环己烯中溴值的测定 应用资料
将已知质量的环己烯溶解于温度维持在0ºC~5ºC的溶剂中,然后用溴化钾-溴酸钾标准溶液滴定。当溶液中出现的游离溴引起电位滴定仪的电位突然改变时,即表示达到滴定终点。
方案详情

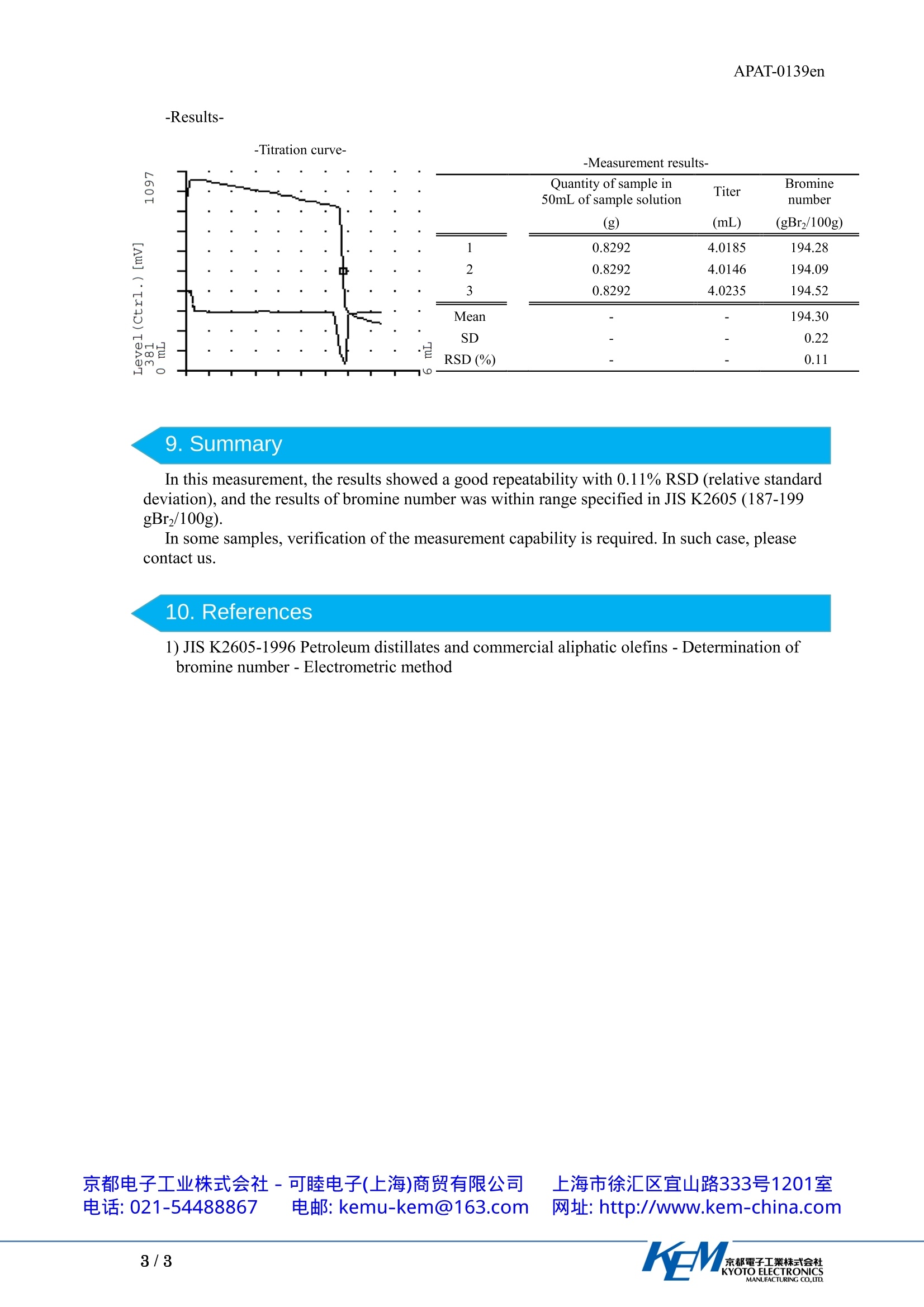
环己烯中溴值的测定 应用资料(英文版)将已知质量的环己烯溶解于温度维持在0ºC~5ºC的溶剂中,然后用溴化钾-溴酸钾标准溶液滴定。当溶液中出现的游离溴引起电位滴定仪的电位突然改变时,即表示达到滴定终点。京都電子工業株式会社KYOTO ELECTRONICSMANUFACTURING CO.,LTD.APAT-0139en APAT-0139en Application Note Bromine number of cyclohexene Industry Petroleum Instrument Automatic potentiometric titrator Measurement method: Polarization titration at constant current Standards JIS K2605,ASTM D1159, GB/T 11135 1.Scope Bromine number of cyclohexene was measured by polarization titration at constant currentbased on“JIS K2605 Petroleum distillates and commercial aliphatic olefins -Determination ofbromine number -Electrometric method”. Bromine number is the number of grams of bromine that is added to carbon-carbon unsatulatedbond in a 100 g of sample and is expressed in“gBrz/100g”. In measurement of bromine number,bromine is released by adding potassium bromide- potassium bromate (KBr-KBrO3) solution intoa sample solution containing strong acid, and then the released bromine is added to carbon-carbondouble bond. The chemical formulas of bromine releasing reaction and addition reaction aregiven in formula (1) and (2). During titration, polarization potential which needs to applyconstant current between twin platinum electrodes is measured and, an equivalent point isdetermined by change of the polarization potential. After the equivalent point, amount of brominein the solution is increased sharply. Polarization potential becomes smaller when amount ofbromine in the solution become larger, therefore a sharp change in potential is observed at theequivalent point. The endpoint of the titration is determined by detecting the change in potential. 5KBr+KBrO3+3HSO4→3Br2+3K2SO4+3HO ...(1) In this measurement, toluene solution of cyclohexene was added to titration solvent whosetemperature was kept in a range of 0-5℃, and then the solution was titrated with 0.25 mol/LKBr-KBrO3 solution. An inflection point on the titration curve was regarded as the end point, andthe bromine number of cyclohexene was calculated from the volume of KBr-KBrO3 solutionconsumed to titrate sample to the end point. 2. Precautions 1) Handle the reagents in a well ventilated room or a draft chamber. 2) Keep a temperature of titration solvent in a range of 0-5°℃ during titration. 3) Discharge titrant between burette and titration nozzle once before a titration. 3. Post-measurement procedure Wash electrode and titration nozzle with ethanol and pure water and then keep them soaked inpure water. If titration nozzle is left standing in the air, it will be clogged with precipitate of thecomponent of titrant. 4. Apparatus Main unit Automatic potentiometric titrator (preamplifier : POT) Electr o d e Twin platinum electrodes Temperature compensation electrode 5.Reagent Titrant 0.25 mol/LKBr-KBrO3 solution Titration solvent : Mixture of glacial acetic acid, toluene, methanol and HSO4(1+5) mixture ratio of them is 714 :134:134:18 (volumetric ratio). 6. Procedure -Preparation of sample solution- 1) Add 10 mL of toluene into a 50 mL volumetric flask. 2) Add 0.6-1.0 g of cyclohexene into the flask and measure mass of it. 3) Add toluene to the mark of the flask and mix it. -Setting of constant current value- 1) Press [Calibration] button. 2) Set Channel/Unit to“Ch3/Pol”. 3) Press [Details] button and set as follows. Calibration Mode :Current Polar Current: 5.00 uA 4) Immerse twin platinum electrode in titration solvent and perform calibration. -Blank test- 1) Add 110 mL of titration solvent into a 200 mL tall beaker. 2) Keep temperature of the titration solvent in a range of 0-5℃ by ice cooling. 3) Add 5 mL of toluene. 4) Titrate with 0.25 mol/L KBr-KBrO3 solution. -Measurement- 1) Add 110 mL of titration solvent into a 200 mL tall beaker. 2) Keep temperature of the titration solvent in a range of 0-5°℃ by ice cooling 3) Add 5 mL of sample solution 4) Titrate with 0.25 mol/L KBr-KBrO3 solution. 7.Calculation : Concentration conversion coefficient=39.95 mg/mL 8. Example Quantity of sample in Titer Bromine 50mL of sample solution number (g) (mL) (gBr2/100g) 1 0.8292 4.0185 194.28 2 0.8292 4.0146 194.09 3 0.8292 4.0235 194.52 Mean 一 一 194.30 SD 一 一 0.22 RSD (%) 一 一 0.11 9. Summary In this measurement, the results showed a good repeatability with 0.11% RSD (relative standarddeviation), and the results of bromine number was within range specified in JIS K2605 (187-199gBr2/100g). In some samples, verification of the measurement capability is required. In such case, pleasecontact us. 10. References 1) JIS K2605-1996 Petroleum distillates and commercial aliphatic olefins -Determination ofbromine number-Electrometric method 京都电子工业株式会社-可睦电子(上海)商贸有限公司电话: 021-54488867 电邮: kemu-kem@163.com 都電子工業株式会社KYOTO ELECTRONICSMANUFACTURING CO.,LTD.
确定


还剩1页未读,是否继续阅读?
可睦电子(上海)商贸有限公司-日本京都电子(KEM)为您提供《环己烯中溴值的测定 应用资料》,该方案主要用于烃中溴值检测,参考标准--,《环己烯中溴值的测定 应用资料》用到的仪器有石油产品自动电位滴定仪、AT-710S豪华型自动电位滴定仪
推荐专场
相关方案
更多
该厂商其他方案
更多