方案详情
文
由荷兰Spark Holland公司生产的系列仪器“全自动在线固相萃取-液相色谱联用系统”为食品检测行业的用户带来了一种全新的样品前处理技术。该仪器与传统意义的离线手动固相萃取以及目前流行的全自动固相萃取仪器相比,具有颠覆性的创新技术含量,目前已在全球拥有超过1500家的实验室用户。自2009年进入中国市场以来,产品销量成大幅上升趋势,目前已有包括各级国家食品检验检疫机构,临床药代实验室,省级公安毒理实验室,烟草集团理化实验室,环境监测站以及中科院研究院所等超过三十家用户使用该设备进行各种检测项目和方法开发并获得了广泛的好评。
方案详情

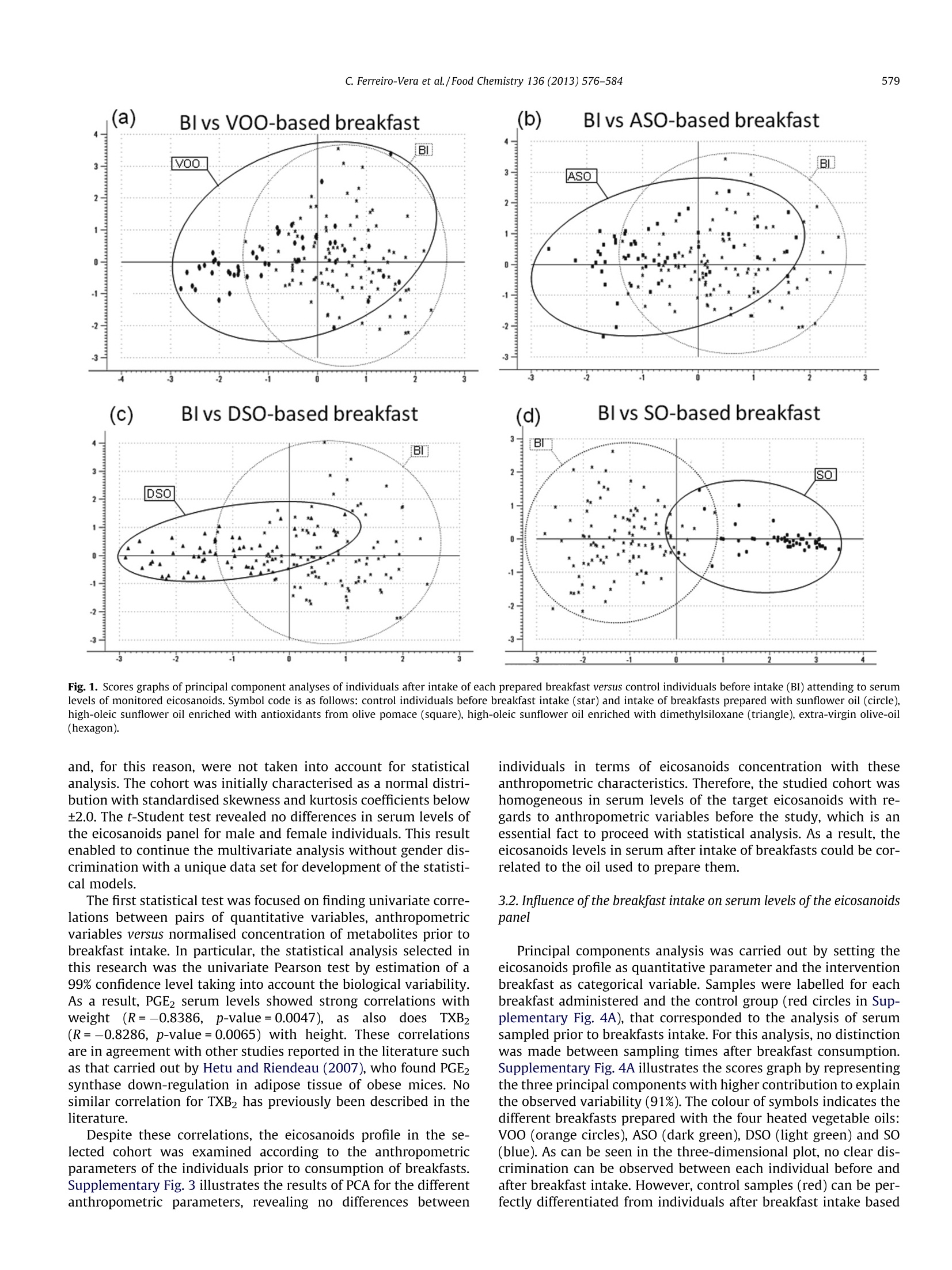
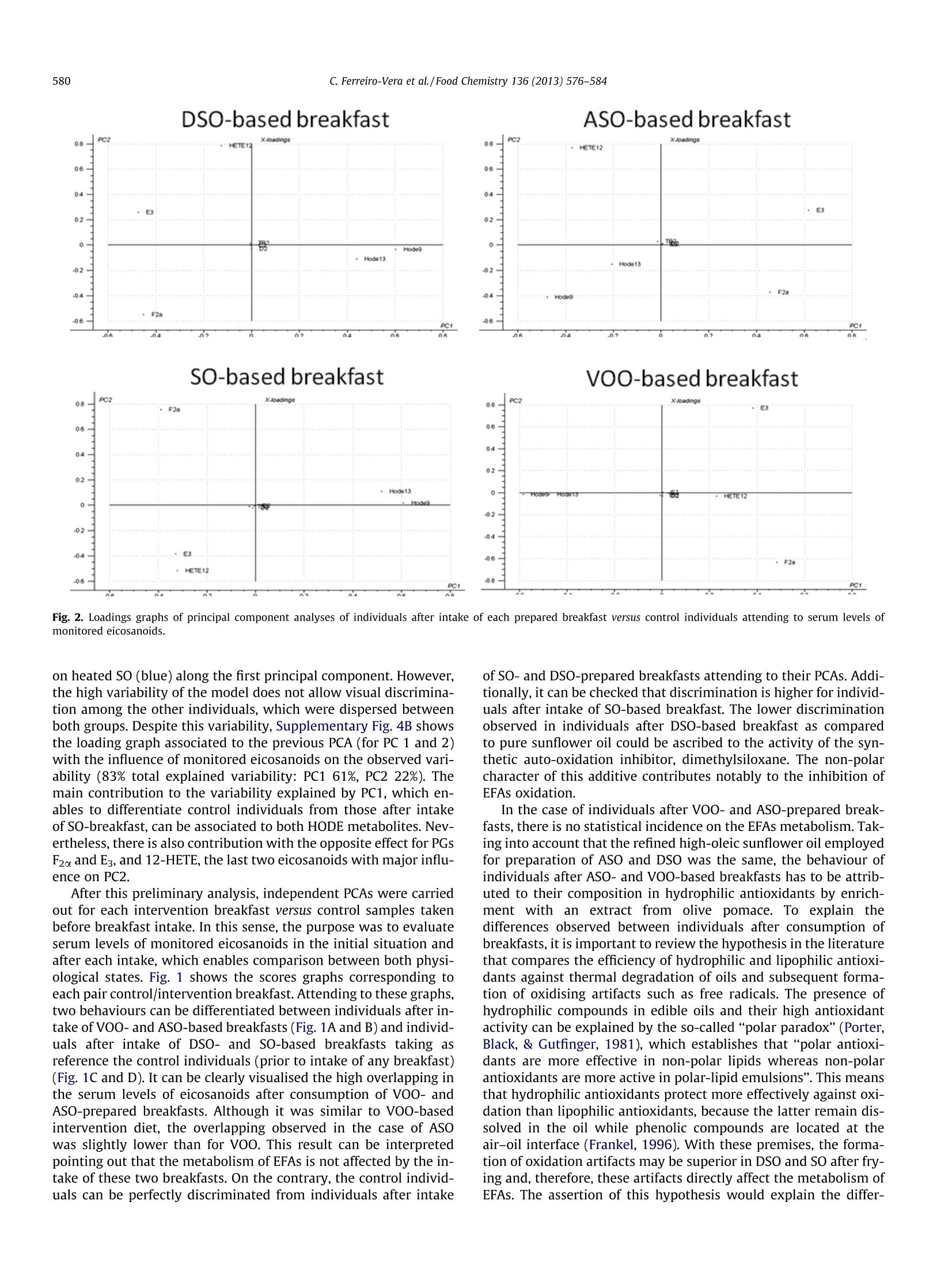
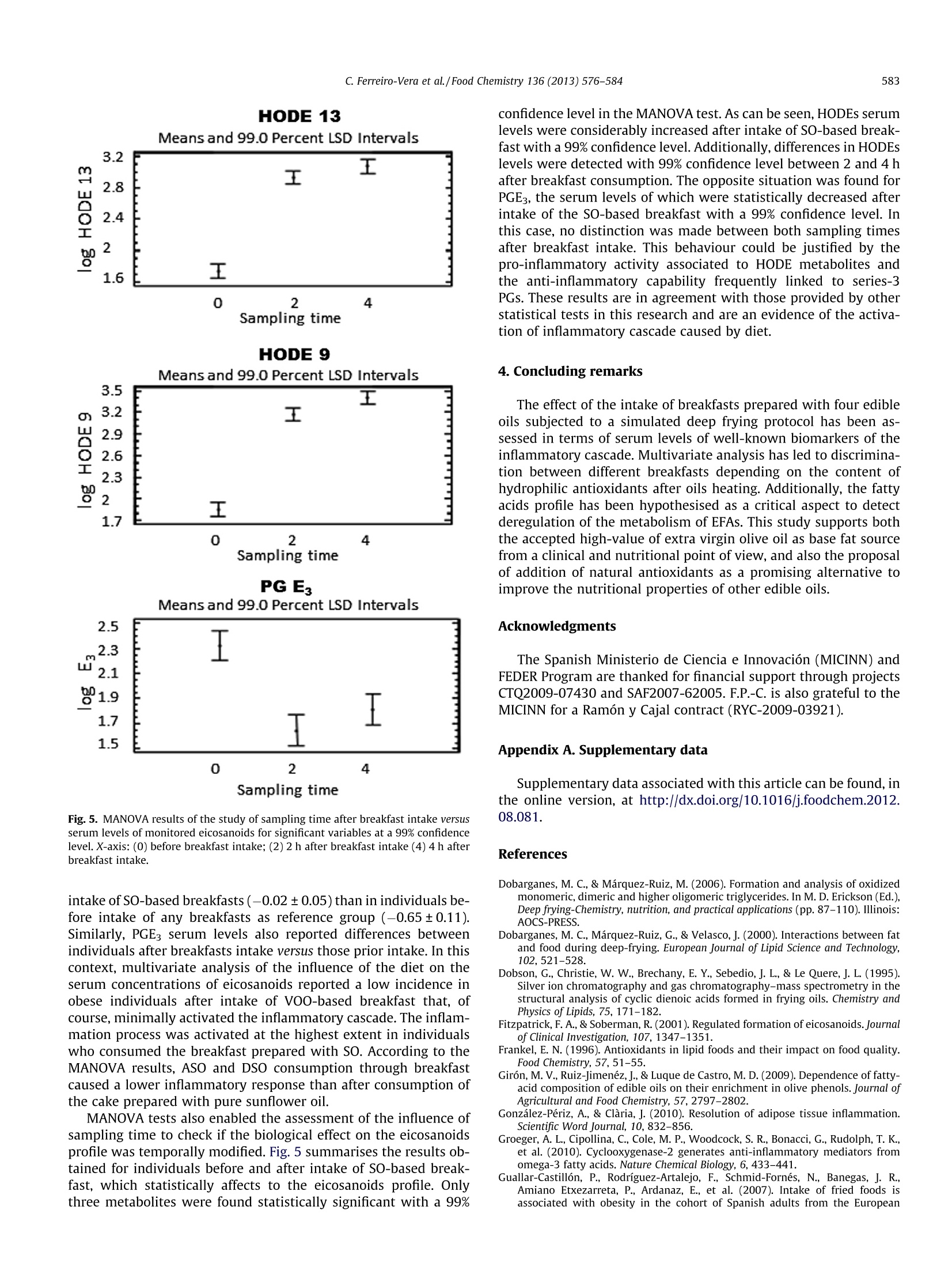
Food Chemistry 136 (2013) 576-584 577C. Ferreiro-Vera et al./Food Chemistry 136 (2013)576-584 Contents lists available at SciVerse ScienceDirect Food Chemistry ELSEVIER journal homepage: www.elsevier.com/locate/fo odchem Short-term comparative study of the influence of fried edible oils intakeon the metabolism of essential fatty acids in obese individuals carlos Ferreiro-Vera ab, Feliciano Priego-Capote a.b,* Jose M. Mata-Granados a.b,c,Maria D. Luque de Castroa,b Department of Analytical Chemistry, Annex C-3, Campus of Rabanales, University of Cordoba, E-14071 Cordoba, SpainDInstitute of Biomedical Research Maimonides (IMIBIC), Reina Sofia Hospital, University of Cordoba, E-14071 Cordoba, Spain Sanyres I+D+i Department, Sanyres Group, E-14012 Cordoba, Spain Article history:Received 16 August 2011Received in revised form 1 March 2012Accepted 31 August 2012Available online 8 September 2012 Keywords:Deep fryingDietary interventionEicosanoidsEssential fatty acidsInflammation biomarkers The effect of breakfast intake of fried oils containing natural antioxidants or a synthetic autooxidationinhibitor on the metabolism of essential fatty acids focused on obese individuals. Serum levels of eicosa-noids were compared in individuals before and after intake of different breakfasts. Univariate descriptiveanalysis was used to characterise the cohort selected for this study and multivariate analysis to revealstatistical differences of normalised eicosanoids concentrations (determined by solid-phase extractioncoupled to LC-MS/MS) depending on the edible oil used for breakfast preparation. The results showedthat the intake of breakfast prepared with pure sunflower oil subjected to deep frying causes an effectover the eicosanoids profile that enables discrimination versus the rest of individuals. The effect was asignificant increase in the concentration of hydroxyoctadecadienoic acid (HODE) metabolites, indicativemarkers of the intake of fried oils. The concentration of HODE metabolites was lower when the oil con-tained either natural antioxidants from olive-oil pomace or a synthetic autooxidation inhibitor asdimethylsiloxane. The comparison of the effect of fried sunflower oils with fried extra virgin olive oilshows the benefits associated to the consumption of the latter. @ 2012 Elsevier Ltd. All rights reserved. 1.Introduction Dietary fat plays a major nutritional role as a key source of en-ergy despite the concerns about fat intake in developed countries(fat provides 9 kcal/g of metabolizable energy versus an averageenergy of 4 kcal/g for carbohydrates and proteins). Dietary fat isalso the source of essential fatty acids (EFAs) and thus, it must bepresent in the diet. EFAs derive from two families of fatty acids,namely,n-6 and n-3 unsaturated fatty acids. Linoleic acid (LA; 18:2 n-6), which accounts for more than 50%of the fatty acids in many vegetable oils (e.g. sunflower oil), is themain n-6 fatty acid in the diet.LA accounts for between 85% and ( A bbreviations: AA, a rachidonic a cid; A SO, refined h i gh-oleic s u nflower oil enriched with a n olive-pomace e x tract of p h enols; B I , b efore i ntake; C Ox, cyclooxygenase; CYP450, cytochrome P450 m o nooxygenase; DSO, refined hi g h- o leic sunflower oil enriched with 400 pg/mL of dimethylsiloxane; E FA, e ssential f atty acid ; HETE, h ydroxyeicosatetraenoic a cid; H ODE, hydroxyoctadecadienoicacid; L A, l inoleic acid; LNA, a-linolenic a c id; L O X, lipoxygenase; LT, leu k otriene; M ANOVA, multivariate analysis of variance; PC, pr i ncipal co m ponent; PCA, prin- cipal component analysis; PG, p r ostaglandin; S F A, saturated f a tty acid; SO, r efined sunflower o il; SRM, selected reaction monitoring; TX, thromboxane; V OO, extra- virgin o live-oil. ) ( * C orresponding author a t : Department of An a lytical C h emistry, A n nex C- 3 , Campus of Rabanales, University of Cordoba,E-14071 Cor d oba, Spain. Tel . /fax: +34 957218615. ) ( E-mail address: q 72prcaf@uco.es (F. Priego-Capote). ) ( 0308-8146/$ -see front ma t ter @ 201 2 Else v ier Ltd. All rights re s erved. h ttp://dx. d oi.org / 10.1 0 16/j. f o od chem.2012.08. 0 81 ) 90% of the n-6 fatty acids in the diet with the balance coming fromarachidonic acid (AA; 20:4 n-6) and y-linolenic acid (18:3 n-6). Bycontrast, o-linolenic acid (LNA; 18:3 n-3) is a member of the n-3family of fatty acids. Like LA, o-linolenic acid is the main n-3 fattyacid in the diet, although common vegetable oils contain LNA con-centrations below 1%. Essential fatty acids are important constituents of biologicalmembranes, which surround cells and1ssubcellular particles(McDonald & Eskin, 2006). EFAs also serve as precursors of a vari-ety of biologically active compounds referred to collectively aseicosanoids (e.g. prostaglandins, thromboxanes, and leukotrienes),which are key regulators of a host of physiological reactions,including constriction and dilation of blood vessels, contractionof smooth muscle,platelet aggregation and regulation of immuneand inflammatory functions (Wang & DuBois, 2007). Eicosanoidsare formed through the action of a set of oxygenase-type enzymessuch as cyclooxygenases (COXs), lipoxygenases (LOXs), and cyto-chrome P450 monooxygenases (CYP450s). Thus, metabolism ofC-20 fatty acids by COX enzymes leads to the formation of prosta-noids, including prostaglandins (PGs) and thromboxanes (TXs),generating three series of compounds depending on the originalfatty acid (Fitzpatrick & Soberman, 2001; Peters-Golden & Brock,2003; Yang et al., 2006) (Supplementary Fig. 1). Eicosanoids ofthe 2- and3-series are ofclinical interest because they derive from two competitive pathways (n-3 and n-6), which could justify theiropposite functions, as suggested by Schmitz and Ecker (2008).Thus, 2-series eicosanoids produced from arachidonic acid possesspro-inflammatory, pro-aggregating, vasoconstriction action andimmunosuppressive properties, as recently reported by Wangand DuBois (2010). On the other hand, eicosanoids of the 3-seriesproduced from eicosapentaenoic acid have anti-inflammatory,anti-aggregating, vasodilatory and anti-arythmic actions andimmunomodulating properties (Gonzalez-Periz & Claria, 2010;Groeger et al., 2010). Lipoxygenases convert AA, LA and other PUFAs into bioactivemetabolites such as leukotrienes (LTs), hydroxyeicosatetraenoicacids (HETEs) and hydroxyoctadecadienoic acids (HODEs). LOX-catalysed products, LTs, HETEs, and HODEs also exert profoundbiological effects on inflammation processes being involved inthe development and progression of specific human cancers suchas colorectal or pancreatic cancer (Xian-Zhong, Wei-Gang, & Tho-mas,2001). Deep fat frying is one of the most common processes usedworldwide for preparation of cooked food. Complex patterns ofoxidative and thermolytic reactions take place in fats and oils dur-ing heating and deep-fat frying, including polymerisation, hydroly-sis, isomerization, and cyclisation (Dobson, Christie, Brechany,Sebedio, & Le Quere,1995; White, 1991; William & Dobson,2000). Secondary oxidation products are mainly oxidised triglycer-ide monomers, dimers, and polymers that define the thermal oxi-dised compounds of the polar material fraction (Dobarganes,Marquez-Ruiz, & Velasco, 2000; Romero, Bastida, & Sanchez-Mu-niz, 2006). Oxidation modifies the organoleptic properties of oilsand affects their shelf life, mainly owing to formation of oxidationproducts of cholesterol and phytosterols. Degradation results inloss of nutritional value of food as well as changes in its physiolog-ical properties (Saguy & Danaa, 2003; Tyagi & Vasishtha, 1996),which cause rejection from the consumers and losses to the targetindustries, as a result. The presence of antioxidants, naturally existing in (or added to)oils, exerts beneficial effects by avoiding or delaying oxidation dur-ing frying of compounds such as sterols, fatty alcohols, triterpenicdialcohols and unsaturated fatty acids. As happens with other foodadditives, natural antioxidants such as phenol compounds havedemonstrated an antioxidant activity superior to that of syntheticoxidation inhibitors; therefore, there is an increased trend to re-place the latter with natural antioxidants. The enrichment of edibleoils with phenols protects them, for example, against oxidationthat means better oil quality and prevention from the formationof toxic products such as cholesterol oxides (Dobarganes & Mar-quez-Ruiz, 2006). The aim of the present research was to evaluate the nutritionalimpact of the intake of four breakfasts prepared with oils subjectedto deep frying on the profile of eicosanoids in human serum. Thetarget metabolites were selected taking into account the directimplication of their metabolism in the inflammatory cascade. Veg-etable edible oils with natural or added content of antioxidantswere selected for this purpose. Breakfast muffins made with theseoils were ingested by 26 obese volunteers who consumed the dif-ferent muffins throughout eight weeks. Liquid-chromatographycoupled to mass detection was used to monitor eicosanoids profilein human serum extracted from all individuals at three differentsampling times. 2. Materials and methods 2.1. Oils and heating procedure The edible oils were: (1) extra-virgin olive oil (VOO) as suchwith a total natural phenols concentration of 400 mg/L, expressed as caffeic acid, 70.5% monounsaturated fatty acids (MUFAs), 11.1%PUFAs, 18.4% saturated fatty acids (SFAs);(2) refined high-oleicsunflower oil enriched with an olive-pomace extract of phenols(ASO) up to 400 mg/L, also expressed as caffeic acid, 76.7%MUFAs,17.6% PUFAs and 5.8%SFAs;(3) refined high-oleic sunflower oil en-riched with 400 mg/L of dimethylsiloxane as a synthetic oxidationinhibitor(DSO), 71.8%MUFAs, 18.0% PUFAs and 10.2% SFAs; and(4)pure refined sunflower oil (SO), 34.3% MUFAs, 58.3% PUFAs and7.3% SFAs. Koipesol (SOS Cuetara S.A., Madrid) provided the oilsfor subsequent enrichment in the laboratory. The composition ofphenolic compounds in VOO and in the extract from olive pomaceis listed in Supplementary Table 1 (Giron, Ruiz-Jimenez,& Luquede Castro, 2009). Two litres of the target oil was placed in a stainless-steel deepfryer without cover (Fagor F-206, Barcelona, Spain). The oil washeated at 180±5 ℃ for 5 min 10 times every day for two days (to-tal heating cycles: 20) with 30 min cooling intervals between heat-ing cycles. This protocol simulates a conventional use of oils forfrying. 2.2. Subjects and samples Twenty six obese individuals with a body mass index between30 and 40 kg/m formed the cohort in this study. All of them gavetheir informed consent and underwent a comprehensive medicalhistory, physical examination and clinical chemistry analysis be-fore enrolment. Participants with evidence of kidney, pancreas,lung, liver or thyroid disease were excluded. All subjects werenon-diabetics,non-smokers, without clinical manifestations of car-diovascular disease and off treatment. The target cohort was com-posed by 17 post-menopausal women, age 48-70 years, and 9men, age 39-70 years. None of the subjects was taking medicationor supplementary vitamins with influential effect on serumlipidome. All volunteers received four breakfasts in muffin format pre-pared with the four different oils (0.45mL of oil per kilogram ofbody weight), previously subjected to the simulated frying process.The administration of each breakfast was held at randomizationand cross following a Latin square design, which increased thepower of the study. The volunteers ate each breakfast every twoweeks (4 oils, 8 weeks). All steps from blood extraction to analysiswere performed in compliance with the guidelines dictated by theWorld Medical Association Declaration of Helsinki (2004), whichwere supervised by the ethical review board of Reina Sofia Hospital(Cordoba, Spain) that approved the experiments. 2.3. Serum extraction Blood extraction was planned just before breakfast intake and 2and 4 h after it. Venous blood was collected into evacuated steriletubes for whole blood haematology determination Vacutainer"(Becton Dickinson, Franklin Lakes, NJ, USA) and centrifuged at1500g 4℃ for 10 min to isolate the serum fraction (processingwithin 2 h after collection), which was placed in a plastic ware tubeand stored at -80 C until analysis. 2.4. Analytical method A fast and automatic method for quantitative analysis of PGE1,PGE2, PGE3, PGD2, PGF20,15keto-PGF2a, TXB2,9-HODE, 13-HODE,5-HETE, 8-HETE,11-HETE, 12-HETE and 15-HETE in serum sampleswas developed. The approach is based on a hyphenated systemcomposed by an SPE workstation (Prospekt-2 unit) on-line coupledto a liquid chromatograph-triple quadrupole-tandem mass-spec-trometer with an ESI source (6410 QqQ Agilent, Palo Alto, CA,USA). Briefly, 100 uL of human serum is injected in the analytical system. Then, the SPE-LC-MS/MS analysis is initiated with the fol-lowing sequence of automatic operations: (1) Solvation of the sta-tionary phase of C183 cartridge with methanol (2mL). (2)Conditioning and equilibration with water (2 mL). (3) Sample load-ing into the cartridge with water (2 mL) as carrier. Under theseconditions, the target compounds are retained in the sorbent and20% methanol aqueous solution (2 mL) is used as washing solutionto remove interferences. The chromatographic step starts byswitching the left clamp valve and eluting for 30 s the content ofthe cartridge into the chromatographic column with the isocraticmobile phase - 76:22:2:0.02 (v/v) methanol-water-acetonitrile-acetic acid - pumped by the chromatographic pump at 0.8 mL/min. The temperature of the column compartment is set at 25℃. Mass-spectrometry detection is performed in negative ESI modewith 4 kV capillary voltage, 315℃ source temperature and 60 psipressure nebulizer. Nitrogen as dessolvation gas is flowed at10 mL/min. The optimum values for parameters involved in thedetermination of target metabolites by selected reaction monitor-ing (SRM) in tandem mass spectrometry are exposed in Supple-mentary Table 2. The entire analytical process is completedwithin 30 min, which enables to synchronise the SPE protocol withthe chromatographic step. MassHunter Quantitative AnalysisWorkstation software version B04.00 from Agilent Technologieswas used to integrate peak areas of the chromatographic signalscorresponding to the target compounds. Due to the relevance of eicosanoids stability on the quality ofthe obtained results, a small experimental planning was done toassess the stability of analytes under experimental conditions.For this propose, a serum sample pool stored at -80 °C was thawedat room temperature and spiked with eicosanoids at concentration(20 ng/mL). Aliquots were analysed every hour up to 8 h. The re-sults obtained for 12-HETE,9-HODE, and PGE3 are exposed in Sup-plementary Fig. 2. No statistical variation was observed (95%confidence limit) for the target eicosanoids in the studied period,which was set as the maximum time for sequences of analyses.Therefore, antioxidants spiking was not required. 2.5. Statistical analysis A total of 312 samples were obtained from the 26 obese volun-teers who received the four breakfasts prepared with the four dif-ferent oils subjected to the simulated frying process. Blood wasextracted before breakfast intake and 2 and 4 h after it. The data for each target analyte were studied by one-variableanalysis, where standardised skewness and kurtosis were used todetermine whether the samples belong or not to a normal distribu-tion. Data distributions were log-transformed for normalisation tomeet the assumption of the statistical tests. Antropometric datanot were statistically transformed. Descriptive statistics are pre-sented as means± SD or as percentages. Univariate and multivari-ate analyses were carried out using the Statgraphics Plus softwareversion 5.10 from Manugistics (Rockville, MD, USA) and TheUnscramblerv7.6 (Oslo,Norway). 3. Results and discussion 3.1. Characteristics of the cohort selected for the study There are several events that can turn on inflammatory re-sponses. The most obvious are microbial invasion, injuries andburns. However, it is important to understand how diet can alsoactivate the same inflammatory responses. Obesity is a multifacto-rial condition resulting from improper balances of hormones andgene expression induced by the diet. It is becoming more evidentthat inflammation plays an important role in the metabolic conse-quences of obesity as well as other chronic degenerative condi-tions, as recently reported by Sears and Ricordi (2011).Inflammation is primarily driven by the production of pro-inflam-matory eicosanoids derived from AA, the levels of which are en-tirely controlled by the diet. Therefore, it is expected thatmonitoring eicosanoid metabolites, with special emphasis on AAmetabolites, can reveal some evidences about the influence ofthe diet. In fact, the intake of fried foods has been linked to obesityin a selected cohort of adult Spaniards (Guallar-Castillon et al.,2007). After preparation of the data set, the first purpose was the sta-tistical characterisation of the cohort selected for this study priorto the experiment. The anthropometric characteristics of the par-ticipants, such as age, weight, height, waist perimeter and bodymass index, depending on the individuals' gender, are exposed inTable 1. Mean, standard deviation and concentration range for eachmetabolite before the intake of the target breakfast, depending onthe individuals' gender, are also included in Table 1, which enablesto set concentration ranges for obese subjects. It is worth empha-sising that PGE1, 15keto-PGF20, 5-HETE, 8-HETE, 11-HETE and15-HETE were below their detection limits in serum samples Table 1 Anthropometric characteristics and average concentrations of the target analytes before the intervention study in the selected cohort of 17 women and 9 men (expressed asmean ± standard deviation). Serum concentrations of target metabolites are expressed as ng/mL. Parameter Mean ± SD Range Mean± SD Range Age (years) 39-63 48-70 Weight (kg) 79-125 69-120 Height (cm) 164-176 146-164 Waist perimeter (cm) 104-145 88-140 Body mass index (kg/m²) 29.4-41.3 30.7-46.9 PGE1 N.D. N.D. PGE2 0.13±0.10 0.01-0.54 0.31±0.43 0.01-2.06 PGE3 12.86±9.95 2.53-55.27 11.12±9.23 0.14-47.83 PGD2 0.33±0.27 0.07-0.87 0.61±0.45 0.17-1.33 PGF2义 12.50±8.25 2.32-55.27 12.37±8.29 2.19-44.3 15keto-PGF20 N.D. N.D. TXB2 0.43±0.48 2.32-50.78 0.75±0.90 0.01-5.87 9-HODE 7.05±4.47 1.99-23.36 7.81±4.23 0.69-26.35 13-HODE 5.99±4.57 1.94-27.23 6.96±4.16 1.5-24.73 5-HETE N.D. - N.D. - 8-HETE N.D. N.D. - 11-HETE N.D. 一 N.D. 一 12-HETE 12.54±6.96 0.98-23.44 12.35±9.76 1.78-33.95 15-HETE N.D. - N.D. 一 (b) Blvs ASO-based breakfast (d) BI vs SO-based breakfast Fig. 1. Scores graphs of principal component analyses of individuals after intake of each prepared breakfast versus control individuals before intake (BI) attending to serumlevels of monitored eicosanoids. Symbol code is as follows: control individuals before breakfast intake (star) and intake of breakfasts prepared with sunflower oil (circle),high-oleic sunflower oil enriched with antioxidants from olive pomace (square), high-oleic sunflower oil enriched with dimethylsiloxane (triangle), extra-virgin olive-oil(hexagon). and, for this reason, were not taken into account for statisticalanalysis. The cohort was initially characterised as a normal distri-bution with standardised skewness and kurtosis coefficients below±2.0. The t-Student test revealed no differences in serum levels ofthe eicosanoids panel for male and female individuals. This resultenabled to continue the multivariate analysis without gender dis-crimination with a unique data set for development of the statisti-cal models. The first statistical test was focused on finding univariate corre-lations between pairs of quantitative variables, anthropometricvariables versus normalised concentration of metabolites prior tobreakfast intake. In particular, the statistical analysis selected inthis research was the univariate Pearson test by estimation of a99% confidence level taking into account the biological variability.As a result, PGE2 serum levels showed strong correlations withweight(R=-0.8386, p-value=0.0047),asalso doesTXB,(R=-0.8286, p-value=0.0065) with height. These correlationsare in agreement with other studies reported in the literature suchas that carried out by Hetu and Riendeau (2007), who found PGE2synthase down-regulation in adipose tissue of obese mices. Nosimilar correlation for TXB2 has previously been described in theliterature. Despite these correlations, the eicosanoids profile in the se-lected cohort was examined according to the anthropometricparameters of the individuals prior to consumption of breakfasts.Supplementary Fig.3 illustrates the results of PCA for the differentanthropometric parameters, revealing no differences between individuals in terms of eicosanoids concentration with theseanthropometric characteristics. Therefore, the studied cohort washomogeneous in serum levels of the target eicosanoids with re-gards to anthropometric variables before the study, which is anessential fact to proceed with statistical analysis. As a result, theeicosanoids levels in serum after intake of breakfasts could be cor-related to the oil used to prepare them. 3.2. Influence of the breakfast intake on serum levels of the eicosanoidspanel Principal components analysis was carried out by setting theeicosanoids profile as quantitative parameter and the interventionbreakfast as categorical variable. Samples were labelled for eachbreakfast administered and the control group (red circles in Sup-plementary Fig.4A), that corresponded to the analysis of serumsampled prior to breakfasts intake. For this analysis, no distinctionwas made between sampling times after breakfast consumption.Supplementary Fig. 4A illustrates the scores graph by representingthe three principal components with higher contribution to explainthe observed variability (91%). The colour of symbols indicates thedifferent breakfasts prepared with the four heated vegetable oils:VOo (orange circles), ASO (dark green), DSO (light green) and SO(blue). As can be seen in the three-dimensional plot, no clear dis-crimination can be observed between each individual before andafter breakfast intake. However, control samples (red) can be per-fectly differentiated from individuals after breakfast intake based DSO-based breakfast SO-based breakfast VOO-based breakfast E3 HETE12 . F2a Fig. 2. Loadings graphs of principal component analyses of individuals after intake of each prepared breakfast versus control individuals attending to serum levels ofmonitored eicosanoids. on heated SO (blue) along the first principal component. However,the high variability of the model does not allow visual discrimina-tion among the other individuals, which were dispersed betweenboth groups. Despite this variability, Supplementary Fig. 4B showsthe loading graph associated to the previous PCA (for PC 1 and 2)with the influence of monitored eicosanoids on the observed vari-ability (83% total explained variability: PC1 61%, PC2 22%). Themain contribution to the variability explained by PC1, which en-ables to differentiate control individuals from those after intakeof SO-breakfast, can be associated to both HODE metabolites. Nev-ertheless, there is also contribution with the opposite effect for PGsF2o and E3, and 12-HETE, the last two eicosanoids with major influ-ence on PC2. After this preliminary analysis, independent PCAs were carriedout for each intervention breakfast versus control samples takenbefore breakfast intake. In this sense, the purpose was to evaluateserum levels of monitored eicosanoids in the initial situation andafter each intake, which enables comparison between both physi-ological states. Fig. 1 shows the scores graphs corresponding toeach pair control/intervention breakfast. Attending to these graphs,two behaviours can be differentiated between individuals after in-take of VOO-and ASO-based breakfasts (Fig. 1A and B) and individ-uals after intake of DSO-and SO-based breakfasts taking asreference the control individuals (prior to intake of any breakfast)(Fig. 1C and D). It can be clearly visualised the high overlapping inthe serum levels of eicosanoids after consumption of VOO- andASO-prepared breakfasts. Although it was similar to VOO-basedintervention diet, the overlapping observed in the case of ASOwas slightly lower than for VOO. This result can be interpretedpointing out that the metabolism of EFAs is not affected by the in-take of these two breakfasts. On the contrary, the control individ-uals can be perfectly discriminated from individuals after intake of SO-and DSO-prepared breakfasts attending to their PCAs.Addi-tionally, it can be checked that discrimination is higher for individ-uals after intake of SO-based breakfast. The lower discriminationobserved in individuals after DSO-based breakfast as comparedto pure sunflower oil could be ascribed to the activity of the syn-thetic auto-oxidation inhibitor, dimethylsiloxane. The non-polarcharacter of this additive contributes notably to the inhibition ofEFAs oxidation. In the case of individuals after VOO- and ASO-prepared break-fasts, there is no statistical incidence on the EFAs metabolism. Tak-ing into account that the refined high-oleic sunflower oil employedfor preparation of ASO and DSO was the same, the behaviour ofindividuals after ASO- and VOO-based breakfasts has to be attrib-uted to their composition in hydrophilic antioxidants by enrich-ment with an extract from olive pomace. To explain thedifferences observed between individuals after consumption ofbreakfasts, it is important to review the hypothesis in the literaturethat compares the efficiency of hydrophilic and lipophilic antioxi-dants against thermal degradation of oils and subsequent forma-tion of oxidising artifacts such as free radicals. The presence ofhydrophilic compounds in edible oils and their high antioxidantactivity can be explained by the so-called “polar paradox" (Porter,Black, & Gutfinger, 1981), which establishes that“polar antioxi-dants are more effective in non-polar lipids whereas non-polarantioxidants are more active in polar-lipid emulsions". This meansthat hydrophilic antioxidants protect more effectively against oxi-dation than lipophilic antioxidants, because the latter remain dis-solved in the oil while phenolic compounds are located at theair-oil interface (Frankel, 1996). With these premises, the forma-tion of oxidation artifacts may be superior in DSO and SO after fry-ing and, therefore, these artifacts directly affect the metabolism ofEFAs. The assertion of this hypothesis would explain the differ- Fig. 3. Scores graphs of principal component analyses of individuals after intake of each prepared breakfast versus control individuals attending to serum levels of monitoredeicosanoids. Distinction was made between the two sampling times after breakfast intake. Symbol code is as follows: control individuals before breakfast intake (circle) andsampling time 2 h after breakfast intake (square), sampling time 4 h after breakfast intake (triangle). ences in serum levels of individuals after consumption of the fourbreakfasts prepared with fried edible oils. Similar results werefound by Quiles et al.(2002) by analysis of liver microsomes fromrats exposed to the intake of fried sunflower and olive oils. The lev-els of lipid peroxidation in rats after intake of fried sunflower oilwere higher than after intake of olive oil. Scores graphs plotted in Fig.1 were complemented with loadinggraphs in these PCA tests for individual breakfasts. Fig. 2 presentsthe loadings graphs obtained by qualitative data analysis of controlindividuals together with individuals after intake of SO- and DSO-prepared breakfasts. Similarly to Supplementary Fig. 4B, the influ-ence of eicosanoids can be evaluated from these graphs. Thus, inthe case of intervention breakfasts based on SO y DSO with a cleardiscrimination from control individuals, this variability, expressedmainly along PC1, can be equally ascribed to HODE metaboliteswith an opposite trend to other less significant metabolites suchas 12-HETE, PGs F2o and E3. On the contrary, no contribution tothe statistical model can be attributed to TBX2 and PGD2. Eicosanoids are synthesised by the action of two different en-zymes,COX and LOX, and are derived from two different biosyn-thetic pathways: n-6 and n-3. The two compounds with highercontribution to explain the variability of the model, HODE metab-olites, are synthesised from linoleic acid (C18:2n-6) by activity ofan LOX enzyme. Linoleic acid is the main fatty acid present in sun-flower oil as triglyceride, with concentrations normally above 50%(w|w). However, this concentration falls down in high-oleic sun-flower oil to values of 20%(w/w) or below. Therefore, linoleic acidpasses to a second place in high-oleic sunflower oil being its posi-tion occupied by oleic acid. With these premises,a second hypoth- esis to complete the discussion dealing with the role of hydrophilicantioxidants is the source of EFAs in the diet. Thus, the precursorfor biosynthesis of HODE metabolites, linoleic acid, is more concen-trated in the pure sunflower oil used to prepare the SO-basedbreakfast than in the high-oleic sunflower oil used to prepare theDSO-based breakfast. In summary, the levels of linoleic acid inthe edible oil used for preparation of breakfast could support thediscrimination between individuals after intake of SO- and DSO-prepared breakfasts. The up-regulation in the synthesis of HODEmetabolites seems to be accompanied by a down-regulation ofthe activity of LOX and COX enzymes using arachidonic acid assubstrate. This could explain the apparent contrary activity of12-HETE and PGF2a. The deregulation of the biosynthetic pathwaysnot only affects to the n-6 route, but also to the n-3 route. In fact,an opposite effect of PGE3 metabolites versus HODE metaboliteswas also found as a consequence of the intake of SO-and DSO-pre-pared breakfasts. This result could be explained by the inflamma-tory properties attributed to eicosanoids synthesised in n-6 andn-3 pathways, pro-inflammatory and anti-inflammatory proper-ties, respectively. 3.3. Influence of sampling time after intake of breakfasts prepared withfried edible oils After comparison of individuals subjected to interventionbreakfasts versus control physiological state, the next study wasaimed at evaluating the sampling time, defined as the intervalwithin breakfast intake and blood extraction. Two sampling timeswere set, 2 and 4 h, to assess the initial response as a consequence HODE 13 PGE3 Means and 99,0 Percent LSD Intervals Fig. 4. MANOVA results of the study breakfast type versus serum levels of monitored eicosanoids for significant variables at a 99% confidence level. X-axis: (1) SO-basedbreakfast; (2) DSO-based breakfast; (3) ASO-based breakfast; (4)VOO-based breakfast; and (5)before breakfast intake. of intake. Fig. 3 shows the scores graphs obtained after PCAs ofeicosanoids levels in serum. In this case, serum analysis of individ-uals prior to breakfast intake is plotted by circles. Squares and tri-angles are used for serum analysis after 2 and 4 h, respectively, ofbreakfast intake. Similarly to the results in Fig. 1, a high overlap-ping is observed with VOO- and ASO-breakfasts. Attending to theresults obtained by PCAs in the evaluation of the sampling time,there are not significant differences in the serum levels of eicosa-noids 2 and 4h after the intake of breakfasts. Therefore, the timescale selected for this experimental planning is not enough to havean impact on the serum levels of eicosanoids for the two samplingtimes selected. 3.4. Multivariate analysis of variance (MANOVA) General linear multivariate models were used to evaluate theinfluence of breakfast intake and sampling time together withanthropometric variables on the serum concentration of targetmetabolites as response variables. Breakfasts were categorisedaccording to type of oils used to prepare the muffins as VOO-,ASO-, DSO-, and SO-breakfast. Time for serum extraction was cate-gorised as previous to the intake, 2 or 4 h after it. Analysis of vari-ance with a lack-of-fit test was used to determine whether themodel was adequate to describe the observed data. Statistical sig-nificance was defined as a p value less than 0.05. A multivariatemodel variance analysis (MANOVA) was used to evaluate theparameters that may have an effect on the eicosanoids concentra-tion. Fisher’s least significant difference procedure was used to dis-criminate among the means of eicosanoids concentration after the different breakfasts in MANOVA studies for 99% of confidence level.Fig. 4 summarises the MANOVA results obtained for those signifi-cant variables correlated with breakfast intake that coincide withthose deregulated eicosanoids (9-HODE, 13-HODE, PGs D2 and E3)according to PCA loadings graphs excepting for PGF2a. As can be: of1seen, serum concentrations of HODEs were clearly increased inindividuals after SO-breakfast intake versus individuals after intakeof breakfasts with the other oils. Means of logarithm values (log) of13-HODE serum levels in obese individuals after SO-based break-fast were statistically higher (1.84±0.04) than in the other break-fasts with a 99% confidence level. Additionally, logarithmic valuesof 13-HODE serum levels were not significantly different in individ-uals treated with the DSO diet (0.98±0.04), ASO diet (1.19±0.04)and VOO diet (1.09±0.04) with a confidence level of 99%. The sametrend was observed for 9-HODE, the logarithmic levels of whichwere statistically higher in obese individuals after intake of SO pre-pared breakfast(1.89±0.04) as compared to the other individualswith a 99% confidence level. Logarithmic values of 9-HODE serumlevels were significant higher in individuals after intake of ASO-based breakfast (1.32±0.04) versus individuals after intake ofDSO-based breakfast (1.17±0.04)and VOO-based breakfast(1.13±0.04), with a confidence level of 99%. Thus, serum levels ofHODE metabolites in SO-based breakfast reveal that LOX enzymeusing linoleic acid as substrate is up-regulated after intake of thisprepared breakfast. By contrast, no discrimination was observedafter intake of DSO-, ASO- and VOO-based breakfasts, althoughthe eicosanoid profile versus control individuals was affected. For the rest of metabolites, differences in serum levels were de-tected for PGD2 with a confidence level of 99% in individuals after HODE 13Means and 99.0 Percent LSD Intervals 3.2 工 2.8 2.4 2 Fig.5. MANOVA results of the study of sampling time after breakfast intake versusserum levels of monitored eicosanoids for significant variables at a 99% confidencelevel. X-axis: (0) before breakfast intake; (2) 2h after breakfast intake (4) 4 h afterbreakfast intake. intake of SO-based breakfasts (-0.02±0.05) than in individuals be-fore intake of any breakfasts as reference group (-0.65±0.11).Similarly, PGEs serum levels also reported differences betweenindividuals after breakfasts intake versus those prior intake. In thiscontext, multivariate analysis of the influence of the diet on theserum concentrations of eicosanoids reported a low incidence inobese individuals after intake of VOO-based breakfast that, ofcourse, minimally activated the inflammatory cascade. The inflam-mation process was activated at the highest extent in individualswho consumed the breakfast prepared with SO. According to theMANOVA results, ASO and DSO consumption through breakfastcaused a lower inflammatory response than after consumption ofthe cake prepared with pure sunflower oil. MANOVA tests also enabled the assessment of the influence ofsampling time to check if the biological effect on the eicosanoidsprofile was temporally modified. Fig. 5 summarises the results ob-tained for individuals before and after intake of SO-based break-fast, which statistically affects to the eicosanoids profile. Onlythree metabolites were found statistically significant with a 99% confidence level in the MANOVA test. As can be seen,HODEs serumlevels were considerably increased after intake of SO-based break-fast with a 99% confidence level. Additionally, differences in HODEslevels were detected with 99% confidence level between 2 and 4 hafter breakfast consumption. The opposite situation was found forPGE, the serum levels of which were statistically decreased afterintake of the SO-based breakfast with a 99% confidence level. Inthis case, no distinction was made between both sampling timesafter breakfast intake. This behaviour could be justified by thepro-inflammatory activity associated to HODE metabolites andthe anti-inflammatory capability frequently linked to series-3PGs. These results are in agreement with those provided by otherstatistical tests in this research and are an evidence of the activa-tion of inflammatory cascade caused by diet. 4. Concluding remarks The effect of the intake of breakfasts prepared with four edibleoils subjected to a simulated deep frying protocol has been as-sessed in terms of serum levels of well-known biomarkers of theinflammatory cascade. Multivariate analysis has led to discrimina-tion between different breakfasts depending on the content ofhydrophilic antioxidants after oils heating. Additionally, the fattyacids profile has been hypothesised as a critical aspect to detectderegulation of the metabolism of EFAs. This study supports boththe accepted high-value of extra virgin olive oil as base fat sourcefrom a clinical and nutritional point of view, and also the proposalof addition of natural antioxidants as a promising alternative toimprove the nutritional properties of other edible oils Acknowledgments The Spanish Ministerio de Ciencia e Innovacion (MICINN) andFEDER Program are thanked for financial support through projectsCTQ2009-07430 and SAF2007-62005. F.P.-C. is also grateful to theMICINN for a Ramon y Cajal contract (RYC-2009-03921). Appendix A. Supplementary data Supplementary data associated with this article can be found, inthe online version, at http://dx.doi.org/10.1016/j.foodchem.2012.08.081. References Dobarganes, M. C., & Marquez-Ruiz, M. (2006). Formation and analysis of oxidizedmonomeric, dimeric and higher oligomeric triglycerides. In M. D. Erickson (Ed.),Deep frying-Chemistry, nutrition, and practical applications (pp. 87-110). Illinois:AOCS-PRESS. Dobarganes, M. C., Marquez-Ruiz, G., & Velasco,J.(2000). Interactions between fatand food during deep-frying. European Journal of Lipid Science and Technology,102, 521-528. Dobson, G., Christie, W. W., Brechany, E. Y., Sebedio,J. L., & Le Quere, J. L. (1995).Silver ion chromatography and gas chromatography-mass spectrometry in thestructural analysis of cyclic dienoic acids formed in frying oils. Chemistry andPhysics of Lipids, 75,171-182. Fitzpatrick, F. A., & Soberman, R. (2001). Regulated formation of eicosanoids. Journalof Clinical Investigation, 107, 1347-1351. Frankel, E.N.(1996). Antioxidants in lipid foods and their impact on food quality.Food Chemistry, 57,51-55. Giron, M.V., Ruiz-Jimenéz, J., & Luque de Castro, M. D.(2009). Dependence of fatty-acid composition of edible oils on their enrichment in olive phenols. Journal ofAgricultural and Food Chemistry, 57, 2797-2802. Gonzalez-Périz, A., & Claria,J. (2010). Resolution of adipose tissue inflammation.Scientific Word Journal, 10,832-856. Groeger, A. L., Cipollina, C., Cole, M. P., Woodcock, S. R., Bonacci, G., Rudolph, T. K.,et al. (2010). Cyclooxygenase-2 generates anti-inflammatory mediators from. omega-3 fatty acids. Nature Chemical Biology, 6, 433-441. Guallar-Castillon, P., Rodriguez-Artalejo, F., Schmid-Fornes, N., Banegas,J. R.,Amiano Etxezarreta, P., Ardanaz, E., et al. (2007). Intake of fried foods isassociated with obesity in the cohort of Spanish adults from the European prospective investigation into cancer and nutrition. American Journal of ClinicalNutrition, 86, 198-205. Hetu, P. O., &Riendeau, D. (2007).Down-regulation of microsomal prostaglandin E2synthase-1 in adipose tissue by high-fat feeding. Obesity, 15, 60-68. McDonald, B. E., & Eskin, M. N. A. (2006). Role of fat in the diet. In M. D. Erickson(Ed.), Deep frying-Chemistry, nutrition, and practical applications (pp. 167-171).Illinois: AOCS-PRESS. Peters-Golden, M., & Brock, T. G. (2003). 5-Lipoxygenase and FLAP. ProstaglandinsLeukotrienes and Essential Fatty Acids, 69, 99-109. Porter, W. L., Black, E. D., & Gutfinger, T. (1981). Polyphenols in olive oils. Journal ofthe American Oil Chemists’ Society, 58, 966-968. Quiles, J. R., Huertas, J. R., Battino, M., Ramirez-Tortosa, M. C.,Cassinello, M., Mataix,J., et al. (2002). The intake of fried virgin olive or sunflower oils differentiallyinduces oxidative stress in rat liver microsomes. British Journal of Nutrition, 88,57-65. Romero, A., Bastida, S., & Sánchez-Muniz, F. J. (2006). Cyclic fatty acid monomerghnformation in domestic frying of frozen foods in sunflower oil and high oleic acidsunflower oil without oil replenishment. Food and Chemical Toxicology, 44,1674-1681. Saguy, I. S., & Danaa,D. (2003). Integrated approach to deep fat frying: Engineering,nutrition, health and consumer aspects. journal of Food Engineering, 56,143-152. Schmitz, G., & Ecker, J. (2008). The opposing effects of n-3 and n-6 fatty acids.Progress in Lipid Research, 47, 147-155. Sears, B. & Ricordi, c. (2011). Anti-inflammatory nutrition as a pharmacologicalapproach to treat obesity. Journal of Obesity, article ID 431985, 14 pages. Tyagi, V. K.,& Vasishtha, A. K. (1996). Changes in the characteristics andcomposition of oils during deep-fat frying. Journal of the American OilChemists’Society, 73,499-506. Wang, D., & DuBois, R. N. (2007). Measurement of eicosanoids in cancer tissues.Methods in Enzymology, 433, 27-50. Wang, D., & DuBois, R. N. (2010). Eicosanoids and cancer. Nature Reviews Cancer, 10,181-193. White, P. J. (1991). Methods for measuring changes in deep-fat frying oils. FoodTechnology, 45,75-80. William, W. C., & Dobson,G. (2000). Formation of cyclic fatty acids during the fryingprocess. European Journal of Lipid Science and Technology, 102,515-520. Xian-Zhong, D., Wei-Gang, T., & Thomas, E. A. (2001). Cyclooxygenases andlipoxygenases as potential targets for treatment of pancreatic cancer.Pancreatology, 1, 291-299. Yang, P., Chan,D., Felix, E., Madden,T., Klein, R. D.,Shureiqi, I., Chen, X.,Dannenberg,A. J., & Newman, R. A. (2006). Determination of endogenous tissue inflammationprofiles by LC/MS/MS: COX- and LOX-derived bioactive lipids. ProstaglandinsLeukotrienes and Essential Fatty Acids, 75, 385-395. 由荷兰Spark Holland公司生产的系列仪器“全自动在线固相萃取-液相色谱联用系统”为食品检测行业的用户带来了一种全新的样品前处理技术。该仪器与传统意义的离线手动固相萃取以及目前流行的全自动固相萃取仪器相比,具有颠覆性的创新技术含量,目前已在全球拥有超过1500家的实验室用户。自2009年进入中国市场以来,产品销量成大幅上升趋势,目前已有包括各级国家食品检验检疫机构,临床药代实验室,省级公安毒理实验室,烟草集团理化实验室,环境监测站以及中科院研究院所等超过三十家用户使用该设备进行各种检测项目和方法开发并获得了广泛的好评。
确定






还剩7页未读,是否继续阅读?
仪真分析仪器有限公司为您提供《食用油中脂肪酸代谢物检测方案(固相萃取仪)》,该方案主要用于食用植物油中理化分析检测,参考标准--,《食用油中脂肪酸代谢物检测方案(固相萃取仪)》用到的仪器有超高压液相在线SPE色谱联用系统CHRONECT Symbiosis
推荐专场
相关方案
更多
该厂商其他方案
更多
















