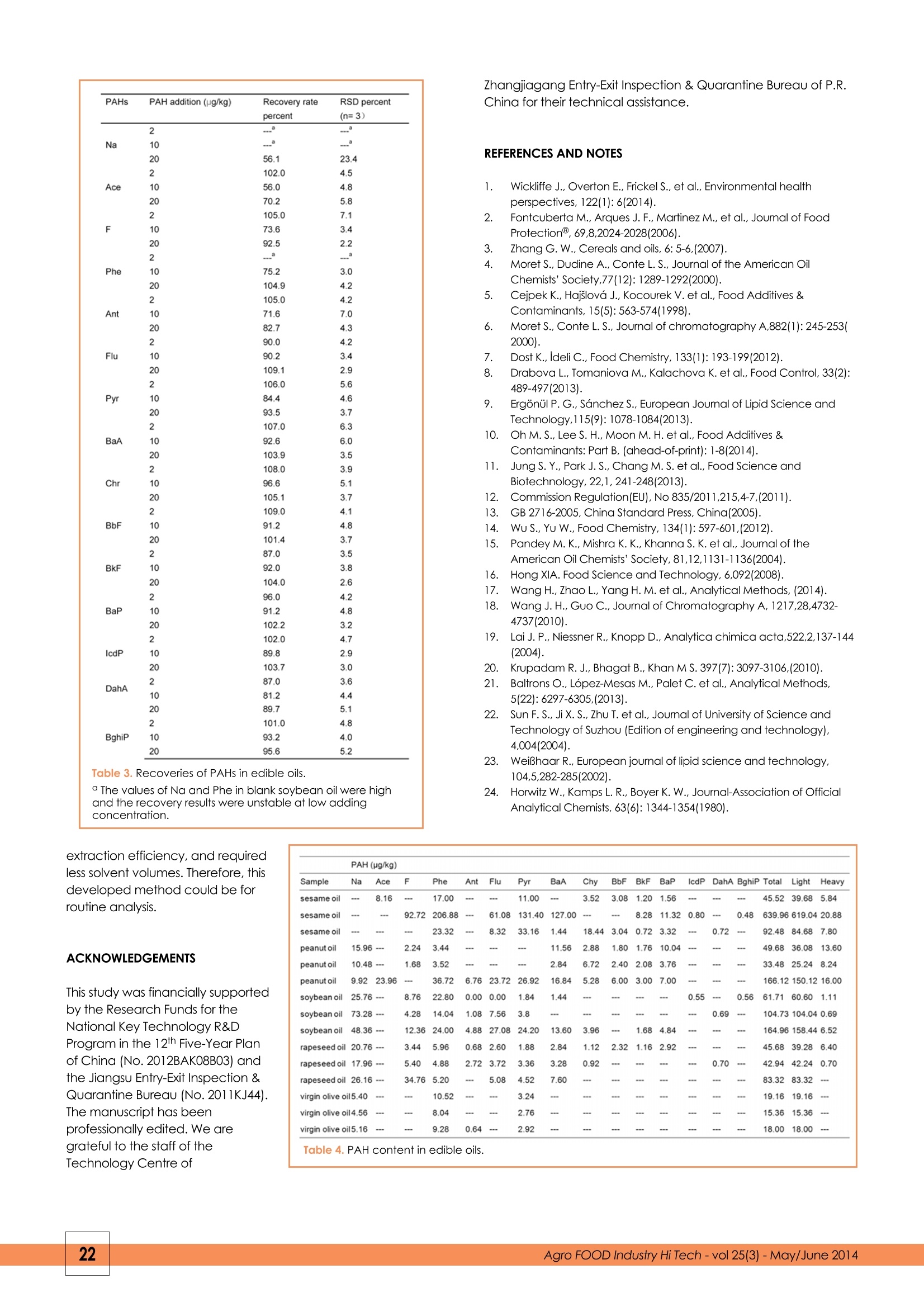
FOOD ANALYSISYE YU1,2,3.4 YUE WANG2, QINZE JIN1,3,4, HUA DONG5, XINGGUO WANG1,3,4**Corresponding author 1. Jiangnan University, State KeyLaboratory of Food Science and Technology, Wuxi 214122, PR China 2. Food Inspection Authority of Zhangjiagang Entry-Exit Inspection and Quarantine Bureau, Zhangjiagang,215600, PR China 3. Jiangnan University, School of Food, Wuxi 214122, PR China 4. Synergetic Innovation Centre of Food Safety and Nutrition, Wuxi 214122,PR China 5. Eastocean Oils & Grains Industries (Zhangjiagang) CO., LTD., Zhangjiagang, 215634, PR China Polycyclic aromatic hydrocarbonsin edible oils: analysis by molecularly imprinted solid-phase extractionwith HPLC-FLD KEYWORDS: polycyclic aromatic hydrocarbons, HPLC-FLD, fats, vegetable oils, molecularly imprinted solid-phase extraction +A new method was developed to determine the presence of polycyclic aromatic hydrocarbons (PAHs) inAbstracltA edible fats and oils. Samples are dissolved in petroleum ether, and PAHs are adsorbed onto a molecularlyimprinted solid-phase extraction (MIPSPE) cartridge and eluted with ethyl acetate. After evaporation of the solvent, PAHs werere-dissolved in acetonitrile and analyzed by RP-HPLC with fluorescence detection. The detection limits of PAHs were 0.04-0.66 pg/kgand the recovery rates ranged from 56.0 percent to 109.1 percent. The total contents of PAHs in edible oils were 18.00-639.96 pg/kg.The developed method was effective due to its high specificity for PAHs. INTRODUCTION Polycyclic aromatic hydrocarbons (PAHs) are carcinogeniccompounds (1), which result from the incomplete combustionof organic matter. Their presence in foods is mainly attributedto environmental pollution and food processing.Thecontamination of edible oils with PAHs (2) occurs during seeddrying, harvesting, transportation, processing, and packaging(3-6). In this way, PAHs have been detected in severalvegetable oils including corn oil, coconut oil, grape seed oil,peanut oil, olive oil,rapeseed oil, palm oil, sunflower oil, andpumpkin seed oil (6-10). Several countries have established maximum residue limitsof PAHs in edible fats and oil. For example, in South Korea,the maximum residue limit of benzo(a)pyrene (BaP) is <2pg/kg (11) in vegetable oil; in the European Union, themaximum residue limit of combined PAHs (e.g., BaP, benz(a)anthracene,benzo(b)fluoranthene, and chrysene) is <10pg/kg in edible fats and oils (COMMISSION REGULATION No835/2011) (12); and in China, the maximum residue limit of BaPin edible oils is 10 pg/kg for Bap (13). The most common methods to determine the presence ofPAHs in oils and fats include saponification (7), liquid-liquidextraction (14,15), cloud point extraction (16), and gelpermeation chromatography (GPC) (17,18). These methodsinvolve several complicated steps that are time consumingand require large volumes of solvents. Additionally, thesurfactant applied in cloud point extraction affects PAHdetection. In recent years, molecular imprinting is atechnique that has been widely studied and applied inchemistry, medical science, and molecular biology. Lai J.P. and Krupadam R. J. have successfully applied molecular imprinting in the detection of PAHs (19-21). The objective ofthis study was to develop a new method for PAH detectionin edible oils by combining molecularly imprinted solid-phaseextraction (MIPSPE) with HPLC-FLD.The developed methodwas then applied to determine the levels of PAHs in edible oilscommercially available in China. EXPERIMENTAL Reagents and materials A mixture of PAH standards was purchased fromAccuStandard Inc. The standards included naphthalene (Na),200 pg/ml; acenaphthene (Ace), 200 pg/ml; fluorene (F), 200pg/ml; phenanthrene (Phe), 200 pg/ml; anthracene (Ant), 200pg/ml; fluoranthene (Flu), 200 pg/ml; pyrene (Pyr), 200 pg/ml;benz[a]anthracene (BaA), 200 pg/ml; chrysene (Chr), 200 pg/ml; benzo[b]fluoranthene (BbF), 200 pg/ml;benzo[k]fluoranthene (BkF), 200 pg/ml;and benzo[a]pyrene (BaP), 200pg/ml. The standard mix was stored at 20°℃ in the dark toprevent volatilization and photodegradation. Stock solutions(200 ng/ml) were prepared by diluting the standard mix withacetonitrile (1:1,v/v). Tetrahydrofuran (THF) and methylenechloride were purchased from Fisher (Fisher Scientific, USA);acetonitrile was acquired from Sigma (Germany); n-hexaneand cyclohexane were obtained from ANPEL (Shanghai,China); diethyl ether was purchased from Tedia (USA); acetonewas purchased from Merck (Germany); petroleum ether wasobtained from Ourchem (40-60℃; Shanghai, China); and ethylacetate was acquired from CNW (Germany). Solvents were ofHPLC grade. Milli-Q ultrapure water was used in all experiments(Millipore, Bedford, MA, USA). Instruments The following instruments were used for samplepreparation: a vortex mixer (WH-866, Huamei Corporation,China), an electronic balance (AL 204, Mettler Toledo,China), an automatic solid phase extraction instrument(Biotage, Rapid Trace SPE2, Sweden), a MIP-PAH cartridge(SBEQ-CA5054; 500 mg, ANPEL, China),and an automaticquantitative concentration instrument (Biotage,TurboVapⅡ, Sweden). Membrane filters (0.22 pm) were used in theexperiments (SCAA-104, ANPEL, China). Samples The edible oils used in this experiment consisted of threebrands of each of the following, sesame oil, peanut oil,soybean oil, rapeseed oil, and virgin olive oil,which werepurchased at local supermarkets in 2013. The edible oil ofthese brands was the typical products in China. 5 sampleswere selected for each brand. Samples were stored in thedark at 20°C. MIPSPE purification Aliquots (0.5 g) of edible oils were dissolved in 2 mlpetroleum ether. MIPSPE cartridges were activated with 5ml of petroleum ether.Each sample was loaded into thecartridges, which were washed with 2 ml of petroleumether. Finally, the sorbent was washed with 5 ml ofpetroleum ether, and PAHs were eluted with 10 ml ofethyl acetate. MIPSPE extracts were evaporated todryness, re-dissolved in 1 ml acetonitrile, filtered,andanalyzed by HPLC. HPLC The HPCL system (Agilent series 1200, Agilent Technologies,Santa Clara, CA, USA), which was coupled to ananalytical column specific for PAHs(250 mm x 4.6 mm, 5um particle size; SupelcosilTM LC-PAH), was equipped witha vacuum degasser, an auto-sampler, a columnthermostat, a binary pump, a diode array, and afluorescence detector. The column temperature wasmaintained at 25°C. The sample (20 pl) was injected at aflow rate of 1.0 ml/min. The mobile phase consisted of A(acetonitrile) and B (water) was used to PAHs separation.The elution conditions were as follows: 0-5 min, 50 percentA isocratic; 5-30 min, linear gradient 50-100 percent A;30-45 min, 100 percent A isocratic. The column wasre-equilibrated for 5 min between samples. RESULTS AND DISCUSSION Optimum detection wavelengths The excitation and emission wavelengths of different PAHswere recorded. The optimum detection wavelengths areshown in Table 1. Time (min) Excitation wavelengths (nm) Emission wavelengths (nm) PAHs 0 212 336 Na 13.5 260 336 Ace/F 18 260 366 Phe 19.6 230 420 Ant/Flu/Pyr 26 260 420 BaA/BbF/BbF/BkF/BaP/lcdP/DahA 38 250 500 BghiP The chromatogram presented in Figure 1 showed a goodresolution obtained with the HPLC conditions used. Figure 1. Chromatogram of a 10 ng/ml standard mixture. Optimized MIPSPE procedure Sample solvent Four organic solvents of weak polarity (i.e., n-hexane,cyclohexane, diethyl ether, and petroleum ether) were usedas solvents in MISPE to elute a PAH standard solution (10 ng/ml). The results (Figure 2) revealed that the adsorption rates ofn-hexane, cyclohexane, diethyl ether, and petroleum etherfor PAHs were 41.7-100 percent, 57.1-100 percent, 52.3-100percent, and 83.8-100 percent, respectively. Therefore,petroleum ether was chosen as the sample solvent. Oil removal by petroleum ether Different volumes of petroleum ether were used to remove oilfrom soybean oil samples. The results are shown in Figure 3. With increasing petroleum ether volumes (from 0 to 5 ml), therate of oil removal increased from 64.8 percent to 98.7percent. Eluent for MIPSPE The PAH standard solution (10 ng/ml) was loaded into theMIPSPE cartridge and eluted with acetone, ethyl acetate,methylene chloride, or tetrahydrofuran (Figure 4). The recoveryrates of PAHs with acetone, ethyl acetate, methylene chloride,and tetrahydrofuran as eluents were 53.5-102.0 percent, 85.0- Figure 3. Oil removal by petroleum ether. 110.6 percent, 7.4-103.2 percent, and 8.9-108.2 percent,respectively. Therefore, ethyl acetate, which has strongeluotropic properties, can effectively break the ionic andhydrogen interactions between imprinted molecules andimprinted sites. Ethyl acetate was chosen as the eluent. Figure 4. Selection of MIPSPE eluent. Volume of ethyl acetate Different volumes of ethyl acetate (2, 5, and 10 ml) were usedas eluents. The results (Figure 5) revealed that with increasingvolume, the elution rate of PAHs increased. At 10 ml ethylacetate, the elution rate of PAHs was >88.9 percent. Figure 5. Effect of different elution volumes of ethyl acetate on PAHelution rate. Validation of the method Limit of detection and quantitation Standard working solutions of PAHs with concentrations rangingfrom 1 to 100 ng/ml (equivalent to a sample concentration of2-200 pg/kg) were prepared. The linear regression equations areshown in Table 2. PAHs Linearity range Equation of linear regression Correlation coefficient(R) LOD (pg/kg) LOQ (ug/kg) (ug/kg) Na 2-200 y=2.13977804*x+0.5063856 0.99991 0.12 0.40 Ace 2-200 y=1.11831955*x+0.2570337 0.99994 0.30 1.01 F 2-200 y=4.47725958*x+1.1974377 0.99995 0.08 025 Phe 2-200 y=2.65904754*x+1.4770131 0.99979 0.12 0.40 Ant 2-200 y=3.01762331*x-0.2975548 0.99999 0.14 0.33 Flu 2-200 v=0.72171687*x+0.2612258 0.99992 0.44 1.48 Pyr 2-200 ym1.85990599*x+0.5085746 0.99993 0.18 0.59 BaA 2-200 y=3.19617992*x+0.4009234 0.99997 0.10 0.34 Chr 2-200 y=1.43579819*x+0.0699002 0.99999 0.24 0.77 BbF 2-200 y=2.35031981"x+0.3058343 0.99995 0.14 0.48 BkF 2-200 y=8.30842409*x+1.6054772 0.99995 0.04 0.14 BaP 2-200 y=7.74496261*x+1.3639059 0.99996 0.06 0.16 lcdP 2-200 y=0.77833764*x-0.0290997 0.99994 0.54 1.82 DahA 2-200 y=1.43417041*x-0.2135457 0.99993 0.38 1.26 Bghip 2-200 y=0.88725282*x-0.7394815 0.99984 0.66 2.25 detection (LOD), and limit of quantitation (LOQ) of PAH in soybean oil. Calibration curves were linear at the tested ranges R2>0.999. TheLOD and LOQ of PAHs, which were calculated with a signal tonoise ratio (S/N) of 3 and 10, were 0.04-0.66 pg/kg and 0.14-2.25ug/kg, respectively, thereby meeting the maximum residue limitsof PAHs in edible oils established in several countries (11-13). Repeatability and recovery To determine absolute recovery of PAHs in edible oil,respectivelyadded standard solution of 3 different concentration (2,10 and20pg/kg) into a refined soybean oil with relatively low PAHs, inwhich the concentration of Na ,F, Phe, Pyr ,BaA and total PAHswas 18.10pg/kg,10.12pg/kg, 20.02pg/kg,1.08pg/kg,1.34 pg/kgand 50.66pg/kg,respectively. Na and Phe in the oil were high toabout 20pg/kg perhaps as a result of contamination by thesolvent used in the process steps, therefore the highconcentration of 20pg/kg was used in our experiments.This testwas repeated 3 times to calculate the average and standarddeviation (SD). The results are shown in Table 3. The recoveries of PAHs ranged from 56.0 percent to 109.1percent. The recovery rates of Na, Ace, and Phe were lowbecause these are volatile and easily lost duringquantification(23-24). The relative standard deviations (RSD) ofPAHs were 2.2-7.1 percent, however, the RSD of Na was 23.4percent due to its high volatility. The values indicate asatisfactory precision of the method for determinations at pg /kglevels (24). PAH content in edible oils Our developed method was applied to detect PAHs in sesameoil, peanut oil, soybean oil, rapeseed oil, and virgin olive oil. Thetotal content of PAHs in the vegetable oils was 18.00-639.96 pg/kg. With the exception of benzopyrene in one sample of peanutoil and in one sample of sesame oil, all other PAHs were withinthe maximum residue limits established in China (Table 4).Thehigh level of PAHs in sesame oil is probably due to the hightemperature during sesame roasting. CONCLUSION A rapid detection method of PAHs in edible oils was developedby combining MIPSPE with HPLC-FLD. Compared with traditionalmethods, this method is simpler and easier to perform.Additionally, the method was less time consuming, had higher a The values of Na and Phe in blank soybean oil were highand the recovery results were unstable at low addingconcentration. Zhangjiagang Entry-Exit Inspection & Quarantine Bureau of P.R.China for their technical assistance. REFERENCES AND NOTES ( 1 . Wickliffe J., Overton E., Frickel S., e t al . , Environmental health perspectives,122(1):6(2014). ) ( 2. Fontcuberta M., A r ques J. F., M artinez M., et al., Journal of Food Protection@, 69,8,2024-2028(2006). ) ( 3. Zhang G. W . , Cereals and oils, 6:5 - 6,(2007). ) ( 4. Moret S., Dudine A., Conte L.S., Journal of the American Oil Chemists' S ociety,77(12):1 2 89-1292(2000). ) ( 5. Cejpek K.,H a jslová J., K ocourek V. et al., Food Additives &Contaminants, 1 5 (5):563-574(1998). ) ( 6. Moret S., Conte L. S., Journal of chromatography A,882(1): 245-253( 2000). ) ( 7. Dost K., Ideli C . , Foo d Chemistry, 133(1) : 193-199(2012). ) ( 8. Drabova L., Tomaniova M., Kalachova K. et al., Food Control,33(2):489-497(2013). ) ( 9. ErgonUl P. G., Sanchez S., European Journal of Lipid Science and Technology,115(9):1078-1084(2013). ) ( 10. Oh M. S., Lee S. H. . Moon M. H.et al..Food Additives & Contaminants: Part B , (ahead-of-print):1 - 8(2014). ) ( 1 1 . J u ng S. Y . , Park J.S., Chang M.S. et al., Food Science and Biotechnology, 22,1, 241-248(2013). ) ( 12. Commission Regulation(EU), No 835/2011,215,4-7,(2011). ) ( 13. G B 2716-2005, China Standard Press, China(2005). ) ( 14. W u S., Yu W . , Food Ch e mistry, 134(1): 597-601,(2012). ) ( 15. P a ndey M. K., Mishra K. K ., Khanna S. K. e t al., Journal of t h e American Oil Chemists' Society, 81,12,113 1 -1136(2004). ) ( 16. H ong XIA. Food Science an d Technology, 6,092(2008). ) ( 17. W a ng H., Zhao L., Yang H. M. et al., Analytical Methods, (2014). ) ( 18. W ang J.H.,Guo C. , Journal of Chromatography A, 1217,28,4732- 4737(2010). ) ( 19. Lai J.P.,Niessner R., Knopp D., Analytica chimica acta,522,2,137-144 (2004). ) ( 20. K r upadam R. J., Bhagat B., Khan M S. 397(7): 3097-3106,(2010). ) ( 21. B a ltrons O., Lopez-Mesas M., Palet C. et al . , Analytical Methods, 5(22):6297-6305,(2013). ) ( 2 2. S u n F.S., Ji X.S., Zhu T. et al., Journal of University of Science andTechnology of Suzhou (Edition of engineering and technology), 4,004(2004). ) ( 23. W e iBhaar R.,Eu r opean journal of lipid s cience and technology, 104,5,282-285(2002). ) ( 24. Horwitz W., K a mps L. R., Boyer K. W., J ournal-Association of OfficialAnalytical Chemists, 63(6): 1344-1354(1980). ) extraction efficiency, and requiredless solvent volumes. Therefore, thisdeveloped method could be forroutine analysis. ACKNOWLEDGEMENTS This study was financially supportedby the Research Funds for theNational Key Technology R&DProgram in the 12th Five-Year Planof China (No. 2012BAK08B03) andthe Jiangsu Entry-Exit Inspection &Quarantine Bureau (No. 2011KJ44).The manuscript has beenprofessionally edited. We aregrateful to the staff of theTechnology Centre of PAH(ug/kg) Sample Na Ace F Phe Ant Flu Pyr BaA Chy BbF BkFBaP lcdP DahA BghiP Total Heavy Light sesame oil --- 8.16 17.00 11.00 3.52 3.08 1.201.56 --- 5.84 45.52 39.68 sesame oil 92.72 206.88 -- 61.08 131.40 127.00 -- -- 8.2811.32 0.80 0.48 639.96 619.04 20.88 sesame oil --- 23.32 8.32 33.16 1.44 18.44 3.04 0.72 3.32 0.72 --- 7.80 92.48 84.68 peanut oil 15.96-- 2.24 3.44 - 11.56 2.88 乍1.80 1.76 10.04 13.60 49.6836.08 peanut oil 10.48--- 1.68 3.52 2.84 6.72 2.40 2.083.76 33.4825.248.24 peanut oil 9.92 23.96 36.72 6.76 23.7226.92 16.84 5.28 6.00 3.007.00 mmm 166.12 150.12 16.00 soybean oil 25.76-. 8.76 22.80 0.00 0.00 1.84 1.44 0.55 0.56 61.7160.601.11 soybean oil 73.28--- 4.28 14.04 1.08 7.56 3.8 0.69 ---. 104.73 104.04 0.69 soybeanoil 48.36--- 12.36 24.00 4.88 27.08 24.20 13.60 3.96 .mm 1.68 4.84 164.96 158.44 6.52 rapeseed oil 20.76 - 3.44 5.96 0.68 2.60 1.88 2.84 1.12 2.32 1.162.92 45.68 6.40 39.28 rapeseed oil 17.96 …- 5.40 4.88 2.72 3.72 3.36 3.28 0.92 --- --- 0.70 0.70 42.9442.24 rapeseedoil 26.16 --- 34.76 5.20 5.08 4.52 7.60 -- 83.3283.32 virgin olive oil5.40 10.52 3.24 19.16 19.16 virgin olive oil4.56 8.04 --- 2.76 -- 15.36 15.36 virgin olive oil5.16 ... 9.28 0.64 --- 2.92 --- --- 18.00 18.00 Table 4. PAH content in edible oils. gro FOOD Industry Hi Tech - vol -May/June
确定


还剩2页未读,是否继续阅读?
上海安谱实验科技股份有限公司为您提供《食用油中多环芳烃检测方案 》,该方案主要用于食用植物油中营养成分检测,参考标准--,《食用油中多环芳烃检测方案 》用到的仪器有Talboys 基本型旋涡混合器、Talboys 数显型旋涡混合器、美国ORGANOMATION氮吹仪
推荐专场
相关方案
更多