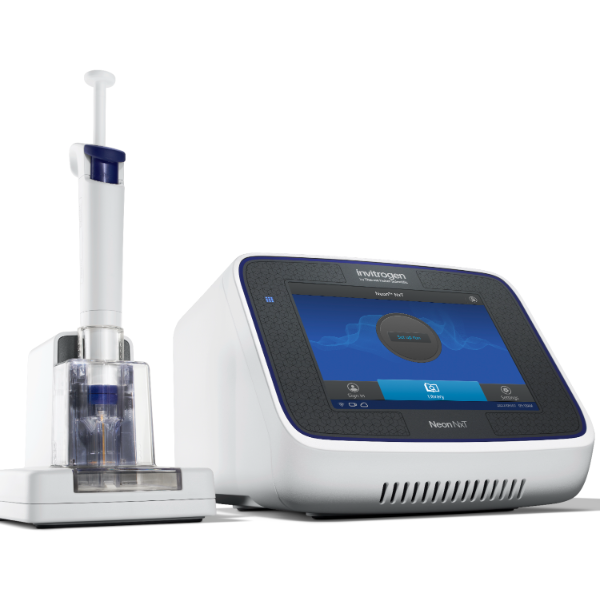
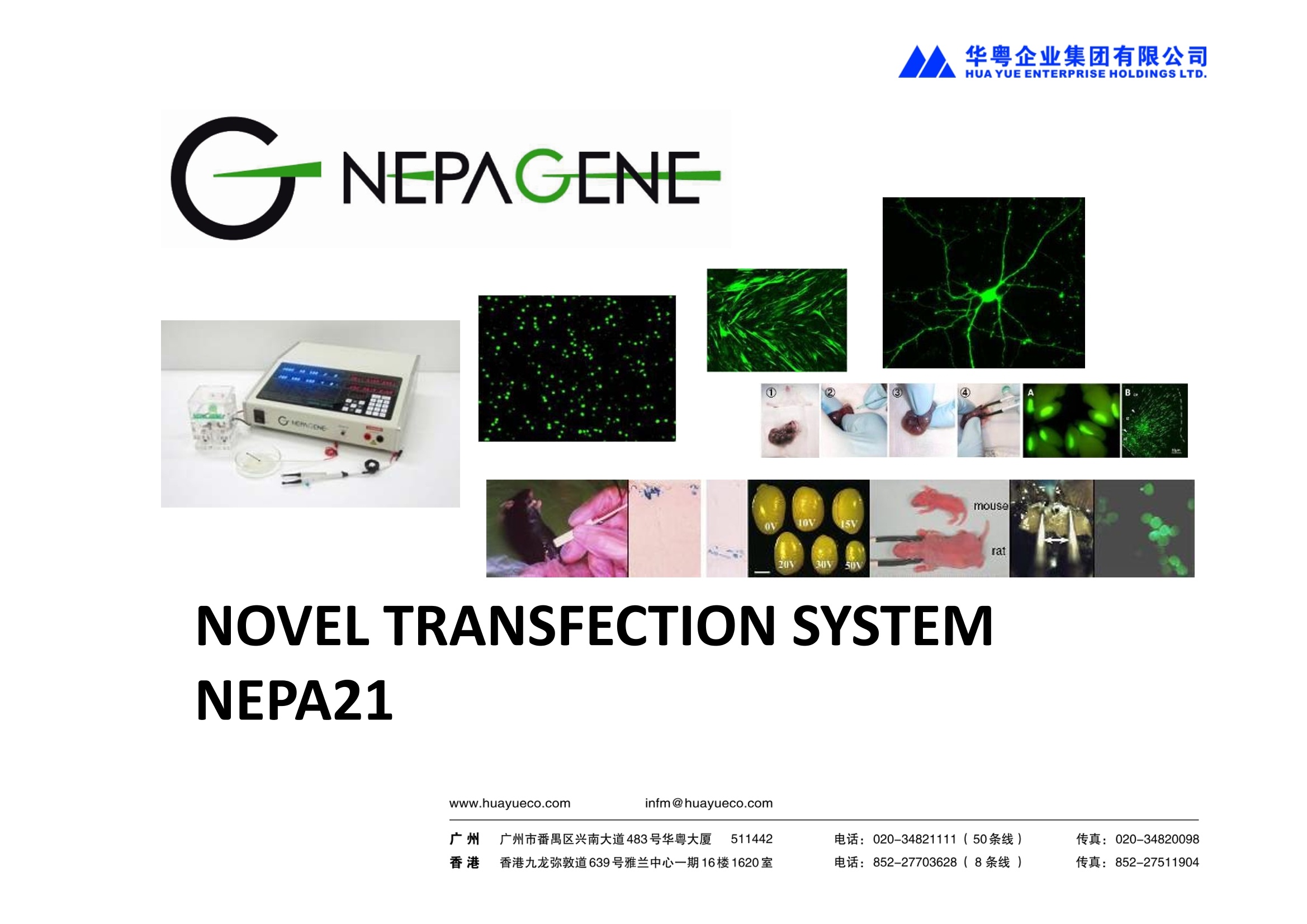
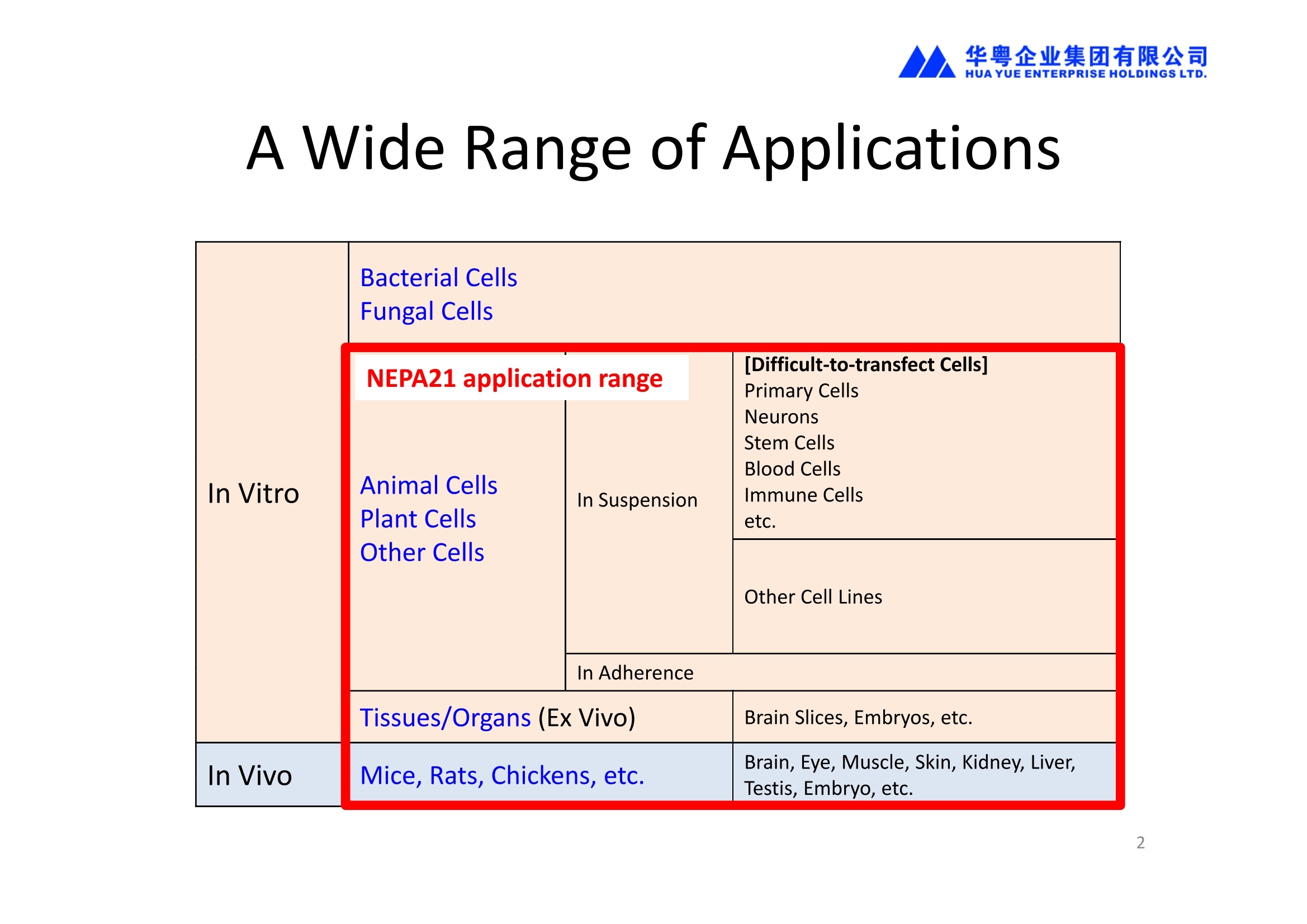
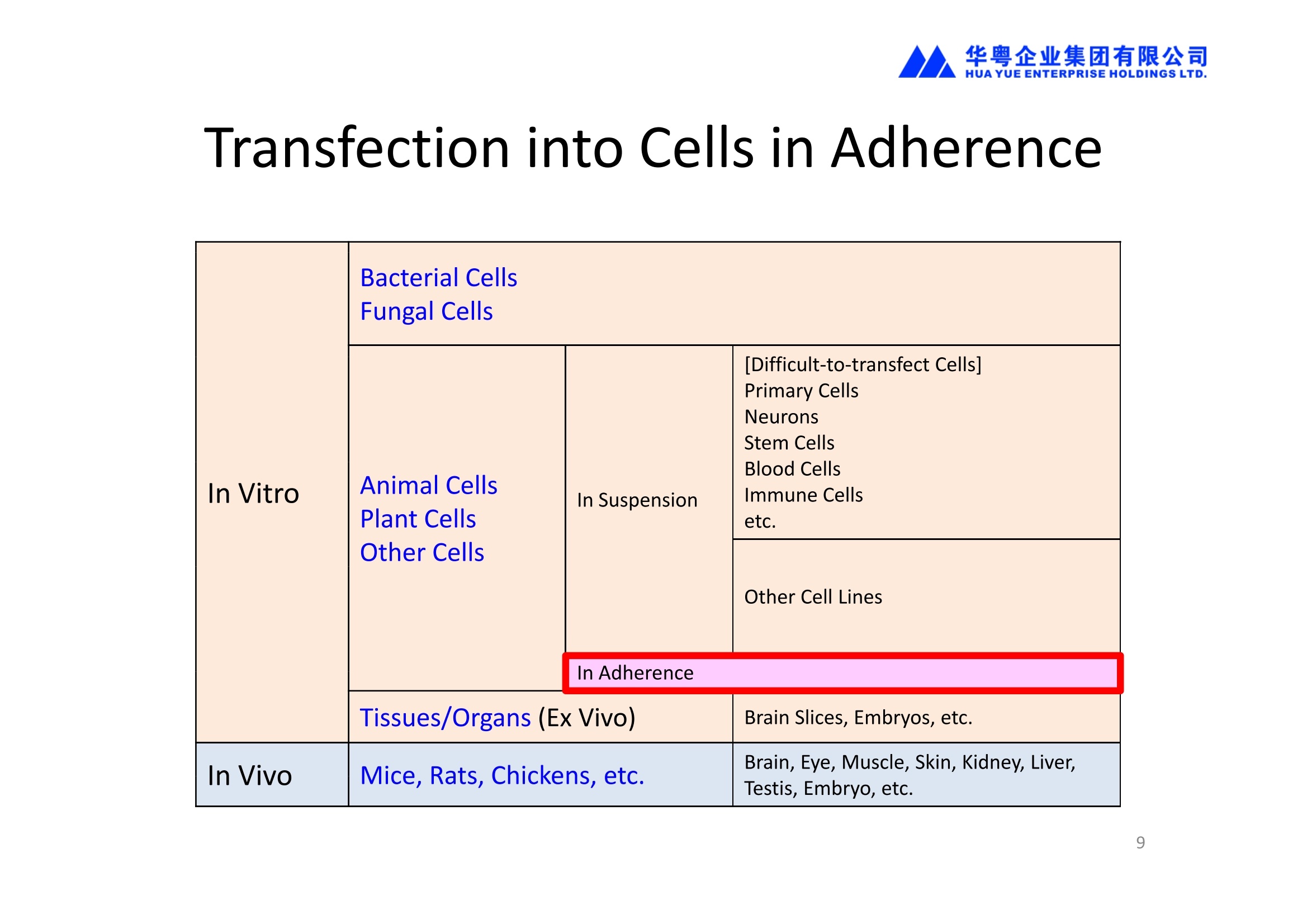
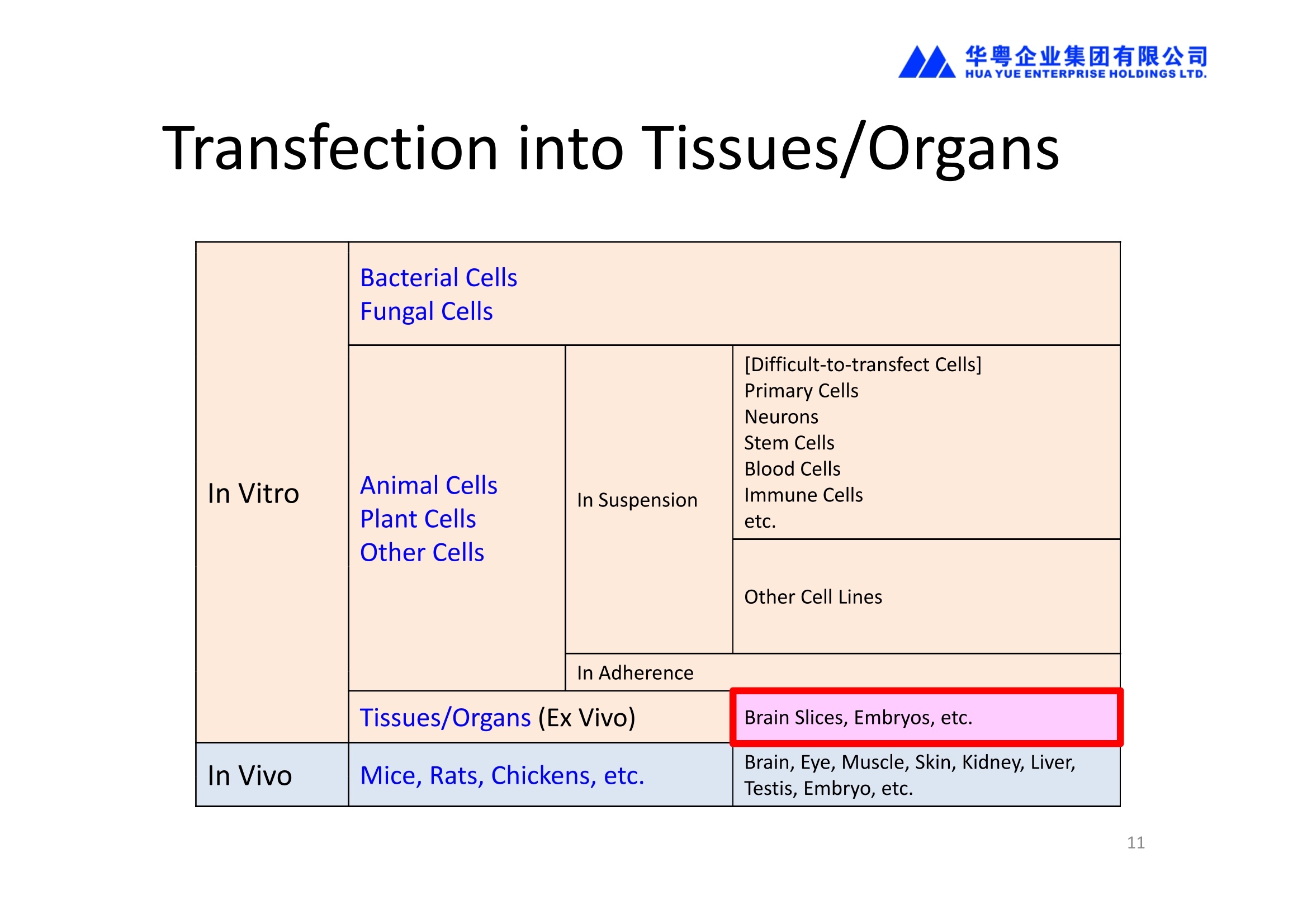
NEPA21高校基因转染的应用范围十分广泛:
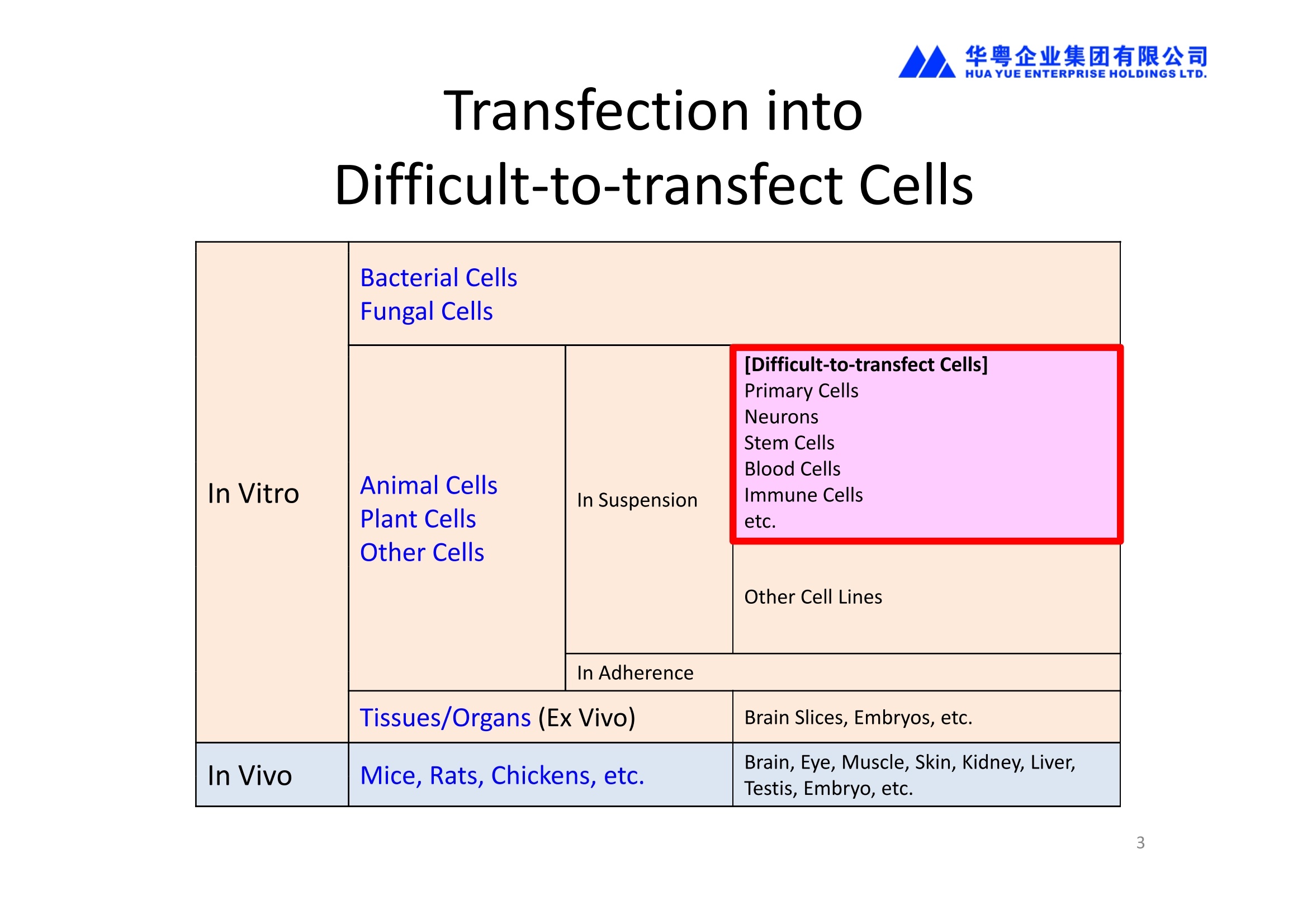
1、能对真核细胞进行快速、高效、高存活率的基因转染(In Vitro Transfection),特别针对难转染的细胞,如原代细胞、神经细胞、干细胞、悬浮细胞等提供细胞特异性的实验方案(protocol)共享服务
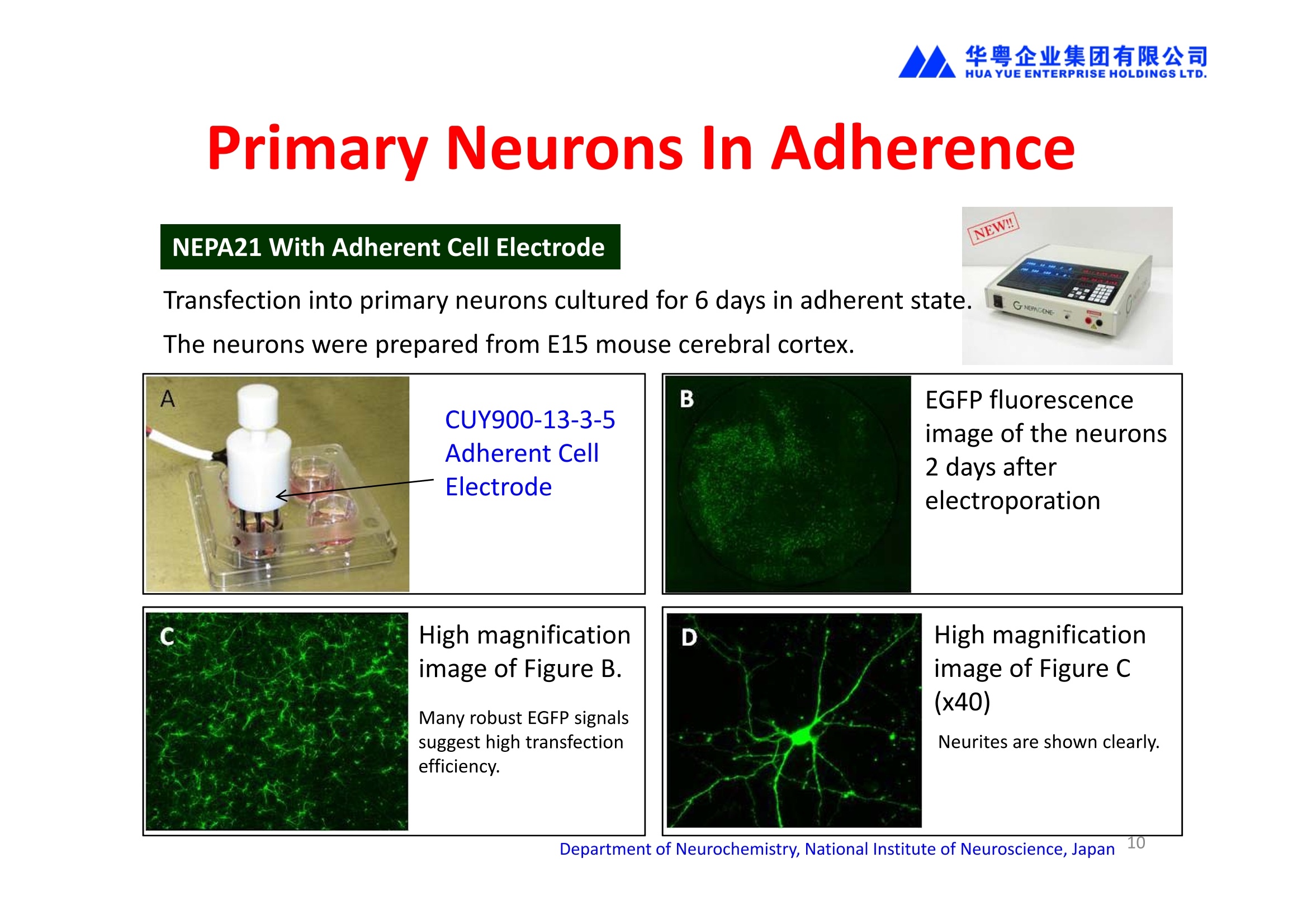
2、能对贴壁状态的细胞直接进行转染,无需经过细胞消化、悬浮的过程,增加了细胞存活率和实验的简便性。
3、能对离体的组织或器官进行转染(Ex Vivo Transfection)。
4、能进行活体动物的基因转染(In Vivo Transfection)。
使用NEPA21进行基因转染,全过程为非病毒介导,具有安全高效的特性,应用于活体转染时,更具有转染部位特异性高的优点;
NEPA21进行细胞转染时,不依赖特殊的转染试剂,而是通过电场作用直接将外源基因(包括DNA、RNA及siRNA等)导入到目标细胞的细胞浆和细胞核中,运维成本低。
方案详情

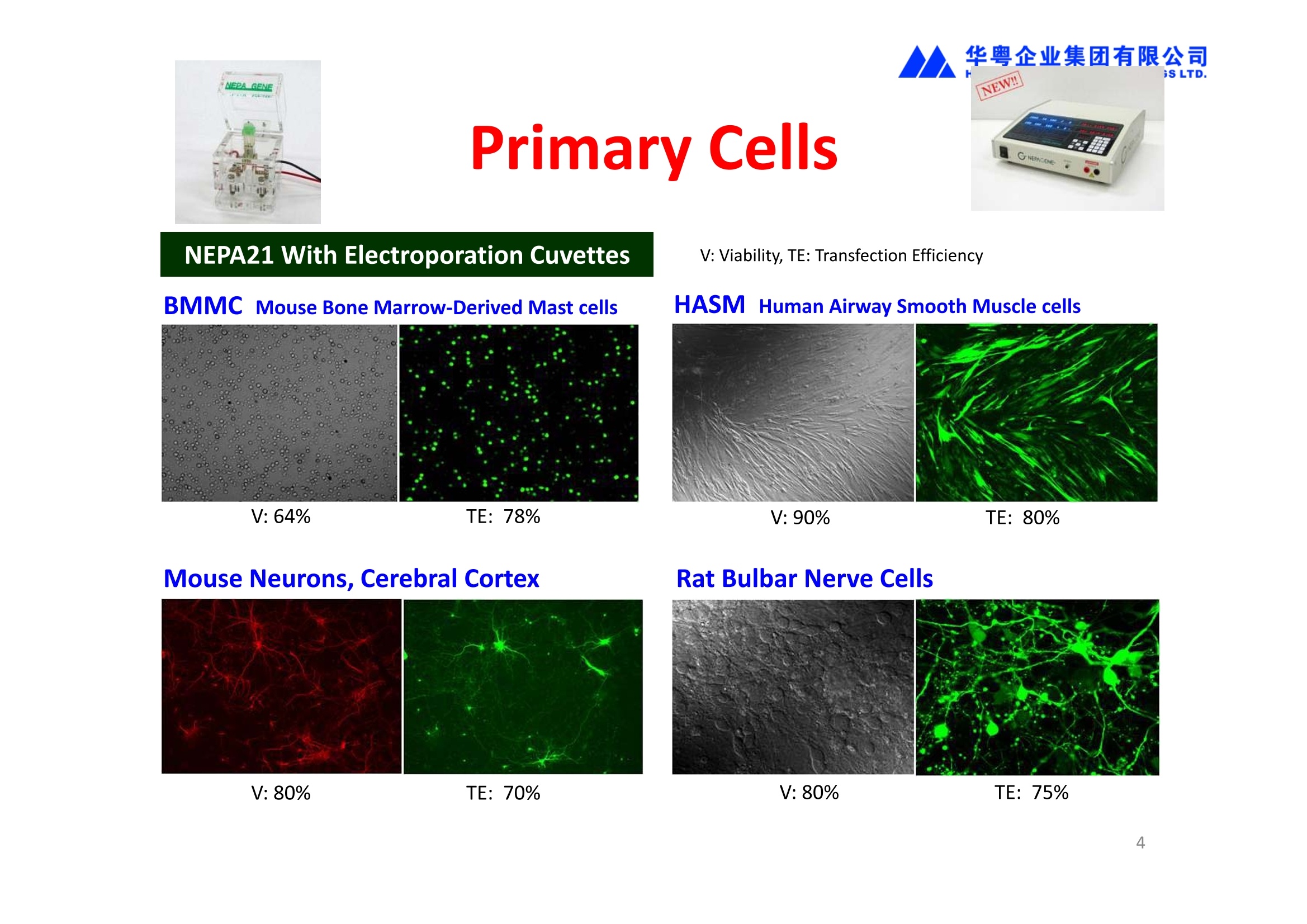
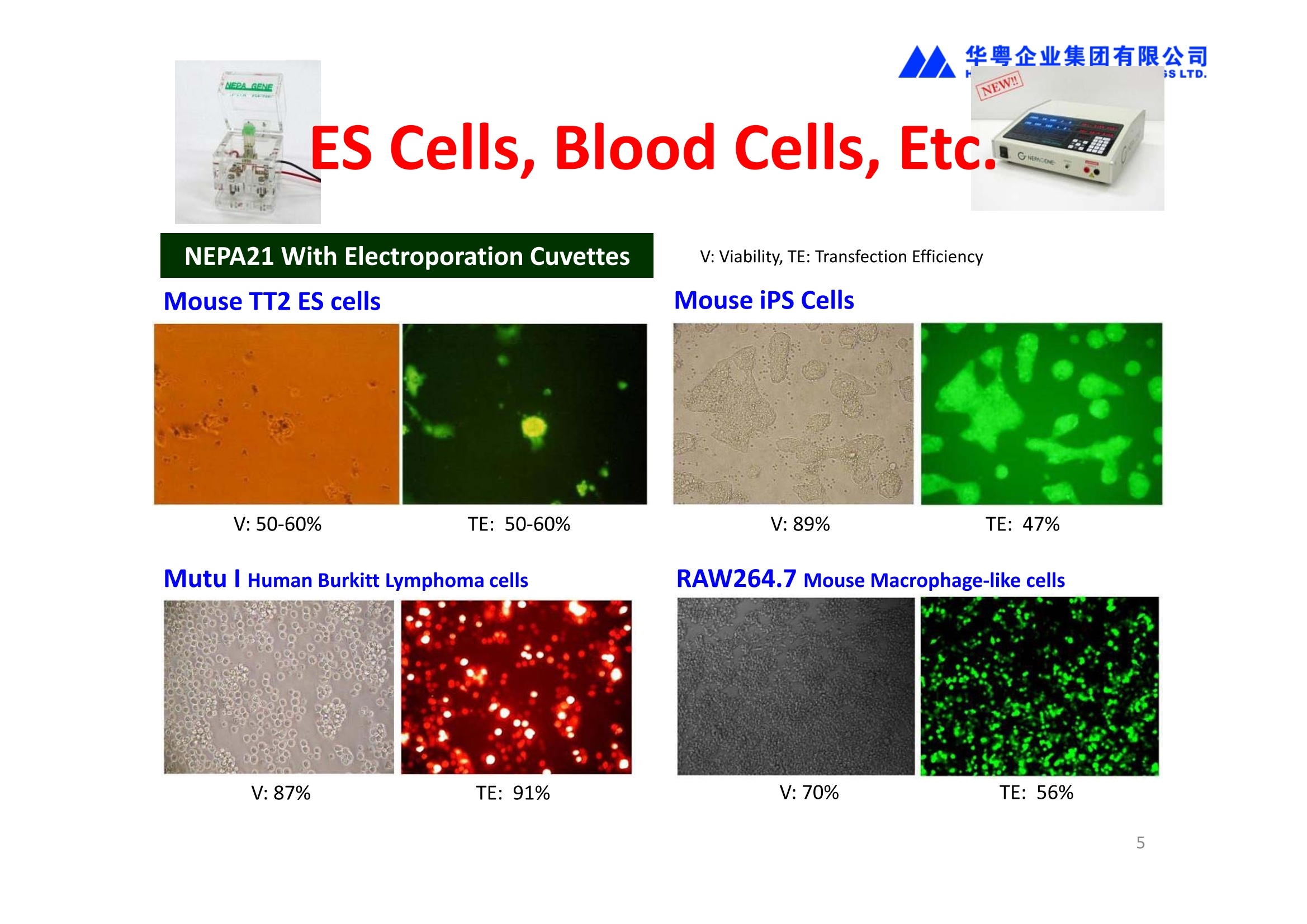
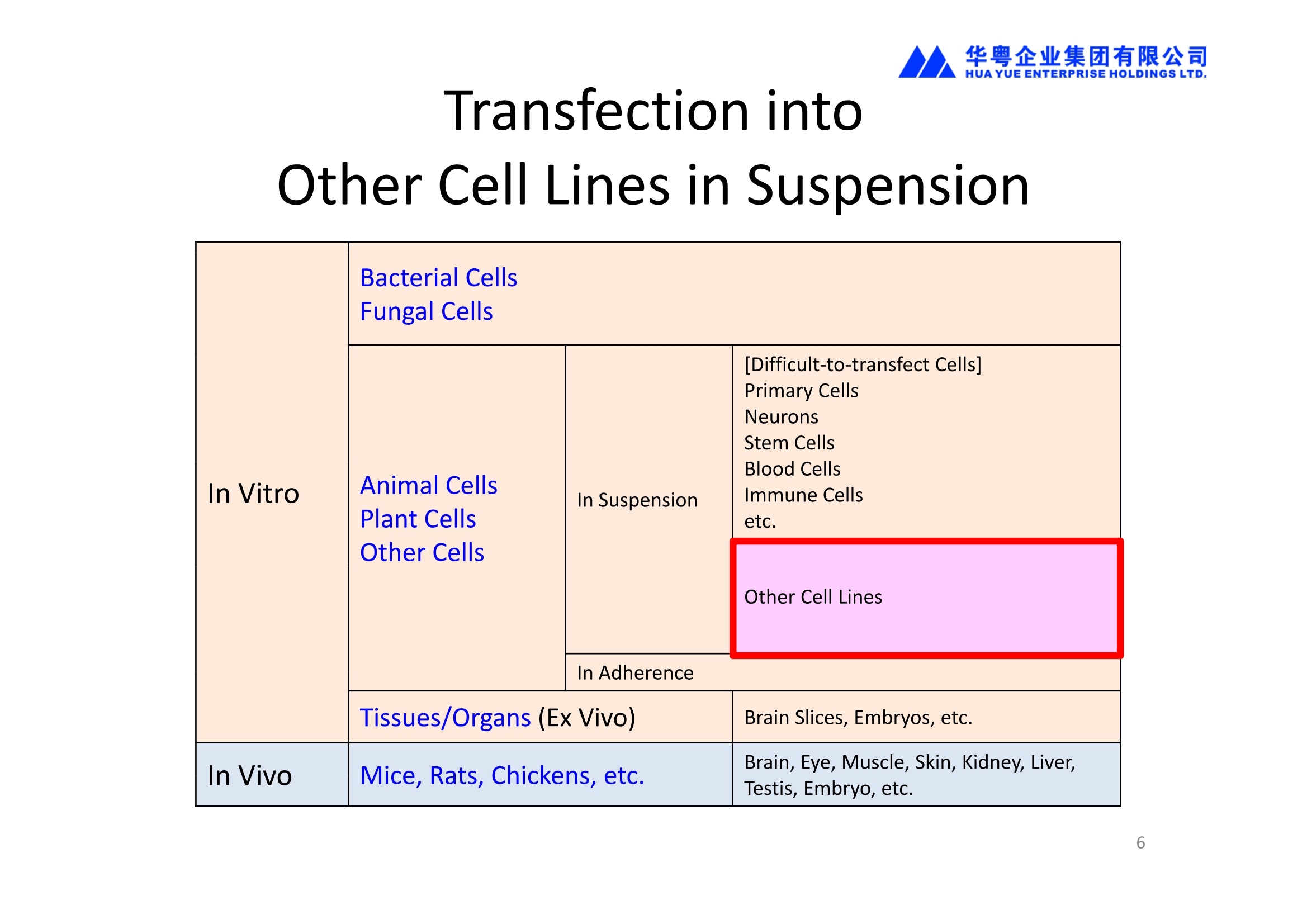
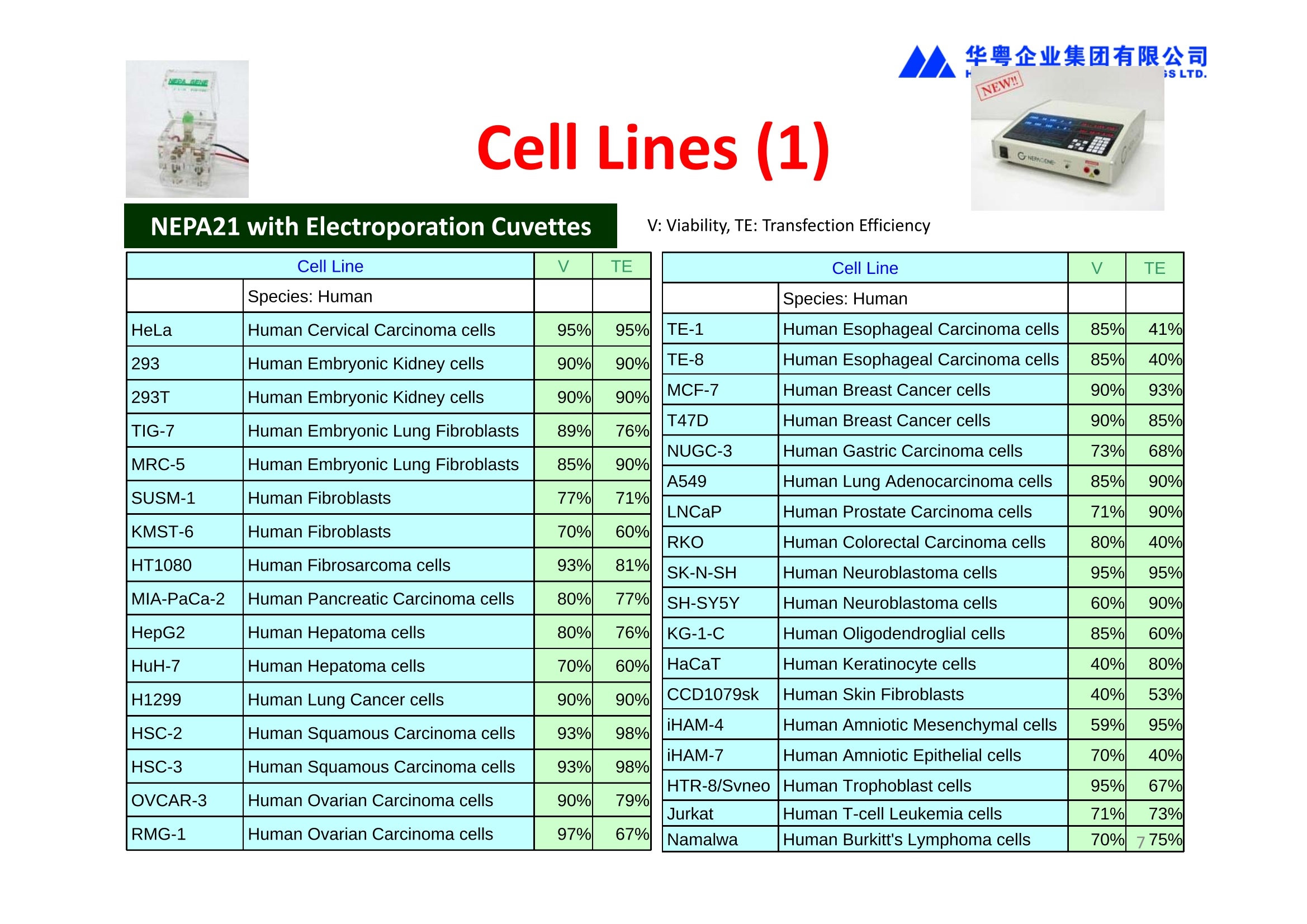
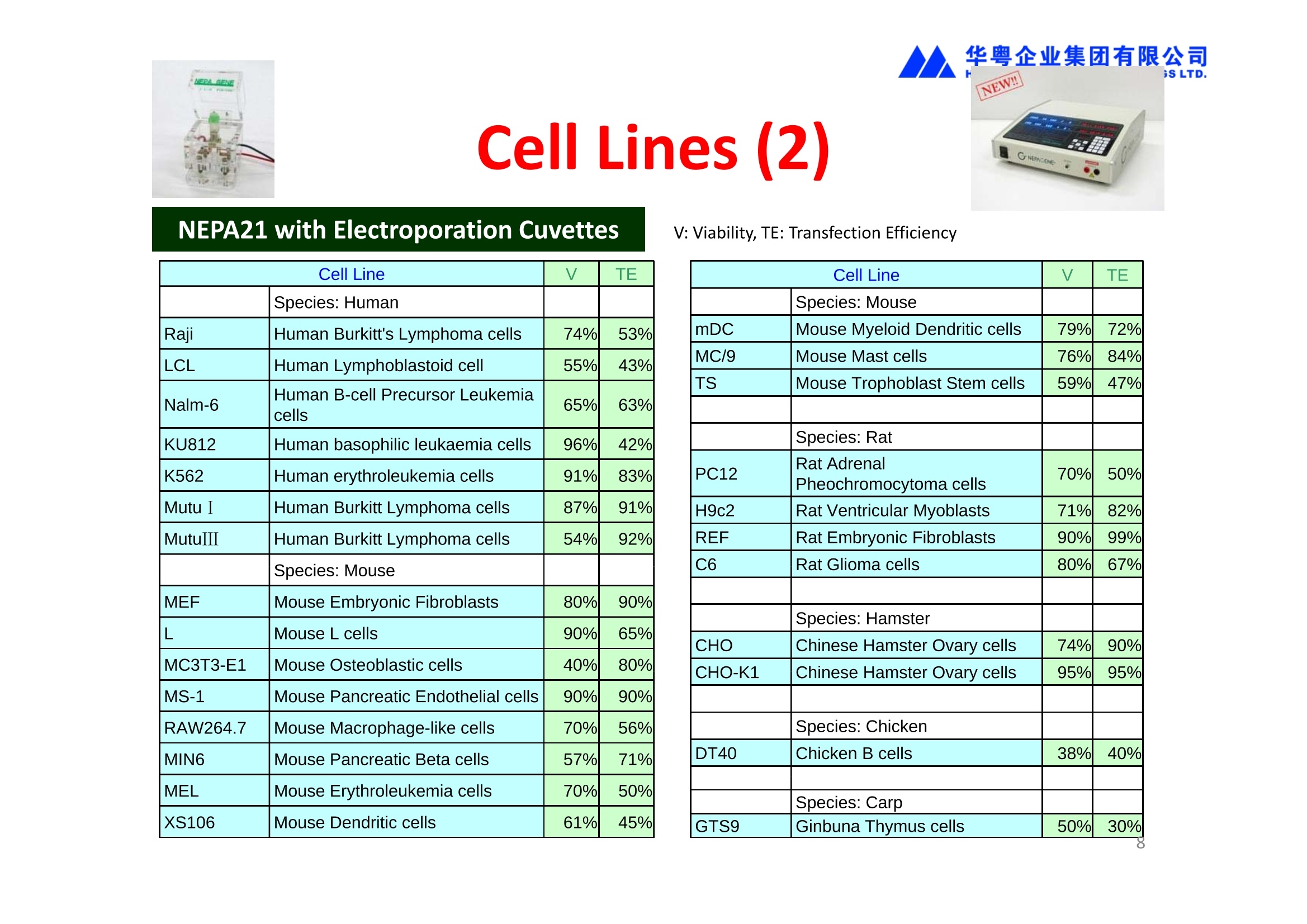
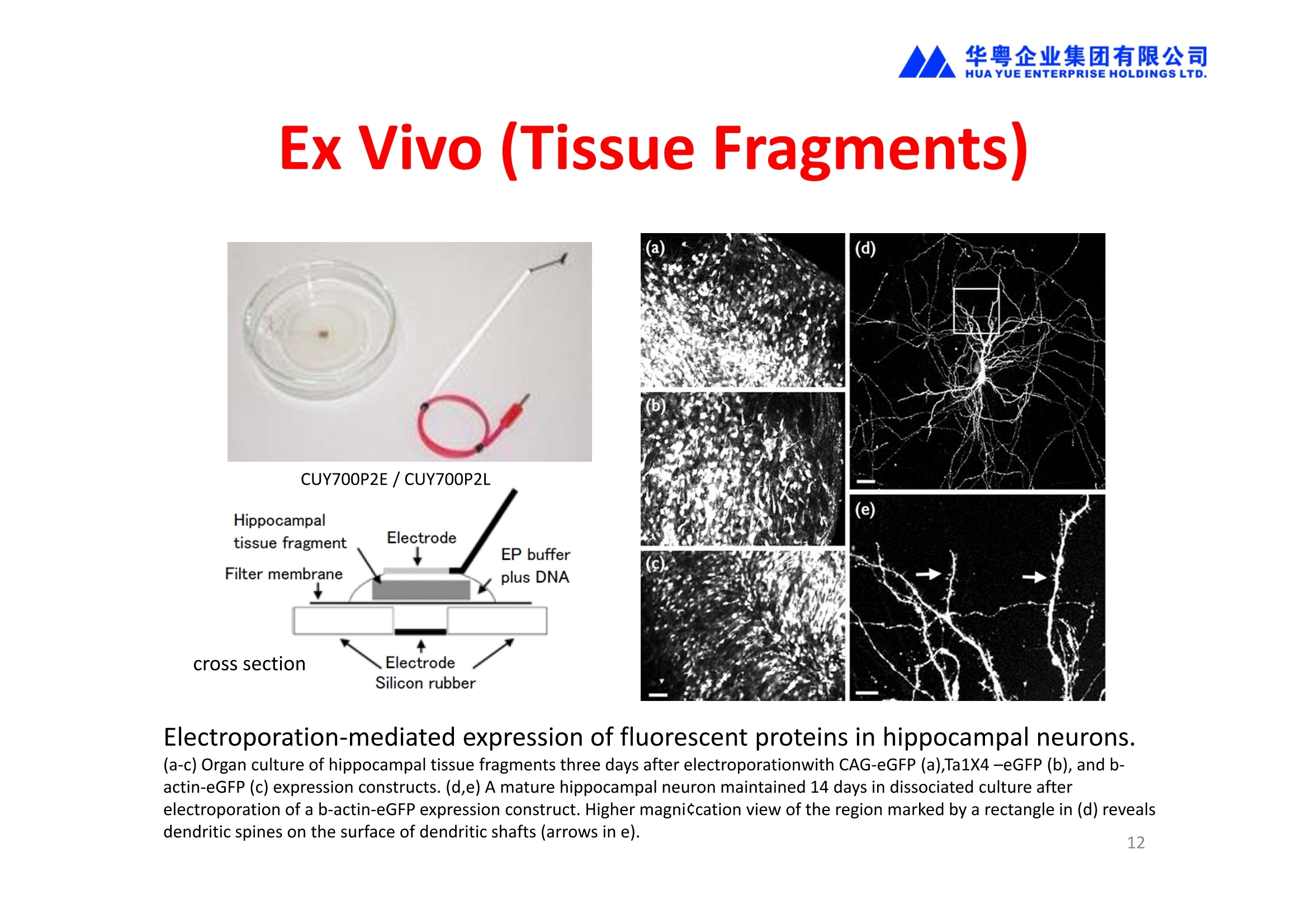
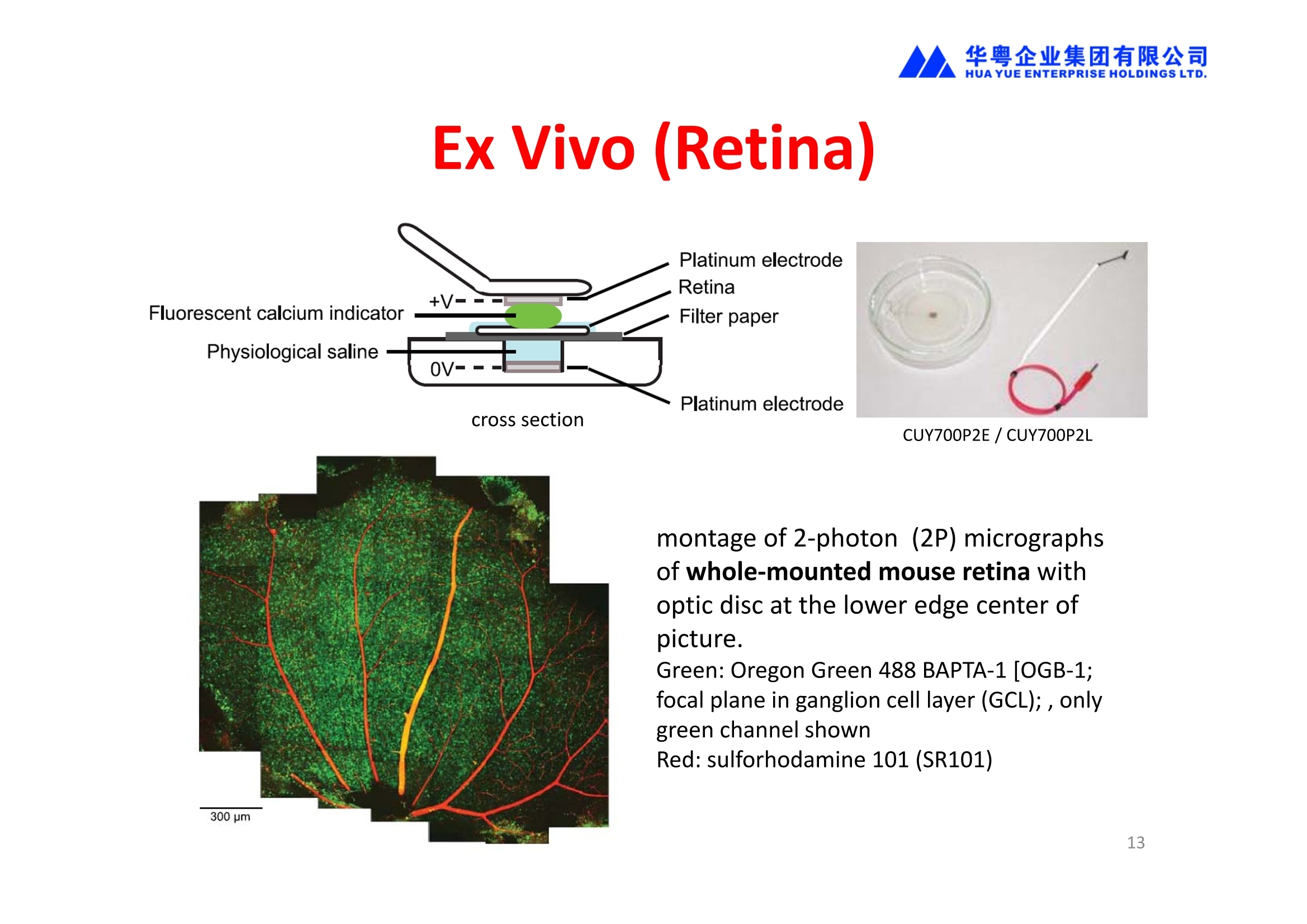
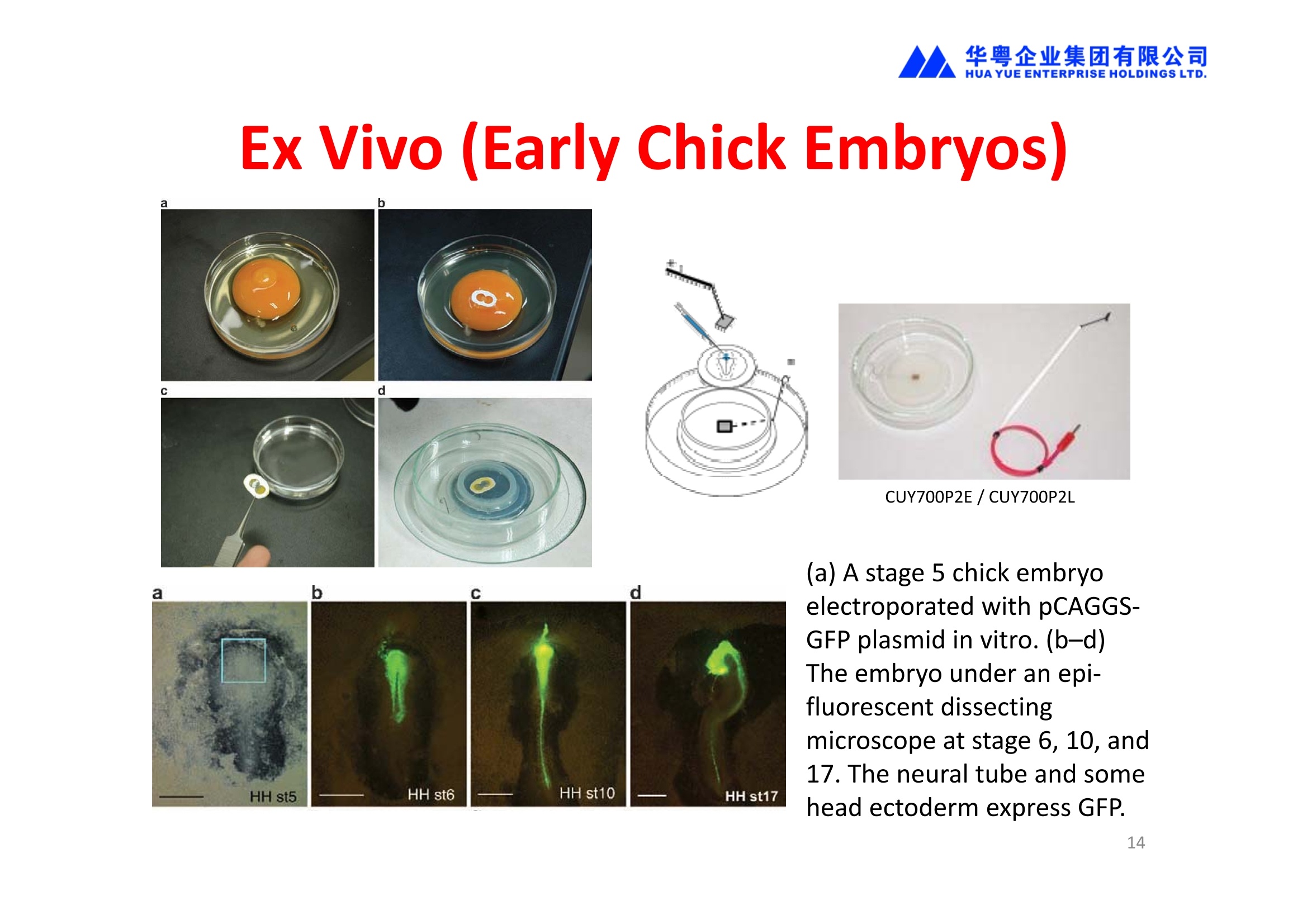
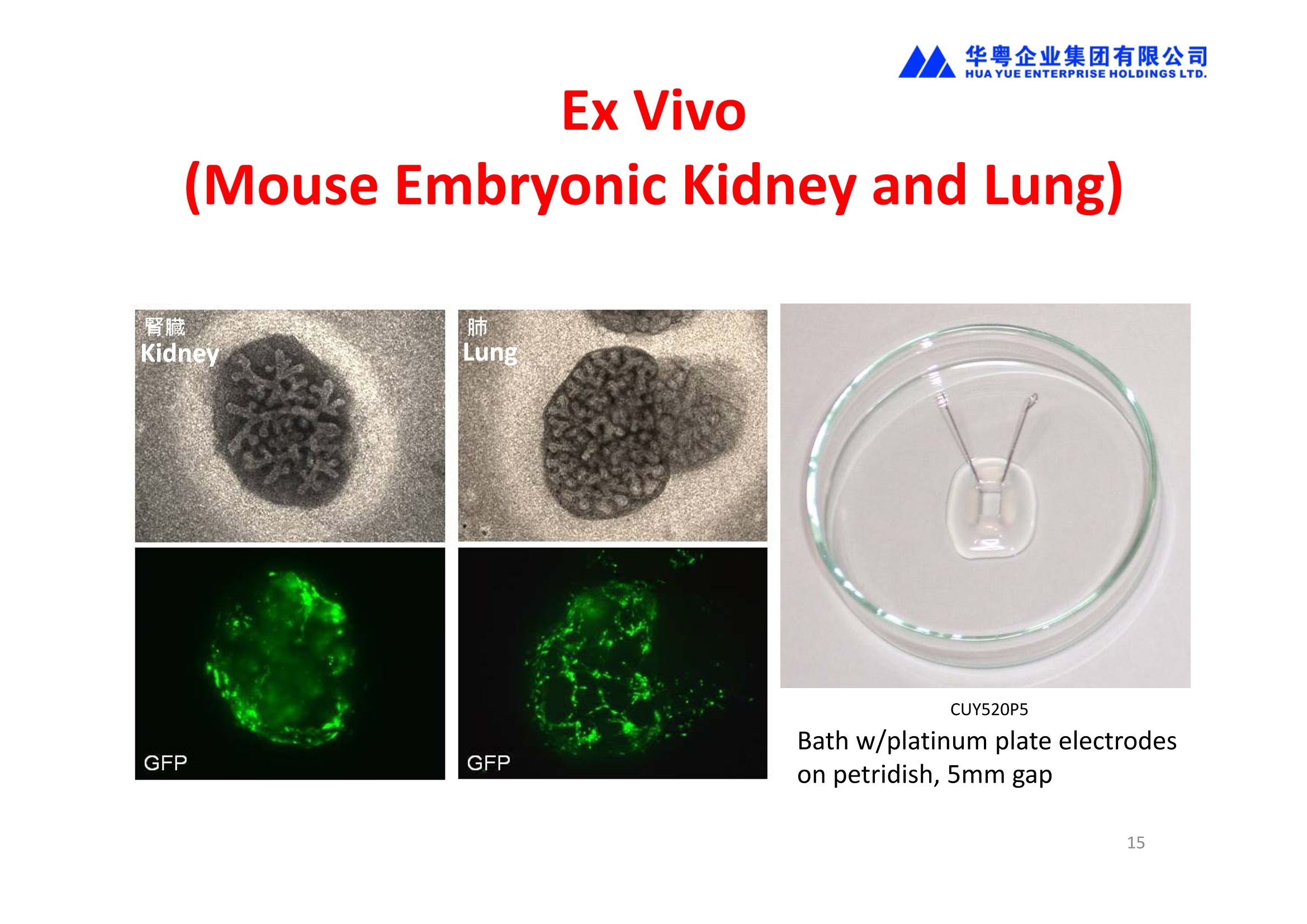
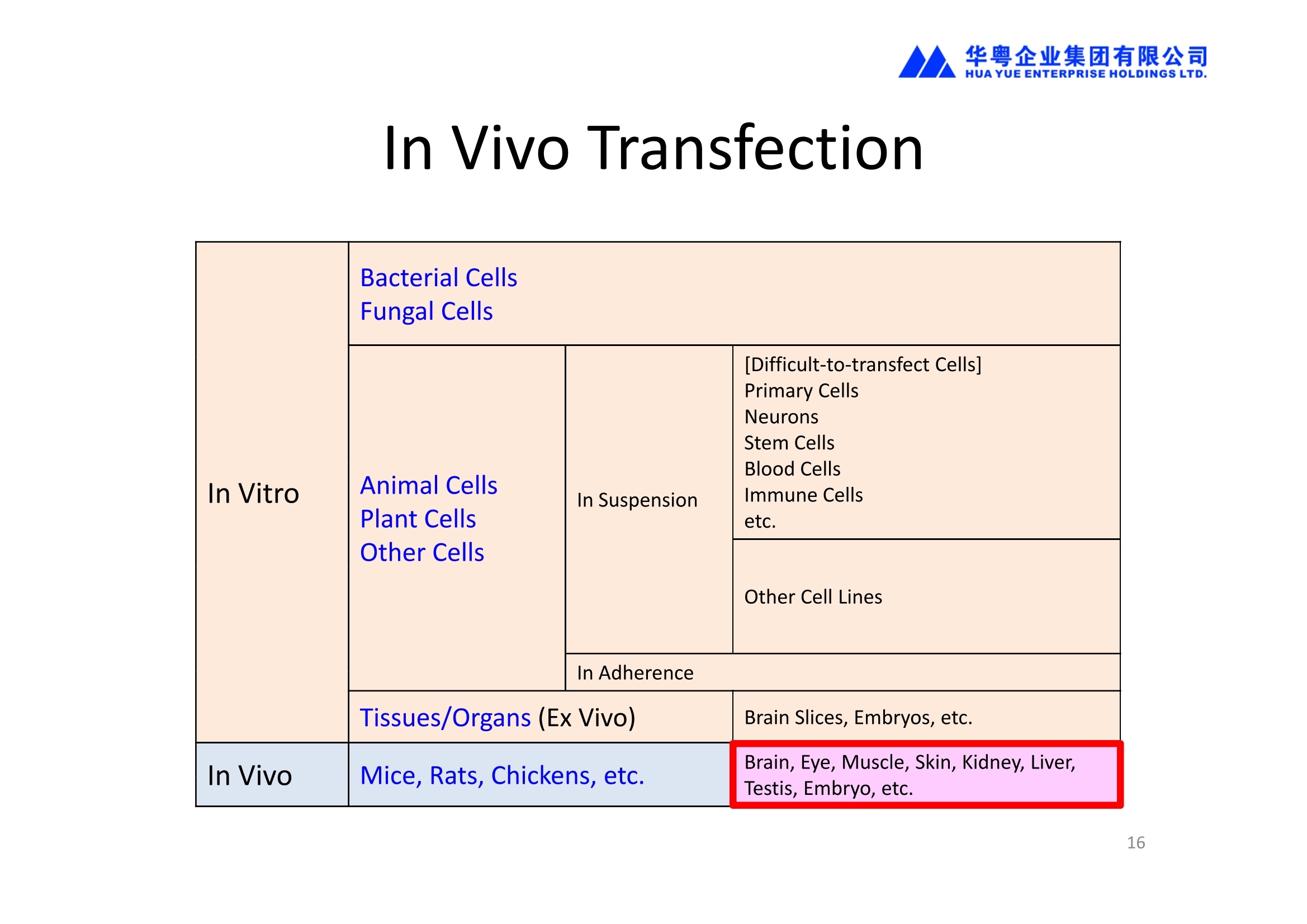
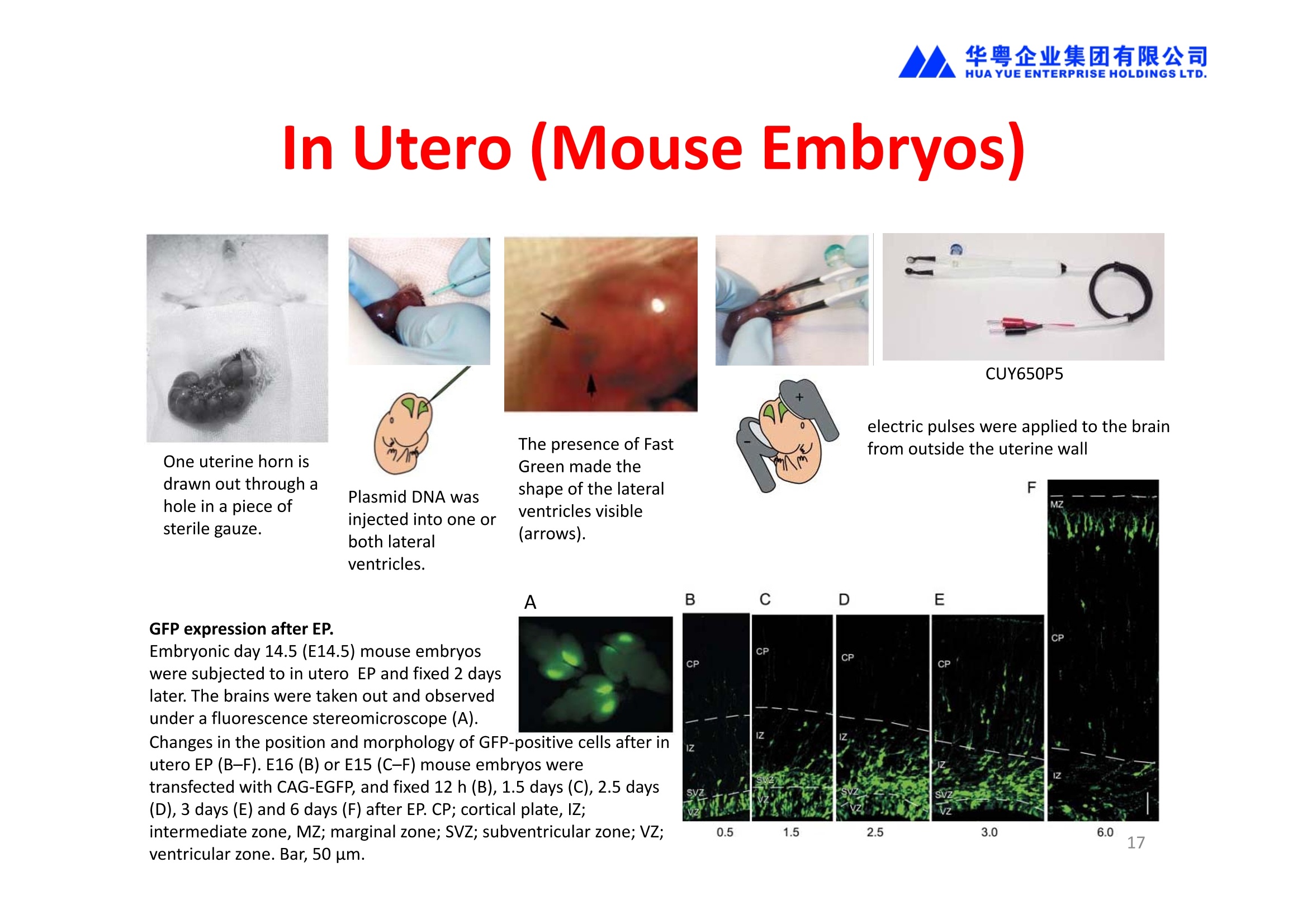
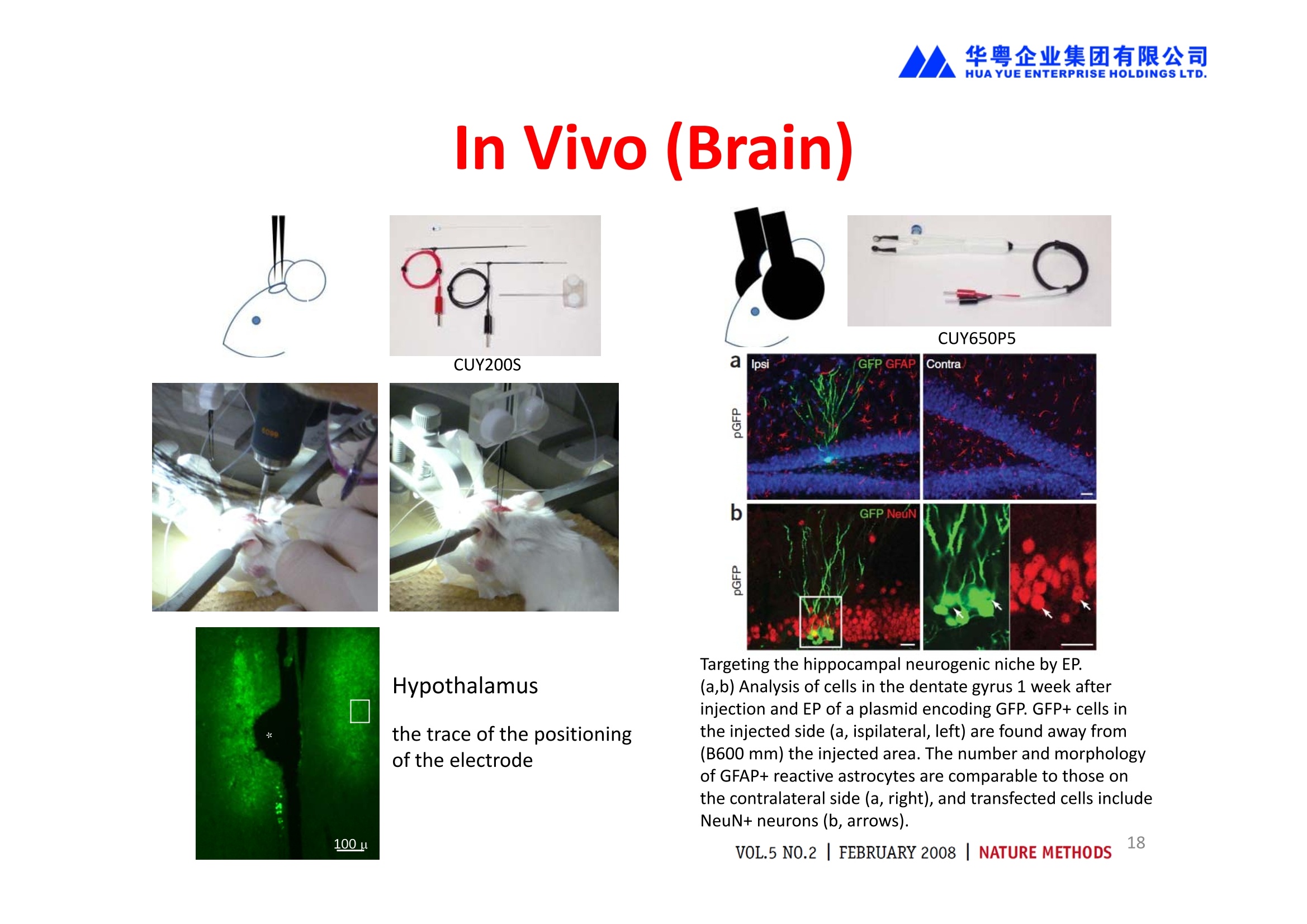
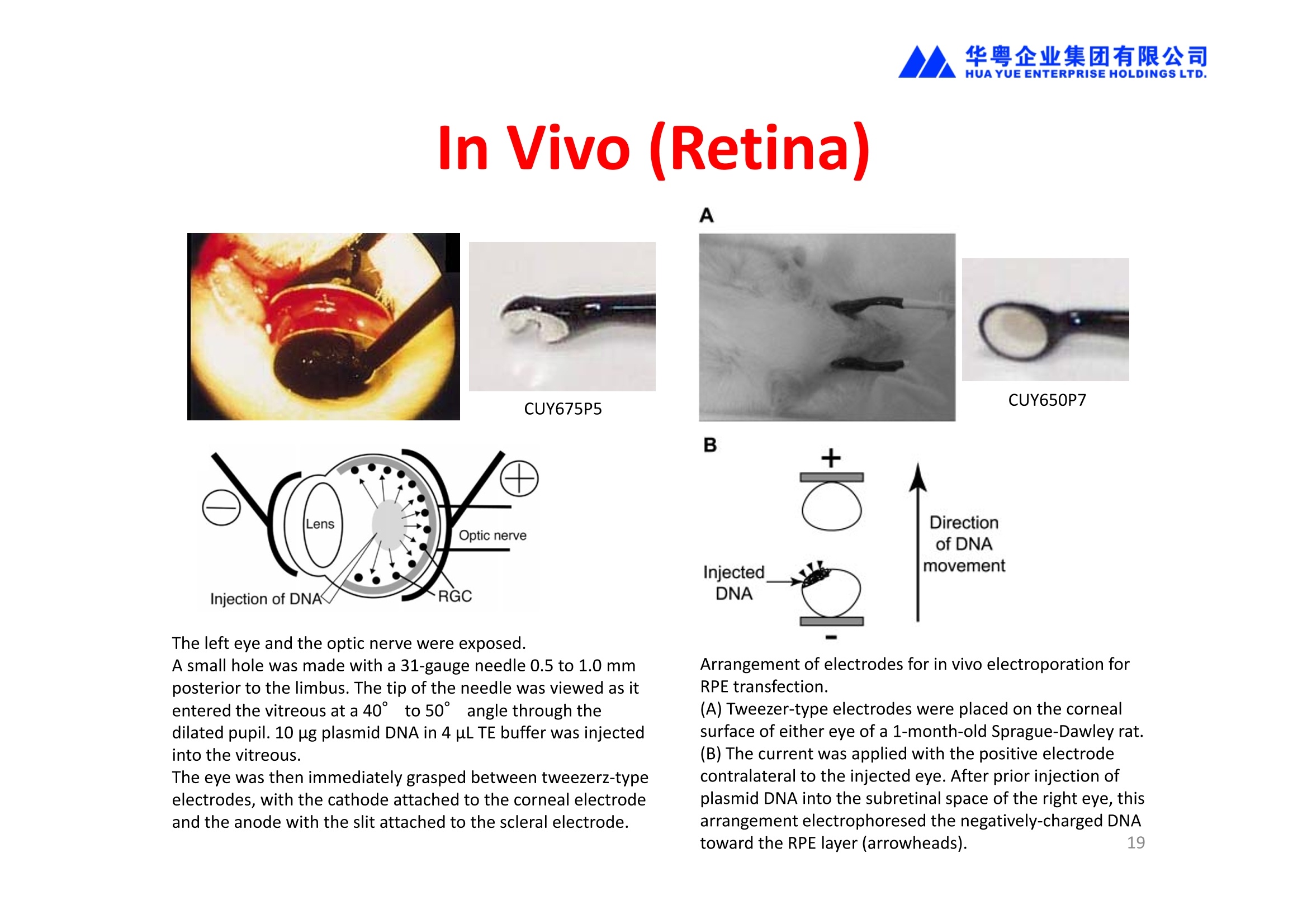
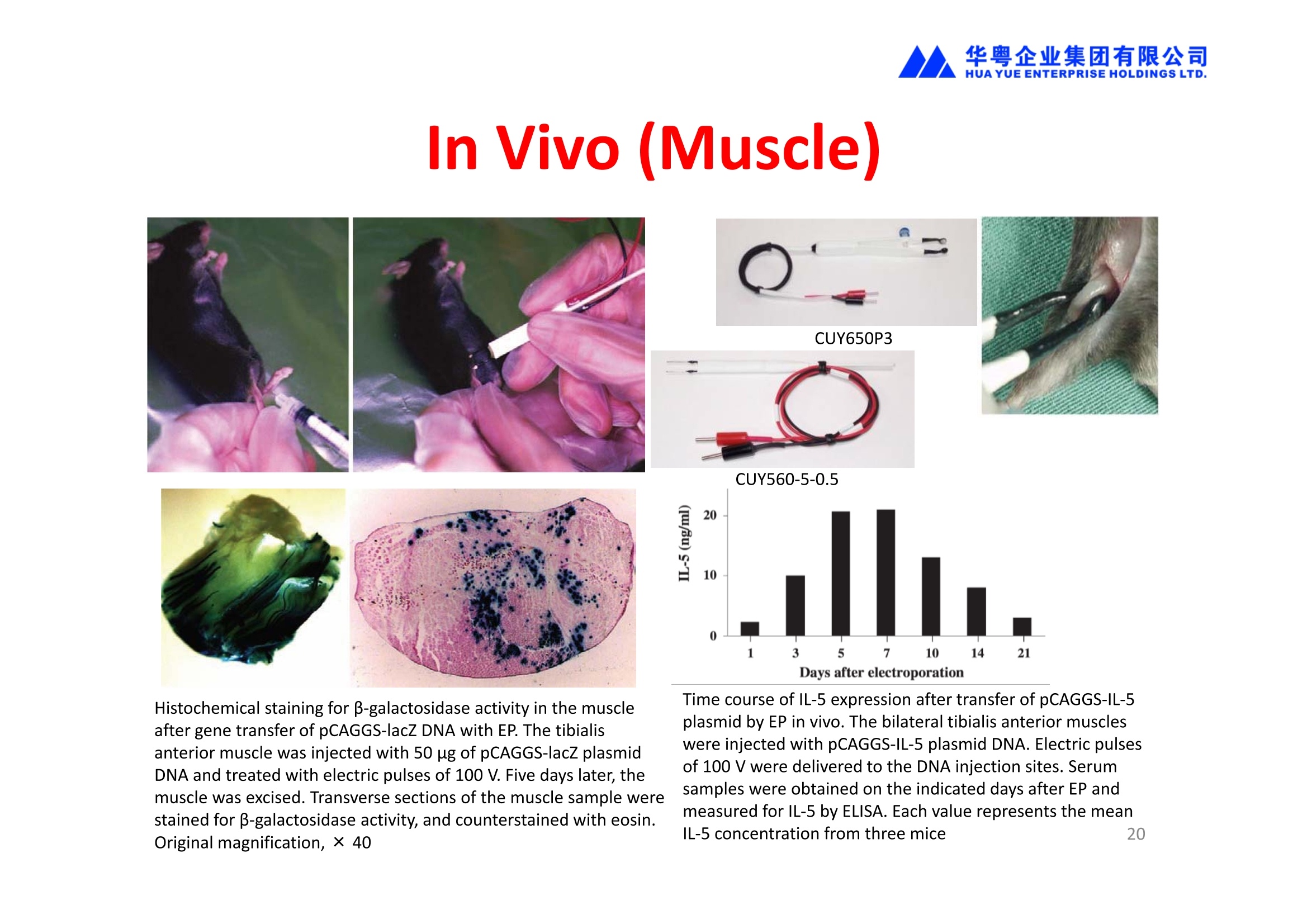
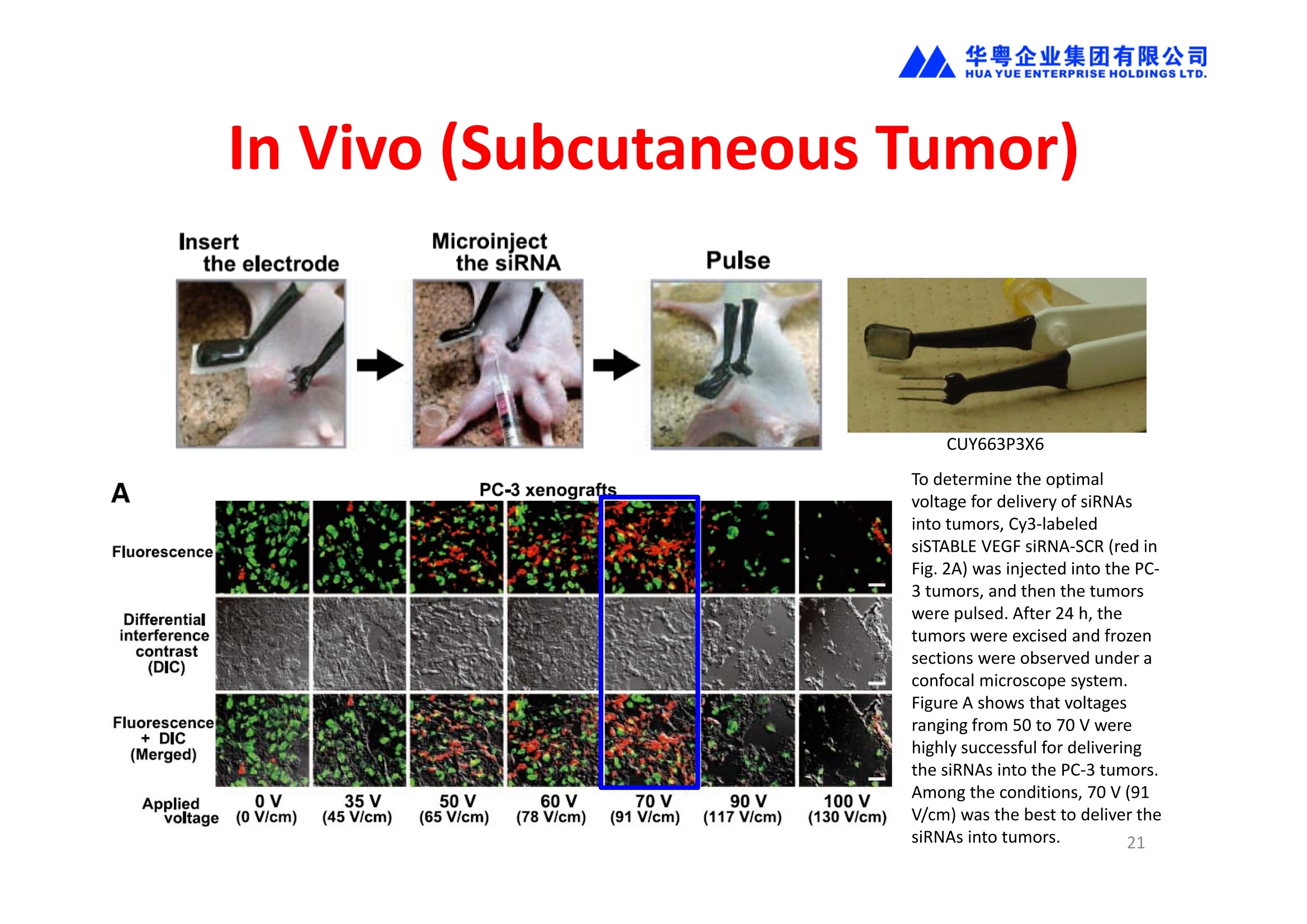
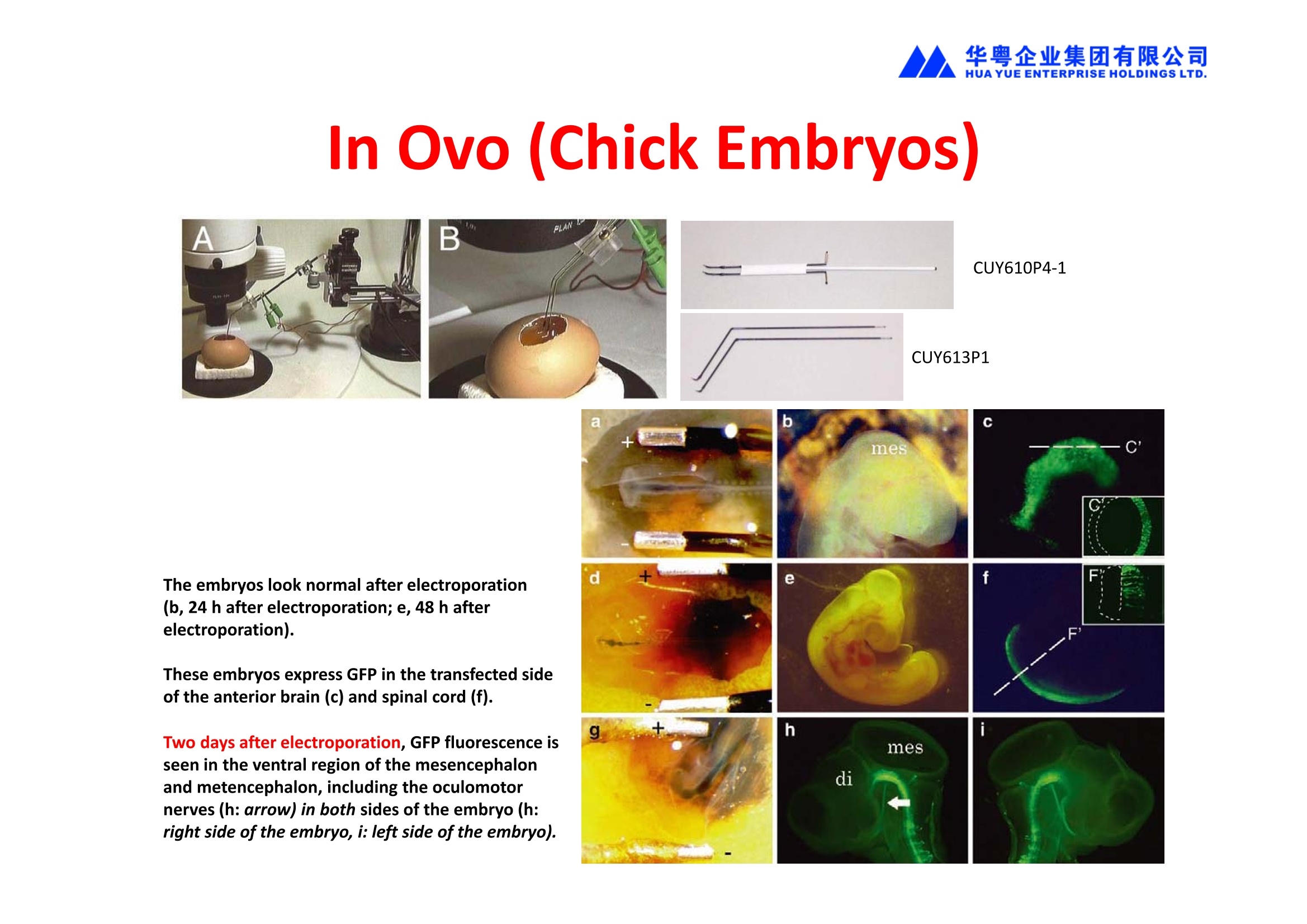
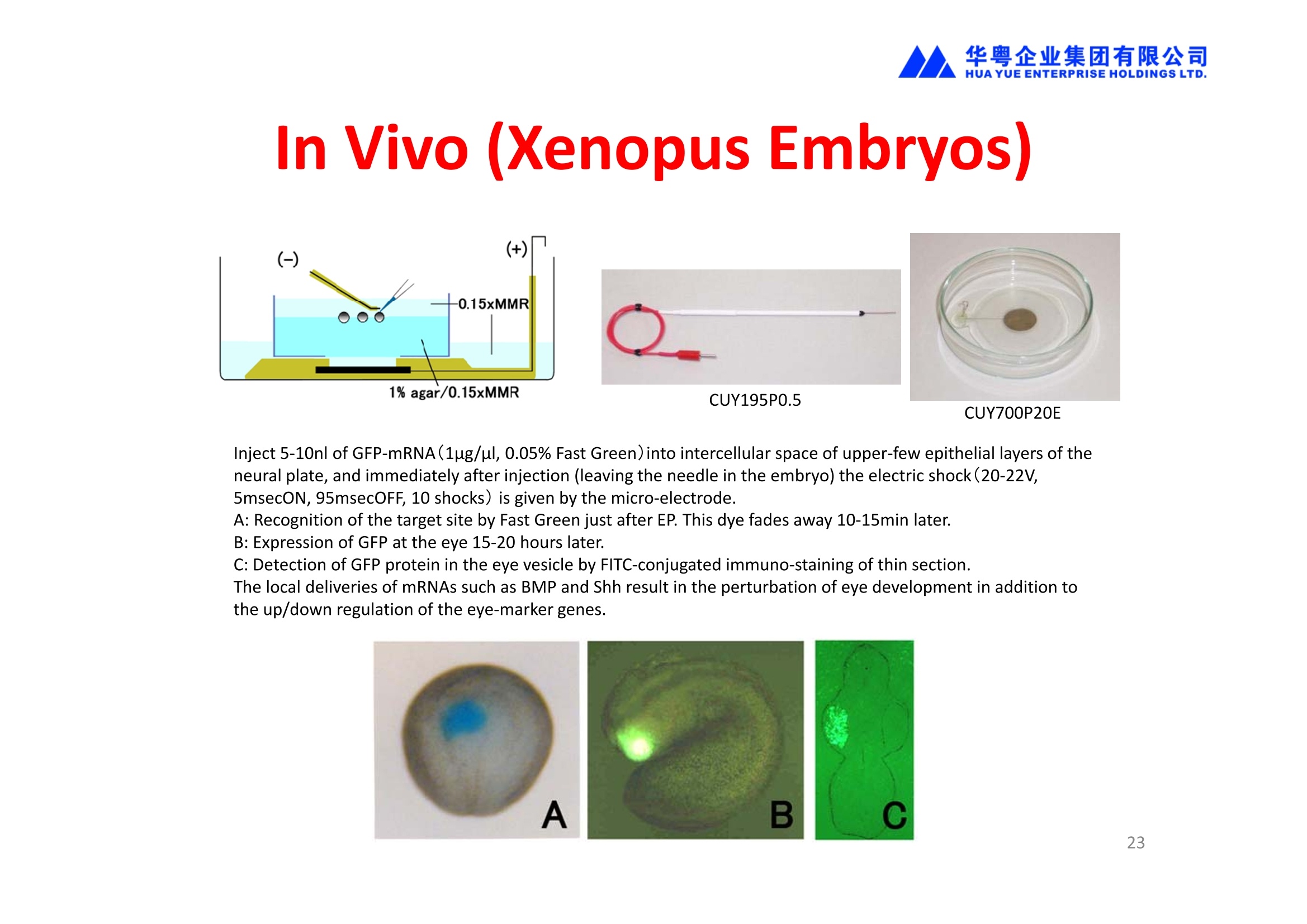
华粤企业集团有限公司HUAYUEENTERPRISE HOLDINGS LTD. 'NEPAGENE NOVEL TRANSFECTION SYSTEMNEPA21 A Wide Range of Applications In Vitro Bacterial Cells Fungal Cells NEPA21 application range 「Difficult-to-transfect Cells] Primary Cells Neurons Stem Cells Blood Cells Immune Cells etc. Animal Cells Plant Cells Other Cells In Suspension Other Cell Lines In Adherence Tissues/Organs (Ex Vivo) Brain Slices, Embryos, etc. In Vivo Mice, Rats, Chickens, etc. Brain, Eye, Muscle, Skin, Kidney, Liver,Testis, Embryo, etc. Transfection intoDifficult-to-transfect Cells In Vitro Bacterial Cells Fungal Cells Animal CellsPlant Cells Other Cells In Suspension 「Difficult-to-transfect Cells1 Primary Cells Neurons Stem Cells Blood Cells Immune Cellsetc. Other Cell Lines In Adherence Tissues/Organs (Ex Vivo) Brain Slices, Embryos, etc. In Vivo Mice, Rats, Chickens, etc. Brain, Eye, Muscle, Skin, Kidney, Liver,Testis, Embryo, etc. NEPA GENE Primary Cells NEPA21 With Electroporation Cuvettes V:Viability, TE: Transfection Efficiency BMMCMouse Bone Marrow-Derived Mast cells HASM IHuman Airway Smooth Muscle cells V:64% TE:78% V: 90% TE: 80% Mouse Neurons, Cerebral Cortex Rat Bulbar Nerve Cells V: 80% TE: 70% V:80% TE: 75% NEPA GENEES Cells, Blood Cells, Etc.GeNDe NEPA21 With Electroporation CuvettesMouse TT2 ES cells V: Viability, TE: Transfection Efficiency Mouse iPS Cells V:50-60% TE: 50-60% V:89% TE: 47% Mutu I Human Burkitt Lymphoma cells RAW264.7 Mouse Macrophage-like cells V: 87% TE:91% V: 70% TE:56% Transfection intoOther Cell Lines in Suspension In Vitro Bacterial CellsFungal Cells Animal CellsPlant Cells Other Cells In Suspension [Difficult-to-transfect Cells] Primary Cells Neurons Stem Cells Blood Cells Immune Cells etc. Other Cell Lines In Adherence Tissues/Organs (Ex Vivo) Brain Slices, Embryos, etc. In Vivo Mice, Rats, Chickens, etc. Brain, Eye, Muscle, Skin, Kidney, Liver,Testis, Embryo, etc. NEPA21 with Electroporation Cuvettes V: Viability, TE: Transfection Efficiency Cell Line V TE Cell Line Species: Human TE TE-1 Human Esophageal Carcinoma cells 85% 41% TE-8 Human Esophageal Carcinoma cells 85% 40% MCF-7 Human Breast Cancer cells 90% 93% T47D Human Breast Cancer cells 90% 85% NUGC-3 Human Gastric Carcinoma cells 73% 68% A549 Human Lung Adenocarcinoma cells 85% 90% LNCaP Human Prostate Carcinoma cells 71% 90% RKO Human Colorectal Carcinoma cells 80% 40% SK-N-SH Human Neuroblastoma cells 95% 95% SH-SY5Y Human Neuroblastoma cells 60% 90% KG-1-C Human Oligodendroglial cells 85% 60% HaCaT Human Keratinocyte cells 40% 80% CCD1079sk Human Skin Fibroblasts 40% 53% iHAM-4 Human Amniotic Mesenchymal cells 59% 95% iHAM-7 Human Amniotic Epithelial cells 70% 40% HTR-8/Svneo Human Trophoblast cells 95% 67% Jurkat Human T-cell Leukemia cells 71% 73% Namalwa Human Burkitt's Lymphoma cells 70% 775% NEPA21 with Electroporation Cuvettes Cell Line V TE Species: Human Raji Human Burkitt's Lymphoma cells 74% 53% LCL Human Lymphoblastoid cell 55% 43% Nalm-6 Human B-cell Precursor Leukemiacells 65% 63% KU812 Human basophilic leukaemia cells 96% 42% K562 Human erythroleukemia cells 91% 83% Mutu I Human Burkitt Lymphoma cells 87% 91% MutuIII Human Burkitt Lymphoma cells 54% 92% Species: Mouse MEF Mouse Embryonic Fibroblasts 80% 90% L Mouse L cells 90% 65% MC3T3-E1 Mouse Osteoblastic cells 40% 80% MS-1 Mouse Pancreatic Endothelial cells 90% 90% RAW264.7 Mouse Macrophage-like cells 70% 56% MIN6 Mouse Pancreatic Beta cells 57% 71% MEL Mouse Erythroleukemia cells 70% 50% XS106 Mouse Dendritic cells 61% 45% V: Viability, TE: Transfection Efficiency Cell Line V TE Species: Mouse mDC Mouse Myeloid Dendritic cells 79% 72% MC/9 Mouse Mast cells 76% 84% TS Mouse Trophoblast Stem cells 59% 47% Species: Rat PC12 Rat Adrenal Pheochromocytoma cells 70% 50% H9c2 Rat Ventricular Myoblasts 71% 82% REF Rat Embryonic Fibroblasts 90% 99% C6 Rat Glioma cells 80% 67% Species: Hamster CHO Chinese Hamster Ovary cells 74% 90% CHO-K1 Chinese Hamster Ovary cells 95% 95% Species: Chicken DT40 Chicken B cells 38% 40% Species: Carp GTS9 Ginbuna Thymus cells 50% 30% Transfection into Cells in Adherence In Vitro Bacterial Cells Fungal Cells Animal CellsPlant Cells Other Cells In Suspension [Difficult-to-transfect Cells] Primary Cells Neurons Stem Cells Blood Cells Immune Cells etc. Other Cell Lines In Adherence Tissues/Organs (Ex Vivo) Brain Slices, Embryos, etc. In Vivo Mice, Rats, Chickens, etc. Brain, Eye, Muscle,Skin, Kidney,Liver,Testis, Embryo, etc. Primary Neurons In Adherence NEPA21 With Adherent Cell Electrode Transfection into primary neurons cultured for 6 days in adherent state.The neurons were prepared from E15 mouse cerebral cortex. CUY900-13-3-5Adherent CellElectrode B EGFP fluorescence image of the neurons2 days after electroporation DHigh magnification image of Figure C (x40) Neurites are shown clearlv. Transfection into Tissues/Organs In Vitro Bacterial Cells Fungal Cells Animal CellsPlant Cells Other Cells In Suspension [Difficult-to-transfect Cells] Primary Cells Neurons Stem Cells Blood Cells Immune Cells etc. Other Cell Lines In Adherence Tissues/Organs (Ex Vivo) Brain Slices, Embryos, etc. In Vivo Mice, Rats, Chickens, etc. Brain, Eye, Muscle, Skin, Kidney, Liver,Testis, Embryo, etc. CUY700P2E/CUY700P2L Electroporation-mediated expression of fluorescent proteins in hippocampal neurons.(a-c) Organ culture of hippocampal tissue fragments three days after electroporationwith CAG-eGFP (a),Ta1X4-eGFP(b),and b-actin-eGFP (c) expression constructs. (d,e) A mature hippocampal neuron maintained 14 days in dissociated culture afterelectroporation of a b-actin-eGFP expression construct. Higher magniccation view of the region marked by a rectangle in (d) revealsdendritic spines on the surface of dendritic shafts (arrows in e). montage of 2-photon (2P) micrographsof whole-mounted mouse retina withoptic disc at the lower edge center ofpicture. Green: Oregon Green 488 BAPTA-1 [OGB-1;focal plane in ganglion cell layer (GCL); , onlygreen channel shownRed: sulforhodamine 101 (SR101) Ex Vivo (Early Chick Embryos) a b m c d 口- CUY700P2E /CUY700P2L a b c d (a) A stage 5 chick embryoelectroporated with pCAGGS-GFP plasmid in vitro. (b-d)The embryo under an epi-fluorescent dissectingmicroscope at stage 6, 10, and17. The neural tube and some HH st5 HH st6 HH st10 HH st17 head ectoderm express GFP. Ex Vivo(Mouse Embryonic Kidney and Lung CUY520P5 Bath w/platinum plate electrodeson petridish, 5mm gap In Vivo Transfection In Vitro Bacterial Cells Fungal Cells Animal CellsPlant Cells Other Cells In Suspension [Difficult-to-transfect Cells] Primary Cells Neurons Stem Cells Blood Cells Immune Cells etc. Other Cell Lines In Adherence Tissues/Organs (Ex Vivo) Brain Slices, Embryos, etc. In Vivo Mice, Rats, Chickens, etc. Brain, Eye, Muscle,Skin, Kidney, Liver,Testis, Embryo, etc. In Utero (Mouse Embryos) CUY650P5 electric pulses were applied to the brainfrom outside the uterine wall One uterine horn isdrawn out through ahole in a piece ofsterile gauze. Plasmid DNA wasinjected into one orboth lateralventricles. The presence of FastGreen made theshape of the lateralventricles visible(arrows). MZ A B C D E GFP expression after EP. CP Embryonic day 14.5 (E14.5) mouse embryoswere subjected to in utero EP and fixed 2 dayslater. The brains were taken out and observedunder a fluorescence stereomicroscope (A). CP CP CP CP Changes in the position and morphology of GFP-positive cells after inutero EP (B-F). E16 (B) or E15 (C-F) mouse embryos were IZ IZ IZ transfected with CAG-EGFP, and fixed 12 h (B), 1.5 days (C), 2.5 days(D), 3 days (E) and 6 days (F) after EP. CP; cortical plate, IZ; intermediate zone, MZ; marginal zone; SVZ; subventricular zone;VZ;ventricular zone. Bar, 50 um. 0.5 1.5 2.5 3.0 6.0 17 In Vivo (Brain) CUY200S Hypothalamusthe trace of the positioningof the electrode Targeting the hippocampal neurogenic niche by EP.(a,b) Analysis of cells in the dentate gyrus 1 week afterinjection and EP of a plasmid encoding GFP. GFP+ cells inthe injected side (a, ispilateral, left) are found away from(B600 mm) the injected area.The number and morphologyof GFAP+ reactive astrocytes are comparable to those onthe contralateral side (a, right), and transfected cells includeNeuN+ neurons (b, arrows). 100u The left eye and the optic nerve were exposed. A small hole was made with a 31-gauge needle 0.5 to 1.0 mmposterior to the limbus. The tip of the needle was viewed as itentered the vitreous at a 40°to 50°angle through thedilated pupil. 10 ug plasmid DNA in 4 uL TE buffer was injectedinto the vitreous. The eye was then immediately grasped between tweezerz-typeelectrodes, with the cathode attached to the corneal electrodeand the anode with the slit attached to the scleral electrode. In Vivo (Retina) A CUY650P7 Arrangement of electrodes for in vivo electroporation forRPE transfection. (A) Tweezer-type electrodes were placed on the corneal surface of either eye of a 1-month-old Sprague-Dawley rat.(B) The current was applied with the positive electrodecontralateral to the injected eye. After prior injection ofplasmid DNA into the subretinal space of the right eye, thisarrangement electrophoresed the negatively-charged DNAtoward the RPE layer (arrowheads). 19 In Vivo (Muscle】 Days after electroporation Histochemical staining for B-galactosidase activity in the muscleafter gene transfer of pCAGGS-lacZ DNA with EP. The tibialisanterior muscle was injected with 50 pg of pCAGGS-lacZ plasmidDNA and treated with electric pulses of 100 V. Five days later, themuscle was excised. Transverse sections of the muscle sample werestained for B-galactosidase activity, and counterstained with eosin.Original magnification, × 40 Time course of IL-5 expression after transfer of pCAGGS-IL-5plasmid by EP in vivo. The bilateral tibialis anterior muscleswere injected with pCAGGS-IL-5 plasmid DNA. Electric pulsesof 100 V were delivered to the DNA injection sites.Serumsamples were obtained on the indicated days after EP andmeasured for IL-5 by ELISA. Each value represents the meanIL-5 concentration from three mice 20 In Vivo (Subcutaneous Tumor) Insert Microinjectthe electrode the siRNA Pulse To determine the optimalvoltage for delivery of siRNAsinto tumors, Cy3-labeledsiSTABLE VEGF siRNA-SCR (red inFig. 2A) was injected into the PC-3 tumors, and then the tumorswere pulsed. After 24 h, thetumors were excised and frozensections were observed under aconfocal microscope system.Figure A shows that voltagesranging from 50 to 70 V werehighly successful for deliveringthe siRNAs into the PC-3 tumors.Among the conditions, 70 V (91V/cm) was the best to deliver the siRNAs into tumors. In Ovo (Chick Embryos) PLANI A B CUY610P4-1 CUY613P1 mes The embryos look normal after electroporation(b, 24 h after electroporation; e, 48 h afterelectroporation). These embryos express GFP in the transfected sideof the anterior brain (c) and spinal cord (f). Two days after electroporation, GFP fluorescence isseen in the ventral region of the mesencephalonand metencephalon, including the oculomotornerves (h: arrow) in both sides of the embryo (h:right side of the embryo, i: left side of the embryo). In Vivo (Xenopus Embryos) CUY700P20E Inject 5-10nl ofGFP-mRNA(1ug/ul, 0.05% Fast Green) into intercellular space of upper-few epithelial layers of theneural plate, and immediately after injection (leaving the needle in the embryo) the electric shock(20-22V,5msecON, 95msecOFF, 10 shocks) is given by the micro-electrode. A: Recognition of the target site by Fast Green just after EP. This dye fades away 10-15min later. B: Expression of GFP at the eye 15-20 hours later. C: Detection of GFP protein in the eye vesicle by FITC-conjugated immuno-staining of thin section. The local deliveries of mRNAs such as BMP and Shh result in the perturbation of eye development in addition tothe up/down regulation of the eye-marker genes. In Vivo (Zebrafish Otocyst) Phalloidin staining of actin filaments with 3 dpf embryos. (A) The 3 dpf embryo injected with control MO has strongstaining of phalloidin in the posterior macula (pm). (B) The embryo which was injected with control MO and thenelectroporated had similar levels of phalloidin staining in pm in the injection only embryos.(C) Injection with mif MOsinto the otic vesicle alone did not cause reduction of phalloidin staining, while injection of mif MOs along withelectroporation caused a dramatic reduction of phalloidin staining in the pm (D). am, anterior macula. PUBLICATIONS 电话:852-27703628(8条线) MultipolarMigration:The Third Mode of Radial Neuronal Migration in the Developing Cerebral Cortex H. Tabata and K. Nakajima Development, Volume 131, Issue 4, Pages 787-796,February 2004 Takahashi et al. Manipulating gene expressions bw electroporation in the developing brain of mammalian embrwos Differentiation, Volume 70, Issue 4-5, Pages 155-162,June 2002 Shibuya et al. Isolation and Characterization of Vasohibin-2 as a Homologue of VEGF-Inducible Endothelium-Derived Angiogenesis Inhibitor Vasohibin Kawabata et al. Electroporationmediated gene transfer swstem applied to cultured CNS neurons Neuroreport, Volume 15, Issue 6, Pages 971-975,29 April 2004 Cell,Volume 114, Issue 4, Pages 469-482,22 August 2003 Masanori Takahashi and Noriko Osumi Pax6 regulates specification of ventral neurone subtyoes in the hindbrain by establishing progenitor domains Tomomi Fukuchi-Shimogori and ElizabethA Grove Emx2 patterns the neocortex by regulating FGF positional simaling Nature Neuroscience, Volume 6. Number 8, Pages 825-831,August 2003 Ohtsuka et al. Roles of the Basic Helix-Loop-Helix Genes Hes1 and Hes5in Expansion of Neural Stem Cells of the Developing Brain NEPA21/CUY21 Publications Yomogida K et at.. Dramatic Expansion of Germinal Stem Cells bw Ectopically Expressed Human Glial Cell Line-Derived Neurotrophic Factor in Mouse Sertoli Cells Suzuki et al. Effect of CaClconcentration on the rate of foreigm gene transfer and exoression by in vivo electroporation in the mouse ovary Sakai et al. Hepatocute-targeted gene transfer by combination of vascularly delivered plasmidDNA and in vivo electroporation. Yazawa et al.Direct transfer of hepatocute growth factor gene into kidney suppresses cuclosporin A nephrotoxicitw in rats Nephrology Dialysis Transplantation.Volume 19. Issue 4. Pages 812-816.April 2004 Deubiquitylation of histone H2A activates transcriptional initiation via trans-histone crosstalk with H3K4 di- and trimethylation Ohta H et al.. cAMPresponsive element in TATA-less core promoter is essential for haploid-specific gene expressionin mouse testis NEPA21/CUY21Publications Hepatocvte growth factor gene therapy accelerates regeneration in cirrhotic mouse livers after hepatectomy Kawaiet al. Ectopic Bone Formation bw Human Bone Morohogenetic Protein-2 Gene Transfer to Skeletal Muscle Using Transcutaneous Electroporation Inoue et al. Anti-Monocyte Chemoattractant Protein-1 Gene Therapy Limits Progression and Destabilization of Established Atherosclerosis in Apolipoprotein E-Knockout Mice Ikeda et al. Anti-monocvte chemoattractant protein-1 gene therapy attenuates pulmonarw hvoertension in rats Xue et al. Attenuated acute liver iniurw in mice by naked hepatocute growth factor gene transfer into skeletalmuscle with electroporation Biology of Reproduction, Volume 67, Issue 3, Pages 712-717,September 2002 Takeshita et al. Toll-Like Receptor Adaotor Molecules Enhance DNA-Raised Adaptive Immune Responses against Influenza and Tumors through Activation of Innate Immunity Journal of Virology, Volume 80, Number 13,Pages 6218-6224,July 2006 Saha et al.Multivalent DNA vaccine protects mice against pulmonary infection caused by Pseudomonas aeruginosa Vaccine, Volume 24, Issues 37-39, Pages 6240-6249,11 September 2006 Kitaguchi et al. Immune deficiencw enhances expression of recombinant human antibody in mice after nonviral in vivo gene transfer Page:5 Inoue et al. Modulation of scratching behavior by silencing an endogenous cyclooxvgenase-1 gene in the skin through the administration of siRNA Inoue et al. Electro-transfer of smallinterfering RNA ameliorated arthritis in rats Epidermis-Targeted Gene Transfer Using In Vivo Electroporation Epidermal Cells Methods and Protocols, Series: Methods in Molecular Biology. Volume 289, Pages 431-436, October 2004 Pharmaceutical Research, Volume 22.Number 6,Pages 883-891.June 2005 International Journal of Molecular Medicine, Volume 8,Number 5, Pages 489-494, November 2001 Takada et al.Infectivitw-Enhancing Antibodies to Ebola Virus Glycoprotein Protection Against Autoimmune Myocarditis by Gene Transfer of Interleukin-10 by Electroporation Guan X et al. Iniection site-dependent induction of immuneresponse by DNA vaccine: comparison of skin and spleen as a target for vaccination. Shibata MA et al.. Combination therapw with short interfering RNA vectors against VEGF-C and VEGF-Asuppresses lmph node and lung metastasis in a mouse immunocompetent mammarw cancer model. NEPA21/CUY21 Publications Page:8 Categories Publications Gonzalez-Estevez et al.. Transgenic planarian lines obtained by electroporation using transposon-derived vectors and an eve-specific GFP marker Yamada et al. ElectricalStimulation Modulates Fate Determination of Differentiating Embryonic Stem Cells Sasagawaet al, Improved mRNA Electroporation Method for Xenopus Neurula Embryos Genesis, Volume 33, Issue 2, Pages 81-85, June 2002 Tsai FM et al.. Induction of apoptosis by the retinoid inducible growth regulator RIG1 depends on the NC motif in HtTA cervical cancer cells. Takekazu Kunieda and Takeo Kubo In vivo gene transfer into the adult honeybee brain by usingelectroporation Biochemical and Biophysical Research Communications, Volume 318, Issue 1, Pages 25-31, 21 May 2004 Shinmyo et al.piggyBac mediated somatictransformation of the two-spotted cricket. Grvllus bimaculatus THANK YOU 电话:852-27703628(8条线) 广州 广州市番禺区兴南大道华粤大厦 电话:线) 传真:港 香港龙弥敦道雅兰中心期 电话:线) 传真:
确定












还剩34页未读,是否继续阅读?
广州市华粤行仪器有限公司为您提供《细胞中基因转染检测方案(基因导入仪)》,该方案主要用于其他中基因转染检测,参考标准--,《细胞中基因转染检测方案(基因导入仪)》用到的仪器有NEPA21 高效基因转染系统
推荐专场
相关方案
更多