氟尼辛大聚胺(FM)是一种非甾体抗炎药,因其对兽医呼吸道和黏膜的刺激而受到限制。本研究旨在采用热熔挤压法(HME)制备一种口感掩盖的FM固体分散体(SD),并选用辅料直接制备口腔崩解片(ODT)压缩。
方案详情

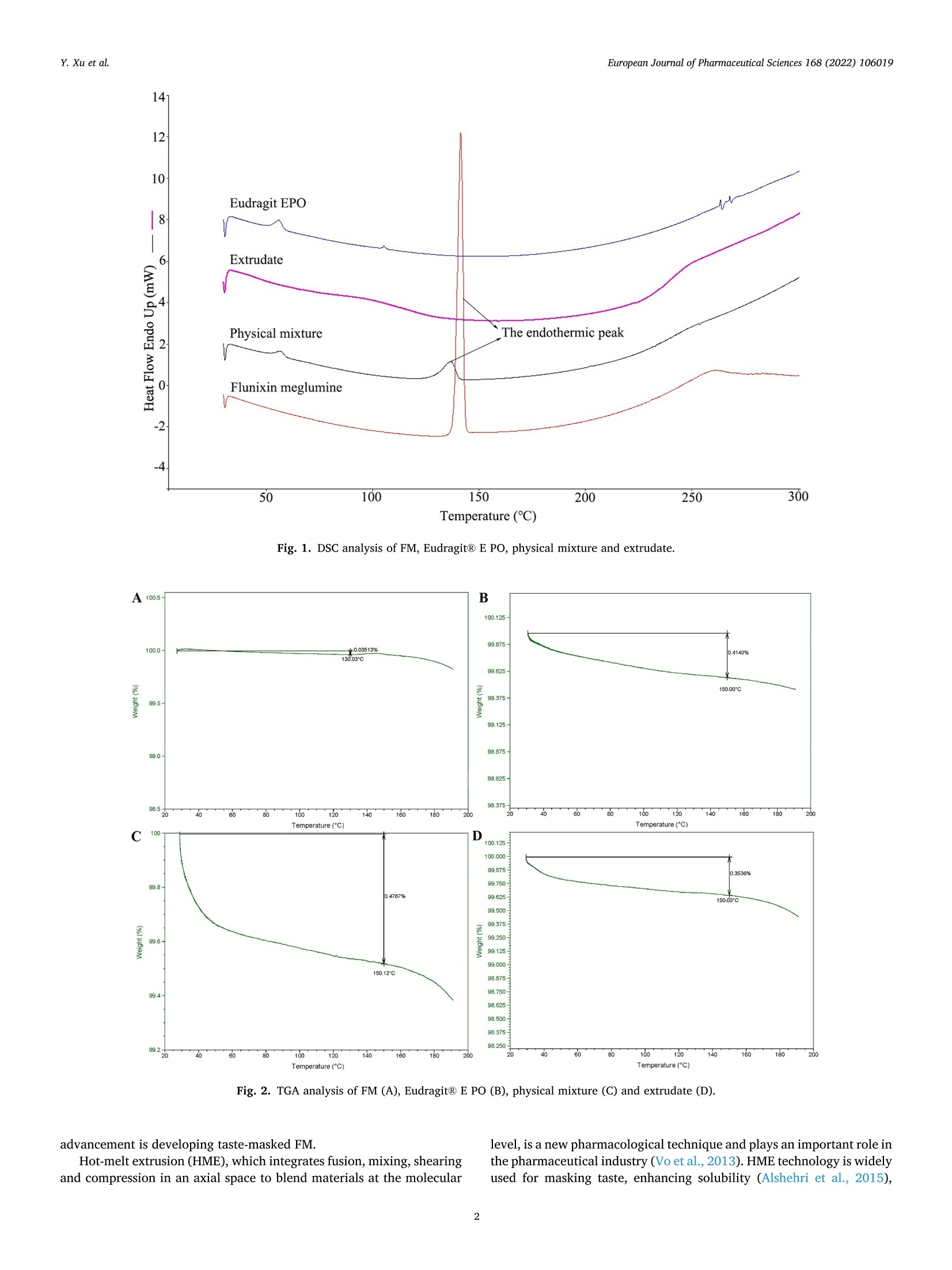
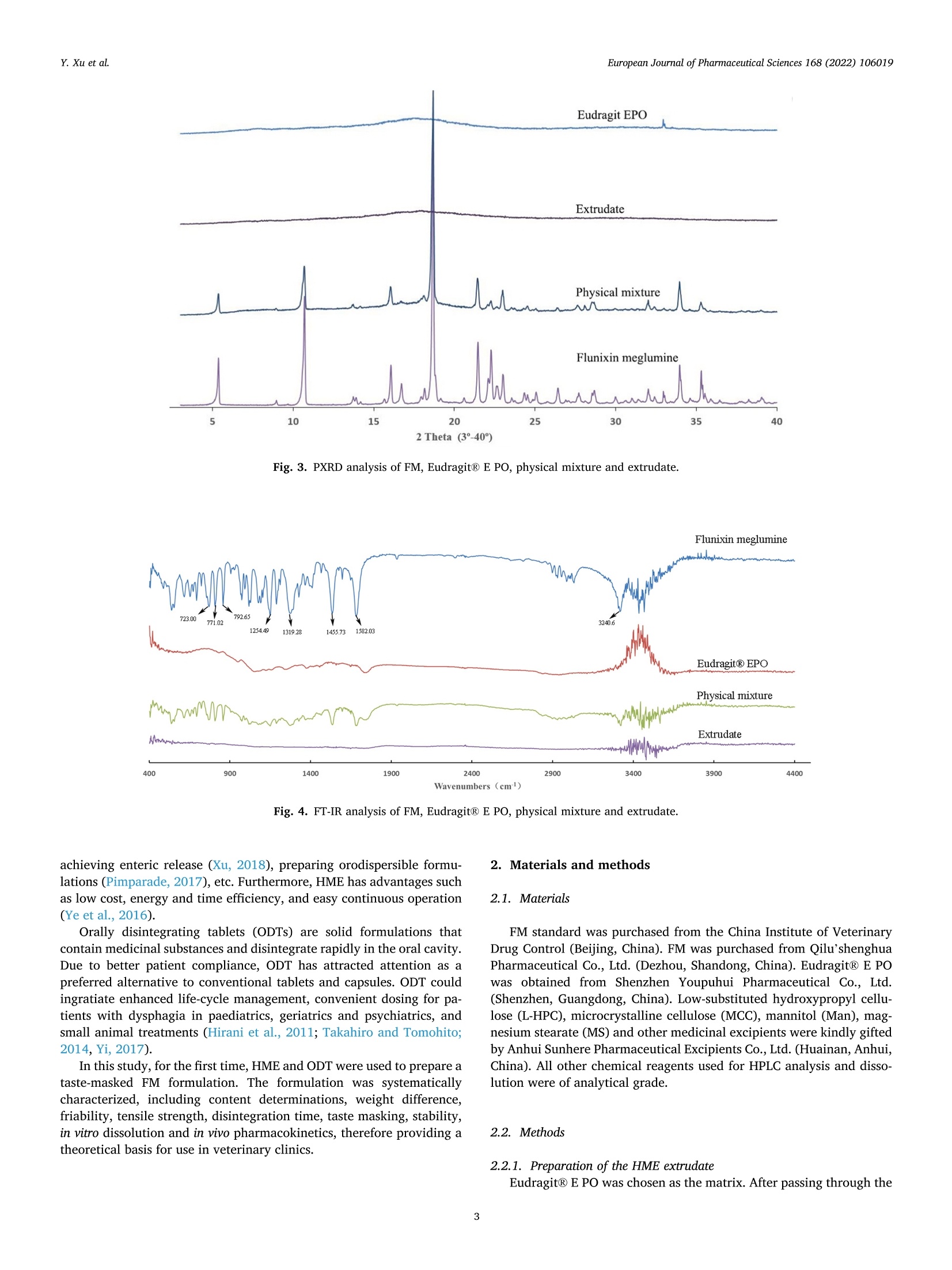
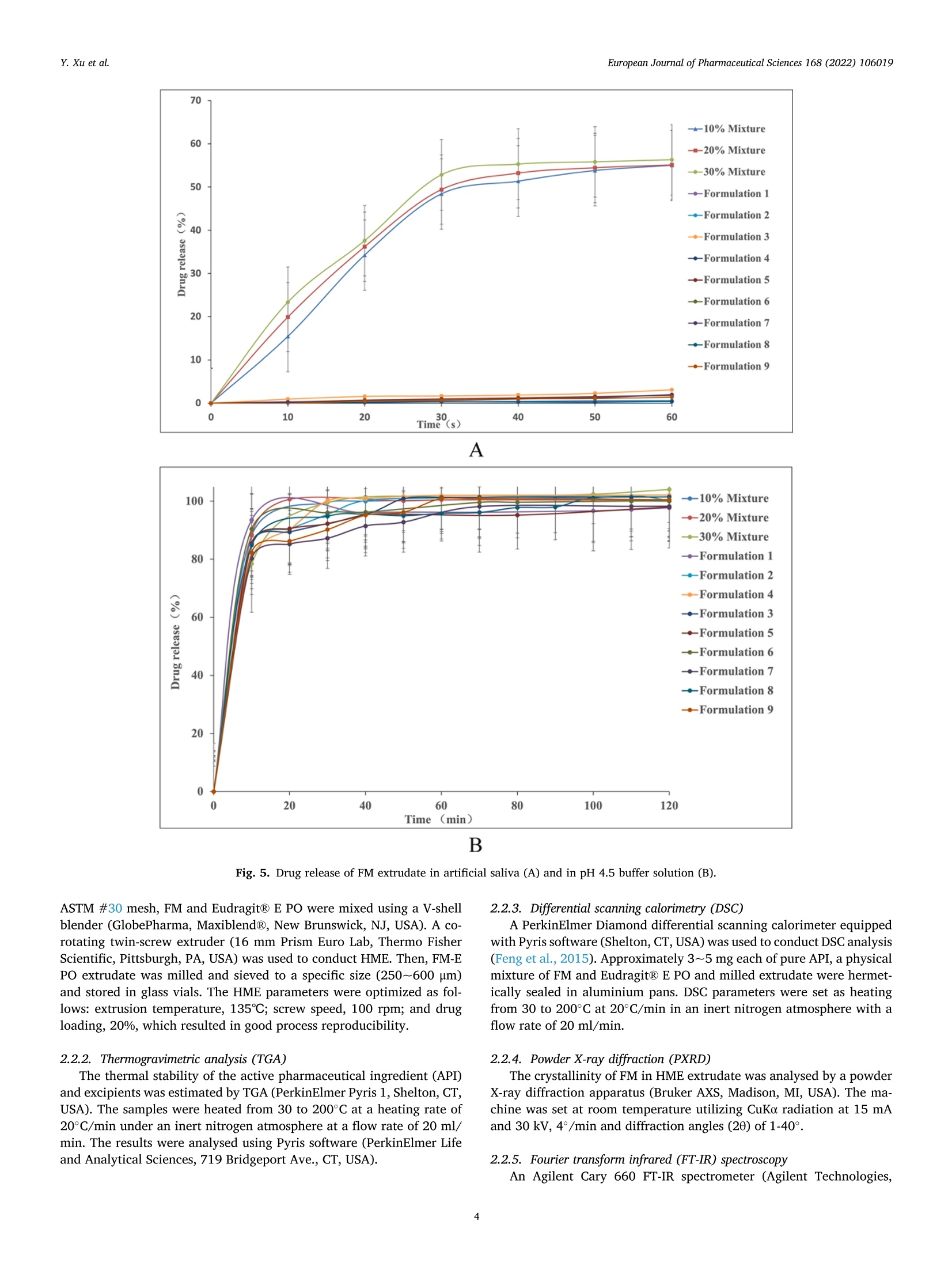
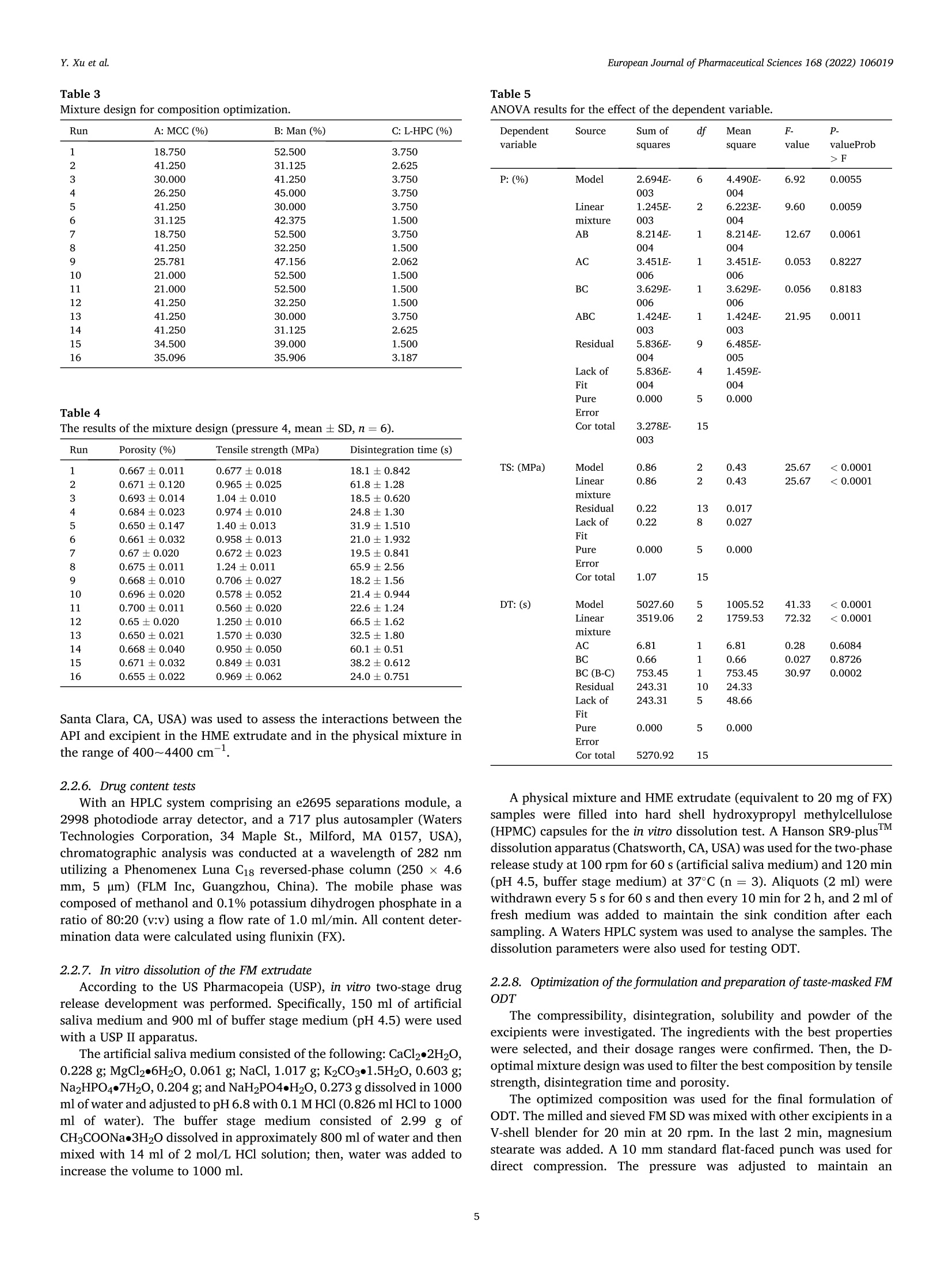
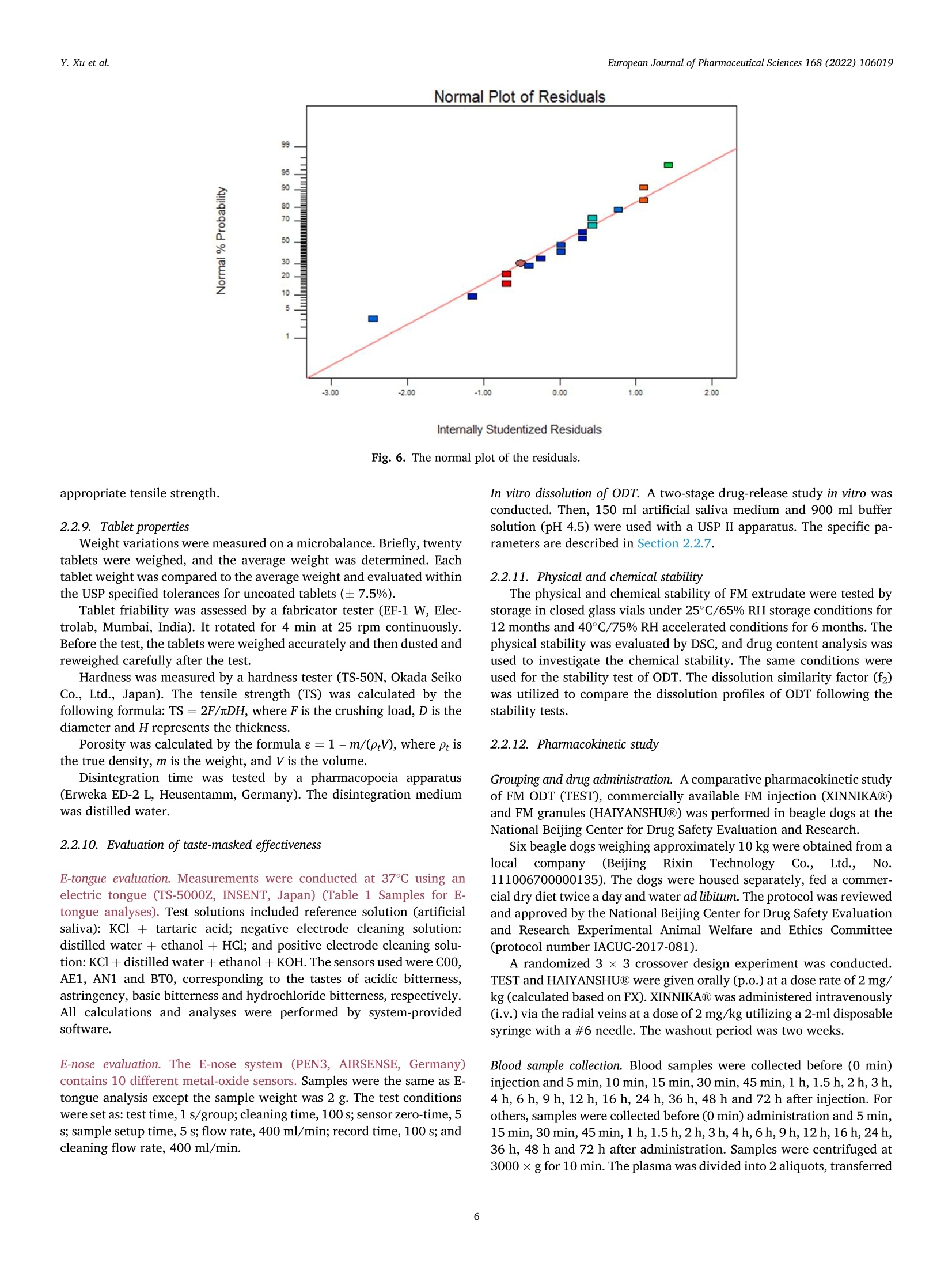
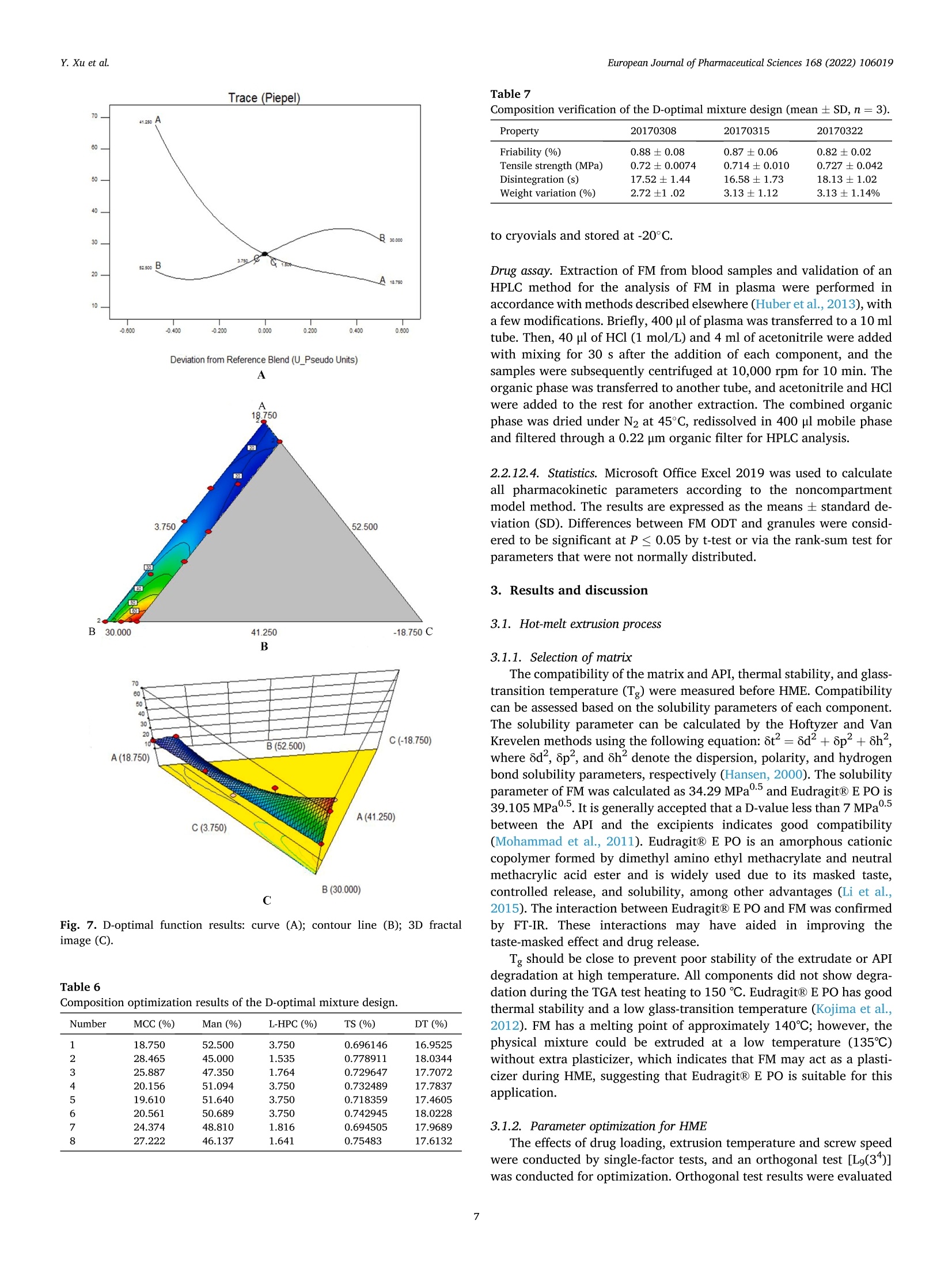
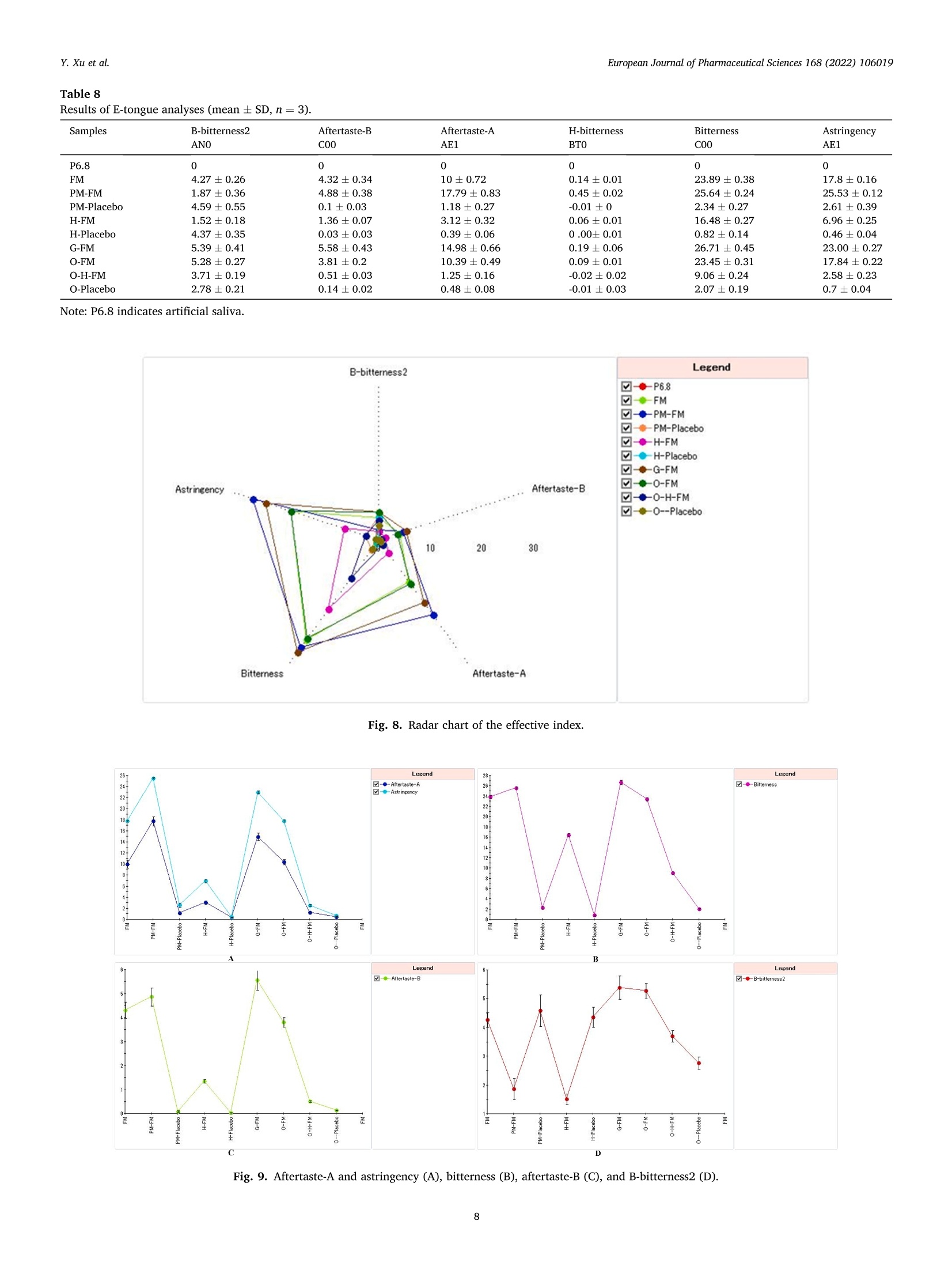
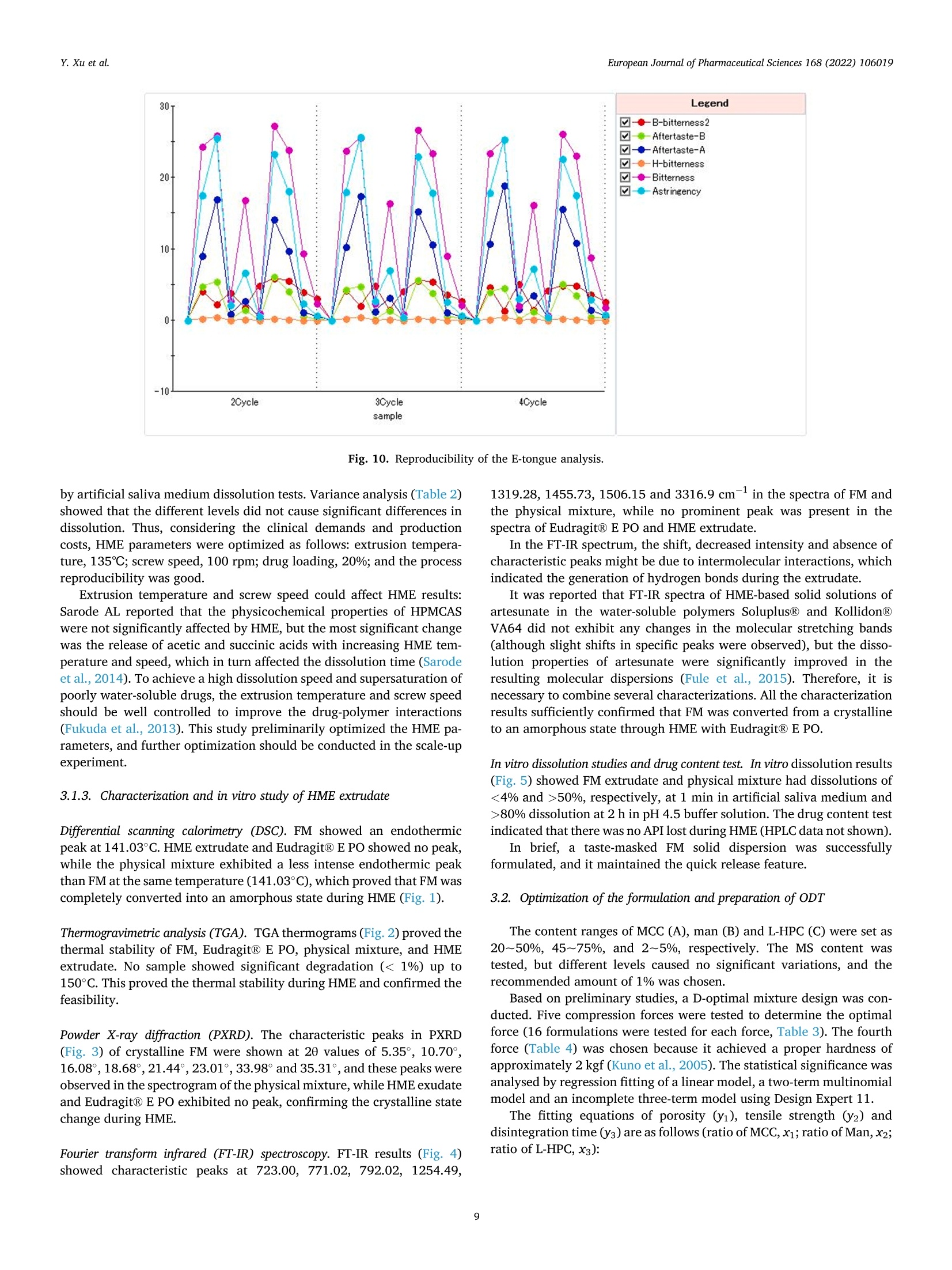
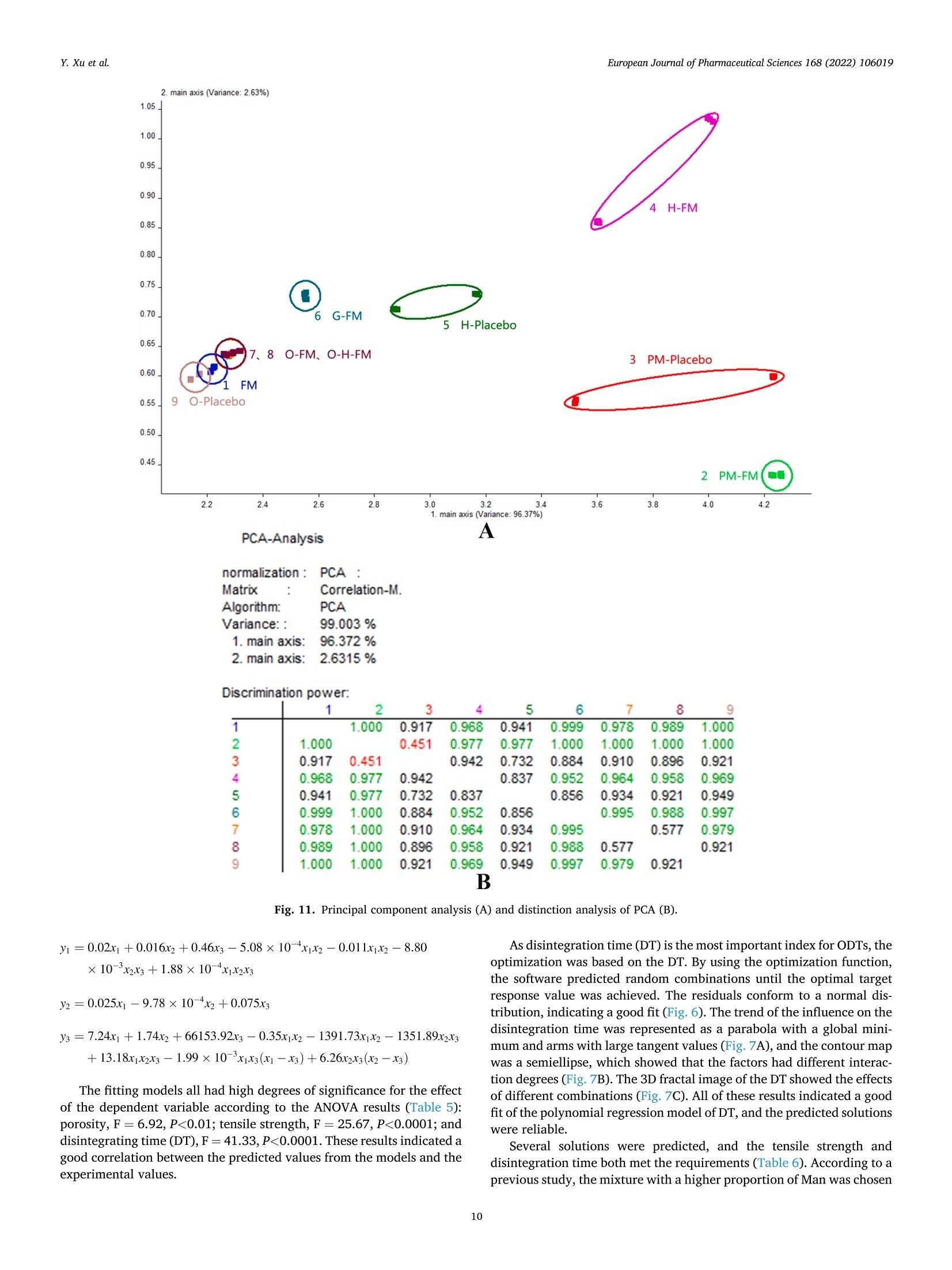
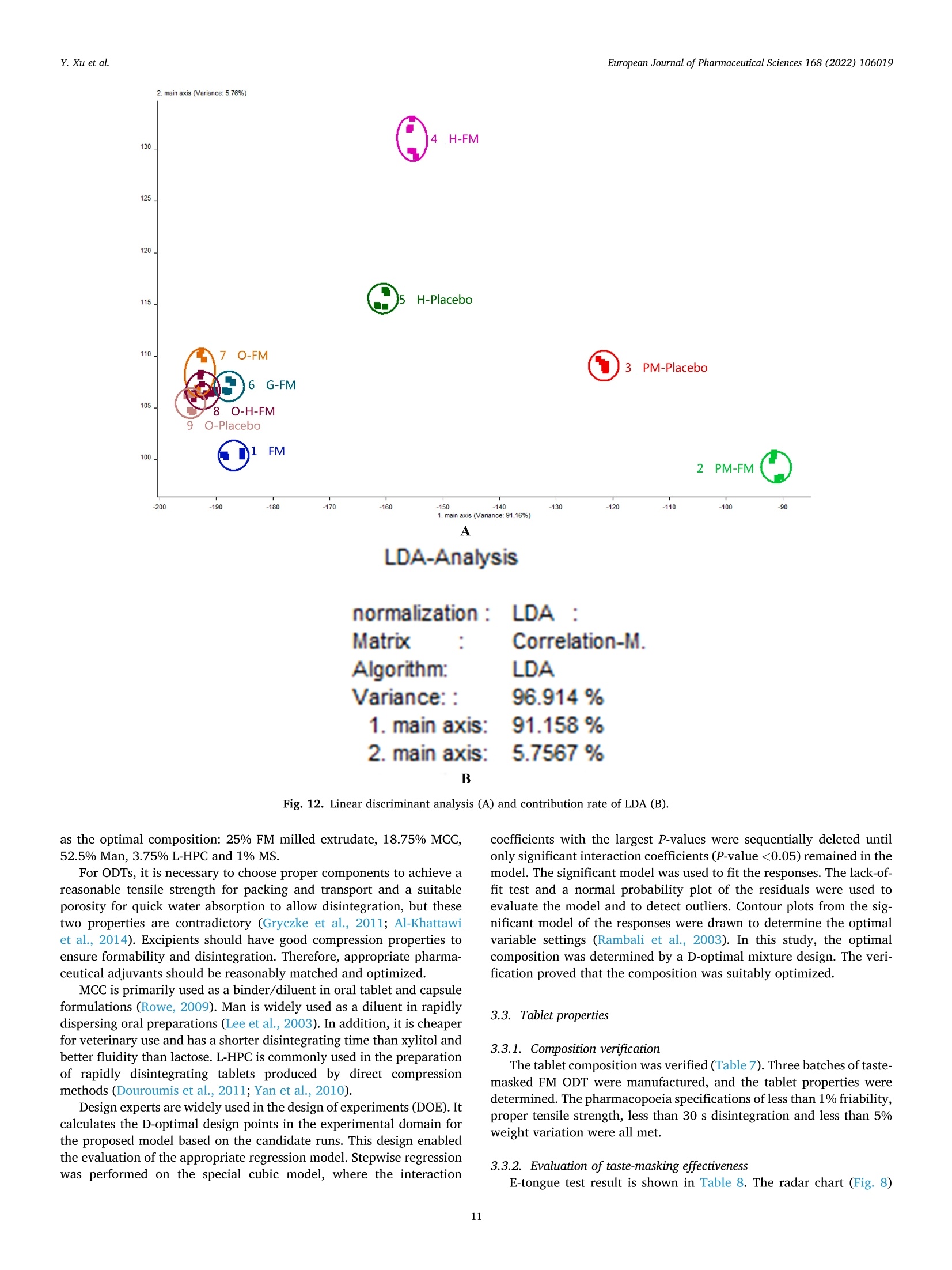
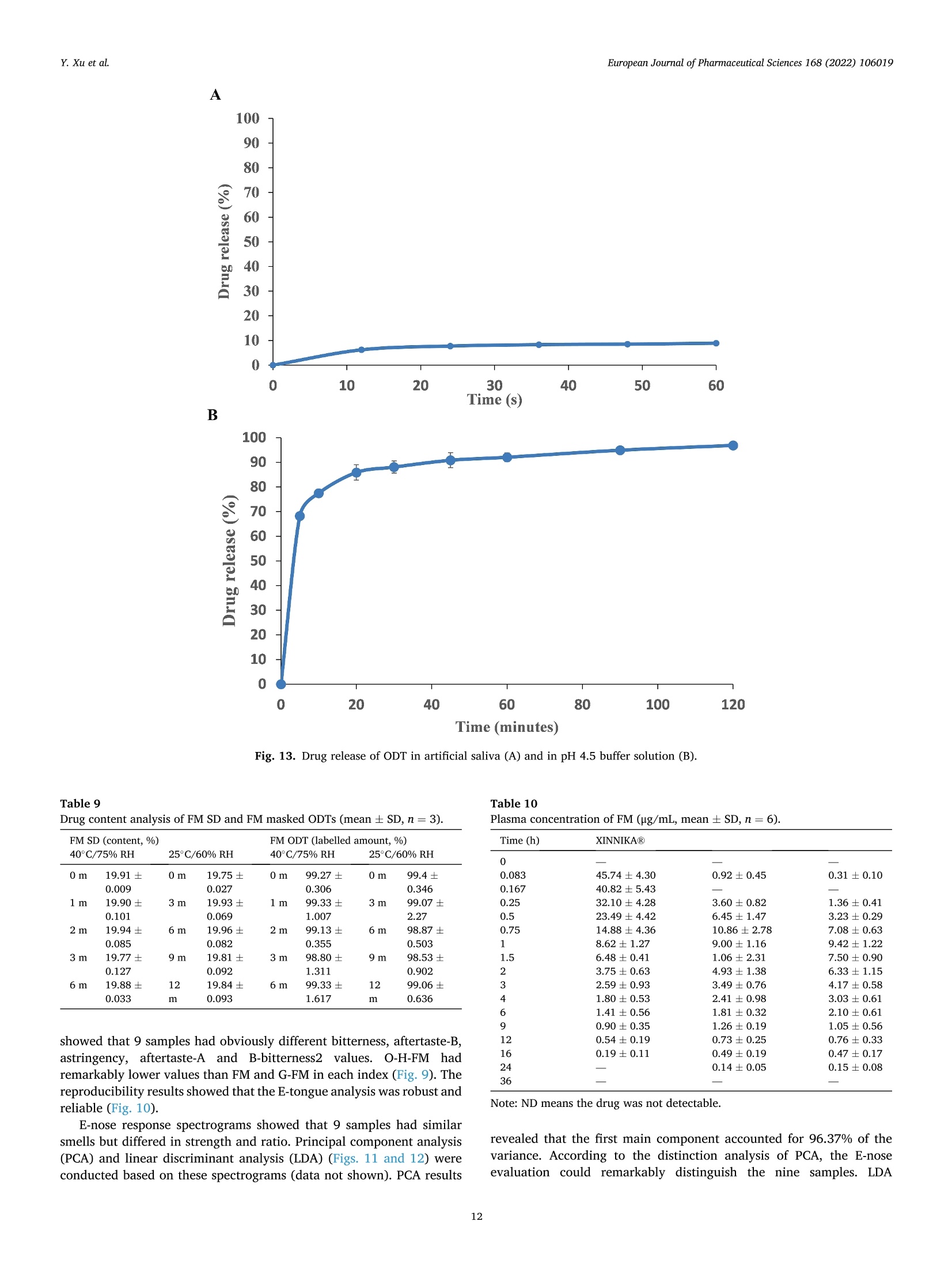
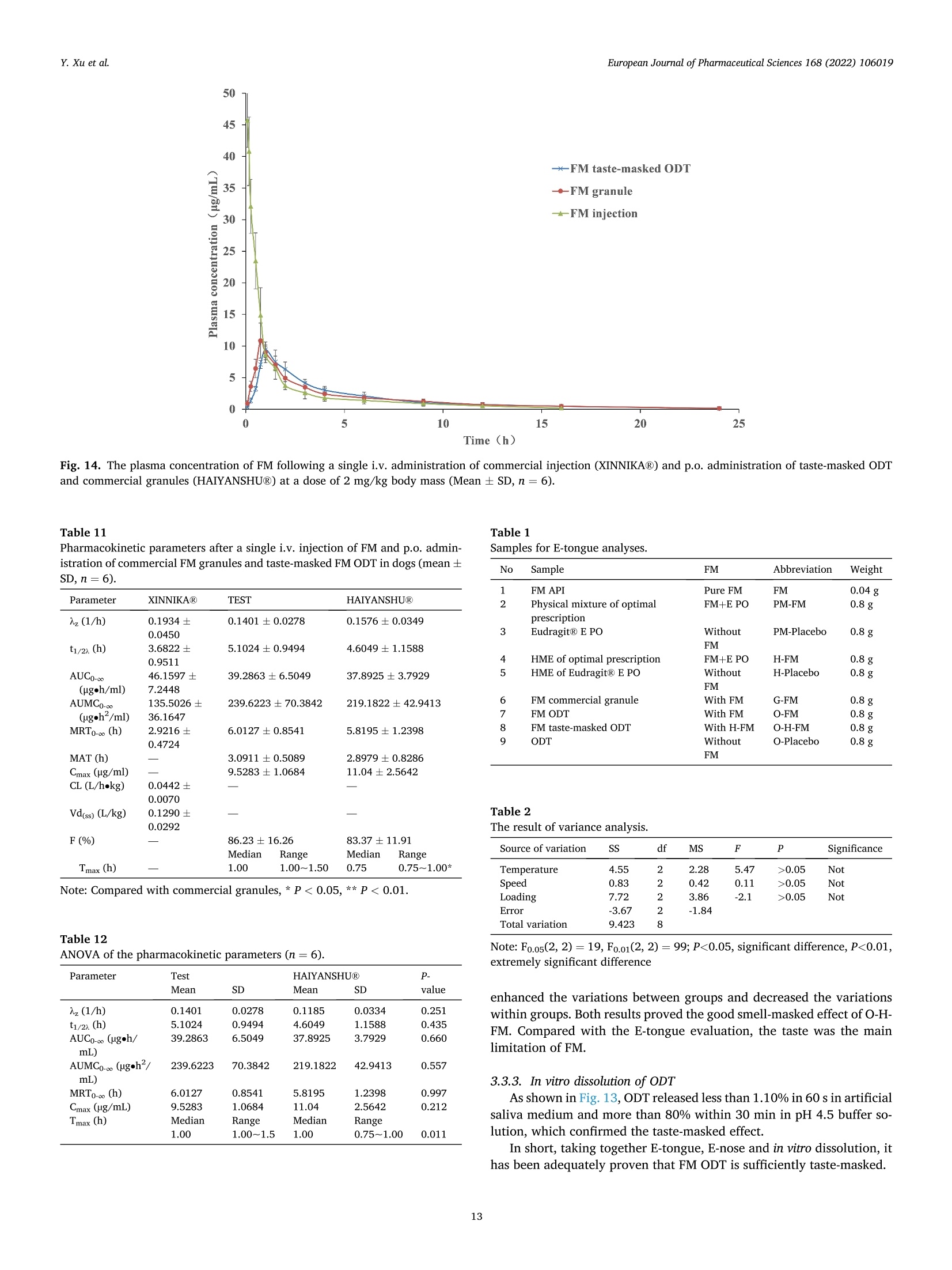
氟尼辛大聚胺(FM)是一种非甾体抗炎药,因其对兽医呼吸道和黏膜的刺激而受到限制。本研究旨在采用热熔挤压法(HME)制备一种口感掩盖的FM固体分散体(SD),并选用辅料直接制备口腔崩解片(ODT)压缩。选择Eudragit®E PO作为基体,对HME参数进行优化:挤出温度135℃;螺杆转速:100rpm;装药量占20%表征技术证明FM在HME挤出物中呈现无定形。体外溶出度研究表明,FM SD在人工唾液中的释放速度明显慢于相应的物理混合物。根据压缩成形性、崩解性和溶解度选择辅料。采用d -最优混合设计优化其组成:25% FM SD、18.75%微晶纤维素、52.5%甘露醇、3.75%低取代羟丙基纤维素、1%硬脂酸镁。掩味FM ODT的抗拉强度为0.7±0.01 MPa,崩解时间为17.6±0.1 s。电子舌和电子鼻分析表明,FM ODT比市售颗粒具有更好的掩味效果。最后一项药代动力学研究证明,FM ODT的主要药代动力学参数与商业颗粒的药代动力学参数没有显著差异,这表明这些配方在比格犬体内具有相似的药代动力学行为。 Preparation, evaluation, and pharmacokinetics in beagle dogs of a taste-masked flunixin meglumine orally disintegrating tablet prepared using hot-melt extrusion technology and D-optimal mixture design发表期刊:European Journal of Pharmaceutical Sciences,发表单位:广西大学掩盖效果的评估仪器:电子舌 日本INSENT公司;电子鼻,德国AIRSENSE公司电子鼻和电子舌实验谱图: 实验结论:用Eudragit®E PO成功挤压氟尼辛聚氨胺。表征研究证实了FM的非晶态转变。采用直接压缩法制备了FM ODT。崩解时间为17.6±0.1s,抗拉强度为0.7±0.01 MPa。各项性能均满足ODT的要求。电子舌、电子鼻和溶出度分析证实了掩味效果。药代动力学研究表明,fmodt在比格犬体内的药代动力学行为与商业颗粒相似。此外,稳定性研究证明了FM SD和ODT的物理和化学稳定性。综上所述,本研究制备的FM ODT达到了掩盖味道的效果和良好的药代动力学行为,可能是一种潜在的临床应用的新配方。European Journal of Pharmaceutical Sciences 168 (2022) 106019 Contents lists available at Sc i enceDirect European Journal of Pharmaceutical Sciences journal homepage: www.elsevier.com/locate/ejps Preparation, evaluation, and pharmacokinetics in beagle dogs of a taste-masked flunixin meglumine orally disintegrating tablet prepared using hot-melt extrusion technology and D-optimal mixture design Yangfeng Xu , Guoqing Yan , Xuemei Wen , Liqin Wu , Ruihan Deng , Qiuling Liang,Linjie Zhang ", Hangping Chen ", Xin Feng , Jiakang He a ,^ A College of Animal Science and Technology, Guangxi University, 100 Daxue Road, Xixiangtang Distric t, Nanning, Guangxi 530005, China DSun Yat-Sen University Guangzhou Nansha Science and Technology Innovation Industrial Park Co., Ltd, Guangzhou, Guangdong 510000, China C Department of Pharmaceutics and Drug De l ivery, School of Pharmacy, The University of Mississippi, MS 38677, USA ARTICLEINFO ABSTRACT Keywords:Flunixin meglumine Hot -melt extrusion Taste-masked Orally dis i ntegrating tablet D-optimal mixture design Flunixin meglumine (FM) is a nonsteroida l anti-inflammatory drug l i mited by irritation of the respiratory tract and mucosa in veterinary t issue. This study aimed to develop a taste-masked FM solid dispersion (SD) by hot -melt extrusion (HME) and formulate an orally disintegrating tablet (ODT) with selected excipients by direct compression. Eudragit@ E PO was chosen as the matrix, and HME parameters were opt i mized: extrusion tem-perature, 135℃; screw speed, 100 rpm; and drug loading, 20%. Characterization techniques proved t hat FM was rendered amorphous in t he HME extrudate. In vitro dissolution studies showed that FM SD released significantly slower than the corresponding physical mixture in artificial sal i va. Exc i pients were selected based on compression formabi l ity, disintegration, and solubility. A D-opt i mal mixture design was used to optimize the composition: 25% FM SD, 18.75% microcrystalline cellulose, 52.5% mannitol, 3.75% low-substituted hydrox-ypropyl cellulose, and 1% magnesium stearate. Taste-masked FM ODT had a tensile strength of 0.7 ±0.01 MPa and a disintegration time of 17.6 ±0.1 s. E-tongue and E-nose analysis showed that FM ODT had a better taste -masked effect than commercial granules. Finally, a pharmacokinetic study proved that the main pharmacokinetic parameters of FM ODT were not significantly different from those of commercial granules, which indicated that these formulations had similar pharmacokinetic behaviours in beagles. 1. Introduction With improvements in living standards, an increasing number of people are keeping pets, and this i ndustry has been growing rapidly (Al v es and R oc h a , 2018). Pets have been continuously kept throughout history. Human-companion animal interactions afford physiological and psychosocial-like cardiovascular benefits and benefits for in-dividuals with psychiatric disorders, nursing home residents, and chi l -dren (Barker a nd W o l en, 2008; P o r esk y e t al ., 2016). However , companion animals, especially older pets and racing ani-mals, often suffer from arthritis, arthralgia and inflammation. These conditions undermine pet health and competitiveness levels. Neverthe-less, there are only a few medications that can currently be used for arthrit i s. FM i s widely used for the treatment of dairy cow mastitis (Y e i se r e t a l., 2012), pain management in the dehorning of calves (Hu ber et al.,2013), postoperative pain management i n horses (N a yl o r e t al ., 2014),etc. However, it is limited by irritant effects, and most formulations are injection, granule, premix, and powder. An irritating taste wil l lead to poor adaptabil i ty and reduce feed intake and curative effects (Mair e t al ., 2010). Conventional injection solvents such as dimethylacetamide and dimethylformamide possess high toxicity, which may cause injury and even food safety issues (W e i n er and K o tk o s k ie , 2000). Furthermore,normal injections result in rapid metabolism, which means higher administration frequencies and labour costs, especially in large and sensi t ive animals, causing stress responses and affecting treatment (K e y ser e t a l ., 2007). Hence, it i s interesting to develop new formula-tions of FM to fully exploit its clinical value. Convenient medication can improve patient compliance, therefore i mproving the overall thera-peutic index (Witchey-Lakshmanan and Li, 2000). The key for this E-mail address: a hong 18@v i p.s in a .c o m (J. He). h t tps://doi.or g/10.1016/j .ejp s.2021.106019 Received 10 Apri l 2021; Received in revised form 19 September 2021; Accepted 22 September 2021 Available online 24 September 2021 0928-0987/C 2021 The Authors. Published byy ElsevierB.V. This is an open access article under the CC BY-NC-ND license (ht t p://c r ea t i v e c o mmons.o r g /l ic enses/b y-n c -n d /4.0/). 14 12 10 Fig. 1. DSC analysis of FM, Eudragit @ E PO, physical mixture and extrudate. Temperature (C ) T e m p er a ture (C) Fig. 2. TGA analysis of FM (A), Eudragit@ E PO (B), physical mixture (C) and extrudate (D). advancement is developing taste-masked FM. Hot-melt extrusion (HME), which integrates fusion, mixing, shearing and compression in an axial space to blend materials at the molecular level , is a new pharmacological technique and plays an important role in the pharmaceutical i ndustry (Vo e t al., 2013). HME technology is wide l y used for masking taste, enhancing solubility (A l sheh r i et al ., 2015), Fig. 3. PXRD analysis of FM, Eudragit @ E PO, physical mixture and extrudate. Fig. 4. FT-IR analysis of FM, Eudragit@ E PO, physical mixture and extrudate. achieving enteric release (Xu, 2018), preparing orodispersible formu-lations (Pim p arade,2017), etc. Furthermore, HME has advantages such as low cost, energy and t ime efficiency, and easy cont i nuous operation (Ye e t a l., 2016). Orally disintegrating tablets (ODTs) are solid formulations that contain medicinal substances and disintegrate rapidly in the oral cavity.Due to better patient compliance, ODT has at t racted attention as a preferred alternative to conventiona l tablets and capsules. ODT could ingratiate enhanced l ife-cycle management, convenient dosing for pa-tients with dysphagia in paediatrics, geriatrics and psychiatrics, and smal l animal treatments (Hir a ni et al., 2011; T a k a hir o a n d T o mohit o;2014, Yi , 2017). In this study, for the f i rst time, HME and ODT were used to prepare a taste-masked FM formulation. The formulation was systematically characterized, including content determinations, weight difference,friability, tensile strength, disintegration time, taste masking, stability,in vitro dissolution and in vivo pharmacokinetics, therefore providing a theoretical basis for use in veterinary clinics. 2.1. Materials FM standard was purchased from the China Institute of Veterinary Drug Control (Bei ji ng, China). FM was purchased from Qilu’shenghua Pharmaceutical Co., Ltd. (Dezhou, Shandong, China). Eudragit E PO was obtained from Shenzhen Youpuhui Pharmaceutical Co., Ltd.(Shenzhen, Guangdong, China). Low-substituted hydroxypropy l cellu-lose (L-HPC), microcrystalline cellulose (MCC), mannitol (Man), mag-nesium stearate (MS) and other medicinal excipients were kindly gifted by Anhui Sunhere Pharmaceutical Excipients Co., Ltd. (Huainan, Anhui,China). All other chemical reagents used for HPLC analysis and disso-lution were of analytical grade. 2.2. Methods 2.2.1. Preparation of the HME extrudate Eudragit@ E PO was chosen as the matrix. After pass i ng through the A B Fig. 5. Drug release of FM extrudate i n artificial saliva (A) and i n pH 4.5 buffer solution (B). ASTM #30 mesh,FM and Eudragit@ E PO were mixed using a V-shell blender (GlobePharma, Maxiblend@, New Brunswick, NJ, USA). A co-rotating twin-screw extruder (16 mm Prism Euro Lab, Thermo Fisher Scientific, Pittsburgh, PA, USA) was used to conduct HME. Then, FM-E PO extrudate was milled and sieved to a specific size (250~600 um)and stored in glass vials. The HME parameters were optimized as fol-lows: extrusion temperature, 135℃; screw speed, 100 rpm; and drug loading, 20%, which resulted in good process reproducibility. 2.2.2. Thermogravimetric analysis (TGA) The thermal stability of the active pharmaceutical ingredient (API)and excipients was estimated by TGA (PerkinElmer Pyris 1, Shelton, CT,USA). The samples were heated from 30 to 200°C at a heating rate of 20°C/min under an inert nitrogen atmosphere at a f low rate of 20 ml/min. The results were analysed using Pyris software (PerkinElmer Life and Analytical Sciences, 719 Bridgeport Ave., CT, USA). 2.2.3. Differential scanning calorimetry (DSC) A PerkinElmer Diamond differential scanning calorimeter equipped with Pyris software (Shelton, CT, USA) was used to conduct DSC analysis (F en g et a l ., 2015). Approximately 3~5 mg each of pure API , a physical mixture of FM and Eudragit@ E PO and milled extrudate were hermet-ically sealed in aluminium pans. DSC parameters were set as heating from 30 to 200°C at 20°C/min in an inert nitrogen atmosphere with a flow rate of 20 ml/min. 2.2.4. Powder X-ray diffraction (PXRD) The crystal li nity of FM i n HME extrudate was analysed by a powder X-ray di f fraction apparatus (Bruker AXS, Madison, MI, USA). The ma-chine was set at room temperature utilizing CuKo radiation at 15 mA and 30 kV, 4°/min and diffraction angles (20) of 1-40°. 2.2.5. Fourier t ransform infrared (FT-IR) spectroscopy An Agilent Cary 660 FT-IR spectrometer (Agilent Technologies, Run A: MCC (%) B: Man (%) C: L-HPC(%) 1 18.750 52.500 3.750 2 41.250 31.125 2.625 3 30.000 41.250 3.750 4 26.250 45.000 3.750 5 41.250 30.000 3.750 6 31.125 42.375 1.500 7 18.750 52.500 3.750 8 41.250 32.250 1.500 9 25.781 47.156 2.062 10 21.000 52.500 1.500 11 21.000 52.500 1.500 12 41.250 32.250 1.500 13 41.250 30.000 3.750 14 41.250 31.125 2.625 15 34.500 39.000 1.500 16 35.096 35.906 3.187 Table 4The results of the mixture design (pressure 4, mean ± SD, n=6). Run Porosity (%) Tensile strength (MPa) Disintegration time (s) 1 0.667±0.011 0.677±0.018 18.1±0.842 2 0.671±0.120 0.965±0.025 61.8±1.28 3 0.693±0.014 1.04±0.010 18.5±0.620 4 0.684±0.023 0.974±0.010 24.8±1.30 5 0.650±0.147 1.40±0.013 31.9±1.510 6 0.661±0.032 0.958±0.013 21.0±1.932 0.67±0.020 0.672±0.023 19.5±0.841 8 0.675±0.011 1.24±0.011 65.9±2.56 9 0.668±0.010 0.706±0.027 18.2±1.56 10 0.696±0.020 0.578±0.052 21.4±0.944 11 0.700±0.011 0.560±0.020 22.6±1.24 12 0.65±0.020 1.250±0.010 66.5±1.62 13 0.650±0.021 1.570±0.030 32.5±1.80 14 0.668±0.040 0.950±0.050 60.1±0.51 15 0.671±0.032 0.849±0.031 38.2±0.612 16 0.655±0.022 0.969±0.062 24.0±0.751 Santa Clara, CA, USA) was used to assess the interactions between the API and excipient in the HME extrudate and in the physical mixture in the range of 400~4400 cm-. 2.2.6. Drug content tests With an HPLC system comprising an e2695 separations module, a 2998 photodiode array detector, and a 717 plus autosampler (Waters Technologies Corporation, 34 Maple St., Milford, MA 0157, USA),chromatographic analysis was conducted at a wavelength of 282 nm utilizing a Phenomenex Luna C18 reversed-phase column (250×4.6mm, 5 um) (FLM Inc, Guangzhou, China). The mobile phase was composed of methanol and 0.1% potassium dihydrogen phosphate in a ratio of 80:20 (v:v) using a flow rate of 1.0 ml/min. Al l content deter-mination data were calculated using flunixin (FX). 2.2.7. In vitro dissolution of the FM extrudate According to the US Pharmacopeia (USP), in vitro two-stage drug release development was performed. Specifically, 150 ml of arti f icial saliva medium and 900 ml of buffer stage medium (pH 4.5) were used with a USP II apparatus. The art i f i cial saliva medium consisted of the following: CaCl2▪2H2O,0.228 g; MgCl26H20,0.061 g; NaCl, 1.017 g; K2CO3·1.5H20,0.603 g;Na2HPO4·7H20,0.204 g; and NaH2PO4●H20,0.273 g dissolved in 1000ml of water and adjusted to pH 6.8 with 0.1 M HCl (0.826 ml HCl to 1000ml of water). The buffer stage medium consisted of 2.99 g of CH3COONae3H2O dissolved in approximately 800 ml of water and then mixed with 14 ml of 2 mol/L HCl solution; then, water was added to increase the volume to 1000 ml. ANOVA results for the effect of the dependent variable. Dependent Source Sum of df Mean F- P- variable squares square value valueProb >F P: (%) Model 2.694E- 6 4.490E- 6.92 0.0055 003 004 Linear 1.245E- 2 6.223E- 9.60 0.0059 mixture 003 004 AB 8.214E- 1 8.214E- 12.67 0.0061 004 004 AC 3.451E- 1 3.451E- 0.053 0.8227 006 006 BC 3.629E- 1 3.629E- 0.056 0.8183 006 006 ABC 1.424E- 1 1.424E- 21.95 0.0011 003 003 Residual 5.836E- 9 6.485E- 004 005 Lack of 5.836E- 4 1.459E- Fit 004 004 Pure 0.000 5 0.000 Error Cor total 3.278E- 15 003 TS: (MPa) Model 0.86 2 0.43 25.67 <0.0001 Linear 0.86 2 0.43 25.67 <0.0001 mixture Residual 0.22 13 0.017 Lack of 0.22 8 0.027 Fit Pure 0.000 5 0.000 Error Cor total 1.07 15 DT:(S) Model 5027.60 5 1005.52 41.33 <0.0001 Linear 3519.06 2 1759.53 72.32 <0.0001 mixture AC 6.81 1 6.81 0.28 0.6084 BC 0.66 1 0.66 0.027 0.8726 BC (B-C) 753.45 1 753.45 30.97 0.0002 Residual 243.31 Lack of 243.31 5 48.66 Fit Pure 0.000 5 0.000 Error Cor total 5270.92 15 A physical mixture and HME extrudate (equivalent to 20 mg of FX)samples were filled into hard shell hydroxypropyl methylcellulose (HPMC) capsules for the in vitro dissolution test. A Hanson SR9-plusTM dissolut i on apparatus (Chatswor t h, CA, USA) was used for the two-phase release study at 100 rpm for 60 s (artificial saliva medium) and 120 min (pH 4.5, buffer stage medium) at 37°℃ (n = 3). Aliquots (2 ml ) were wi t hdrawn every 5 s for 60 s and then every 10 min for 2 h, and 2 ml of fresh medium was added to maintain the sink condition after each sampling. A Waters HPLC system was used to analyse the samples. The dissolution parameters were also used for testing ODT. 2.2.8. Optimization of the formulation and preparation of taste-masked FM ODT The compressibility, disintegration, solubility and powder of the excipients were investigated. The ingredients with the best properties were selected, and their dosage ranges were confirmed. Then, the D-optimal mixture design was used to fi l ter the best composition by tensile strength, disintegration t i me and porosity. The optimized composition was used for the final formulation of ODT. The milled and sieved FM SD was mixed with other excipients in a V-shell blender for 20 min at 20 rpm. In the last 2 min, magnes i um stearate was added. A 10 mm standard flat-faced punch was used for direct compression. The pressure was adjusteddto maintain an Normal Plot of Residuals o d E o In te rna ll y S t u d e n t i ze d R e si d u a l s Fig. 6. The normal plot of the residuals. 2.2.9. Tablet properties Weight variations were measured on a microbalance. Briefly, twenty tablets were weighed, and the average weight was determined. Each tablet weight was compared to the average weight and evaluated within the USP specified tolerances for uncoated tablets (±7.5%). Tablet friability was assessed by a fabricator tester (EF-1 W, Elec-trolab, Mumbai, India). It rotated for 4 min at 25 rpm continuously.Before the test, the tablets were weighed accurately and then dusted and reweighed carefully after the test. Hardness was measured by a hardness tester (TS-50N, Okada Seiko Co., Ltd., Japan). The tensile strength (TS) was calculated by the following formula: TS=2F/nDH, where F is the crushing load, D is the diameter and H represents the thickness. Porosity was calculated by the formula e =1-m/(prV), where pt is the true density, m i s the weight , and V is the volume. Disintegration ICOT t i me was tested by a pharmacopoeia apparatus (Erweka ED-2 L, Heusentamm, Germany). The disintegration medium was distilled water. 2.2.10. Evaluation of taste-masked effectiveness E -tongue eva l uati o n. M e a sure m en t s w e r e c o nd u c t ed a t 37℃ u s i n g an e le ctr i c to n gue (T S -5000Z, I N SE N T, J a p a n ) (T a b le 1 Samples f o r E -t o ng u e a n a l yses). Test solutions included reference solution (artificial saliva): KCl + tartaric acid; negative electrode cleaning solution:dist i lled water + ethanol + HCl ; and positive electrode cleaning solu-tion: KCl + distilled water+ ethanol +KOH. The sensors used were C00,AE1, AN1 and BTO, corresponding to the tastes of acidic bi t terness,astringency, basic bitterness and hydrochloride bitterness, respectively.All calculations and analyses were performed by system-provided software. E -no s e e valua ti on . The E -nos e s ys t em (PE N 3, A I R SE N SE , G e r ma ny )c o n t a i n s 10 diffe re nt m e t a l -o xid e se n s o rs . Samples were the same as E-tongue analysis except the sample weight was 2 g. The test condi t ions were set as : test t ime,1 s/group; cleaning time, 100 s; sensor zero-t i me, 5s; sample setup time , 5 s; flow rate, 400 ml/min; record time, 100 s; and cleaning f low rate, 400 ml/min. In vitro dissolution of ODT. A two-stage drug-release study in vitro was conducted. Then, 150 ml artificial saliva medium and 900 ml buffer solution (pH 4.5) were used with a USP I I apparatus. The specific pa-rameters are described in S e ct ion 2.2.7. 2.2.11. Physical and chemical stability The physical and chemical stability of FM extrudate were tested by storage in closed glass vials under 25°C/65% RH storage conditions for 12 months and 40°C/75% RH accelerated conditions for 6 months. The physica l stabi l ity was evaluated by DSC, and drug content analysis was used to investigate the chemical stabil i ty. The same conditions were used for the stability test of ODT. The dissolution similarity factor (f2)was utilized to compare the dissolution profiles of ODT following the stabi l ity tests. 2.2.12. Pharmacokinetic study Grouping and drug administration. A comparative pharmacokinetic study of FM ODT (TEST), commercially available FM injection (XINNIKAR)and FM granules (HAIYANSHUQ) was performed in beagle dogs at the National Bei ji ng Center for Drug Safety Evaluation and Research. Six beagle dogs weighing approximately 10 kg were obtained from a localcompany(Beijing Rixin Technology y Co.,Ltd., No.111006700000135). The dogs were housed separately, fed a commer-cial dry diet twice a day and water ad libitum. The protocol was reviewed and approved by the Nationa l Beijing Center for Drug Safety Evaluation and Research Experimental Animal Welfare and Ethics Committee (protocol number IACUC-2017-081). A randomized 3 × 3 crossover design experiment was conducted.TEST and HAIYANSHUQ were given oral l y (p.o.) at a dose rate of 2 mg/kg (calculated based on FX). XINNIKAQ was administered intravenous l y (i.v.) via the radial veins at a dose of 2 mg/kg utilizing a 2-ml disposable syringe with a #6 needle. The washout period was two weeks. Blood sample collection. Blood samples were collected before (0 min)injection and 5 min, 10 min, 15 min, 30 min, 45 min,1 h, 1.5 h, 2 h,3h,4 h, 6 h, 9 h, 12 h, 16 h, 24 h, 36 h, 48 h and 72 h after injection. For others, samples were collected before (0 min) administration and 5 min,15 min, 30 min, 45 min, 1 h, 1.5 h,2h,3h,4h,6h,9h,12h,16 h,24h,36 h , 48 h and 72 h after administration. Samples were centrifuged at 3000 × g for 10 min. The plasma was divided into 2 aliquots, transferred B(30.000) C Fig. 7. D-optimal f unction results: curve (A); contour line (B); 3D fractal image (C). Table 6Composition opt i mization results of the D-optimal mixture design. Number MCC (%) Man (%) L-HPC (%) TS (%) DT (%) 1 18.750 52.500 3.750 0.696146 16.9525 2 28.465 45.000 1.535 0.778911 18.0344 3 25.887 47.350 1.764 0.729647 17.7072 4 20.156 51.094 3.750 0.732489 17.7837 5 19.610 51.640 3.750 0.718359 17.4605 6 20.561 50.689 3.750 0.742945 18.0228 7 24.374 48.810 1.816 0.694505 17.9689 8 27.222 46.137 1.641 0.75483 17.6132 Table 7 Composition verification of the D-optimal mixture design (mean ± SD, n =3). Property 20170308 20170315 20170322 Friability (%) 0.88±0.08 0.87±0.06 0.82±0.02 Tensile strength (MPa) 0.72±0.0074 0.714±0.010 0.727±0.042 Disintegration (S) 17.52±1.44 16.58±1.73 18.13±1.02 Weight variation (%) 2.72±1.02 3.13±1.12 3.13±1.14% to cryovials and stored at -20°C. Drug assay. Extraction of FM from blood samples and validation of an HPLC method for the analysis of FM in plasma were performed in accordance with methods described elsewhere (Hub er et a l., 2013), with a few modifications. Briefly, 400 pl of plasma was transferred to a 10 ml tube. Then, 40 pl of HCl (1 mol/L) and 4 ml of acetonitrile were added with mixing for 30 s after the addition of each component, and the samples were subsequently centrifuged at 10,000 rpm for 10 min. The organic phase was transferred to another tube, and acetonitrile and HCI were added to the rest for another extraction. The combined organic phase was dried under N2 at 45°C, redissolved in 400 pl mobile phase and f i l tered through a 0.22 pm organic filter for HPLC analysis. 2.2.12.4. Statistics. Microsoft Office Excel 2019 was used to calculate all pharmacokinetic parameters according to the noncompartment model method. The results are expressed as the means ± standard de-viation (SD). Differences between FM ODT and granules were consid-ered to be signi f icant at P ≤ 0.05 by t-test or via the rank-sum test for parameters that were not normally distributed. 3. Results and discussion 3.1. Hot-melt extrusion process 3.1.1. Selection of matrix The compatibility of the matrix and API, thermal stability, and glass-transition temperature (Tg) were measured before HME. Compatibi l ity can be assessed based on the solubility parameters of each component.The solubility parameter can be calculated by the Hoftyzer and Van Krevelen methods using the following equation: 6t2=8d²+6p²+6h,where 6d,8p, and 8h denote the dispersion, polarity, and hydrogen bond solubi l i ty parameters, respectively (Ha n sen , 2000). The solubil i ty parameter of FM was calculated as 34.29 MPaand Eudragit@ E PO is 39.105 MPa. It is generally accepted that a D-value less than 7 MPa between the API and the excipients indicates good compatibi l ity (Mohamm ad e t al., 2011). Eudragit E PO is an amorphous cationic copolymer formed by dimethyl amino ethyl methacrylate and neutral methacrylic acid ester and is widely used due to its masked taste,controlled release, and solubility, among other advantages (L i et al.,2015). The interaction between EudragitQ E PO and FM was confirmed by FT-IR. These interactions may have aided in improving the taste-masked effect and drug release. Tg should be close to prevent poor stability of the extrudate or API degradation at high temperature. All components did not show degra-dation during the TGA test heating to 150 ℃. Eudragit@ E PO has good therma l stabi l ity and a low glass-transition temperature (K oj i ma et al.,2012). FM has a melting point of approximately 140℃; however, the physical mixture could be extruded at a low temperature (135℃)without extra plasticizer, which indicates that FM may act as a plasti-cizer during HME, suggesting that Eudragit E PO is suitable for this application. 3.1.2. Parameter optimization for HME The effects of drug loading, extrusion temperature and screw speed were conducted by single-factor tests, and an orthogonal test [L9(3")]was conducted for optimization. Orthogonal test results were evaluated Table 8Results of E-tongue analyses (mean ± SD, n =3). Samples B-bitterness2 Aftertaste-B Aftertaste-A H-bitterness Bitterness Astringency ANO C00 AE1 BTO C00 AE1 P6.8 0 0 0 0 0 0 FM 4.27±0.26 4.32±0.34 10±0.72 0.14±0.01 23.89±0.38 17.8±0.16 PM-FM 1.87±0.36 4.88±0.38 17.79±0.83 0.45±0.02 25.64±0.24 25.53±0.12 PM-Placebo 4.59±0.55 0.1±0.03 1.18±0.27 -0.01±0 2.34±0.27 2.61±0.39 H-FM 1.52±0.18 1.36±0.07 3.12±0.32 0.06±0.01 16.48±0.27 6.96±0.25 H-Placebo 4.37±0.35 0.03±0.03 0.39±0.06 0.00±0.01 0.82±0.14 0.46±0.04 G-FM 5.39±0.41 5.58±0.43 14.98±0.66 0.19±0.06 26.71±0.45 23.00±0.27 O-FM 5.28±0.27 3.81±0.2 10.39±0.49 0.09±0.01 23.45±0.31 17.84±0.22 O-H-FM 3.71±0.19 0.51±0.03 1.25±0.16 -0.02±0.02 9.06±0.24 2.58±0.23 O-Placebo 2.78±0.21 0.14±0.02 0.48±0.08 -0.01±0.03 2.07±0.19 0.7±0.04 Note: P6.8 indicates artif i cial saliva. Fig. 8. Radar chart of the effective index. Fig. 9. Aftertaste-A and astringency (A), bi t terness (B), aftertaste-B (C), and B-bitterness2 (D). Fig. 10. Reproducibi l ity of the E-tongue analysis. by arti fi cia l saliva medium dissolution tests. Variance analysis (Tab le 2)showed that the different levels did not cause significant differences in dissolution. Thus, considering the clinical demands and production costs, HME parameters were optimized as follows: extrusion tempera-ture, 135℃; screw speed, 100 rpm; drug loading, 20%; and the process reproducibility was good. Extrusion temperature and screw speed could affect HME results:Sarode AL reported that the physicochemical properties of HPMCAS were not significantly affected by HME, but the most significant change was the release of acetic and succinic acids with increasing HME tem-perature and speed, which in turn affected the dissolution t i me (S arode et a l., 2014). To achieve a high dissolution speed and supersaturation of poorly water-soluble drugs, the extrusion temperature and screw speed should be wel l controlled to improve the drug-polymer interactions (Fu ku d a e t al., 2013). This study preliminarily optimized the HME pa-rameters, and further optimization should be conducted in the scale-up experiment. 3.1.3. Characterization and i n vitro study of HME extrudate Differential scanning calorimetry (DSC). FM showed an endothermic peak at 141.03°C. HME extrudate and Eudragit@ E PO showed no peak,while the physical mixture exhibited a less intense endothermic peak than FM at the same temperature (141.03°C), which proved that FM was completely converted into an amorphous state during HME (Fig .1). Thermogravimetric analysis (TGA). TGA thermograms (Fi g. 2) proved the thermal stabi li ty of FM, Eudragit@ E PO, physical mixture, and HME extrudate. No sample showed significant degradation (< 1%) up to 150C. This proved the therma l stabil i ty during HME and conf i rmed the feasibility. Powder X-ray diffraction (PXRD). The characteristic peaks in PXRD (Fi g . 3) of crystal l ine FM were shown at 20 values of 5.35°,10.70°,16.08°,18.68°,21.44°,23.01°,33.98° and 35.31°, and these peaks were observed in the spectrogram of the physical mixture, while HME exudate and Eudragit@ E PO exhibited no peak, confirming the crystalline state change during HME. Fourier transform infrared (FT-IR) spectroscopy. FT-IR results (F ig . 4)showed characteristic peaks at 723.00, 771.02,792.02,1254.49, 1319.28, 1455.73,1506.15 and 3316.9 cm-in the spectra of FM and the physical mixture, while no prominent peak was present in the spectra of Eudragit@ E PO and HME extrudate. In the FT-IR spectrum, the shift, decreased i ntensity and absence of characteristic peaks might be due to intermolecular interactions, which indicated the generation of hydrogen bonds during the extrudate. It was reported that FT-IR spectra of HME-based solid solutions of artesunate in the water-soluble polymers Soluplus@ and KollidonQ VA64 did not exhibit any changes in the molecular stretching bands (although slight shifts i n specific peaks were observed), but the disso-lution properties of artesunate were significantly improved in the resulting molecular dispersions (F u l e et al ., 2015). Therefore, it is necessary to combine several characterizations. Al l the characterization results sufficiently confirmed that FM was converted from a crystall i ne to an amorphous state through HME with Eudrag i tQ E PO. In vitro dissolution studies and drug content t est. In vitro dissolution results (F i g . 5) showed FM extrudate and physical mixture had dissolutions of <4% and >50%, respectively, at 1 min in artificial saliva medium and >80% dissolution at 2 h in pH 4.5 buffer solution. The drug content test indicated that there was no API l ost during HME (HPLC data not shown). In brief, a taste-masked FM solid dispersion was successfully formulated, and it maintained the quick release feature. 3.2. Optimization of the formulation and preparation of ODT The content ranges of MCC (A), man (B) and L-HPC (C) were set as 20~50%,45~75%, and 2~5%, respectively. The MS content was tested, but different levels caused no signi f icant variations, and the recommended amount of 1% was chosen. Based on prel i minary studies, a D-opt i mal mixture design was con-ducted. Five compression forces were tested to determine the optimal force (16 formulations were tested for each force, T a ble 3). The fourth force (T ab l e 4) was chosen because it achieved a proper hardness of approximately 2 kgf (K u n o e t al ., 2005). The statistical significance was analysed by regression f i t ting of a l inear model, a two-term multinomial model and an incomplete three-term model using Design Expert 11. The fitting equations of porosity (yi), tensile strength (y2) and disintegration time (y3) are as follows (ratio of MCC, x1; ratio of Man,x2;ratio of L-HPC, X3): 1. m a in a xi s (V a r i a nc e : 96.37%) PC A -A n a l ys i s A n o r m a li za t io n ::P C A : Ma t r ix Co r r e l a ti o n -M . A l g or i t hm : P CA V a r i a n c e :: 99.003% 1. m a in ax i s : 96.372% 2. m a i n a x i s : 2.6315% D i s cr i m in a t i o n p o w e r : 2 3 5 6 7 8 9 1.000 0.917 0.968 0.941 0.999 0.978 0.989 1.000 1.000 0.451 0.977 0.977 1.000 1.000 1.000 1.000 0.917 0.451 0.942 0.732 0.884 0.910 0.896 0.921 0.968 0.977 0.942 0.837 0.952 0.964 0.958 0.969 0.941 0.977 0.732 0.837 0.856 0.934 0.921 0.949 0.999 1.000 0.884 0.952 0.856 0.995 0.988 0.997 0.978 1.000 0.910 0.964 0.934 0.995 0.577 0.979 0.989 1.000 0.896 0.958 0.921 0.988 0.577 0.921 1.000 1.000 0.921 0.969 0.949 0.997 0.979 0.921 Fig. 11. Principal component analysis (A) and distinction analysis of PCA (B). The f itt ing models all had high degrees of significance for the effect of the dependent variable according to the ANOVA results (Table 5):porosity, F = 6.92, P<0.01; tensile strength, F=25.67, P<0.0001; and disintegrating t ime (DT), F= 41.33,P<0.0001. These results indicated a good correlation between the predicted values from the models and the experimental values. As disintegration t i me (DT) is the most important i ndex for ODTs,the opt i mization was based on the DT. By using the opt i mization function,the software predicted random combinations until the optimal target response value was achieved. The residuals conform to a normal dis-tributio n , indicating a good fit (Fi g.6). The trend of the influence on the disintegration t i me was represented as a parabola with a global mini -mum and arms with large tangent values (F ig. 7A), and the contour map was a semiellipse, which showed that the factors had different interac-tion degrees (F i g. 7B). The 3D fracta l i mage of t he DT showed the effects of different combinations (Fi g.7C). All of these results i ndicated a good fit of the polynomial regression model of DT, and the predicted solutions were reliable. Several solutions were predicted, and the tensile strength and disintegration time both met the requirements (T a bl e 6). According to a previous study, the mixture with a higher proportion of Man was chosen A LDA -A n aly s i s Fig. 12. Linear discriminant analysis (A) and contribution rate of LDA (B). as the optimal compos i tion: 25% FM milled extrudate, 18.75% MCC,52.5% Man, 3.75% L-HPC and 1% MS. For ODTs, it is necessary to choose proper components to achieve a reasonable tensile strength for packing and transport and a suitable porosity for quick water absorption to allow disintegration, but these two properties are contradictory (Grycz k e et al., 2011; A l -K hatta w i e t a l., 2014). Excipients should have good compression properties to ensure formabil i ty and disintegration. Therefore, appropriate pharma-ceutical adjuvants should be reasonably matched and optimized. MCC is primar i ly used as a binder/diluent in oral tablet and capsule formulations (Row e , 2009). Man is widely used as a diluent in rapidly dispersing oral preparations (L ee et a l ., 2003). In addition, i t i s cheaper for veterinary use and has a shorter disintegrating time than xylitol and better fluidity than lactose. L-HPC is commonly used in the preparation of rapidly disintegrating tablets produced by direct compression methods (D ou r o u mi s et al ., 2011; Ya n e t a l ., 2010). Design experts are widely used in the design of experiments (DOE). It calculates the D-optimal des i gn points in the experimental domain for the proposed mode l based on the candidate runs. This design enabled the evaluation of the appropriate regression model. Stepwise regression was performed on the special cubic model, where the i nteraction coefficients with the largest P-values were sequentially deleted until only significant interaction coefficients (P-value <0.05) remained in the model. The significant model was used to fit the responses. The lack-of-fit test and a normal probabil i ty plot of the res i duals were used to evaluate the model and to detect outliers. Contour plots from the sig-nificant model of the responses were drawn to determine the optimal variable settings (R a m bal i e t a l., 2003). In this study, the optimal composition was determined by a D-optimal mixture design. The veri -fication proved that the composition was suitably optimized. 3.3. Tablet properties 3.3.1. Composition verification The tablet composition was verified (T ab le 7). Three batches of taste-masked FM ODT were manufactured, and the tablet properties were determined. The pharmacopoeia speci f ications of less than 1% friabi l ity,proper tensile strength, less than 30 s disintegration and less than 5%weight variation were all met. 3.3.2. Evaluation of taste-masking effect i veness E-tongue test result is shown in T ab le 8. The radar char t (F i g . 8) Fig. 13. Drug release of ODT in artificial saliva (A) and in pH 4.5 buffer solution (B). Table 9Drug content analysis of FM SD and FM masked ODT s (mean ± SD, n=3). FM SD (content,%) FM ODT (labelled amount, %) 40°C/75% RH 25°C/60% RH 40°C/75% RH 25°C/60% RH 0m 19.91土 0m 19.75± 0m 99.27土 0m 99.4± 0.009 0.027 0.306 0.346 lm 19.90± 3m 19.93土 1m 99.33± 3m 99.07± 0.101 0.069 1.007 2.27 2m 19.94土 6m 19.96± 2m 99.13土 6m 98.87土 0.085 0.082 0.355 0.503 3m 19.77土 9m 19.81± 3m 98.80± 9m 98.53土 0.127 0.092 1.311 0.902 6m 19.88± 12 19.84± 6m 99.33± 12 99.06± 0.033 m 0.093 1.617 m 0.636 showed that 9 samples had obviously different bitterness, aftertaste-B,astringency, aftertaste-A and B-bitterness2 values. O-H-FM M had remarkably lower values than FM and G-FM in each index (Fi g. 9). The reproducibility results showed tha t the E-tongue analysis was robust and reliable (F ig. 10). E-nose response spectrograms showed that 9 samples had similar smells but differed in strength and ratio. Principal component analysis (PCA) and l inear discriminant analysis (LDA) (F i gs. 11 a nd 12) were conducted based on these spectrograms (data not shown). PCA results Table 10Plasma concentration of FM (ug/mL, mean ±SD,n=6). Time (h) XINNIKAQ 0 一 0.083 45.74±4.30 0.92±0.45 0.31±0.10 0.167 40.82±5.43 0.25 32.10±4.28 3.60±0.82 1.36±0.41 0.5 23.49±4.42 6.45±1.47 3.23±0.29 0.75 14.88±4.36 10.86±2.78 7.08±0.63 1 8.62±1.27 9.00±1.16 9.42±1.22 1.5 6.48±0.41 1.06±2.31 7.50±0.90 2 3.75±0.63 4.93±1.38 6.33±1.15 3 2.59±0.93 3.49±0.76 4.17±0.58 4 1.80±0.53 2.41±0.98 3.03±0.61 1.41±0.56 1.81±0.32 2.10±0.61 0.90±0.35 1.26±0.19 1.05±0.56 0.54±0.19 0.73±0.25 0.76±0.33 0.19±0.11 0.49±0.19 0.47±0.17 0.14±0.05 0.15±0.08 Note: ND means the drug was not detectable. revealed that the first main component accounted for 96.37% of the variance. According to the distinction analysis of PCA, the E-nose evaluation could remarkably distinguish the nine samples. LDA Table 11 Pharmacokinetic parameters after a single i .v . i njection of FM and p.o. admin-istration of commercial FM granules and taste-masked FM ODT in dogs (mean ± Parameter XINNIKAR TEST HAIYANSHUQ A(1/h) 0.1934± 0.1401±0.0278 0.1576±0.0349 0.0450 t1/2A(h) 3.6822± 5.1024±0.9494 4.6049±1.1588 0.9511 AUC0-o 46.1597± 39.2863±6.5049 37.8925±3.7929 (ug·h/ml) 7.2448 AUMCo-。 135.5026土 239.6223±70.3842 219.1822±42.9413 (ug·h²/ml) 36.1647 MRT0-oo(h) 2.9216± 6.0127±0.8541 5.8195±1.2398 0.4724 MAT (h) 3.0911±0.5089 2.8979±0.8286 Cmax (ug/ml) 9.5283±1.0684 11.04±2.5642 CL (L/hekg) 0.0442± 二 0.0070 Vd(ss) (L/kg) 0.1290± 0.0292 — — F(%) 86.23±16.26 83.37±11.91 Median Range Median Range Tmax (h) 一 1.00 1.00~1.50 0.75 0.75~1.00* Note: Compared with commercial granules,* P < 0.05, **P<0.01. Table 12ANOVA of the pharmacokinetic parameters (n =6). Parameter Test HAIYANSHUR P- Mean SD Mean SD value A2(1/h) 0.1401 0.0278 0.1185 0.0334 0.251 t1/2x(h) 5.1024 0.9494 4.6049 1.1588 0.435 AUC0-o.(ug·h/ 39.2863 6.5049 37.8925 3.7929 0.660 mL) AUMCo..o(ug·h²/ 239.6223 70.3842 219.1822 42.9413 0.557 mL) MRTo-.(h) 6.0127 0.8541 5.8195 1.2398 0.997 Cmax (ug/mL) 9.5283 1.0684 11.04 2.5642 0.212 Tmax (h) Median Range Median Range 1.00 1.00~1.5 1.00 0.75~1.00 0.011 Table 1 Samples for E-tongue analyses. No Sample FM Abbreviation Weight 1 FM API Pure FM FM 0.04 g 2 Physical mixture of optimal FM+E PO PM-FM 0.8g prescription 3 Eudragit@ E PO Without PM-Placebo 0.8g FM 4 HME of optimal prescription FM+E PO H-FM 0.8g 5 HME of Eudragit@ E PO Without H-Placebo 0.8g FM 6 FM commercial granule With FM G-FM 0.8g 7 FM ODT With FM O-FM 0.8g 8 FM taste-masked ODT With H-FM O-H-FM 0.8g 9 ODT Without O-Placebo 0.8g FM Table 2 The resul t of variance analysis. Source of variation SS df MS F P Significance Temperature 4.55 2 2.28 5.47 >0.05 Not Speed 0.83 2 0.42 0.11 >0.05 Not Loading 7.72 2 3.86 -2.1 >0.05 Not Error -3.67 2 -1.84 Total variation 9.423 8 Note: Fo.05(2, 2)=19,Fo.01(2,2) =99; P<0.05, significant difference, P<0.01,extremely significant difference enhanced the variations between groups and decreased the variations within groups. Both results proved the good smel l -masked effect of O-H-FM. Compared with the E-tongue evaluation, the taste was the main limitation of FM. 3.3.3. In vitro dissolution of ODT As shown in F ig . 13, ODT released less than 1.10% in 60 s in artificial saliva medium and more than 80% within 30 min in pH 4.5 buffer so-lution, which confirmed the taste-masked effect. In short, taking together E-tongue, E-nose and in vitro dissolution, it has been adequately proven that FM ODT is sufficiently taste-masked. Amorphous solid dispersions tend to recrystallize during storage,causing physical instability due to high-energy states. Therefore, it is necessary to select a proper matrix to form hydrogen bonds with the API,solving this problem and physically stabilizing the API over longer storage periods (P ap ageor g i o u , 2009). The PXRD characterization and HPLC results showed that FM SD and ODT were physically and chemi-cally stable during storage. These results showed that API was dispersed within the matrix and formed intermolecular interactions with Eudra-gitQ E PO. In addition, the dissolution behaviour of FM ODT after 12months of storage was similar (f2=86) to that of the fresh tablets, which indicated stability (Tab le 9). 3.4. Pharmacokinetics of the FM formulations i n beagle dogs The FM concentration in plasma was tested at various time points (T a bl e 10). Concentration-time curves were generated (F ig. 14). The pharmacokinetic parameters were calculated (T a ble 11), and statistical analysis was performed (T a ble 12). The Tmax values of the two formulations were signif i cantly di f ferent (p>0.05), 1.00 h and 0.75 h, respectively. This result indicated ODT had a slower absorption. FM granules were dissolved in water and then administered by gavage. This may lead to rapid absorption, with a smaller Tmax and a larger Cmax. FM ODT underwent water uptake,disintegration, dissolution, and absorption after administration. The particle size of the FM mi l led extrudate was 300~600 um, which may lead to prolonged drug release and cause a larger Tmax and a smaller Cmax. However, t1/2 and MRT were not significantly different, which may be due to the properties of FM. FM has a lower solubil i ty in acidic media , so it is mainly absorbed in the intestine. FM ODT disintegrated quickly, and the matrix (Eudragit@E PO) dissolved at pH<5. Thus, these two formulations were mainly absorbed in the intestinal t ract and had analogous distribution, supersession and elimination pathways, leading to similar t1/2 and MRT values. The values of the area under the concentration-time curve (AUC0-o)of granules and ODT were 39.29±6.5 ugh/ml and 37.89±3.79rgh/ml, and their F values were 86.23 ±16.26% and 83.37±11.91%,respectively, which indicated complete absorption and high bioavail-ability. (Tables 1 and 2) 4. Conclusion Flunixin meglumine was successfully extruded with Eudragit@ E PO by HME. Characterization studies proved the amorphous transition of FM. Then, direct compression was used to formulate the FM ODT. The main tablet properties were as follows: disintegration time of 17.6±0.1s and tensile strength of 0.7 ± 0.01 MPa. All the proper t ies met the re-quirements of ODT. E-tongue, E-nose and dissolution analyses confirmed the taste-masked effectiveness. The pharmacokinetic study showed that FM ODT had pharmacokinetic behaviours similar to com-mercial granules in beagle dogs. In addi t ion, a stability study proved the phys i cal and chemical stabil i ty of FM SD and ODT. In summary, FM ODT prepared in this study achieved a taste-masked effect and good pharmacokinetic behaviour and can be a potentially new formulation for clinica l use. Moreover, there is a need to conduct further trials that involve target animals to ensure convenience, safety and ef-ficacy of treatment. CRediT authorship contribution statement Yangfeng Xu: Conceptualization, Investigation, Formal analys i s,Writing - original draft. Guoqing Yan: Conceptualization, Investiga-tion. Xuemei Wen: Conceptualization, Investigation. Liqin Wu:Conceptualization, Investigation. Ruihan Deng: Conceptualization,Investigation. Qiuling Liang: Conceptual i zation, Investigation. Linjie Zhang: Conceptualization, Investigation. Hangping Chen: Conceptual i zation, Investigation . Xin Feng: Formal analysis, Writing -original draft, Wri t ing - review & edit i ng. Jiakang He: Conceptualiza-tion, Formal analysis, Wri t ing-review & edi t ing. Declaration of Competing Interest The authors have no conflicts of interest to disclose. Contributed reagents/materials/analysis tools: H.C. Wrote the paper: Y.X., J.H. Acknowledgements This study was supported by the National Natural Science Founda-tion of China (No. 31960717) and the Technology Research & Devel-opment Program of Nanning, Guangxi (No. 20175171). We wish to thank Dr. Xilong Xiao and Dr. Yafen Guo for their technical assistance. References A l ves, R R N , Roc ha , L A , 2018. F aun a a t home: A n imals as pe t s[M]//Ethn o zoolo g y . A c a d e m i c P r e s s , p p. 303-321. Barker , S.B., W o l e n , A .R., 2008. T he ben ef its o f hum an-co mp an i on an im a l i nt e r act ion : a review. J . V et. Med . Educ . 35 (4), 487-495. Papageo r g i o u , G.Z., et al ., 2009. Impr ove me n t i n ch em i c al a n d ph y s i ca l s t a bi l i ty of f l u va st a t i n d ru g thr ou gh hy d r o gen bo ndi n g i n ter ac t i o n s w i t h diffe r en t polyme r matr i ces. Curr . Dr ug Deliv. 6(1),101-112. Pim p arade, M .B ., et al ., 2017. Develo p men t and eva l ua t io n o f a n oral f a s t dis i n t egrat in g an t i-a l lergic f i l m u sin g h ot -me l t e xt r u sio n t e chn o logy. Eur. J . P h a rm . Bi opha r m. 119,S I I 81-90. Of f icia l Jou r n al o f Arbe it s g e mei nschaf t Fur P h ar m az eut i sc h e Verfa h renstech ni k E V . Po r esky, R.H ., e t al ., 2016. The c o m panio n an i m al bo nd i ng sca l e: i n ter n a l re li a bi l i ty a nd c o ns t ruc t v a l i dit y. Psych o l. R ep . 60 (3), 743-746. Y e i s er, E .E ., et a l ., 2012. T he e ff ec t s of e xpe ri me nt a l ly ind u ced E s c h er i chi a c ol i m a s t i t is an d fl u ni xi n meg l u m ine ad m in i s t ra t i on on ac t i v i ty mea s ures, feed i nta k e, a n d m ilk pa r ameters . J. D ai r y Sc i . 95(9),4939. H u be r , J ., e t a l ., 2013. P ai n man ag e me n t w it h f lu nixi n m e g l u mine at d e h o rn ing o f calves. J . Da i ry S c i . 96 (1), 132-140. Na y l or, R.J ., et al., 2014. C om p arison o f f l u n i x i n meg l umi n e an d m e loxica m for pos t ope r a t ive man a gemen t o f h orses wi th stran g u l at i ng small i n t es t ina l lesion s. E q u i ne V e t . J . 46 (4),427-434. Ma i r, T .S ., H owart h , S., Lane , J .G ., 2010. Evalua t ion of s o me prop h y l ac t i c t h era pi es f or the id i op ath ic h ea d s h a k er syndrome. Equine Ve t . J . 24 (S11), 10-12. Wei n er , M .L., K o tk oski e , L.A ., 2000. Exci pi e n t Tox i ci t y and Sa f ety. Mar c el D ekker . Ke y se r, S ., e t a l ., 2007. Eff ects of Saccha r omy ce s ce r ev i s ia e s ubs pe cies bou l a rd i i CN C M I -1079 on fe e d i ntake by heal thy b eef ca tt le t r eated with f l orfe ni c ol an d on h e al t h a n d perfo r m a nce of n ewly rece i ved beef hei f ers1. J . Anim . S ci . 85 (5), 1264. Witc h ey -L aks h man an, L .C ., Li , Y ., 2000. C h a p t e r 9-Cont r o l l ed drug d el i very and t h e c omp an i on a n imal . Con t ro l led R e l ease Vete r i n ary D ru g D eli v e ry . E l se v i er ,p p. 249-267. V o , C.L .N., Pa r k, C ., L ee, B .J ., 2013. C u rr en t t re nds a nd fu t ure pe rsp ecti ves of so l id d C I i s S p e r s ion s con tai n i n g poo rl y wa t e r -solubl e dr ugs. Eu r . J . Ph arm. B i oph ar m . 85 (3),799-813. A l sh ehri , S.M ., e t a l ., 2015. Me fenami c acid t a st e -mas k ed ora l di s in t eg r at i ng tabl e t s w i th enhanced s o lubi l ity v i a mo l ec ul ar i nt eract io n p roduced b y ho t m el t extrusion tech n ology. J . Drug D e l iv. S c i . Tec hn ol. 27, 18-27. Ye, X ., et al., 2016. Co n j u g a t i on o f h ot -me lt e x t ru si on wi t h hi gh -p r es su re homogeniza t i o n : a novel m et h o d of con ti nuous l y prep ar i n g na n o crysta l sol id di spe rsion s . AAPS Pharm S ciTec h 17 (1),78-88. H i rani, J .J., Ratho d, D.A., V a d a lia, K.R ., 2011. O r a l ly disintegra t i n g tablet s : a r ev i ew. I nt .R es. J. P ha rm. 2 (4), 81-88. Takahiro H, Tomohito O, 2014. Rapidly disintegrating tablet suitable for administration to smal l animals and s i mple production method therefor:, WO/2014/171306[P]. Yi , T., 2017. T he a p p l icat i on pros p ec t s an d deve l o p me n t tre nd s of o r a l l y d i s i n t eg r a t in g tab l e t s to d og s . Cl i n . R u r a l 47 (4), 1-6. Feng , X., e t a l., 2015. T he e f f ects of polyme r c arr ier , h o t melt extrus i on proces s an d down s trea m p r o c essing p aram et e r s on t h e m o isture sorption p r opert ie s of am or p ho u s s ol id dispe rsions . J. P h a rm . Ph a rm aco l . 68 (5),692-704. H anse n, C.M ., 2000. H a ns en Solu bi l i ty Pa r a m eters . S pr i n ger , N ew Y o rk , pp. 289-303. M oh a m m ad,M.A., A l h al awe h, A., Ve la ga , S ., 2011. H a n s e n so l ub il i t y p a r am et er as a t oo l to pre d i c t cocrys t al f ormat i on. I n t . J . P h a r m. 407 (1-2), 63-71. L i , J ., e t a l ., 2015. C urcu min -Eu d ragi t B E PO s o li d di s pe r s i o n: a sim pl e a nd po t en t me t ho d to so l ve t he problems of curcu m in . Eu r . Ph ar m . Bio p harm. 94, 322-332. Kojima, T ., e t a l., 2012. E rratum to : s t a b i li zat i on o f a s up ersa t u r a t e d so l ut i on o f me f ena m ic a c id fr om a so l i d d i spe r sio n w i th EU DRA GIT (@) EPO . P harm. Res. 29(10),2777. Rowe, R.C ., et a l ., 2009. H a n d boo k o f p har m aceut i cal exc i pien t s I n : P harma c e u t ica l Developm e n t & Te ch n o l ogy , 7t h Ed ., 18. Li bro s D igit a l es P h a r ma c eu t ica l P r ess,p . 544.-544. Sa r od e , A .L ., et a l ., 2014. Stab i l it y assessm en t of hypr om el l ose ac e tate su c c i na t e C C T (H P MCAS) NF for app l ica t ion i n ho t me l t extr u s i o n (HME). Ca rboh ydr. P olym. 101(1),146. Lee , K .J ., et a l ., 2003. Eva l ua t ion o f c r i t i ca l f ormu l at i on f a cto r s in t he development o f a rap idl y dispe r si n g c ap t opr i l o r a l d osage fo rm . D r ug D e v. I nd. P h a rm . 29 (9),967-979. D o uro u mi s , D .D ., G r yczke, A ., Sc hm i n ke , S., 2011. D eve l op men t a n d ev a l u a t ion o f ce t i ri zine H C l tas t e-masked ora l d i s i ntegra t i ng t a b lets. AAPS P har mSciTec h 12 (1),141-151. X u , Y ., et a l.,2018. P r epa r a t io n, chara c t er iza tion , an d p har macok i net i cs in sw i n e of a florfe ni co l en t e ri c f o r mulat ion pr e p ared u s i n g ho t -me l t ex t rusio n t ech no logy . J . V e t .Ph arm a c ol . Th er . 41 (4), 572-580. Yan, Y.D., e t a l., 2010. Pr epa r at i o n and eva l u at io n of tas t e -masked d o nep e z i l h y d r ochloride o r al l y di s i n t eg r a t i n g t a b let s . B i o l . P harm. Bul l . 33 (8), 1364. n y 101. Ramba l i , B., e t a l ., 2003. It ra c ona z ole f orm u lat i o n st u d i e s o f t h e mel t -e x tru s i o n p r ocess wi t h mix tur e d e sign. Drug D ev . I nd. Pha r m . 29 (6), 641-652.
确定



还剩13页未读,是否继续阅读?
北京盈盛恒泰科技有限责任公司为您提供《采用热熔挤压和D-最优混合设计制备的掩味氟尼辛大聚胺口腔崩解片的制备、评价及在比格犬体内的药代动力学研究》,该方案主要用于中药材和饮片中药物掩味效果评估检测,参考标准--,《采用热熔挤压和D-最优混合设计制备的掩味氟尼辛大聚胺口腔崩解片的制备、评价及在比格犬体内的药代动力学研究》用到的仪器有德国AIRSENSE品牌PEN3电子鼻
推荐专场
感官智能分析系统(电子鼻/电子舌)
相关方案
更多
该厂商其他方案
更多