方案详情
文
锂电池鼻祖J.-M. Tarascon《ARTICLES METAL HYDRIDES FOR LITHIUM ION BATTTERIES》
方案详情

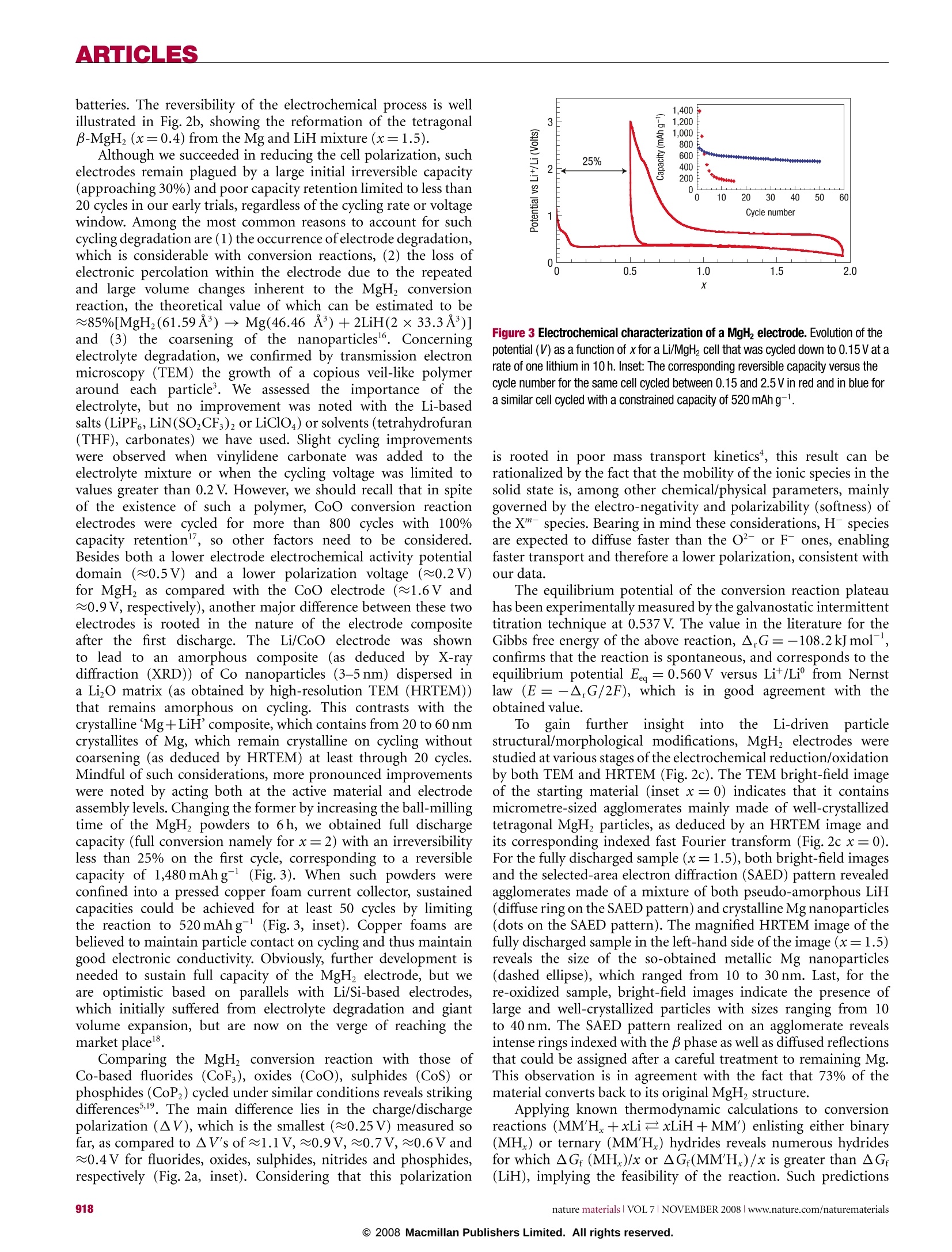
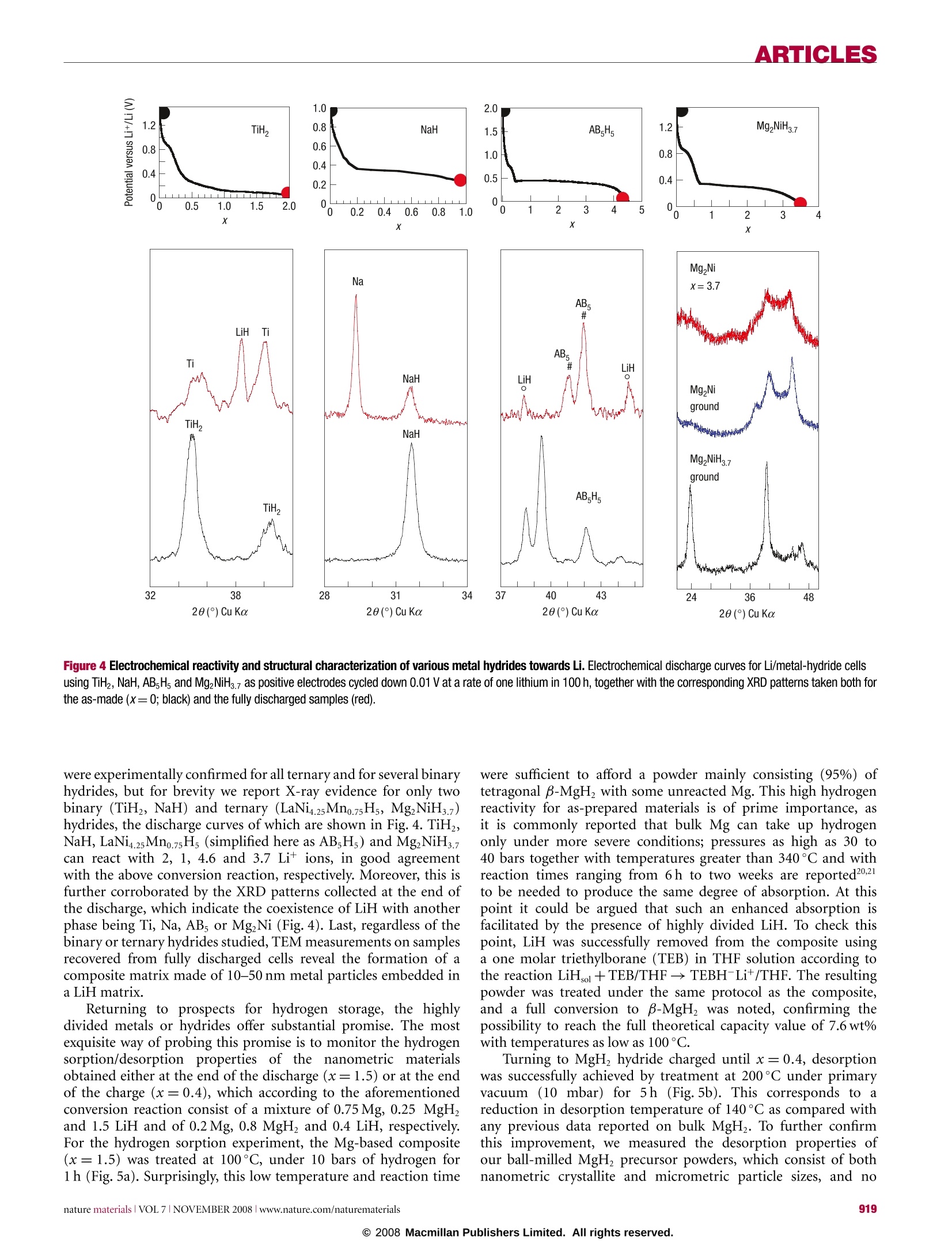
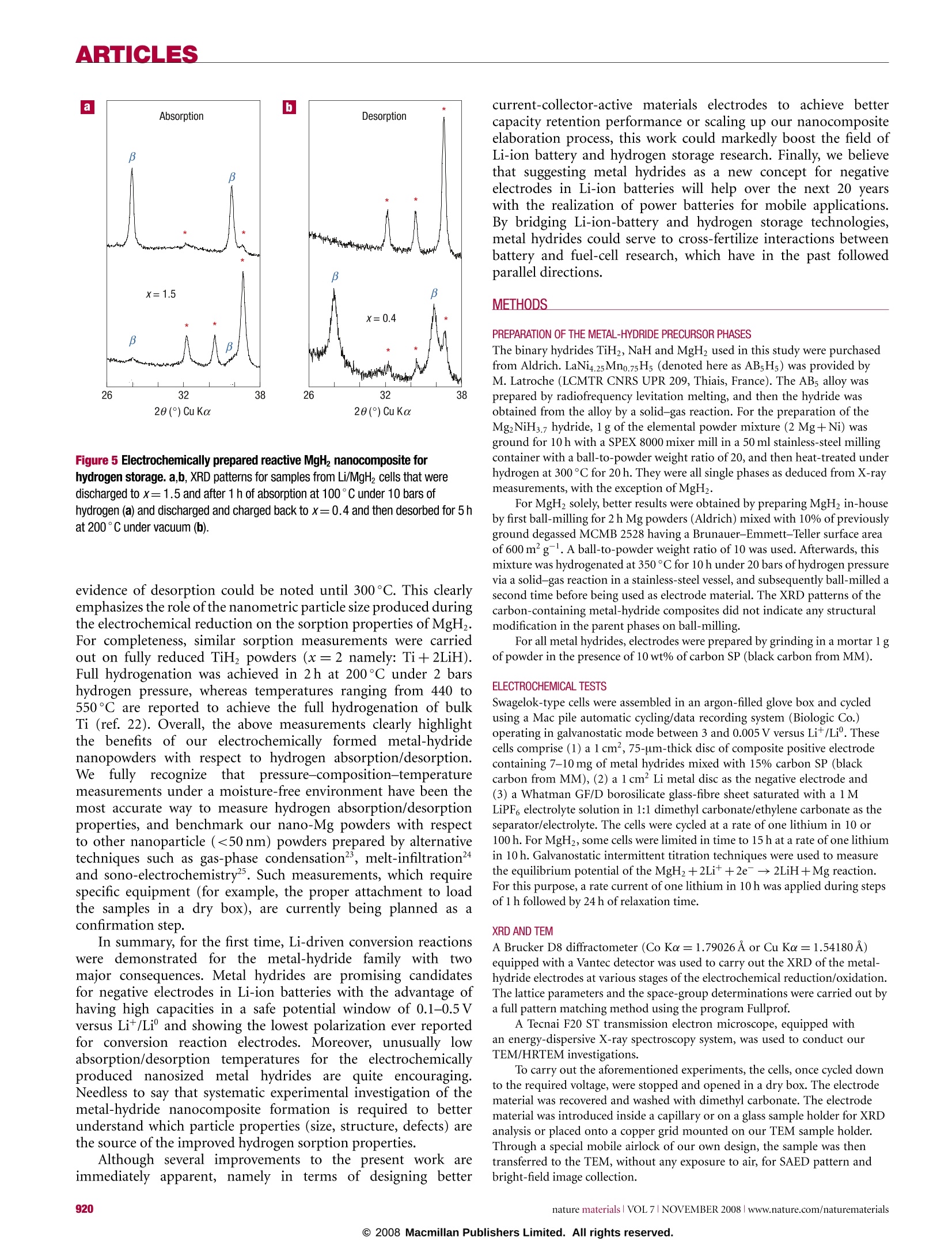
ARTICLES ARTICLES Metal hydrides for lithium-ion batteries Y. OUMELLAL1, A. ROUGIER1, G. A. NAZRI2, J-M. TARASCON1 AND L. AYMARD1* 1Laboratoire de Reactivite et de Chimie des Solides UMR CNRS 6007, 100 rue Saint Leu, Amiens, France General Motors R & D and Planning Center, Warren, Michigan 48090, USA *e-mail:luc.aymard@u-picardie.fr Published online: 12 October 2008; doi:10.1038/nmat2288 Classical electrodes for Li-ion technology operate via an insertion/de-insertion process. Recently, conversion electrodes have shownthe capability of greater capacity, but have so far suffered from a marked hysteresis in voltage between charge and discharge, leadingto poor energy efficiency and voltages. Here, we present the electrochemical reactivity of MgH2 with Li that constitutes the first use ofa metal-hydride electrode for Li-ion batteries. The MgH electrode shows a large, reversible capacity of 1,480 mAhgat an averagevoltage of 0.5V versus Li*/Li° which is suitable for the negative electrode.In addition, it shows the lowest polarization for conversionelectrodes. The electrochemical reaction results in formation of a composite containing Mg embedded in a LiH matrix, which oncharging converts back to MgH. Furthermore, the reaction is not specific to MgH, as other metal or intermetallic hydrides showsimilar reactivity towards Li. Equally promising, the reaction produces nanosized Mg and MgH, which show enhanced hydrogensorption/desorption kinetics.We hope that such findings can pave the way for designing nanoscale active metal elements withapplications in hydrogen storage and lithium-ion batteries. Finite fossil-fuel supplies, nuclear waste and global warminglinked to CO emissions have made the development ofalternative/green’ methods of energy production, conversion andstorage popular topics in today's energy-conscious society. Thesecrucial environmental issues, together with the rapid advance andeagerness from the electric automotive industry (for example,electric vehicles and hybrid electric vehicles) have combinedto make the development of radically improved energy storagesystems a worldwide imperative. Within the myriad of available battery chemistries, Ni-MHbattery technology, which is nearly a substitute for Ni-Cd, currentlypowers hybrid vehicles. Nevertheless, Li-ion battery technology,which powers most of today's portable electronic devices, stands asa serious contender for the years to come to take over the hybrid andplug-in hybrid electric vehicle market. Although the performanceof Li-ion batteries has improved with time even beyond initialexpectations, the technology now stands at a point where, owingto an intrinsic limitation associated with insertion reactions(forexample, le per3d-metal), it will have difficulty in meeting futuremarket demands 2.An alternative solution’could be providedby conversion reactions ithattenlist the complete electrochemicalreduction of metal oxides,sulphides, nitrides, phosphides andfluorides into a composite material consisting of nanometricmetallic particles (2-8 nm) dispersed in an amorphous LimX matrixaccording to the overall reaction M,X,+ne +nLi+xM'+yLimX(m=n/y), especially because electrodes based on these reactionsoffer a staggering capacity enhancement over classical insertionelectrodes due to the fact that two or more electrons per redoxcentre can be used.Although quite attractive,such conversionreactions suffer from poor energy efficiency; thisis due to a largepolarization between charge/discharge curves tthat is not alleviatedby acting on the electrode kinetics even via the elaboration ofnanostructured current collectors. In contrast, such polarizationwasshown to depend onthe iono-covalencyofthe M-Xbond,but regardless of the chemistries so far studied (fluorides, oxides, sulphides, nitrides and phosphides) remained quite high.Alternative chemistries are then necessary if we ever want suchconversion electrodes to reach the market place rather than remaina laboratory curiosity. Besides batteries, fuel cells are an appealing direction to powerfuture generations of green’vehicles. Their positive attributes arebased on the use of hydrogen as the fuel. Although attractive,the implementation of fuel-cell technology in electric vehicletransportation is beset with formidable technical and economicaldifficulties linked, among other issues, to the lack of efficientmeans to safely store large amounts of hydrogen. Intensiveresearch is currently being pursued-9 to find a solid hydrogen-absorbing material meeting the performance required for vehicularapplications, namely an absorbing material that should have agravimetric capacity of at least 6wt%, a volumetric capacityhigher than 70gland fast absorption/desorption kinetics attemperatures below 100°C. Among the large variety of hydrogenstorage materials (for example, intermetallics, alanates, imides)currentlystudied, none is able to meet all of these targets. For stablelight hydrides such as TiH, and MgH2, most researchers believe thatlower working temperatures would result from highly divided (forexample, nanosize) particles, but chemical/physical attempts haveremained unsuccessful so far10-12. Here, we address a type of reaction with implications forboth Li-ion batteries and fuel cells. The electrochemical reactivityof metal hydrides with Li is studied as being the basis for anew concept for the negative electrode of Li-ion batteries as wellas a novel route for the production of nanosized metals andhydrides for hydrogen storage. MgH was investigated as the firstexample because of its high theoretical capacity versus Li*/Li° (2eper unit formula, that is,2,038 Ahkg2,878 Ahl) and bothhigh gravimetric (7.6 wt%) and volumetric capacities (108g H/l)for H. Turning first to battery electrodes, a typical voltage-composition(x: mole fraction of Li) trace for a Li/MgH2 cell discharged a b Figure 1 Voltage profile and XRD patterns of MgH electrode at various stagesof the conversion reaction. a, The evolution of the potential (V) as a function of x(mole fraction of Li) for a MgH electrode cycled between 3 and 0.005V at a rate ofone lithium in 100 h. Inset: The discharge-charge trace as a function of x for asimilar cell cycled with a rate of one Li in 10 h. b, The recorded XRD patternsassociated with the various stages of the discharge, denoted by the numbers (1), (2),(3), (4) and (5) in a and corresponding to x=0 (starting electrode material),x=0.5, 1, 1.5 and 1.8, respectively. The X-ray peaks marked by an asterisk, β,yand green circle correspond to Mg, B-MgH2,y-MgH2 and LiH, respectively. Inset:Magnification of the XRD pattern in the region where LiH peaks are prominent.c, The XRD patterns (6), (7), and (8)corresponding to x=2.1, 2.35 and 2.9. TheBragg peaks marked by an asterisk and plus sign correspond to Mg (h.c.p.) and Li(b.c.c.) solid solutions, respectively. at a current of one lithium for 100h between 3 and 0.005 Vis shown in Fig. la. Initially the potential abruptly drops toreach a first plateau located near 0.44V before showing anotherfast decay around x=2 to reach a lower voltage plateau (near0.095V) that extends up to x=2.5. From a structural point ofview (Fig. 1b), the starting electrode material consists mainly oftetragonal B-MgH2 with the presence of orthorhombic y-MgHphases as results from ball milling. As Li reacts with MgH, theintensity of the main Bragg peaks decreases at the expense oftwo well-defined sets of peaks located at 20=37.56°,40.16°,42.80° and 20= 44.80°,52.30° corresponding to the main(100,002,101) and (111, 200) peaks of Mg and LiH that reachtheir maximum intensity around x=2. This suggests that MgHreacts with Li according to the following conversion reactionMgH+2Lit+2e →Mg+2LiH. Pursuing further lithiation(Fig.1c) leads to a progressive shift in the position of the Braggpeaks corresponding to Mg (a=3.2090(3) A, c=5.2100(4) A,Fig. 1b(5)), whereas those of LiH remain unaffected, indicativeofthe onset of hexagonal Mg-type solid solution (a=3.1970(2) Aand 5.1410(6) A,Fig. 1c(6)). Afterwards, the X-ray evolves with theappearance ofa two-phase process corresponding to the coexistence a b x=0.4 hu mMW x= 1.5 c O X=0 B LLLLLLL 26 2830323436384042 20(°) Cu Ka Figure 2 Electrochemical and structural analysis proof for reversibleconversion/formation of MgH2 in Li/MgH2 cell. a, Evolution of the potential (V) asa function of xfor a Li/MgH cell that was cycled down to x=1.5 at a rate of onelithium in 10 h. Inset: The evolution of the polarization in volts (AV) for variousLi/M Xz cells having X=H, P,N, S, O, F and run under similar current rateconditions; M= metals. b, The XRD patterns taken at various stages of thedischarge, x=0 (starting electrode material), x=1.5 (end of the first discharge)and x=0.4 (end of the first charge). The Bragg peaks marked by an asterisk, B, yand green circle correspond to Mg, B-MgH2, y-MgH2 and LiH, respectively. c, TheHRTEM observations of the MgH2 electrode at various stages of the electrochemicaldischarge/charge process corresponding to the filled black circles in a. Morespecifically, both the HRTEM image and bright-field image (inset) of the MgH2sample, together with the fast Fourier transform or the SAED pattern, are reportedfor the fresh (x=0), fully discharged (x=1.5) and fully charged (x=0.4)materials. The presence of tetragonal MgH2 particles for thex=0 sample is clearas compared with the existence for the x=1.5sample of pseudo-amorphous LiHand crystallized Mg particles (circled by the dashed white line) ranging from 10 to30 nm or of 10 to 40 nm MgH2 particles for the recharged sample. of Mg h.c.p.- and Li b.c.c.-type solid solutions and one singlephase (Li b.c.c. solid solution, Fig. lc (8), ab.c.c. =3.5168A(4))corresponding to the sloping voltage curve from x=2.5 to2.9. Thereversibility of both processes is shown in the inset of Fig. la withan overall discharge capacity of 4,000mAhg , and a reversiblecapacity of 2,700 mAhgat a rate corresponding to one lithium in10 h. At this juncture, it is beyond the scope of this article to furtherdeal with the Li-Mg alloying process previously studied13-15, butto rather focus on the conversion process; therefore, from nowon, cycling curves will be limited to x=1.5 (Fig.2a) so as to befree ofthe Li-Mg alloying reaction. Within this domain of use,MgH, electrodes lead to a discharge capacity of 1,500 mAhgfor a reversible capacity of 1,125 mAhgas compared with370 mAhgfor graphite currently commercialized for Li-ion batteries. The reversibility of the electrochemical process is wellillustrated in Fig. 2b, showing the reformation of the tetragonalB-MgHz(x=0.4) from the Mg and LiH mixture (x=1.5). Although we succeeded in reducing the cell polarization, suchelectrodes remain plagued by a large initial irreversible capacity(approaching 30%) and poor capacity retention limited to less than20 cycles in our early trials, regardless of the cycling rate or voltagewindow. Among the most common reasons to account for suchcycling degradation are (1) the occurrence ofelectrode degradation,which is considerable with conversion reactions, (2) the loss ofelectronic percolation within the electrode due to the repeatedand large volume changes inherent to the MgH conversionreaction, the theoretical value of which can be estimated to be~85%[MgHz(61.59A3)→Mg(46.46 A)+2LiH(2×33.3 A’)]andi (3) the coarsening of the nanoparticles6. Concerningelectrolyte degradation, we confirmed by transmission electronmicroscopy (TEM) the growth of a copious veil-like polymeraround eachi particle. We assessed the importance of theelectrolyte, but no improvement was noted with the Li-basedsalts (LiPF,LiN(SOzCF)2 or LiClO4) or solvents (tetrahydrofuran(THF), carbonates) we have used. Slight cycling improvementswere observed when vinylidene carbonate was added to theelectrolyte mixture or when the cycling voltage was limited tovalues greater than 0.2 V. However, we should recall that in spiteof the existence of such a polymer, CoO conversion reactionelectrodes were cycled for more than 800 cycles with 100%capacity retention", so other factors need to be considered.Besides both a lower electrode electrochemical activity potentialdomain (~0.5V) and a lower polarization voltage (≈0.2V)for MgH as compared with the CoO electrode (≈1.6V and≈0.9V, respectively), another major difference between these twoelectrodes is rooted in the nature of the electrode compositeafter the first discharge. The Li/CoO electrode was shownto lead to an amorphous composite (as deduced by X-raydiffraction (XRD)) of Co nanoparticles (3-5 nm) dispersed ina LizO matrix (as obtained by high-resolution TEM (HRTEM))that remains amorphous on cycling. This contrasts with thecrystalline Mg+LiH'composite, which contains from 20 to 60 nmcrystallites of Mg, which remain crystalline on cycling withoutcoarsening (as deduced by HRTEM) at least through 20 cycles.Mindful of such considerations, more pronounced improvementswere noted by acting both at the active material and electrodeassembly levels. Changing the former by increasing the ball-millingtime of the MgHz powders to 6h, we obtained full dischargecapacity (full conversion namely for x=2) with an irreversibilityless than 25% on the first cycle, corresponding to a reversiblecapacity of 1,480 mAhg (Fig.3). When such powders wereconfined into a pressed copper foam current collector, sustainedcapacities could be achieved for at least 50 cycles by limitingthe reaction to 520 mAhg(Fig.3, inset). Copper foams arebelieved to maintain particle contact on cycling and thus maintaingood electronic conductivity. Obviously, further development isneeded to sustain full capacity of the MgH, electrode, but weare optimistic based on parallels with Li/Si-based electrodes,which initially suffered from electrolyte degradation and giantvolume expansion, but are now on the verge of reaching themarket place. Comparing the MgH2 conversion reaction with those ofCo-based fluorides (CoF), oxides (CoO), sulphides (CoS) orphosphides (CoP2) cycled under similar conditions reveals strikingdifferences,9. The main difference lies in the charge/dischargepolarization (AV), which is the smallest (≈0.25V) measured sofar, as compared to ▲V's of≈1.1V,~0.9V,≈0.7V,~0.6 V and≈0.4V for fluorides, oxides, sulphides, nitrides and phosphides,respectively (Fig. 2a, inset). Considering that this polarization Figure 3 Electrochemical characterization of a MgH2 electrode. Evolution of thepotential (V) as a function of x for a Li/MgH2 cell that was cycled down to 0.15 V at arate of one lithium in 10 h. Inset: The corresponding reversible capacity versus thecycle number for the same cell cycled between 0.15 and 2.5 V in red and in blue fora similar cell cycled with a constrained capacity of 520 mAh g. is rooted in poor mass transport kinetics, this result can berationalized by the fact that the mobility of the ionic species in thesolid state is, among other chemica/physical parameters, mainlygoverned by the electro-negativity and polarizability (softness) ofthe X"- species. Bearing in mind these considerations, H- speciesare expected to diffuse faster than the O-or F- ones, enablingfaster transport and therefore a lower polarization, consistent withour data. The equilibrium potential of the conversion reaction plateauhas been experimentally measured by the galvanostatic intermittenttitration technique at 0.537V. The value in the literature for theGibbs free energy of the above reaction, A.G=-108.2kJmol,confirms that the reaction is spontaneous, and corresponds to theequilibrium potential Eeg=0.560V versus Lit/Li’ from Nernstlaw (E=-A,G/2F), which is in good agreement with theobtained value. Togain ffurther insight intoy theLi-driveni particlestructural/morphological modifications, MgH electrodes werestudied at various stages of the electrochemical reduction/oxidationby both TEM and HRTEM (Fig.2c). The TEM bright-field imageof the starting material (inset x=0) indicates that it containsmicrometre-sized agglomerates mainly made of well-crystallizedtetragonal MgH2 particles, as deduced by an HRTEM image andits corresponding indexed fast Fourier transform (Fig. 2c x=0).For the fully discharged sample (x=1.5), both bright-field imagesand the selected-area electron diffraction (SAED) pattern revealedagglomerates made of a mixture of both pseudo-amorphous LiH(diffuse ring on the SAED pattern) and crystalline Mg nanoparticles(dots on the SAED pattern). The magnified HRTEM image of thefully discharged sample in the left-hand side of the image (x=1.5)reveals the size of the so-obtained metallic Mg nanoparticles(dashed ellipse), which ranged from 10 to 30 nm. Last, for there-oxidized sample, bright-field images indicate the presence oflarge and well-crystallized particles with sizes ranging from 10to 40 nm. The SAED pattern realized on an agglomerate revealsintense rings indexed with the p phase as well as diffused reflectionsthat could be assigned after a careful treatment to remaining Mg.This observation is in agreement with the fact that 73% of thematerial converts back to its original MgH, structure. Applying known thermodynamic calculations to conversionreactions (MM'H+xLixLiH +MM') enlisting either binary(MH) or ternary (MM'H) hydrides reveals numerous hydridesfor which ▲Gr (MH.)/x or ▲Gr(MM'H)/x is greater than ▲G(LiH), implying the feasibility of the reaction. Such predictions Figure 4 Electrochemical reactivity and structural characterization of various metal hydrides towards Li. Electrochemical discharge curves for Li/metal-hydride cellsusing TiH, NaH, AB,Hs and MgNiHs.7 as positive electrodes cycled down 0.01 V at a rate of one lithium in 100 h, together with the corresponding XRD patterns taken both forthe as-made (x=0; black) and the fully discharged samples (red). were experimentally confirmed for all ternary and for several binaryhydrides, but for brevity we report X-ray evidence for only twobinary (TiH, NaH) and ternary (LaNi4.25Mno.75Hs, MgNiHs.7)hydrides, the discharge curves of which are shown in Fig. 4. TiHz,NaH,LaNi4.25Mno.75H, (simplified here as AB,Hs)and MgNiH.7can react with 2, 1, 4.6 and 3.7 Lit ions, in good agreementwith the above conversion reaction, respectively. Moreover, this isfurther corroborated by the XRD patterns collected at the end ofthe discharge, which indicate the coexistence of LiH with anotherphase being Ti, Na, AB, or MgzNi (Fig. 4). Last, regardless of thebinary or ternary hydrides studied, TEM measurements on samplesrecovered from fully discharged cells reveal the formation of acomposite matrix made of 10-50 nm metal particles embedded ina LiH matrix. Returning to prospects for hydrogen storage, the highlydivided metals or hydrides offer substantial promise. The mostexquisite way of probing this promise is to monitor the hydrogensorption/desorption properties of the nanometric materialsobtained either at the end of the discharge (x=1.5) or at the endof the charge (x=0.4), which according to the aforementionedconversion reaction consist of a mixture of 0.75 Mg, 0.25 MgH2and 1.5 LiH and of 0.2 Mg, 0.8 MgH and 0.4 LiH, respectively.For the hydrogen sorption experiment, the Mg-based composite(x=1.5) was treated at 100°C, under 10 bars of hydrogen forl h (Fig.5a). Surprisingly, this low temperature and reaction time were sufficient to afford a powder mainly consisting(95%) oftetragonal B-MgH2 with some unreacted Mg. This high hydrogenreactivity for as-prepared materials is of prime importance, asit is commonly reported that bulk Mg can take up hydrogenonly under more severe conditions; pressures as high as 30 to40 bars together with temperatures greater than 340°C and withreaction times ranging from 6h to two weeks are reported20.21to be needed to produce the same degree of absorption. At thispoint it could be argued that such an enhanced absorption isfacilitated by the presence of highly divided LiH. To check thispoint, LiH was successfully removed from the composite usinga one molar triethylborane (TEB) in THF solution according tcthe reaction LiHsol+TEB/THF→ TEBH-Li+/THF. The resultingpowder was treated under the same protocol as the compositeand a full conversion to B-MgH was noted, confirming thepossibility to reach the full theoretical capacity value of 7.6wt%with temperatures as low as 100°C. Turning to MgH hydride charged until x=0.4, desorptionwas successfully achieved by treatment at 200C under primaryvacuum (10 mbar) for 5h (Fig.5b). This corresponds to areduction in desorption temperature of 140°C as compared withany previous data reported on bulk MgH. To further confirmthis improvement, we measured the desorption properties ofour ball-milled MgH2 precursor powders, which consist of bothnanometric crystallite and micrometric particle sizes, and no a Figure 5 Electrochemically prepared reactive MgHz nanocomposite forhydrogen storage. a,b, XRD patterns for samples from Li/MgH2 cells that weredischarged to x= 1.5 and after 1 h of absorption at 100°C under 10 bars ofhydrogen (a) and discharged and charged back to x=0.4 and then desorbed for 5 hat 200 °C under vacuum (b). evidence of desorption could be noted until 300°C. This clearlyemphasizes the role of the nanometric particle size produced duringthe electrochemical reduction on the sorption properties of MgH2.For completeness, similar sorption measurements were carriedout on fully reduced TiH powders (x=2 namely: Ti+2LiH).Full hydrogenation was achieved in 2 h at 200°C under 2 barshydrogen pressure, whereas temperatures ranging from 440 to550°C are reported to achieve the full hydrogenation of bulkTi (ref. 22). Overall, the above measurements clearly highlightthe benefits of our electrochemically formed metal-hydridenanopowders with respect to hydrogen absorption/desorption.We fullyrecognizeethatpressure-composition-temperaturemeasurements under a moisture-free environment have been themost accurate way to measure hydrogen absorption/desorptionproperties, and benchmark our nano-Mg powders with respectto other nanoparticle (<50 nm) powders prepared by alternativetechniques such as gas-phase condensation23, melt-infiltration24and sono-electrochemistry2. Such measurements, which requirespecific equipment (for example, the proper attachment to loadthe samples in a dry box), are currently being planned as aconfirmation step. In summary for the first time, Li-driven conversion reactionswere demonstrated for the metal-hydride family withn twomajor consequences. Metal hydrides are promising candidatesfor negative electrodes in Li-ion batteries with the advantage ofhaving high capacities in a safe potential window of 0.1-0.5 Vversus Lit/Liand showing the lowest polarization ever reportedfor conversion reaction electrodes. Moreover, unusually lowabsorption/desorption temperatures for the electrochemicallyproduced nanosized metal hydrides are quite encouraging.Needless to say that systematic experimental investigation of themetal-hydride nanocomposite formation is required to betterunderstand which particle properties (size, structure, defects) arethe source of the improved hydrogen sorption properties. Although several improvements to the present work areimmediately apparent, namely in terms of designing better current-collector-active materials electrodes to achieve bettercapacity retention performance or scaling up our nanocompositeelaboration process, this work could markedly boost the field ofLi-ion battery and hydrogen storage research. Finally, we believethat suggesting metal hydrides as a new concept for negativeelectrodes in Li-ion batteries will help over the next 20 yearswith the realization of power batteries for mobile applications.By bridging Li-ion-battery and hydrogen storage technologies,metal hydrides could serve to cross-fertilize interactions betweenbattery and fuel-cell research, which have in the past followedparallel directions. METHODS PREPARATION OF THE METAL-HYDRIDE PRECURSOR PHASES The binary hydrides TiH2, NaH and MgH2 used in this study were purchasedfrom Aldrich. LaNi4.25Mn0.75Hs (denoted here as ABsHs) was provided byM. Latroche (LCMTR CNRS UPR 209, Thiais, France). The ABs alloy wasprepared by radiofrequency levitation melting, and then the hydride wasobtained from the alloy by a solid-gas reaction. For the preparation of theMgNiH3.7 hydride, 1 g of the elemental powder mixture (2 Mg+Ni) wasground for 10 h with a SPEX 8000 mixer mill in a 50 ml stainless-steel millingcontainer with a ball-to-powder weight ratio of 20, and then heat-treated underhydrogen at 300 °C for 20 h. They were all single phases as deduced from X-raymeasurements, with the exception of MgH2. For MgH solely, better results were obtained by preparing MgHz in-houseby first ball-milling for 2 h Mg powders (Aldrich) mixed with 10% of previouslyground degassed MCMB 2528 having a Brunauer-Emmett-Teller surface areaof 600 m’g. A ball-to-powder weight ratio of 10 was used. Afterwards, thismixture was hydrogenated at 350°C for 10 h under 20 bars of hydrogen pressurevia a solid-gas reaction in a stainless-steel vessel, and subsequently ball-milled asecond time before being used as electrode material. The XRD patterns of thecarbon-containing metal-hydride composites did not indicate any structuralmodification in the parent phases on ball-milling. For all metal hydrides, electrodes were prepared by grinding in a mortar 1 gof powder in the presence of 10 wt% of carbon SP (black carbon from MM). ELECTROCHEMICAL TESTS Swagelok-type cells were assembled in an argon-filled glove box and cycledusing a Mac pile automatic cycling/data recording system (Biologic Co.)operating in galvanostatic mode between 3 and 0.005 V versus Lit/Li. Thesecells comprise (1) a 1 cm², 75-um-thick disc of composite positive electrodecontaining 7-10 mg of metal hydrides mixed with 15% carbon SP (blackcarbon from MM), (2) a 1 cm’ Li metal disc as the negative electrode and(3) a Whatman GF/D borosilicate glass-fibre sheet saturated with a 1 MLiPF6 electrolyte solution in 1:1 dimethyl carbonate/ethylene carbonate as theseparator/electrolyte. The cells were cycled at a rate of one lithium in 10 or100 h. For MgH2, some cells were limited in time to 15 h at a rate of one lithiumin 10h. Galvanostatic intermittent titration techniques were used to measurethe equilibrium potential of the MgH2+2Li++2e一→2LiH+Mg reaction.For this purpose, a rate current of one lithium in 10 h was applied during stepsof 1 h followed by 24 h of relaxation time. XRD AND TEM A Brucker D8 diffractometer (Co Kα=1.79026 A or Cu Ko=1.54180A)equipped with a Vantec detector was used to carry out the XRD of the metal-hydride electrodes at various stages of the electrochemical reduction/oxidation.The lattice parameters and the space-group determinations were carried out bya full pattern matching method using the program Fullprof. A Tecnai F20 ST transmission electron microscope, equipped withan energy-dispersive X-ray spectroscopy system, was used to conduct ourTEM/HRTEM investigations. To carry out the aforementioned experiments, the cells, once cycled downto the required voltage, were stopped and opened in a dry box. The electrodematerial was recovered and washed with dimethyl carbonate. The electrodematerial was introduced inside a capillary or on a glass sample holder for XRDanalysis or placed onto a copper grid mounted on our TEM sample holder.Through a special mobile airlock of our own design, the sample was thentransferred to the TEM, without any exposure to air, for SAED pattern andbright-field image collection. SOLID-GAS REACTIONS Sorption reactions were carried out in a hermetically sealed reactor with avalve enabling us to fill the container in the presence of hydrogen (absorption)or to evacuate (desorption). The reactor was filled with 0.1 g of a samplerecovered from Li/hydride cells. The sample loadings were done inside anargon glove box to avoid oxygen contamination. Then, the reactor was placedinside a tubular furnace. The temperature and the duration of the heattreatment were selected to achieve a complete absorption or desorption in goodagreement with the XRD of the powder carried out after the treatment undervacuum or hydrogen. Received 1 February 2008; accepted 8 September 2008; published 12 October 2008. ( R e fere n ces ) ( 1. T arascon, J.-M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414,359- 3 67(2001). ) ( 2. T 7 irado, J . - L . I norganic m a teria l s f o r the negative electrode o f lithium-io n ba t terie s : State-o f -t h e a r t and future prospects. Mater. Sci. Eng. R 40,103-136( 2 003). ) ( 3. ) . Poizot, P, Laruelle, S., Grugeon, S. , Dupont, L . & Tarascon, J.-M. Nano-sized transition metal oxidesas negative electrode materials for lithium - ion batteries. Nature 407, 496 - 499 (2000). ) ( 4. 上 . Taberna, P-L.,Mitra, S., Poizot, P, Simon, P . & Tarascon,J.-M. High rate capabilities Fe, O, -based Cu ) ( nano-architectured electrodes for lithium-ion battery applications. Nature Mater. 5,567-573 ( 2006). 5. . B ruce, P. G., Scrosati , B. & Tarascon, J.-M. Nanomaterials f o r rechargeable lithium ba t teries. Angew. Chem. Int. Ed. 47,2930- 2 946 (2008). ) ( 6 ) . . . Schapbach, L. & Zuttel, A. Hydrogen-storage materials for mobile applications. Nature 414, 353-358(2001). ) ( 7. S eayad,A.-M . & Antonelli, M.-D . Recent advances in hydrogen storage in metal-containing inorganic n anostructures and r e lated materials. Adv. M ater. 16,765-77 7 (2004). ) 8. 2Zuttel, A. Materials for hydrogen storage. Mater. Today 24-33 (2003). ( 9. S androck, G. P a noramic overview of hydrogen storage alloys from a gas reaction poi n t of v iew. . J. Alloys Compounds 293-295,877 - 888 (1999). ) ( 10. H uot, J. , L i ang, G., Boily, S., Neste, A. V. & Schulz, R. Structural study and hydrogen so r ption kinetics o f ball-milled magnesium hydride. J. Allo y s Compounds 495,293-295 (1999). ) ( 11. Fatay, D., Revesz, A. & Spassov, T. Particle size and cataly t ic ef f ect on the dehydriding of MgH J . Alloys Compounds 399, 237-2 4 1 (2005). ) 12. Oelerich, W., Klassen, T. & Bormann, R. Metal oxides as catalysts for improved hydrogen sorption in nanocrystalline Mg-based materials. J. Alloys Compounds 315,237-242(2001).13. Herbstein, F. H.& Averbach,B. L. The structure of lithium magnesium solid solutions. Acta Metall. 4,407-413(1956). 14. Shi, Z., Naik, D. & Gole, J. L. Electrochemical properties of Li-Mg alloy electrodes for lithiumbatteries. J. Power Sources 92,70-80 (2001). 15. Park, M., Kim, Y. U., Kim, H. & Sohn, H. J. Enhancement of the rate capability and cyclability of anMg/C composite electrode for Li secondary batteries. J. Power sources 158, 1451-1455 (2006). 16. Larcher, D. et al. Recent findings and prospects in the field of pure metals as negative electrodes for. Li-ion batteries. J. Mater. Chem. 17,3759-3772(2007). 17. Tarascon, J.-M. et al. New concepts for the search of better electrode materials for rechargeable ( l ithium b a tteries. C. R. Chimie 8, 9-15(2005). ) 18. Obrovac, M.N et al. Alloy design for lithium-ion battery anodes. J Electrochem. Soc. 154,A103-A108 (2007). ( 19. Tarascon, J.-M., Grugeon, S. , L a ruelle,S. , Larcher, D. & Po i zot, P. in Lithium Batteries-Scien c e and T echnology (eds Nazri, G. A. & Pistoia, G.) C h . 7 (Kluwer-Academic, 20 0 3). ) ( 2 0. G erard,N. & Ono, S. in Hydrogen in Intermetallic Compounds II Vol. 67, 44 (ed. Schlapbach, L.) 1 65-195 (Springer, 1 992). ) ( 2 1. Reimann, A . -L. Clean-up of hydrogen by magnesium. Phil. Mag. 16, 673-686 (1933). ) 22. Sandim, H. R. Z., Morante, B. V.& Suzuki, P. A. Kinetics of thermal decomposition of titanium ( h ydride powder using in situ h igh-temperature X-ray diffraction (HTXRD). Mater. Re s. 3, 293-297(2005). ) ( 23. F riedrichs,O.,Kolodziejczyk, L., Sanchez-Lopez, J. C., Lopez-Cartes, C. & Fernandez, A. Synthesis of n anocrystalline MgHzpowder by gas-phase condensation and in situ hydridation: TEM, XPS and X RD study.J. A lloys Compounds 434-435,721-7 24 (2007) ) ( 24. de J ongh, P. E. et al. The preparation o f carbon-supported magnesium nanoparticles usi n g ) ( m elt-infiltration. Chem. Mater. 19, 6052-6057 ( 2007) ) ( 25. H aas, I . & Gedanken, A . Synthesis of metallic magnesium nanoparticles by sonoelectrochemistry. Chem. Commun. 1795-1797(2008). ) ( Reprints and p e rmissions information is available online at http://npg.nature.com/reprintsandpermissions.Correspondence and requests for materials should be addressed to L.A A. ) Jump to main content Jump to navigation nature.com homepage Publications A-Z index Browse by subject Login reprints & permissions@npg goAdvanced search Reprints and permissions About this site Nature Publishing Group offers a range of reprints and permissions services for authors, readers, writers and commercial companies. Browse reprints & permissions Home : Reprints and permissions Author reprints Published authors in any Nature Publishing Group journal can order reprints of their article for distribution to their peers and colleagues. Author reprints are offered at a competitiverate, in quantities ranging from 100 to 500 copies. Authors can order reprints in print or as an encrypted PDF, and can place an order either before or after publication of their paper. More information or to order Commercial reprints Commercial reprints are an excellent way for companies to promote and inform the medical community about their activities and products. They are recognized as a credible andtimely source of information, and the Nature Publishing Group portfolio contains some of the most highly cited and influential journals in the world. More information or to order Permissions requests Nature Publishing Group grants permission for authors, readers and third parties to reproduce material from its journals and online products as part of another publication or entity.This includes, for example, the use of a figure in a presentation, the posting of an abstract on a web site, or the reproduction of a full article within another journal. Certain permissionscan be granted free of charge;others incur a fee. For more information or to request a permission Other services Details of how to order single issues of Nature Publishing Group journals, posters of covers from recent issues of selected journals, or translated reprints. For more information or to order Frequently asked questions If you are unsure of the service you require please go to frequently asked questions. Contact details For more information or to order. Main navigation 。 Site content Reprints and permissions homepage Author reprints Commercial reprints Permission requests Other services FAQs Contact details 。Resources@npg Advertising work@npg For librarians Authors and referees NPG Journals by Subject Area ● Chemistry 口Chemistry Drug discovery 口Biotechnology Materials Methods & Protocols ● Clinical Practice & Research Cancer Cardiovascular medicine Dentistry Endocrinology Gastroenterology & Hepatology Methods & Protocols Pathology & Pathobiology Urology ●Earth & Environment Earth sciences Evolution & Ecology Life sciences Biotechnology Cancer Development 口Drug discovery Evolution & Ecology Genetics Immunology Medical research Methods & Protocols Microbiology Molecular cell biology Neuroscience Pharmacology Systems biology ·Physical sciences Physics Materials byA-ZIndex Home : Reprints and permissions Extra navigation naturenanotechnology Table of contents e-alerts The latest issue highlights straight to your desktop Sign up here lop AboutNPGhttp://www.nature.com/reprints/index.html (第6/7页)2008-11-49:41:50 Home : Reprints and permissions Contact NPG Nature jobs.com Privacy policy Legal notice Accessibility statement ●RSS web feeds Help ature materials|VOL |NOVEMBER www.nature.com/naturematerialsC Macmillan Publishers Limited. All rights reserved. ature materials|VOL|NOVEMBER|www.nature.com/naturematerials@ Macmillan Publishers Limited. All rights reserved.
确定






还剩11页未读,是否继续阅读?
武汉科思特仪器股份有限公司为您提供《ARTICLES METAL HYDRIDES FOR LITHIUM ION BATTTERIES》,该方案主要用于其他中--检测,参考标准--,《ARTICLES METAL HYDRIDES FOR LITHIUM ION BATTTERIES》用到的仪器有CS2350H双单元电化学工作站(双恒电位仪)、CS350M电化学工作站/电化学测试系统、CS310M电化学工作站/电化学测试系统
推荐专场
相关方案
更多
该厂商其他方案
更多


















