方案详情
文
池塘循环流水养殖和传统池塘养殖中草鱼的生长和肌肉质量Growth and Muscle Quality of Grass Carp (Ctenopharyngodon idella) in In-Pond Raceway Aquaculture and Traditional Pond Culture
上海海洋大学教育部水生遗传资源开发利用重点实验室
方案详情

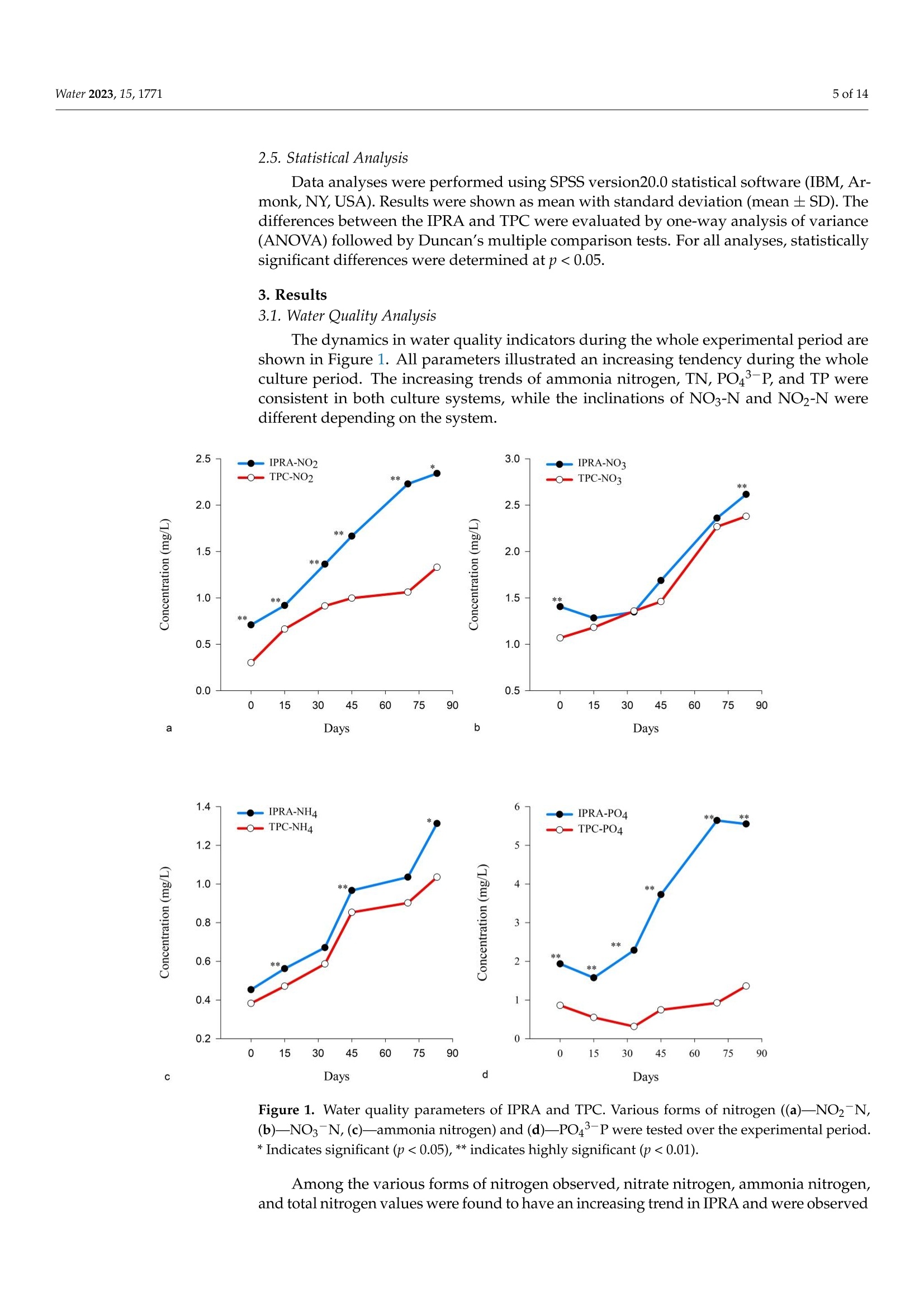
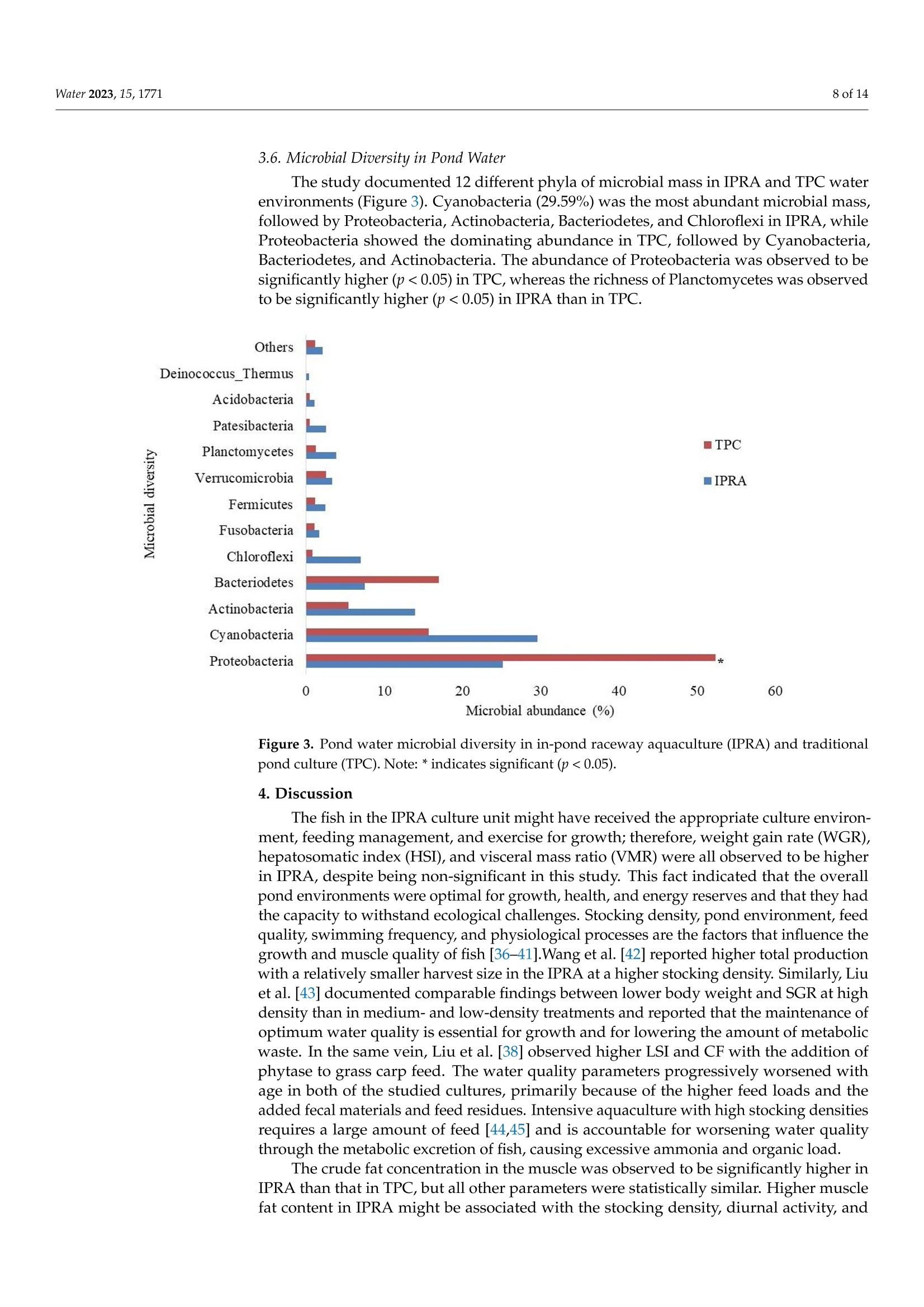
池塘循环流水养殖和传统池塘养殖中草鱼的生长和肌肉质量Growth and Muscle Quality of Grass Carp (Ctenopharyngodon idella) in In-Pond Raceway Aquaculture and Traditional Pond Culture上海海洋大学教育部水生遗传资源开发利用重点实验室Water 2023, 15,17712 of 14 池塘循环流水养殖和传统池塘养殖中草鱼的生长和肌肉质量 Citation: Ghart i , K.; Yan, L .; Li , K.; Boonpeng, N.; Liu, L. Growth and Muscle Quality of Grass Carp (Ct e nopharyng o don id ella) i n In-Pond Raceway Aquacult u re and Traditional Pond Culture. Water 2023,15,1771. https://doi .org/10.3390/ w15091771 Academic Editor: Dapeng Li Received: 31 March 2023 Revised : 27 April 2023 Accepted: 30 April 2023 Publ i shed: 5 May 2023 Copyright: @ 2023 by the authors. L i censee MDPI, Basel, Switzerland. This article is an open access ar t icle distributed under the terms and conditions of the Crea ti ve Commons At tri bution (CC BY ) l icense (https://creativecommons.org/licenses/by/4.0/). Abstract: In-pond raceway aquaculture (IPRA) i s the rational prescription for water eutrophication and improves the pond environment, enhancing production and the quality of f ish. This experi-ment explored the growth performances and muscle quality of grass carp with stocking densities of 32 t ail/m’ and 0.07 tail/m²i n IPRA and tradit i onal pond culture (TPC), respectively. The hepatoso-matic i ndex, visceral mass ratio, and correction factor were statistically similar in IPRA compared to TPC. While the weight gain rate (p<0.001) and the content of crude l ipid (p < 0.05) i n t he f lesh of grass carp were observed to be statistically promising in IPRA, the pH and water holding capacity, as well as hardness and chewiness, in grass carp muscle were not significantly di f ferent between the two culture systems. However, the 2-MIB concentration in the muscle was observed to increase cont i nuously for the complete culture period in IPRA. The abundance of Proteobacteria was found to be higher in TPC (p <0.05), while the richness of Planctomycetes was superior in IPRA (p<0.05). Despite the high stocking density, the of f -flavor in IPRA-produced grass carp had l ess of an impact on the flesh aesthetic quality compared to TPC. Considering all these fac t s, the results of t his study show that grass carp with a better muscle quality can be produced from IPRA. Keywords: grass carp; muscle qual it y; in-pond raceway aquaculture; of f -f lavor 1. Introduction Production intensification is an alternative for increasing production and produc-tivity and for ensuring the sustainable supply of aquacul t ure production [1,2]. In fac t , pond culture is a key practice globally for maximizing production, protein supply, and in-come [3]. The production of grass carp (Ctenopharyngodon i della) ranks highly among the freshwater aquaculture industries [4], accounting for11.8% of global aquaculture production [5]. The growth of fish is affected by multiple factors, from the rearing environment and food nutrition to genetics [6,7]. IPRA ensures the better control of water movement, enhances water quality, and allows for high-density stocking, thus increasing production and productivity compared to TPC. Wang et al. [8] documented a high potential for more controllable and efficient production of bluntnose black bream, channel catfish, yellow catfish, and largemouth bass in IPRA, but information on the production efficiency of grass carp in IPRA is scarce. Similarly, Fatima et al. [9] reported a higher average biomass (57.33 kg/m³) of GIFT tilapia from IPRA and 0.38 kg/m’from traditional ponds in Pakistan. Wholesomeness, freshness, and i ntegrity are the major aesthetic characteristics that describe fish muscle quality [10]. In other terms, these characteristics define the hygienic, nutritional , and sensory features, as well as the serviceability of the fish. The freshness of fish muscle i s ref l ected in i ts appearance, flavor, and texture, while nutritional consti t uents and water-holding capacity (WHC) also have considerable i mpacts on fish muscle quality. These characteristics are affected by the species, age, and size of f ish and other factors (available nutrients, period, water condition and environment, and consumers) as well as the methods of slaughter, storage, and processing [7,11-13]. Thus, modulation of the culture struc t ure and environment to enable higher productivity with i mproved quality i s a prime concern of aquaculture at present [7,14]. The nutri t iona l characteristics of fish include protein, lipid, fatty acids, amino acids, and minerals [15]. Wen et al. [16] reported elevated muscle protein content and WHC with depressed moisture, lipid, and ash contents, as well as improved dietary phosphorus in grass carp. In addition, muscle pH has a l inear relationship with softness and WHC [13], which describes the characteristics of muscle for avoiding moisture loss. Similarly, water quality parameters were allied with the growth, nutritional, and sensory values of aquatic species, including f ish [17,18]; thus, grass carp cultured in poor-quality water yields a low grade of fish quality [19]. With the rapid development of aquaculture, water pollution that negatively affects the water environment and fish quality is becoming an important concern [20,21]. Therefore, water qual i ty deterioration and eutrophication are serious concerns in intensive aquacul-ture, as t hey encourage cyanobacterial blooms and result in the production of earthy-musty compounds in f ish flesh [14,22]. The common odorous compounds in fish muscle are 2-methylisoborneol (MIB; 1,2,7,7-tetramethylexo-bicyclo[2.2.1]heptan-2-ol) and geosmin (trans-1,10-dimethyl-trans-9-decalol), which degrade quality and reduce the consumer acceptability and market development [23]. Qin et al. [24] and Tang et al . [13] reported mi-crobial diversity of pond water and described Proteobacteria, Bacteriodetes, Actinobacteria, and Planctomycetes as the dominant microorganisms in different culture systems, while Lukassen et al. identified Proteobacteria as a l eading geosminproducer [25]. On such a backdrop, improved culture models (such as a split-plot/partition aquaculture system and in-pond raceway system) have been i mplemented in t raditional culture ponds with more efficient water usage, l ower env i ronmental impacts, and high-quality aquatic products with no off-flavor complaints; these models have received more attention i n aquaculture [26-28]. IPRA has been used i n recent years as an aquaculture engineering approach and environ-mental remediation key for water eutrophication. Thus, IPRA is modulated in just 2-5% of pond areas for intensive culture, making the remaining spaces for water purification more productive and easily manageable, ul t imately resul t ing in better water quality [29,30]. Grass carp is a popular fish species with a large global freshwater production, pro-viding an inexpensive source of high-class animal protein for consumers [4,28], and is a good candidate for IPRA. In recent years, the muscle quality of cultured grass carp has been declining , mainly due to pol l ution and compromised pond water quality [7]. Poor water quality can be a significant driver for reductions in production, product quality, and profit [17,31]; thus, shifting to and adopting IPRA could purify the eutrophic water and improve t he culture environment, yielding a higher f ish quality. Furthermore,proper feeding management in IPRA for monitoring the amount of residual feed can be a useful approach for enhancing the muscle quality of grass carp [32,33].Thus, this work aimed to investigate the growth and muscle quality of grass carp in IPRA and TPC. 2. Materials and Methods 2.1. Experimental Layout Set-Ulp The experiment was carried out in IPRA and TPC for 83 days a t Songjian, Shanghai, with the experimental species being grass carp (Ctenopharyngodon idella). The IPRA was installed in a 2.0 ha traditional pond and consisted of three rectangular raceways (out of ten raceways; 25 m×5m×2.5 m) that were interrelated with each other viaa common pathway. Each raceway unit had airl i ft, f ish culturing, and waste t ransfer compartments. The fish culture area was separated by barriers (1.0 cm × 1.0 cm mesh) that were set up at the two ends of each cell . Stocking density was maintained at 32 t ail/m ; surface and bottom aeration were provided. By contrast, TPC was maintained in three 0.33 ha ponds, each of which had the same water depth and a stocking density of 0.07 tail/m2. For all ponds, t he standard feed and feeding techniques were used, and occasional topping of a smal l quantity of water was carried out to compensate for evaporation loss. 2.2. Water Quality Measurement Water sample collection was performed in an integrated manner using a Plexiglas water collector before 8 a.m. in a fortnightly interval for physicochemical parameters, and the water was subsequently transported to the laboratory for analysis in a cool box. The dissolved oxygen level of both culture systems was more than 6 mg/L for the whole experimental period, which was opt i mal for the water temperatures of 22-34 °C and pHs of 7.2 to 7.8. For chemical analysis, water samples were f iltered with Whatman GF/C glass fiber (0.45 um), and nitrite nitrogen (NO2-N) was examined by using spectrophotometry; nitrate nitrogen (NO3-N) was examined by using sulfamic acid ultraviolet spectrophotometry; ammonia nitrogen was examined by using nesslerization colorimetry; and phosphorus (PO4-P) was examined by using phospho-molybdenum blue spectrophotometry. Unfil-tered samples were analyzed for total phosphorus (TP) by using ammonium molybdate spectrophotometry method and total nitrogen (TN)was examined by using the alkaline potassium persulfate digestion UV spectrophotometry method. Total suspended solids (TSS) were determined per APHA (2005) and l chemical oxygen demand (COD D)1by using the magnesium persulfate method (GB 1189289). Similar l y, chlorophyll-a analysis was performed according to ISO (1992) and WHO (1999). The details of the water quality status in IPRA and TPC are depicted in Table 1. Table 1. Water quality indicators of complete culture period in in-pond raceway aquaculture (IPRA) and tradi t ional pond culture (TPC). Water Quality Indicators IPRA TPC Min Max Mean±SD Min Max Mean±SD Temperature (°C) 22.00 32.60 26.9±4.29 22.80 34.00 27.4±4.58 Dissolve oxygen (mg/L) 6.50 8.10 7.4±0.63 6.90 8.00 7.7±0.18 pH 7.20 7.60 7.4±0.12 7.50 7.90 7.7±0.18 Total nitrogen (mg/L) 3.05 9.51 5.69±2.33 2.01 8.58 5.13±2.65 Total phosphorus (mg/L) 2.11 7.71 4.32±2.29 0.66 2.67 1.35±1.00 CODMn(mg/L) 4.62 13.22 10.03±4.79 6.39 10.96 7.74±2.24 Chlorophyll-a (mg/L) 112.06 441.09 268±155.41 80.96 351.49 173.91±116.17 Total soluble solid (mg/L) 57.50 82.49 71.83±50.0 64.67 108.90 85.85±42.90 2.3. Sample Collection and Analysis Three grass carp fish from each raceway and pond (n =18) were sampled and trans-ported to t he laboratory at 4C. The morphological trai t s of the fish were evaluated, and the Condition Factor (CF) was determined by CF=(TW/SL3)×100. Weight gain was determined by final weight/initial weight. The hepatosomatic i ndex and the visceral mass ratio were calculated as (LW/TW) × 100 and (VW/TW) × 100, respectively, after identifying the liver weight (LW) and visceral mass weight (VW). Immediate dissection from the dorsal white muscle of the body was performed for proximate nutrient anal-ysis of the fish, and subsamples (54, triplicates of each sample) were preserved frozen (-20°C) till investigation. The chemica l composi t ion of muscle was analyzed according to the National Food Safety Standard, China. Moisture content was determined by using method GB 5009.3-2016; ash by using method GB 5009.4-2016;; crude protein by using the Kjeldahl method (GB 5009.5-2016); and thelipid content by using the Soxtherm extraction system (SOX 416 Macro; Gerhardt, Germany). Moisture and ash content were determined within 12 h of postmortem by using the standard method . The f ish homogenate, prepared by thoroughly mixing 2 g of muscle in 10 mL of distilled water, was used to measure pH using a Cyberscan model 500 pH meter (Euteon Instruments, Jurong, Singapore) and WHC by using the f ilter paper method. Muscle quality was evaluated via a t exture analyzer (Universal TA, Shanghai, China) using (1×1×1 cm’) pieces from t he dorsal part of the fish. The hardness (g), springiness (mm), cohesiveness, stickiness (g), chewiness (g xmm), and adhesiveness were evaluated through exponential l inking software. For off-flavor (GSM and 2-MIB) detection, samples were taken out at -80 C (f ish f lesh was collected and stored in the f reezer) and thawed, a 5 g fillet sample was homogenized and analyzed [34]. Brief details of the analytical procedures are shown i n Table 2. Notes: Detect i on l imi t of t his method was 4.72 ng/L and 5.48 ng/L for GSM and 2-MIB, respec t ively [34] 2.4. Microbial Diversity in Pond Water The water sample (500 mL) was f iltered through a 0.2 um polycarbonate filter with a diameter of 47 mm (Mil l ipore , Billerica, MA, USA) and then stored at -20 °C until processing. The DNA was extracted and purified from f iltered samples using the soil DNA kit (Omega Biotech, Norcross, GA, USA). A nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, NC, USA) was used to evaluate the nucleotide concentration, and the DNA quality was checked by 1% agarose gel electrophoresis. The purified genomic DNA (20 ng/mL) was sent to Majorbio Bio-pharm Biotechnology Co., Ltd. (Shanghai, China), and the MiSeq benchtop sequencer (Illumina, San Diego, CA, USA) was used for 16S rRNA gene-based amplicon sequencing. The bacterial 16S rRNA gene was analyzed via polymerase chain reaction (PCR) using bacterial primers (338F-ACTCCTACGGAGCAGCAG and 806R-GGACTACHVGGGTWTCTAAT) [35]. PCR amplification in triplicate was performed using a 4.0 uL 5FastPfu buffer, 2.0 uL deoxyribonucleotide t riphosphate (dNTP) (2.5 mM), 0.8 uL forward and reverse primers (5.0 mM), 0.4 uL FastPfu polymerase, and 10.0 ng template DNA, respectively. Finally, PCR-grade water was added to the above mixture to obtain a f inal volume of 20.0 uL. The PCR thermal program was set at 95 ℃ for 3 min; then 27 cycles were performed at 95℃,55°C, and 72 °C for 30, 30, and 45 s, respectively. The PCR product was extracted from a 2% agarose gel and further purified using the AxyPrep DNA ge l extraction kit (Axygen Biosciences, Union City, CA,USA). The MiSeq technology was used to analyze microbial communities by high-throughput pyrosequenc-ing (I l lumina, San Diego, CA, USA). The 16S rDNA gene cloning and sequencing were performed in accordance with the instructions provided by Majorbio Bio-pharm Biotech-nology Co., Ltd. (Shanghai, China). The right-sized PCR products were chosen at random, and the National Center for Biotechnology Information (NCBI’s) search engine was used to compare the sequenced 16S rDNA genes and f ind the gene that most closely matched it. The sequence was compared with the sequence in the GenBank database, and the basic local alignment search tool algorithm was used to determine t he approximate phylogenetic attribution. All the sequences used in this study are available from the NCBI Sequence Read Archive (SRA) under accession number MJ20190808066. 2.5. Statistical Analysis Data analyses were performed using SPSS version20.0 statistical software (IBM, Ar-monk,NY, USA). Results were shown as mean with standard deviation (mean ± SD). The differences between the IPRA and TPC were evaluated by one-way analysis of variance (ANOVA) followed by Duncan’s multiple comparison tests. For all analyses, statistically significant differences were determined at p < 0.05. 3. Results 3.1. Water Quality Analysis Water 2023, 15, x FOR PEER REVIEW The dynamics in water quality indicators during the whole experimental period are shown in Figure 1. All parameters i llustrated an increasing tendency during the whole culture period. The increasing trends of ammonia nitrogen, TN, PO46 of 15-P, and TP were consistent i n both culture systems, while the inclinations of NO3-N and NO2-N were different depending on the system. Figure 1. Water quality parameters of IPRA and TPC. Various forms of nitrogen ((a )—NO2−N,(b )—NO3−N, (c )—ammonia nitrogen) and (d )—PO43−P were tested over the experimental period. *Indicates significant (p < 0.05), ** indicates highly significant (p < 0.01). Among the various forms of nitrogen observed, nitrate nitrogen, ammonia nitrogen, and total nitrogen values were found to have an increasing trend in IPRA and were ob- served to have a highly significant difference (p < 0.01), except for a few samplings in to have a highly significant difference (p<0.01), except for a few samplings in TPC. The concentrations of nitrite nitrogen were significantly higher (p<0.01) in IPRA at al l sampling days, except at 83 days (p<0.05). The ascending trend of nitrate nitrogen concentrations in IPRA showed a highly significant difference (p <0.01) at 0 and 83 days and a significant difference (p<0.05) at 70 days compared to TPC. Similarly, concentrations of ammonia nitrogen also showed a rising trend in both culture systems; however, it was significantly higher (p < 0.01) at15, 45, and 83 days (p<0.05) i n IPRA. The phosphate concentration was significantly higher (p<0.01) for the complete culture period in IPRA. Likewise, the concentrations of total nitrogen, total phosphorous, and chemical oxygen demand were significantly higher (p <0.01) in IPRA. However, chlorophyll-a was higher (p<0.01) in TPC (Table 1). 3.2. Growth Performance The growth performances of grass carp were recorded to be higher in TPC than in IPRA during the experimental period. The harvest weight (p<0.05) and the length (p <0.05) were significant (p <0.01) in TPC, while the weight gain rate was significantly better in IPRA. The non- significant differences were observed for the HSI, VMR, and CF of fish in IPRA and TPC systems, but higher values of HSI and VMR were observed in IPRA. The growth performances and biometric measurements of grass carp in IPRA and TPC are depicted in Table 3. Table 3. Growth performance of grass carp from in-pond raceway aquaculture (IPRA) and traditional pond culture (TPC). IPRA (Means ± SD) TPC (Means ± SD) Stocking weight (Kg.) 0.42±0.02 2.72±0.14 Weight (Kg.) 0.86±0.03 3.28±0.02* Weight gain rate (%) 117.93±16.79** 22.34±4.85 Specific growth rate (%/d) 1.35±0.20 1.67±0.87 Length (cm) 34.25±0.50 53.05±3.80 Visceral mass ratio (VMR) 11.82±1.03 11.5±1.06 Hepato somatic index (HSI) 2.89±0.18 2.50±0.91 Condition factor (CFg/cm') 2.14±0.075 2.21±0.52 Note: * Indicates signif i cant (p<0.05), ** indicates highly significant (p<0.01). 3.3. Nutrient Composition of Muscle The crude l ipid content in grass carp muscle was found to be statistically higher (p <0.05) in the IPRA (1.71±0.47) as compared to the TPC (1.38 ±0.21). However, no statistical di f ferences in moisture, mineral ash, and crude protein between the two culture systems during the whole experimental period were documented. The nutri t ional composition of grass carp flesh is depicted in Table 4. Table 4. Nutrient Composition of grass carp in IPRA and TPC. IPRA (n=24) TPC (n=27) Moisture% 79.64±0.81 78.49±2.33 Ash% 1.469±0.24 1.326±0.20 Crude lipid (CL%) 1.707±0.47* 1.383±0.21 Crude protein (CP%) 18.66±1.93 18.71±0.94 Note: Values represent means ±SD, * indicates significant difference (p<0.05). 3.4. Muscle Texture Profiles The comprehensive texture characteristics of grass crap muscle are presented in Table 5. The fl esh adhesiveness was evidently statistically higher (p <0.05) in TPC t han in IPRA. Water 2023, 15, x FOR PEER REVIEW There were no significant differences in physicochem 8 of 15ical characteristics (pH and WHC) in grass carp muscle between the two culture systems. The fish muscles from the TPC were observed to have a higher pH level , while the WHC was recorded as being higher in IPRA Table 5. pH, Water holding capacity, and texture profile analysis of grass carp muscle from in-pond raceway aquaculture (IPRA) and traditional pond culture (TPC). pH, WHC, and Texture Profile Attributes Table 5. pH, Water holding capacity, and texture profile analysis of grass carp muscle from in-pond IPRA TPC pH pH, WHC, and Texture Profile Attributes 6.26 ± 0.07 IPRA 6.34 ± 0.22 TPC Water Holding Capacity (%) 0.25 ± 0.07 0.24 ± 0.068 Hardness 411.75 ± 183.27 582.52 ± 398.45 Springiness 0.51 ± 0.04 0.50 ± 0.07 0.50±0.07 4.17 ± 2.14 Stickiness 4.02 ± 1.78 Chewiness 122.67 ± 49.82 180.28 ± 142.29 Adhesiveness 237.25 ± 91.71 344.14 ± 243.82 * Cohesiveness 0.59 ± 0.05 0.59 ± 0.06 Note: Values represent means ± SD, * indicates significant difference (p < 0.05). 3.5. 2-MIB and Geosmin in Muscle Off-flavor compounds, 2-MIB, and geosmin levels in grass carp muscle from the IPRA and TPC are shown in Figure 2. The muscles were observed to contain significantly higher (p < 0.01) 2-MIB in TPC (3.88 ± 0.24 µg/kg) than that in IPRA (2.09 ± 0.16 µg/kg) at s fi a rs m t pling (0 day/July) but were statistically similar during second (33 days/September) and days/September) and third sampling (83 days/October). Similarly, the geosmin content of flesh was found to be significantly higher (p < 0.01) in IPRA (0.79 ± 0.05 µg/kg) than in TPC (0.53 ± 0.01 µg/kg) at the second sampling (33 days) but was not significantly dif-ferent during the first (0 day) and third sampling (83 days). The2-MIB content in the grass carp muscle gradually increased during the whole culture period in IPRA, but in TPC, the geosmin content in the muscle got more depressed in September than in July and October. Figure 2. 2-MIB and geosmin l evels in flesh of grass carp f rom in-pond raceway aquaculture (IPRA) and t raditional pond culture (TPC). Note: ** indicates highly significant (p <0.01). Figure 2. 2-MIB and geosmin levels in flesh of grass carp from in- pond raceway aquaculture (IPRA) and traditional pond culture (TPC). Note: ** indicates highly significant (p < 0.01). 3.6. Microbial Diversity in Pond Water The study documented 12 different phyla of microbial mass in IPRA and TPC water environments (Figure 3). Cyanobacteria (29.59%) was the most abundant microbial mass, followed by Proteobacteria, Actinobacteria, Bacteriodetes, and Chloroflexi in IPRA, while Proteobacteria showed the dominating abundance in TPC, followed by Cyanobacteria, Bacteriodetes, and Actinobacteria. The abundance of Proteobacteria was observed to be significantly higher(p < 0.05) in TPC, whereas the richness of Planctomycetes was ob-served to be significantly higher (p < 0.05) in IPRA than in TPC. Figure 3. Pond water microbial diversity in in-pond raceway aquaculture (IPRA) and traditional pond culture (TPC). Note: * indicates significant (p < 0.05). 4. Discussion The crude fat concentration in the muscle was observed to be significantly higher in IPRA than that in TPC, but all other parameters were statistically similar. Higher muscle fat content in IPRA might be associated with the stocking density, diurnal activity, and feeding behavior of fish. Zhao et al. and Zhang et al. [7,19] found that water quality and diet affect the nutritional characteristics of grass carp muscle; f ish turn sluggish, and a l esser amount of lipids are burned as energy, resulting in an accumulation in the muscles [46]. The differences in fish fat concentration were reported to be diverse according to different rearing systems [7]. Pyzlukasik et al. [47] reported that rainbow trout from intensive farming contained higher amounts of fat (5.39% compared to 3.13%) and less protein (19.23% compared to 20.34%) than t rout from extensive culture. This f i nding is in agreement with Yang et al. [46] and Younis et al. [48], who reported a higher lipid content and low protein content in tilapia from ponds and in-pond cultured grass carp [49]. However, ref. [50] the documented increase in lipid metabolism and carbohydrate oxidation in grass carp reared at 40 kg/m. In general, muscle fat content depends on the rearing conditions, reared fish species, and feed provided to the aquatic animals [18,51]. However, lower fat contents have been documented in wild f ish species [52] and may be associated with higher alertness and activity. I nterestingly, Valente et al. [53] reported an i ncrease i n lipid content in accordance with the degree of intensification in culture conditions in sea bream (Sparus aurata) and in other fish [54]. Muscle pH and WHC, a set of attributes defining muscle characteristics, are important aspects to evaluate muscle quality [55] and are associated with good mouthfeel [56,57]. Glycolytic potential , some biological reactions, and fatty acid concentrations and composi-tion affec t muscle pH [58] and strongly control the WHC. High muscle WHC may be due to compromised protein breakdown [59]. In this study, the pH and WHC of fish muscles were found to be statistically similar in both culture systems. This observation is similar to t he f indings of El Rammouz et al . [60], who reported anon-significant difference in pH and WHC after 2 to 6 h of postmortem i n rainbow trout (O. mykiss) and stated that a higher WHC was observed at muscle pH 6.33. Texture is an important characteristic for al l members of the value chain, from produc-ers to customers, in terms of satisfaction and acceptance of fish products [61]. The texture of fish muscle, especially hardness and juiciness, is affected mainly by the amount of moisture, fat, and protein, especially the collagen content [62]. Andersoen et al. [63] documented the correlation of fish muscle with a softer texture and fat content, while increased WHC might be linked with muscle hardening [62,64] and lower muscle pH with higher softness. Therefore, the higher hardness of muscle in TPC grass carp might be related to their lower fat content and higher pH. Fuentes et al . [61] demonstrated tha t muscle from wild stock had a higher hardness and springiness as their habitat was better than a cultured aquatic environment. The better adhesiveness of f ish from the TPC was probably related to their higher muscle pH [59]. In this study, muscle texture profi l e analysis showed that al l the tex-tural attributes are statistically similar except for muscle adhesiveness, which was observed to be higher in IPRA fish muscle. These f indings might be due to the better water quality and higher freedom of movement in TPC, which probably affected f ish growth, muscle development, and the characteristic texture. This is because the low stocking density puts less stress on the fish in clean water. This result is i n line with the findings of Zhao et al. [7], who reported a higher f illet lipid content with poor texture parameters such as hardness, resilience, and shear force in grass carp on artificial food. Furthermore, Refaey et al. [65] discussed the relationship between water quality and muscle texture and stated that low water quality spikes chronic stress in f ish, disturbs homeostasis, and negatively affects the size and number of myofibers, influenc i ng the muscle texture [66]1.. Concentration of geosmin and 2-MIB in fish muscle results in "earthy" and "musty"off-flavors [67], and Actinomycetes and a diverse array of blue-green algae are the major culprits [68] for this characteristic’s development . In this study grass carp muscles were observed to contain an insignificantly higher amount of 2-MIB and geosmin content in TPC than those in IPRA at harvest, which might be due to a significantly higher Proteobacteria dominance. The 2-MIB content in the grass carp muscle showed an increasing trend for the whole culture period in both IPRA and TPC; however, muscle geosmin content was observed to be lower in September than in July and October in TPC, which correlated with a higher abundance of Cyanobacteria, Proteobacteria, Actinobacteria, Bacteriodetes, Chloroflexi, Planctomycetes, etc. Actinomycetes, Cyanobacteria, Proteobacteria, fungi, etc., were reported to produce geosmin and 2-MIB as secondary metabolites [25,68].In line with the results of this study, Qin et al. [24] and Tang et al. [13] reported comparable pictures of microbia l diversity in pond water and described Proteobacteria, Bacteriodetes, Actinobacteria, and Planctomycetes as the dominant microorganisms in al l types of culture systems and Proteobacteria as a leading geosmin producer [25]. 5. Conclusions The growth performances and muscle quality of grass carp cultured in TPC and IPRA were assessed and compared in this study. Along with aging culture duration, water quality parameters showed an increasing trend. With the exception of a few samples in TPC, the various forms of nitrogen, including nitrite nitrogen, ammonia, and total nitrogen, phosphate, and total phosphorus levels, were found to have a rising trend in IPRA and were discovered to have a very significant difference. Phosphate and total phosphorous were also significantly higher i n IPRA. The crude lipid was observed to be significantly higher in IPRA fish muscle when compared to TPC during the experimental period. In terms of the physicochemical characteristics (pH and WHC), there were no appreciable variations in t he muscle of grass carp between the two culture systems. The difference was evident upon inspecting flesh adhesiveness. TPC and IPRA c l early differed from one another, with TPC having superior f l esh adhesiveness. At the first sampling in July, it was found that TPC had higher levels of 2-MIB than IPRA did, but these levels were similar at the second sampling in September and at harvest in October. However, at the second sampling, it was discovered that the IPRA had higher levels of geosmin than the TPC did. While the 2-MIB content in fish muscle steadily increased throughout the IPRA culture period, the geosmin content in muscle in TPC declined more in September than i t did i n July and October. In IPRA and TPC water habitats, the study found 12 different phyla of microbial mass. In IPRA, Cyanobacteria (29.59%) had the highest dominance, followed by Proteobacteria, Actinobacteria, Bacteriodetes, and Chloroflexi, while in TPC, Proteobacteria showed the highest concentration, followed by Cyanobacteria, Bacteriodetes, and Actinobacteria. The abundance of Proteobacteria was observed to be higher in TPC, while the richness of Planctomycetes was higher in IPRA than TPC. Despite a high stocking density in IPRA, the i mpact on nutrient composition was low, meaning that a low-fat content in grass carp is produced, and the textura l quality of muscle is non-significant, aside from its adhesiveness. These things considered, the off-flavor in IPRA-produced fish had less of an impact on the aesthetic quality of flesh than TPC did with the aging of the culture duration. Taking into account all of these details, the study's findings demonstrate that IPRA's grass carp culture produces better-quality muscle t han TPCs. Author Contributions: Conceptualization, K.G. and L.L.; methodology, K.G., L.Y., K.L. and L.L .; soft-ware, K.G., L.Y., K.L. and N.B.; validation, K.G. and L.L.; formal analysis, K.G. and K.L.; investigation, K.G.; resources, L.L.; data curation, K.G., K.L.,N.B. and L.L.; writing-origina l draft preparation, K.G.; writing-review and editing, K.G., K.L.,N.B. and L.L.; visualization, K.G. and L.Y.; supervision, L.L.; project administration, L.L. and L.Y.; f unding acquisition, L.L. All authors have read and agreed to the published version of the manuscript. Funding: This research was funded by Shanghai Agriculture Applied Technology Development Programme grant number [X20210301] and The National Key Research and Development Programme of China grant number [2019YFD0900303]. Institutional Review Board Statement: All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of Shanghai Ocean University [SHOU-DW-2019-058], Shanghai,China. Informed Consent Statement : Not applicable. Data Availability Statement: The data that support the findings of this study are available from the corresponding authors upon reasonable request . Acknowledgments: We thank J ustice Frimpong Amankwah for editing and fine-tuning the manusc r ip language. The authors also thank Shanghai Agriculture Applied Technology Development Pro-gramme and The National Key Research and Development Programme of China for thei r f inancial assistance to this work. Conflicts of Interest: The authors declare no conflict of interest. References Subasinghe, R.; Soto, D.; Jia, J. Global aquaculture and i t s role in sustainable development. Rev. Aquac. 2009,1,2-9. [CrossRef ] Crab, R.; Defoirdt, T.; Bossier, P.; Verstraete, W. Biofloc technology in aquaculture: Beneficial effects and future challenges. Aquaculture 2012,356-357,351-356. [CrossRef ] 4 Xie, C.; Li, J .; Li, D.; Shen, Y.; Gao, Y.; Zhang, Z. Grass carp: The fish that feeds half of china. In Aquaculture i n China; Gui , J.-F ., Tang, Q., Li, Z., Liu, J ., De Silva, S.S., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2018; pp. 93-115. FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022. 5 6 Gisbert, E.;Mozanzadeh, M.T .; Kotzamanis, Y.; Estevez, A. Weaning wild f lathead grey mullet (Mugil cephalus) fry with diets with different l evels of fish meal substitution. Aquaculture 2016,462,92-100. [CrossRef ] 7. Zhao,H.; Xia, J.; Zhang, X.; He, X.; Li, L.; Tang, R.; Chi, W.; Li, D. Diet Affects Muscle Quality and Growth Trai t s of Grass Carp (Ctenopharyngodon idellus): A Comparison between Grass and Artificial Feed . Front. Physiol. 2018,9, 283. [CrossRef ] [PubMed] 8. Wang, Y.; Xu, P.; Nie, Z.; Li, Q.; Shao , N.; Xu, G. Growth, digestive enzymes activities, serum biochemical parameters and antioxidant status of juvenile genetically improved farmed tilapia (Oreochromis niloticus) reared at different stocking densities in in-pond raceway recirculating culture system. Aquacult. Res. 2019,50,1338-1347.[CrossRef ] 9 Fatima, S.; Komal , W.; Manzoor, F; Latif, A.A.; Liaqat, R.; Ameen, S.; Janjua, R.S. Analysis of the growth performance, stress, profile of fatty acids and amino acids and cortisol i n T i lapia (Oreochromis niloticus), cultured a t high stocking density using in-pond raceway system. Saudi J. Biol. Sc i . 2021,28,7422-7431. [CrossRef ] Martin, T.E. Are microhabitat preferences of coexisting species under selection and adaptive? Ecology 1998,79,656-670. [CrossRe f ] Nielsen, J ; Hyldig, G.; Larsen, E. 'Eating Quality'of Fish-A Review. J. Aquat. Food Prod. Technol . 2002,11,125-141.[CrossRef] Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How Muscle Structure and Composition Influence Meat and Flesh Quality. Sci. World J. 2016, 2016,3182746.[CrossRef ] [PubMed ] 14. Alamri, S.A.; Mohamed, Z.A. Selective inhibition of toxic cyanobacteria by B-carboline-containing bacterium Bacillus f lexus isolated from Saud i freshwaters. Saudi J. Biol. Sci. 2013,20, 357-363.[C rossRef] [PubMed ] 17. Granada, L.; Lopes, S.; Novais, S.C.; Lemos, M.F.L. Modelling integrated multi-trophic aquaculture: Optimizing a three t rophic level system. Aquaculture 2018, 495, 90-97. [CrossRef ] 18. Zhang, X.; Wang, J ; Tang, R.; He, X.; Li , L .; Takagi , Y.; Li, D. Improvement of Muscle Quality of Grass Carp (Ctenopharyngodon idellus) With a Bio-Floating Bed in Culture Ponds. Front. Physiol. 2019, 10, 683. [CrossRef ] 19. Zhang, X.; Shen, Z.; Qi, T.; Xi, R.; Liang, X.; Li , L.; Tang, R.; Li, D. Slight Increases in Salinity Improve Muscle Quality of Grass Carp (Ctenopharyngodon idellus). Fishes 2021, 6, 7. [CrossRef] Edwards, P. Aquaculture environment interactions: Past, present and 20.l ikely future trends. Aquaculture 2015, 447, 2-14. [CrossRef ] Dauda, A.B.; Ajadi , A.; Tola-Fabunmi, A.S.; Akinwole, A.O. Waste production in aquaculture: Sources, components and managements in different culture systems. Aquac. Fish. 2019,4, 81-88. [CrossRef ] 22. Gutierrez, R.; Itayama, T .;Iwami, N.; Whangchai, N. Analysis of Geosmin and 2-Methylisoborneol Off-flavors In Tilapia Cage-Cultures in Thailand. In Proceedings of the 2nd MJU-Phrae National Research Conference, Phrae, Thailand, 1-2 September 2011.23. Hathurusingha, P.I.; Davey, K.R. A predictive mode l for taste taint accumulation in Recirculating Aquaculture Systems (RAS) farmed -fish-demonstrated with geosmin (GSM) and 2-methylisoborneol (MIB). Ecol. Model. 2014, 291,242-249. [CrossRef ] 24. Qin, Y .; Hou, J;Deng, M.; Liu, Q.; Wu, C.; Ji , Y.; He, X. Bacterial abundance and diversity in pond water supplied with different feed s . Sc i . Rep. 2016,6,35232. [CrossRef ] [PubMed ] 25. Lukassen, M.B.; de Jonge, N.; Bjerregaard , S.M.; Podduturi , R.; Jorgensen, N.O.G.; Petersen, M.A.; David, G.S.; da Silva, R.J .; Nielsen, J.L . Microbia l Production of the Off-Flavor Geosmin in T i lapia Production in Brazil i an Water Reservoirs: Importance of Bacteria in the Intestine and Other Fish-Associated Environments. Front. Microbiol. 2019,10,2447. [CrossRef ] [P ubMed] 26. Brown, T.W.; Tucker, C.S. Pumping performance of a slow-rotating paddlewhee l for split-pond aquaculture systems. N. Am. J. Aquacult. 2013,75,153-158.[CrossRef ] 27.. Chopin, T .; Buschmann, A.H.; Halling, C.; Troell, M.; Kautsky, N.; Neori, A.; Kraemer, G.P.; Zertuche-Gonzalez, J.A.; Yarish, C.; Neefus, C. Integrat i ng seaweeds i nto marine aquaculture systems: A key toward sustainabil i ty. J. Phycol . 2001, 37,975-986. I CrossRe f 28. Schrader, K.K.; Tucker, C.S.; Brown, T.W.; Whi t is, G.N. Earthy and Musty Off-Flavor Episodes in Catfish Spli t -Pond Aquaculture Systems. N. Am. J . Aquacult. 2018,80,26-41. [CrossRef ] 29. Wang, H.; Zhu, Y.; Zhang, J.; Wang, X.; Shi, W. Study on changes in t he quality of grass carp in the process of postmortem. J . Food Biochem. 2018, 42,e12683. [CrossRef ] 30. Brown, T.W.; Chappell,J.A.; Boyd, C.E . A commerc i al-scale, in-pond raceway system for Ictalurid catfish production. Aquacult. Eng. 2011,44,72-79.[CrossRef ] 31. Zhang, X.; Zheng, W.; Zhang, H.; Chai, Y.; Ruan, G. Comparison of Muscle Qual i ty of the Yellow Catfish Cultured in In-Pond Raceway Systems and Traditional Ponds. Water 2022,14,1223. [CrossRef ] 32. Akinwole, A.; Dauda, A.;Ololade, A. Haematological response of Clarias gariepinus j uveniles reared in treated wastewater after waste solids removal using alum or Moringa olei f era seed powder. Int. J. Aquacult . 2016,6,1-8. 33. Zhang, J; Kaneko, G.; Sun, J.; Wang, G.; Xie,J.; Tian,J; Li, Z.; Gong, W.; Zhang, K.; Xia, Y.;et al. Key Factors Affecting the Flesh Flavor Quality and the Nutritional Value of Grass Carp i n Four Cul t ure Modes. Foods 2021, 10, 2075. [CrossRef ] 34. Wang, Z.; Xu, Y.; Shao, J .; Wang, J.; Li, R. Genes associated with 2-methylisoborneol biosynthesis in cyanobacteria: Isolation, characterization, and expression i n response to l i ght. PLoS ONE 2011, 6,e18665. [CrossRef ] [PubMed ]35. Fadrosh, D.W.; Ma, B.; Gajer, P; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the I llumina MiSeq platform. Microbiome 2014, 2, 6. [CrossRef] [PubMed ] 36. Johnston, I .A. Genetic and environmental determinants of muscle growth patterns. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 2001; Volume 18, pp. 141-186. 37. Hua, K.; Koppe, W.; Fontanillas, R. Effects of dietary protein and l ipid l evels on growth, body composition and nutrient utilization of Channa striata. Aquaculture 2019, 501, 368-373. [CrossRef ] 38. Liu, L.; Zhou, Y.; Wu, J.; Zhang, W.; Abbas, K.; Xu-Fang, L.; Luo, Y. Supplemental graded levels of neutral phytase using pretreatment and spraying methods in the diet of grass carp, Ctenopharyngodon idellus. Aquacult. Res. 2014, 45, 1932-1941. I CrossRe f Masser, M.P. In-pond raceways. In Aquaculture Production Systems; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012;pp. 387-394.29. Yuan, J .; Ni, M.; Liu, M.; Wang, H.; Zhang, C.; Mi, G.; Gu, Z. Analysis of the growth performances, muscle quality, blood biochemistry and antioxidant status of Micropterus salmoides farmed in in-pond raceway systems versus usual-pond systems. Aquaculture 2019,511,734241.[CrossRef ] 41. Brown, T .W.; Boyd, C.E.; Chappell,J .A. Approximate Water and Chemical Budgets for an Experimental , In-pond Raceway System. J. World Aquacult. Soc . 2012, 43,526-537. [CrossRef ] 43. Liu, Q.; Hou, Z .; Wen, H.; L i , J; He, F; Wang, J ; Guan, B.; Wang, Q. Effect of stocking density on water qual i ty and (Growth, Body Composition and Plasma Cortisol Content) performance of pen-reared rainbow trout (Oncorhynchus mykiss). J. Ocean. Univ. China 2016,15,667-675. [CrossRef ] 44. Li, W.; Cheng, X.; Xie,J; Wang, Z.; Yu, D. Hydrodynamics of an in-pond raceway system with an aeration plug-flow device for application in aquaculture: An experimental study. R. Soc. Open Sc i . 2019, 6, 182061.[CrossRef ] 45. Ni , M.; Liu, M.; Lou, J.; Mi , G.; Yuan, J .; Gu, Z. Stocking density alters growth performance, serum biochemistry, digestive enzymes, immune response, and muscle quality of largemouth bass (Micropterus salmoides) in in-pond raceway system. F i sh Physiol. Biochem. 2021,47, 1243-1255. [CrossRef ] 46. Yang, H.; He, J.; Lv, M.;Huang, G.; Wen, L.; Bi , X.; Hu, T.; Ma, H. Preliminary effects of dietary protein levels on muscle quality and digestive enzyme ac t ivities in GIFT-Oreochromis niloticus. Aquac. Stud. 2018,18,1-10.[CrossRef ] [PubMed ] 47. Pyz-Lukasik,R.; Chalabis-Mazurek, A.; Gondek, M. Basic and functional nutrients in the muscles of fish : A review. Int. J . Food Prop. 2020, 23,1941-1950. [CrossRef ] 49. Pyz-Lukasik, R.; Paszkiewicz, W. Species Variations in the Proximate Composi t ion, Amino Acid Profile, and Protein Qual i ty of the Muscle Tissue of Grass Carp, Bighead Carp, Siberian Sturgeon, and Wels Catfish. J. Food Qual. 2018,2018,2625401.[CrossRef] 50. He, Y.; Yu, H.; Zhao,H.; Zhu, H.; Zhang, Q.; Wang, A.; Shen, Y.; Xu, X.; Li , J . Transcriptomic analysis to elucidate t he effects of high stocking density on grass carp (C t enopharyngodon i della). BMC Genom. 2021, 22, 620. [CrossRef ] 51. Orban, E.; Lena, G.D.; Nevigato, T .;Casini, I.; Santaroni , G.; Marzetti, A.; Caproni , R. Quality characteristics of sea bass intensively reared and from lagoon as affected by growth conditions and the aquatic environment. J. Food Sci. 2002,67,542-546.[CrossRef ] 52. Grigorakis, K.; Taylor, K.; Alexis, M. Organoleptic and volatile aroma compounds comparison of wild and cultured gilthead sea bream (Sparus aurata): Sensory differences and possible chemica l basis. Aquaculture 2003,225, 109-119.[CrossRef ] 53. Valente, L.M.P .; Cornet, J; Donnay-Moreno, C.; Gouygou, J.P.; Berge, J.P.; Bacelar, M.; Escorcio, C.; Rocha, E.; Malhao, F.; Cardinal , M. Quality differences of gilthead sea bream from distinct production systems i n Southern Europe: I ntensive, integrated, semi-intensive or extensive systems. Food Control 2011,22,708-717. [CrossRef ] 54. Flos, R.; Reig, L.;Oca,J.; Ginovart, M. Influence of marketing and different l and-based systems on gilthead sea bream (Sparus aurata) quality. Aquacult. Int. 2002,10,189-206. [CrossRef] 55. Rasmussen, R.S. Quality of farmed salmonid s with emphasis on proximate composition, yield and sensory characteristics. Aquacult . Res.2001,32,767-786. [C rossRef ] 56. Wang, Y.; Lu, Y.; Zhang, Y;Ning, Z.;Li, Y.; Zhao, Q.; Lu, H.; Huang, R.; Xia,X.; Feng, Q.; et al. The draft genome of the grass carp (Ctenopharyngodon idellus) provides insights into i ts evolution and vegetarian adaptation. Nat . Genet. 2015,47,625-631. [CrossRef][PubMed l 57. Xu,J;Feng, L.; Jiang, W.-D.; Wu, P.; Liu, Y; Jiang, J;Kuang, S.-Y.; Tang, L .; Zhou,X.-Q. Different dietary prote i n levels affect flesh quality, fatty acids and al t er gene expression of Nrf2-mediated antioxidant enzymes i n t he muscle of grass carp (Ctenopharyngodon idella). Aquaculture 2018, 493,272-282. [CrossRef ] 58. Fernandez, X.; MAGARd, M.; Tornberg, E.V.A. Glycolytic Potential in Porcine Longissimus Musc l e before and after Transport:An in Vivo Study. J. Muscle Foods 1992, 3,83-89. [CrossRef ] 59. Bee, G.; Anderson, A.L .; Lonergan, S.M.; Huff-Lonergan, E. Rate and extent of pH decline affect proteolysis of cytoskeletal proteins and water-holding capacity in pork. Meat Sci . 2007, 76,359-365.[CrossRef ] 60. El Rammouz, R.; Abboud,J.; Abboud, M.; El Mur, A.; Yammine, S.; Jammal, B. pH, rigor mortis and physical properties of f i l let in fresh water fish: The case of rainbow trout (Oncorynchus mykiss). J. Appl.Sci. Res. 2013,9,5746-5755. 61. Fuentes, A.; Fernandez-Segovia, I.; Serra, J.A.; Barat, J.M. Comparison of wild and cul t ured sea bass (Dicentrarchus labrax ) quality. Food Chem. 2010,119,1514-1518.[CrossRef ] 622..Cheng,J.H.; Sun, D.W.; Han, Z.; Zeng, X.A. Texture and Structure Measurements and Analyses for Evaluation of F i sh and Fillet Freshness Quality: A Review. Compr. Rev. Food Sc i. Food Saf. 2014,13,52-61.[CrossRef ] 63. Andersen, U.B.; Thomassen, M.S.; Rora, A.M.B. Texture properties of farmed rainbow trout (Oncorhynchus mykiss): Effects of diet, muscle fat content and t i me of storage on ice. J. Sc i . Food Agric. 1997,74, 347-353.[CrossRef ] 64. Pearce, K.L .; Rosenvold, K.; Andersen, H.J .; Hopkins, D.L. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on f resh meat quality attributes-A review. Meat Sci. 2011,89,111-124. [CrossRef] 65. Refaey, M.M.; Li, D.; Tian, X.; Zhang, Z.; Zhang, X.; Li, L.; Tang, R. High stocking density alters growth performance, blood biochemistry, intestinal histology, and muscle quality of channel catfish Ictalurus punctatus. Aquaculture 2018,492,73-81.[CrossRef ] 67. Tucker, C.S. Off-flavor problems in aquaculture. Rev. Fish. Sci. 2000,8,45-88. [CrossRef] 68. Guttman, L.; van Rijn, J .2-Methy l isoborneol and geosmin uptake by organic sludge derived from a recirculating aquaculture system. Water Res. 2009, 43, 474-480. [CrossRef ] [PubMed ] 69. Robertson, R.F.; Hammond, A.; Jauncey, K.; Beveridge, M.C.M.; Lawton, L.A. An i nvestigation into the occurrence of geosmin responsible for earthy-musty taints in UK farmed rainbow trout , Onchorhynchus mykiss. Aquaculture 2006,259,153-163.[CrossRef ] 70. Persson, P.-E. Sensory proper t ies and analysis of two muddy odour compounds, geosmin and 2-methylisoborneol , in water and fish. Water Res. 1980,14, 1113-1118.[CrossRef ] 71..Gutierrez, R.; Whangchai,N.; Sompong, U.; Prarom, W.; Iwami, N.; Itayama, T.; Nomura, N.; Sugiura, N. Off-flavour in Nile tilapia (Oreochromis niloticus) cultured in an i ntegrated pond-cage culture system. Maejo I nt. J . Sci. Technol . 2013, 7,1. 72. Vallod, D.; Cravedi, J.; Hillenweck, A.;Robin, J. Analysis of the off-flavor risk in carp production in ponds in Dombes and Forez (France). Aquacult . Int. 2007,15,287-298. [CrossRef ] 73. Lukassen, M.B.; Saunders, A.M.; Sindilariu, P.-D.; Nielsen, J.L. Quantification of novel geosmin-producing bacteria in aquaculture systems. Aquaculture 2017, 479,304-310. [CrossRef ] Disclaimer/Publisher's Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or t he editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, method s , instructions or products referred to in the content .
确定












还剩12页未读,是否继续阅读?
中国格哈特为您提供《池塘循环流水养殖和传统池塘养殖中草鱼中总脂肪和蛋白质含量的检测,水质化学需氧量(COD)的检测》,该方案主要用于渔业中总脂肪、蛋白质、化学需氧量(COD)检测,参考标准《GB 5009.6 食品中脂肪的测定》,《池塘循环流水养殖和传统池塘养殖中草鱼中总脂肪和蛋白质含量的检测,水质化学需氧量(COD)的检测》用到的仪器有格哈特全自动超级总脂肪测定系统HT6+SOX416、格哈特平底高温电热板 EV16、格哈特快速干燥仪STL56、格哈特自动升降凯氏定氮电热消解仪KT-L 20s、格哈特带自动进样器凯氏定氮仪VAP500C、德国移液器MM、凯氏定氮催化剂5.0g K2SO4+0.5g CuSO4 x 5H2O
相关方案
更多
该厂商其他方案
更多


































