方案详情
文
吲哚-3-乙酸(IAA)作为一种典型的植物激素,可以调节植物细胞的分裂、生长和分化等生物活性。在本文中,通过自组装程序制备了一种 MXene和多壁碳纳米管复合材料,并在丝网印刷电极 (SPE) 上对其进行了改性,从而构建了一种无线便携式电化学传感器。通过循环伏安法研究了 IAA 的电化学研究,并且可以观察到其不可逆的氧化过程。在SPE修饰电极上实现了 IAA 优异的电分析方法,该方法具有较宽的检测范围为 0.05-125.0 μmol/L和较低的检测限(16.7 nmol/L)。将该传感器用于豌豆幼苗不同部位的IAA含量分析,结果满意。
方案详情

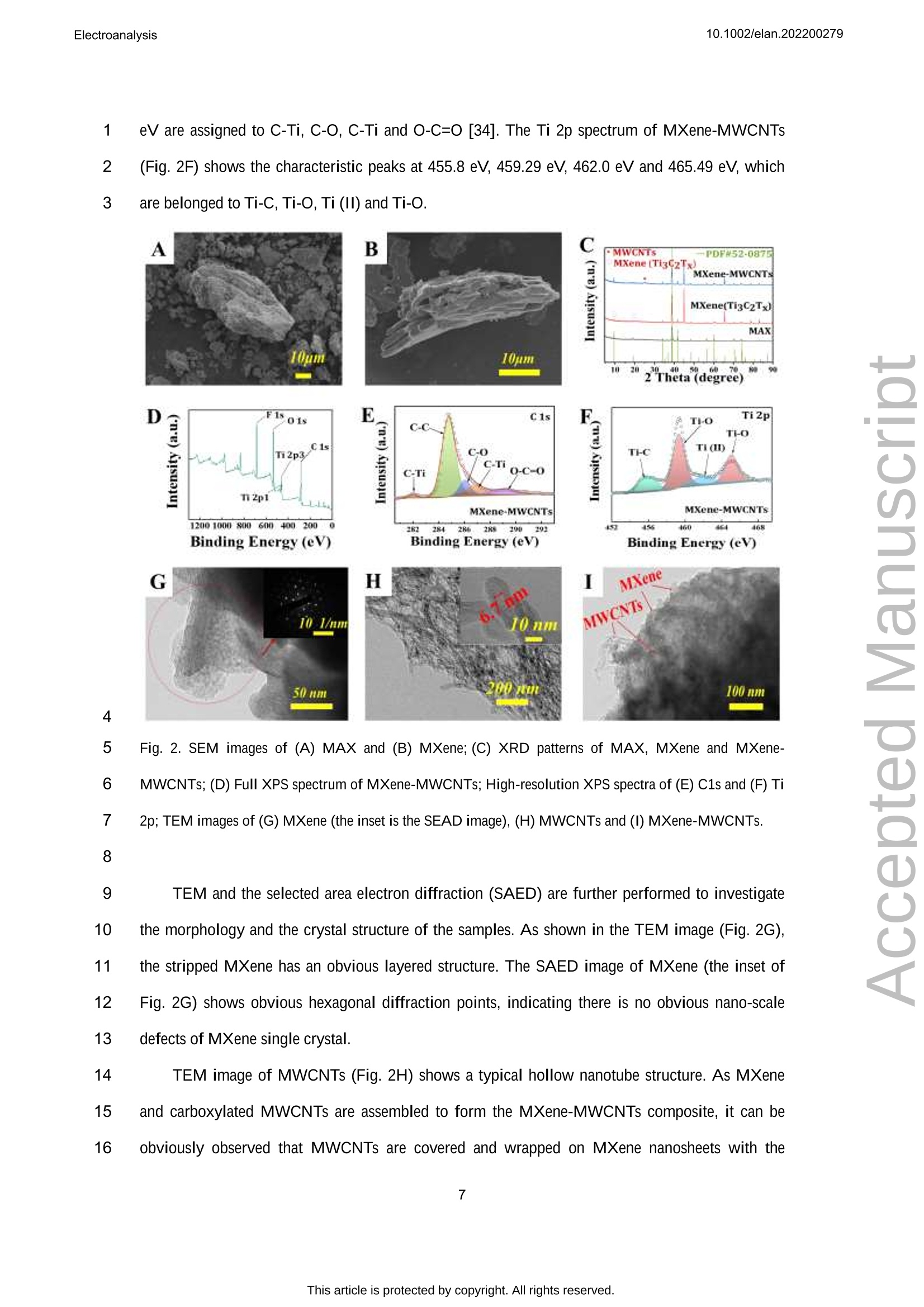
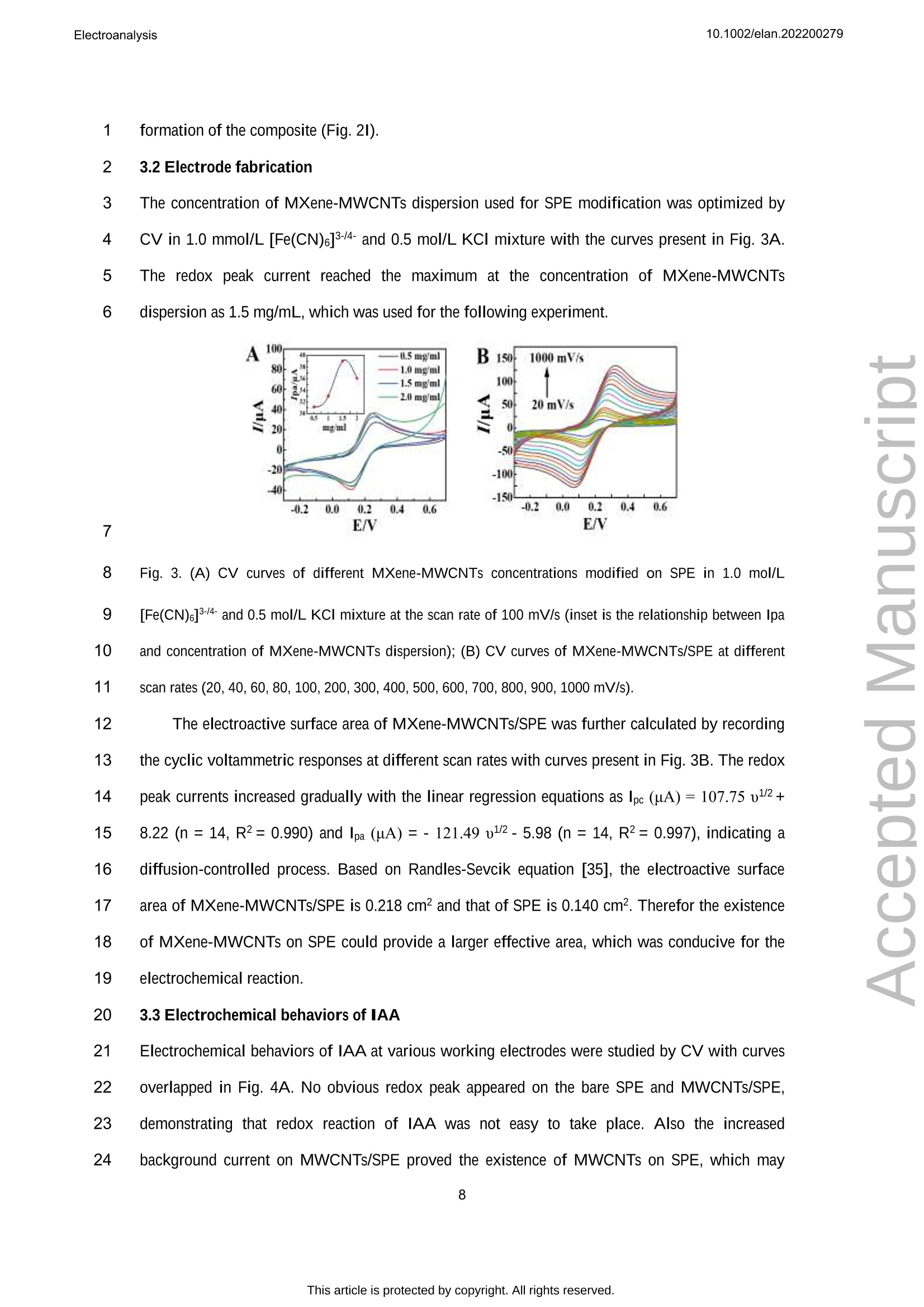
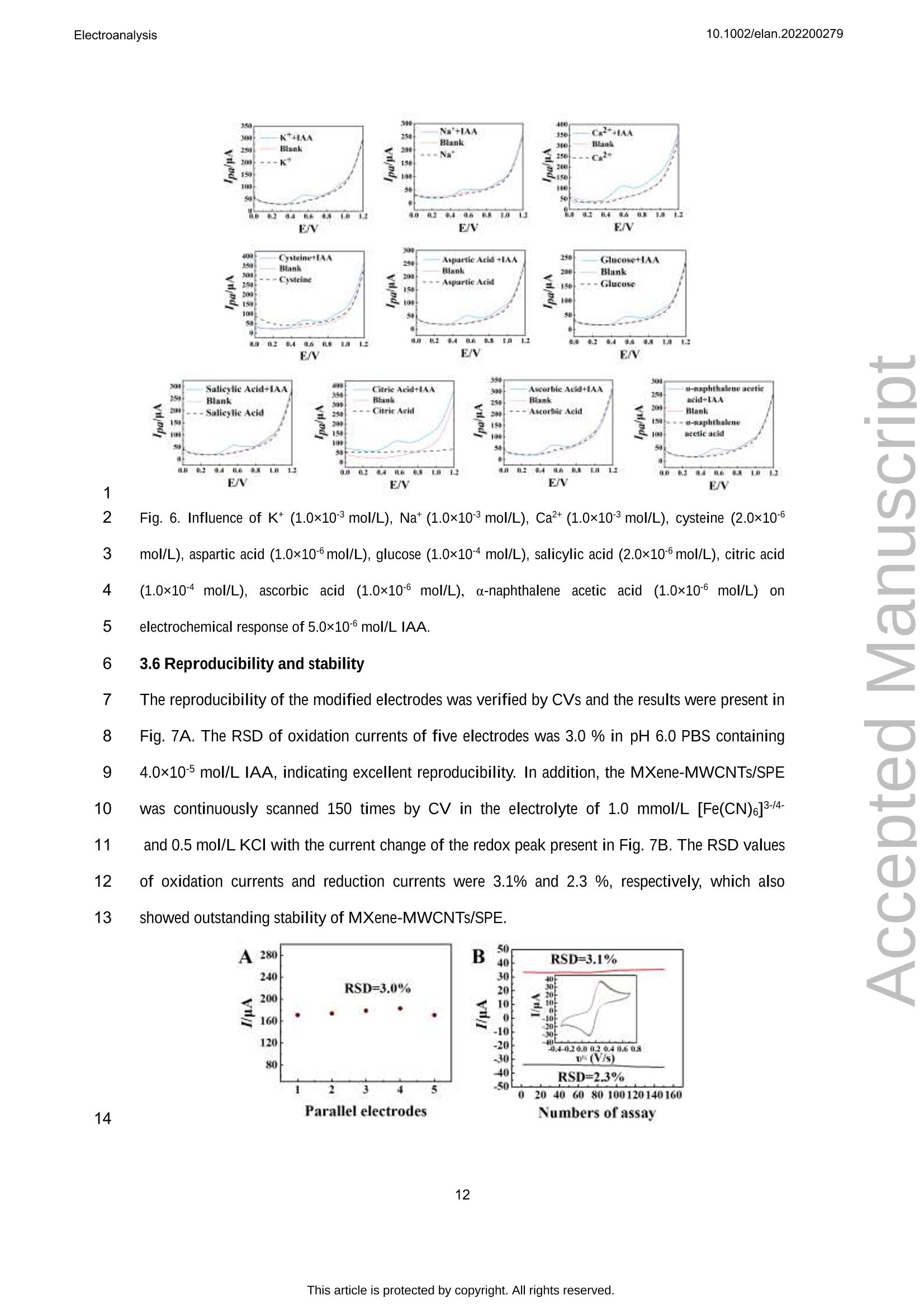
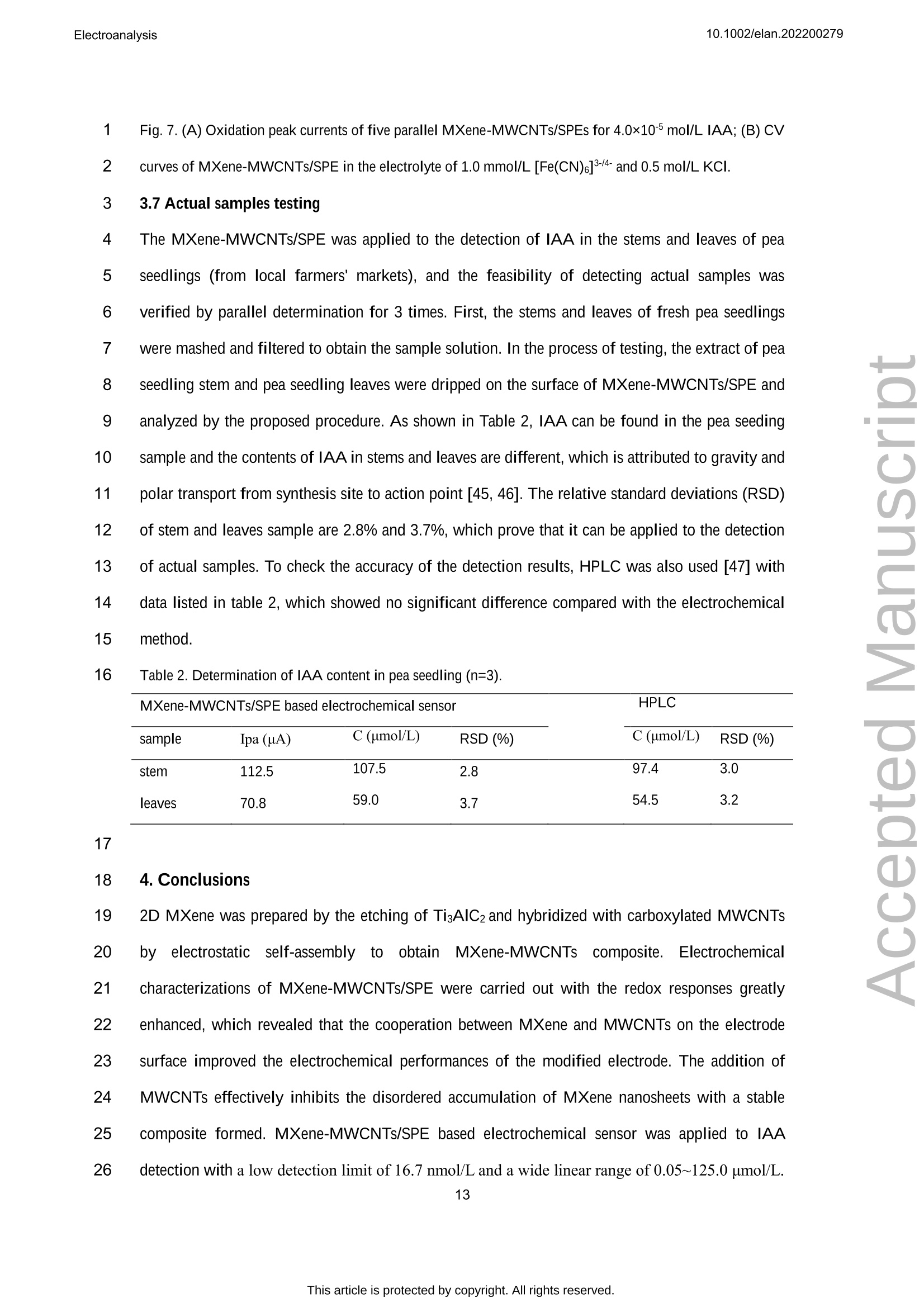
吲哚-3-乙酸(IAA)作为一种典型的植物激素,可以调节植物细胞的分裂、生长和分化等生物活性。在本文中,通过自组装程序制备了一种 MXene和多壁碳纳米管复合材料,并在丝网印刷电极 (SPE) 上对其进行了改性,从而构建了一种无线便携式电化学传感器。通过循环伏安法研究了 IAA 的电化学研究,并且可以观察到其不可逆的氧化过程。在SPE修饰电极上实现了 IAA 优异的电分析方法,该方法具有较宽的检测范围为 0.05-125.0 μmol/L和较低的检测限(16.7 nmol/L)。将该传感器用于豌豆幼苗不同部位的IAA含量分析,结果满意。原文链接:https://doi.org/10.1002/elan.202200279 本文中进行电化学测试的仪器为荷兰PalmSens,型号:EmStat3Blue便携式电化学分析仪,由雷迪美特中国有限公司提供。10.1002/elan.202200279Electroanalysis An International Journal Devoted to Electroanalysis, Sensors and Bioelectronic Devices Accepted Article Title: Portable Electrochemical Sensing of Indole-3-acetic Acid Basedon Self-assembled MXene and Multi-walled Carbon NanotubesComposite Modified Screen-printed Electrode Authors: Yuxue Chen, Yunxiu Sun, Yanyan Niu, Baoli Wang, ZejunZhang, Lina Zeng, Lin Li, and wei sun This manuscript has been accepted after peer review and appears as anAccepted Article online prior to editing, proofing, and formal publicationof the final Version of Record (VoR). This work is currently citable byusing the Digital Object Identifier (DOI) given below. The VoR will beoublished online in Early View as soon as possible and may be differentto this Accepted Article as a result of editing. Readers should obtainthe VoR from the journal website shown below when it is publishedto ensure accuracy of information. The authors are responsible for thecontent of this Accepted Article. To be cited as: Electroanalysis 10.1002/elan.202200279 Link to VoR: https://doi.org/10.1002/elan.202200279 WILEY-VCH Portable Electrochemical Sensing of Indole-3-acetic 2 Acid Based on Self-assembled MXene and Multi- 3 walled Carbon Nanotubes Composite Modified 4 Screen-printed Electrode 5 Yuxue Chen, a# Yunxiu Sun, a# Yanyan Niu, a Baoli Wang, Zejun Zhang, a Lina Zeng, b* Lin Li, 6 Wei Sun a* 7 aKey Laboratory of Laser Technology and Optoelectronic Functional Materials of Hainan Province, Key 8 Laboratory of Functional Materials and Photoelectrochemistry of Haikou, College of Chemistry and 9 Chemical Engineering, Hainan Normal University, Haikou 571158, China 10 b College of Physics and Electronic Engineering, Hainan Normal University, Haikou 571158, China 11 # These authors contributed to the work equally as co-first authors 12 * e-mail: sunwei@hainnu.edu.cn and zenglinahainan@126.com 13 Received: ((will be filled in by the editorial staff)) 14 Accepted: ((will be filled in by the editorial staff)) 15 16 Abstract 17 As a typical plant hormone, indole-3-acetic acid (IAA) can regulate the biological activities including 18 division, growth and differentiation of plant cells. In this paper, a MXene and multi-walled carbon nanotubes 19 composite was prepared by self-assembly procedure, which was modified on screen-printed electrode (SPE) 20 to construct a wireless portable electrochemical sensor. Electrochemical investigations of IAA were studied 21 by cyclic voltammetry andd an11irreversible oxidation processCcould1 be observed. The excellent 22 electroanalytical method of IAA was realized on the modified SPE with the detection range as 0.05-125.0 23 umol/L and the detection limit as 16.7 nmol/L. The sensor was used for IAA content analysis in different 24 part of pea seedlings with satisfactory results. 25 Keywords: MXene, Multi-walled carbon nanotubes, Electrochemistry, Indole-3-acetic acid 26 DOI: 10.1002/elan.((will be filled in by the editorial staff)) 27 28 1. Introduction 2 Plant hormones are usually divided into four categories including plant auxin, gibberellin, 3 cytokinin and inhibitor [1]. As an auxin, indole-3-acetic acid (IAA) is first discovered at the 1930s, 4 which is released from the terminal bud and formed in different parts such as leaves, stems and 5 seeds [2]. As a genuine chemical messenger, IAA can regulate important biological activities 6 including division, growth and differentiation of plant cells [3]. Therefore, IAA content analysis in 7 plants is of great significance to explore its biological function. However, the IAA content is 8 usually too low to be measured in general, and thus highly sensitive method is necessary to be 9 established for IAA analysis. 10 At present, many reports for IAA analysis have been proposed, including chromatography [4- 11 6], colorimetric method [7], capillary electrophoresis [8, 9] and enzyme-linked immunosorbent 12 assays [10]. However, these methods have disadvantages such as inconvenient device movement, 13 expensive instruments, complex operation, long detection procedure and so on. Electrochemical 14 analysis has the advantages such as fast detection, simple operation, portability, low cost and can 15 be used in combination with a variety of technologies [11-14], which has gradually been research 16 hotspot. Currently, modifying electrodes with different nanomaterials has been a common and 17 effective strategy to improve the sensitivity and selectivity of detection [15]. Various functional 18 nanomaterials can be used for electrode modifications, such as graphene, carbon nanotubes, 19 graphite carbon nitride, biomass carbon, MXene, MOFs etc. [16-21]. Among them, MXene with 20 layer structure and large surface area make them outstanding charge-transfer promoters and 21 catalysts for electrochemical sensors. For example, Sun et al. [16] constructed a MXene modified 22 electrode by drop-coating for the voltammetric analysis of quercetin in the concentration from 0.1 23 to 40.0 mM and the detection limit as 33 nM. Myndrul et al. [22] fabricated the ZnO 24 tetrapods/MXene-based electrode for on-body qualitative glucose monitoring in sweat. Zhang et al. 25 [23] prepared MXene/holey graphene composites modified electrode for dopamine detection with 26 the detection limit of 0.058 puM and the detection range of 0.1-225pM. 27 In recent years portable, compact and flexible screen-printed electrode (SPE) has been 28 developed and successfully applied to electrochemical determinations [24]. SPE is a kind of 29 electrode made by printing conductive active material on insulating substrate by film technology, which is flexible and easy to be mass-produced without any complex preparation steps. For 2 example, Beitollahi et al. [25] developed polypyrrole nanotubes modified SPE for facile detection 3 of amaranth with the detection limit as 0.01 uM. Hernandez-Saravia et al. [26] fabricated a 4 copper-nanoporous gold structure anchored SPE to examine the electrochemical behavior of 5 glucose. Santos et al. [27] developed a newV1method for ethinylestradiol detection1with 6 functionalized graphene and graphene quantum dots modified SPE. Besides, SPE can be 7 combined with simple and portable electrochemical devices using various electrochemical 8 methods to achieve a rapid detection and on-site in-situ testing. 9 Portable electrochemical sensing strategies have been proved to be powerful tools for the 10 point-of-care test and rapid on-site analysis by integrating with smartphone, which can be used in 11 food safety, environment and agriculture science. Rao et al. [15] developed a portable 12 electrochemical sensor for hyperin using MoS2 and biochar nanocomposite modified flexible 13 electrodeaandd U-diskpotentiostatwith1smartphone. Schrameetal. [28]fabricateda 14 paraformaldehyde-based sensing platform for amphetamine in street samples by combining the 15 SPE and hand-held electrochemical workstation. Raziq et al. [29] constructed a portable MIP- 16 based electrode for SARS-CoV-2 antigen by portable potentiostat with the linear concentration 17 range from 2.22 to 111 fM. Pungjunun et al. [30] developed a bismuth nanoparticle modified SPE 18 electrode for the simultaneous Sn(II) and Pb(II) analysis with a portable potentiostat with 19 excellent analytical characteristics. 20 In this paper, MXene and multi-walled carbon nanotubes composite with good electrical 21 conductivity was obtained by a simple self-assembly procedure, whichh was used for SPE 22 modification and applied to the determination of plant hormone IAA. As shown in Fig. 1A, 23 Ti3AlC2 was etched in a mixed solution of LiF and HCl to obtain MXene (Ti3C2Tx) nanosheets, 24 which then interacted with carboxylated MWCNTs to form self-assembled MXene-MWCNTs 25 composite. Due to the fine assembly and synergism between one-dimensional MWCNTs and two- 26 dimensional MXene nanosheets, MXene-MWCNTs composite exhibits a large specific surface 27 area with a lot of active sites. The addition of MWCNTs effectively suppresses the disordered 28 accumulation of MXene nanosheets, provides enough space for the adsorption of IAA molecules, 29 and promotes electron transfer. The MXene-MWCNTs composite modified SPE was constructed by modifying the composite on the surface of SPE, and its electrochemical performance was 2 checked by a hand-held wireless electrochemical workstation for practical application (Fig. 1B). 3 The sensor showed good electrochemical response to IAA, which was developed for analysis of 4 standard IAA or IAA concentrations in different parts of pea seedlings with satisfactory results. 5 6 Fig. 1. (A) Synthesis procedure of MXene-MWCNTs composite; (B) Preparation of MXene-MWCNTs/SPE 7 for electrochemical detection of IAA. 8 9 2. Experimental 10 2.1 Reagents 11 Indole-3-acetic acid (IAA, > 97%, Shanghai Macklin Biochemical Co., China), MAX titanium 12 aluminum carbide powder (Ti3AlC2, Nanjing XFNANO Tech. Co., China), multi-walled carbon 13 nanotubes (MWCNTs, Nanjing XFNANO Tech. Co., China), LiF (99%, Shanghai Aladdin 14 Biochem. Tech. Co., China) were used directly. Experimental electrolyte was 0.1 mol/L phosphate 15 buffer solution (PBS, pH 6.0). Ultra-pure water (IQ7000, Merck Millipore Co., Ltd., USA) was 16 used throughout. 17 2.2 Instruments Electrochemical experimentswere carried out with12a hand-held wireless electrochemical 2 workstation (EmStat3+Blue, PalmSens BV, Netherlands) on SPE (Weihai Poten Technology Co., 3 China). The working electrode, reference electrode and counter electrode were composed of 4 carbon disk electrode (小=5mm), Ag/AgCl electrode and carbon ring electrode, respectively. 5 Scanning electron microscope (SEM) and transmission electron microscope (TEM) were 6 characterized by JSM-7100F and JEM-2010F (JEOL, Japan) electron microscopy, respectively. X- 7 ray diffraction (XRD) was recorded using a D/Max-2500V X-ray diffractometer (Rigaku, Japan) 8 under Cu-Ka1 radiation with X-ray photoelectron spectroscopy (XPS) on an AXIS HIS165 X-ray 9 photoelectron spectrometer (Kratos Analytical, UK). An Agilent 1200 high performance liquid 10 chromatography (HPLC) system (Agilent Technologies, USA) was used with the wavelength of 11 detector as 220 nm and the automatic injection loop as 20-uL under the ambient temperature 12 during detection. 13 2.3 Materials preparation 14 Firstly, MWCNTs were pretreated according to the reference [31]. 0.2 g MWCNTs was added to 15 the mixed solution of 5.0 mL H2SO4 (98.3%) and 0.2 mL HNO3(68%) with magnetic stirring for 4 16 h, then 0.3 g potassium permanganate was slowly added and stirred for another 2 h. The 17 precipitate was washed to pH 6 with ultra-pure water and centrifuged at 9000 rpm for 1 h, then 18 redispersed in water by ultrasonic for 30 min to obtain a stable carboxylated MWCNTs dispersion. 19 Subsequently, MXene was prepared according to the references [32]. 3.2 g LiF was slowly 20 mixed with 10.0 mL HCl (36%) solution, and stirred for 10 min until completely dissolved. Then21 2.0 g Ti3AlC2 powder was added and stirred for 48 h. After washing the mixture with ultra-pure22 water, the precipitate was dispersed in ultra-pure water again. Then the dark green solution was23 obtained by ultrasonic for 80 min in an inert gas at 4 C. After centrifuging at 3500 rpm for 30 min,24 the precipitate was dried in vacuum at 60 ℃ to obtain MXene (Ti3C2Tx). 25 Finally, MXene-MWCNTs composite was obtained based on the former report with small26 modifications [31]. 1.0 mL MWCNTs dispersion was added into 50 mL 1.0 mg/mL MXene27 solution. After uniformly dispersed by ultrasonic for 1 h and stirred by magnetic force for 2 h,28 MXene-MWCNTs composites were obtained by centrifugation, washing and filtration. 29 2.4 Construction of electrochemical sensor 25.0 pL 1.5 mg/mL MXene-MWCNTs composite dispersion was directly casted on the working 2 electrode of SPE and dried at room temperature to obtain the MXene-MWCNTs/SPE, which was 3 connected with 1hand-held wireless electrochemical workstation. In order to optimize the 4 concentration of MXene-MWCNTs composite, 0.5 mg/mL, 1.0 mg/mL and 2.0 mg/mL MXene- 5 MWCNTs composite dispersion were used for the same preparation method. 6 2.5 Electrochemical detection 7 Plant hormone IAA was measured by the constructed MXene-MWCNTs/SPE with the following 8 detection process. Different concentrations of IAA solution or sample solution were dropped on 9 the surface of MXene-MWCNTs/SPE, and cyclic voltammetry (CV) or differential pulse 10 voltammetry (DPV) measurement of IAA was achieved by the portable wireless electrochemical 11 workstation. 12 13 3. Results and discussion 14 3.1 Characterization of MXene-MWCNTs composite 15 The morphological features of MAX and MXene were recorded by SEM. The bulk MAX 16 exhibited typical multilayer nanosheet structure (Fig. 2A), which became a more smoothly surface 17 after HCl and LiF etching to MXene (Fig. 2B), and the excess impurities on the surface of MAX 18 were washed off. The XRD spectra of MAX Ti3AlC2 (PDF#52-0875) before and after etching 19 were exhibited in Fig. 2C. It can be seen that the characteristic diffraction peaks of MAX appear at 20 9.5°,19.2°,34.0°,36.8°,39.0°,41.8°,44.9°,48.5°,52.4°,56.6°,60.2°,65.7°,70.5°,74.1°,83.8°, 21 which are corresponded to the (002),(004),(101),(103),(104),(105),(106),(107),(108),(109), 22 (110),(1011),(200),(118), (208) crystal faces of Ti3AlC2 [33]. 23 After etching, most of the Al was removed with the weakened MAX phase peak intensities of 24 (002) and (004), indicating that MXene with larger interlayer spacing than MAX has been 25 synthesized successfully. The chemical composition and surface electronic state of MXene- 26 MWCNTs were analyzed by XPS. The coexistence ofF, O, C and Ti can be clearly observed from 27 the total spectrum of MXene-MWCNTs (Fig. 2D). Fig. 2E and 2F show the high-resolution XPS 28 spectra of C 1s and Ti 2p, respectively. In the C1s spectrum (Fig. 2E), the main peak at 285.37 eV 29 is corresponded to the C-C bond, and the other peaks locate at 282.0 eV, 285.9 eV, 286.9 eV,289.1 eV are assigned to C-Ti, C-O, C-Ti and O-C=O [34]. The Ti 2p spectrum of MXene-MWCNTs 2 (Fig. 2F) shows the characteristic peaks at 455.8 eV, 459.29 eV, 462.0 eV and 465.49 eV, which 3 are belonged to Ti-C, Ti-O, Ti (II) and Ti-O. 4 5 Fig. 2. SEM images of (A) MAX and (B) MXene; (C) XRD patterns of MAX, MXene and MXene- 6 MWCNTs; (D) Full XPS spectrum of MXene-MWCNTs; High-resolution XPS spectra of (E) C1s and (F) Ti 7 2p; TEM images of (G) MXene (the inset is the SEAD image), (H) MWCNTs and (I) MXene-MWCNTs. 8 9 TEM and the selected area electron diffraction (SAED) are further performed to investigate 10 the morphology and the crystal structure of the samples. As shown in the TEM image (Fig. 2G), c11 the stripped MXene has an obvious layered structure. The SAED image of MXene (the inset of 12 Fig. 2G) shows obvious hexagonal diffraction points, indicating there is no obvious nano-scale13 defects of MXene single crystal. 14 TEM image of MWCNTs (Fig. 2H) shows a typical hollow nanotube structure. As MXene 15 and carboxylated MWCNTs are assembled to form the MXene-MWCNTs composite, it can be16 obviously observed that MWCNTs are covered and wrapped on MXene nanosheets with the formation of the composite (Fig.2I). 2 3.2 Electrode fabrication 3 The concentration of MXene-MWCNTs dispersion used for SPE modification was optimized by 4 CV in 1.0 mmol/L [Fe(CN)6]3-4-and 0.5 mol/L KCl mixture with the curves present in Fig. 3A. 5 The redox peak current reached the maximum at the concentration of MXene-MWCNTs 6 dispersion as 1.5 mg/mL, which was used for the following experiment. 7 8 Fig. 3. (A) CV curves of different MXene-MWCNTs concentrations modified on SPE in 1.0 mol/L 9 [Fe(CN)6]-4-and 0.5 mol/L KCl mixture at the scan rate of 100 mV/s (inset is the relationship between Ipaand concentration of MXene-MWCNTs dispersion); (B) CV curves of MXene-MWCNTs/SPE at different 1011 scan rates (20, 40,60,80,100,200,300,400,500,600,700,800,900,1000mV/s). 12 The electroactive surface area of MXene-MWCNTs/SPE was further calculated by recording 13 the cyclic voltammetric responses at different scan rates with curves present in Fig. 3B. The redox14 peak currents increased gradually with the linear regression equations as Ipc (uA)=107.75u1/2+ 15 8.22 (n=14, R2=0.990) and Ipa (uA)=-121.49 u12-5.98(n=14, R2=0.997), indicating a 16 diffusion-controlled process. Based on Randles-Sevcik equation [35], the electroactive surface 17 area of MXene-MWCNTs/SPE is 0.218 cm² and that of SPE is 0.140 cm². Therefor the existence 18 of MXene-MWCNTs on SPE could provide a larger effective area, which was conducive for the 19 electrochemical reaction. 20 3.3 Electrochemical behaviors of IAA 21 Electrochemical behaviors of IAA at various working electrodes were studied by CV with curves 22 overlapped in Fig. 4A. No obvious redox peak appeared on the bare SPE and MWCNTs/SPE, 23 demonstrating that redox reaction of IAA was not easy to take place. Also the increased24 background current on MWCNTs/SPE proved the existence of MWCNTs on SPE, which may overlap the small oxidation peak from IAA. On MXene/SPE an oxidation peak (12pA) appeared 2 without the reduction peak. Notably, an obvious oxidation peak (114 pA) was observed on 3 MXene-MWCNTs/SPE, proving the synergistic effects of MXene and MWCNTs, including the 4 larger specific surface area and better conductivity of MXene-MWCNTs composites. Since no 5 reduction peak appeared on the working electrode, the results indicated that IAA took place an 6 irreversible electrochemical process, which was in accordance with the references [36, 37]. 7 Therefore, the conductivity of the modified electrode had been obviously improved with a fast 8 path for the electron transfer between the IAA molecule and the working electrode. 9 The influence of different pH PBS on the direct oxidation behavior of IAA was checked in 10 pH range from 4.0 to 9.0 with curves shown in Fig. 4B. The oxidation peak potential shifted 11 negatively with pH value, and the oxidation peak current reached the maximum at pH 6.0. 12 Therefore, pH 6.0 PBS was selected as the best electrolyte in the following experiment. The linear 13 regression equation between the oxidation peak potential and pH is obtained as Ep=-0.038 pH+ 14 0.76 (n=6, R2=0.984). According to the slope value and the references [38, 39], the electron- 15 proton ratio of IAA involved in the oxidation reaction is 2:1. 16 In pH 6.0 PBS, the effect of scan rate on the oxidation behavior of IAA was investigated by 17 CV with curves shown in Fig. 4C. The oxidation current increased with scan rate and the potential 18 shifted positively. A linear relationship between the oxidation current and scan rate was got from 19 20 to 600 mV/s with the linear equation as Ipa (uA)=354.04 u (V/s)+37.59 (n=7,R2=0.983), 20 demonstrating an adsorption-controlled process. For the irreversible system controlled by 21 adsorption, the relationship between E, and lnu is as follows: 22 23 Where E is the formal potential, a is the electron transfer coefficient, n is the electron transfer 24 number, k" is the multiphase electron transfer rate constant, R is the mole gas constant, T is the25 absolute temperature, and F is the Faraday constant. Based on the linear relationship between Ep26 and lm with equation as Epa (V)=0.0328 lnu (V/s) + 0.615 (n=5,R2=0.987), the value of o is 27 set to 0.5, and n is approximately equal to 2. 2 Fig. 4 (A) CV curves of 4.0x10° mol/L IAA on different working electrodes in pH 6.0 PBS with the scan 3 rate of 100 mV/s (Insert is CV curves on SPE and MXene /SPE); (B) CV curves of MXene-MWCNTs/SPE 4 in PBS containing 4.0×10 mol/L IAA with different pH (from 4.0 to 9.0); (C) CV curves of 4.0×105mol/L 5 IAA on MXene-MWCNTs/SPE at different scan rates (20, 100, 200,300,400,500,600mV/s); (D)Typical6 DPV curves of different concentrations IAA (a→f: 0.05, 0.2,35,75,100,125 umol/L) on MXene-7 MWCNTs/SPE. 8 Therefore, IAA undertakes an irreversible oxidation reaction on the surface of MXene- 9 MWCNTs/SPE with two electrons and one hydrogen involved, and the oxidation mechanism is 10 expressed in Fig. 5 [40]. 11 12 Fig. 5. Electrochemical oxidation mechanism of IAA 13 3.4 Working curve 14 At the optimal conditions, the detection of different concentrations of IAA on MXene- 15 MWCNTs/SPE was investigated by DPV. As shown in Fig. 4D, the oxidation current increases 16 with the increase of IAA concentration, and a good linear relationship was got from 0.05 to 125.0 17 umol/L with the regression equation as Ipa (uA)=0.86 C (umol/L)+20.09 (n=10,R2=0.996) 18 and the detection limit as 16.7 nmol/L (3o). Compared with other reported modified electrodes for 19 IAA detection (Table 1), MXene-MWCNTs/SPE has wider detection range and lower detection limit. 2 Table 1. Comparison of analytical parameters of IAA by different modified electrodes. Modified electrodes Linear range Detection Limit Ref. (umol/L) (nmol/L) hemin/rGO/GCE [a] 0.1-183 74 [41] PMDTO/RGO/GCE [b] 0.1-10 30 [42] CeCl3-DHP/Au [c] 0.1-20 30 [431 BDD [d] 5-50 1220 [441 CB-MWNT-Nafion/Fc/CB-MWNT/GCE [e,f 25-1000 1990 [36] MXene-MWCNTs/SPE 0.05-125.0 16.7 This work 3 [a] rGO, reduced graphene oxide; [b] PMDTO, poly(N-methyl-3,4-dihydrothieno[3,4-b] [1,4] oxazine); [c] DHP,4 dihexadecyl hydrogen phosphate; [d] BDD, boron-doped diamond; [e] CB, carbon black; [f] Fc, Ferrocene. 5 3.5 Selectivity 6 Many substances such as common inorganic cations, organic acids, vitamins, amino acids etc. 7 may exist in crops, therefore the selectivity was checked. As shown in Fig. 6, there is no oxidation 8 peak in PBS (Blank) and PBS electrolyte control group with only interfering ions. When IAA was 9 added, an obvious oxidation peak appeared on the cyclic voltammetric curve. In addition, easily 10 oxidizable substances such as salicylic acid, citric acid, ascorbic acid, a-naphthalene acetic acid 11 did not significantly interfere with the analysis of IAA. It’s worth noting that the presence of a- 12 naphthylacetic acid, which is also a plant hormone, does not interfere with the detection of IAA. c13 Therefore MXene-MWCNTs/SPE has good selectivity for the real sample analysis. 2 Fig. 6. Influence of K* (1.0×10mol/L), Na* (1.0×10mol/L), Ca+(1.0×10mol/L), cysteine (2.0×106 3 mol/L), aspartic acid (1.0×10mol/L), glucose (1.0×10 mol/L), salicylic acid (2.0×10mol/L), citric acid 4 (1.0×10 mol/L), ascorbic acid (1.0×10 mol/L), a-naphthalene acetic acid (1.0×10 mol/L) on 5 electrochemical response of 5.0×10mol/L IAA. 6 3.6 Reproducibility and stability 7 The reproducibility of the modified electrodes was verified by CVs and the results were present in 8 Fig. 7A. The RSD of oxidation currents of five electrodes was 3.0 % in pH 6.0 PBS containing9 4.0×10 mol/L IAA, indicating excellent reproducibility. In addition, the MXene-MWCNTs/SPE10 was continuously scanned 150 times by CV in the electrolyte of 1.0 mmol/L [Fe(CN)6]3-/4-11 and 0.5 mol/L KCl with the current change of the redox peak present in Fig. 7B. The RSD values12 of oxidation currents and reduction currents were 3.1% and 2.3 %, respectively, which also 13 showed outstanding stability of MXene-MWCNTs/SPE. Fig. 7. (A) Oxidation peak currents of five parallel MXene-MWCNTs/SPEs for 4.0×10 mol/L IAA; (B)CV 2 curves of MXene-MWCNTs/SPE in the electrolyte of 1.0 mmol/L [Fe(CN)6]3-/4-and 0.5 mol/L KCl. 3 3.7 Actual samples testing 4 The MXene-MWCNTs/SPE was applied to the detection of IAA in the stems and leaves of pea 5 seedlings (from local farmers’ markets), and the feasibility of detecting actual samples was 6 verified by parallel determination for 3 times. First, the stems and leaves of fresh pea seedlings 7 were mashed and filtered to obtain the sample solution. In the process of testing, the extract of pea 8 seedling stem and pea seedling leaves were dripped on the surface of MXene-MWCNTs/SPE and 9 analyzed by the proposed procedure. As shown in Table 2, IAA can be found in the pea seeding 10 sample and the contents of IAA in stems and leaves are different, which is attributed to gravity and 11 polar transport from synthesis site to action point [45, 46]. The relative standard deviations (RSD) 12 of stem and leaves sample are 2.8% and 3.7%, which prove that it can be applied to the detection 13 of actual samples. To check the accuracy of the detection results, HPLC was also used [47] with 14 data listed in table 2, which showed no significant difference compared with the electrochemical 15 method. 16 Table 2. Determination of IAA content in pea seedling (n=3). MXene-MWCNTs/SPE based electrochemical sensor HPLC sample Ipa (uA) C(umol/L) RSD (%) C (umol/L) RSD (%) stem 112.5 107.5 2.8 97.4 3.0 leaves 70.8 59.0 3.7 54.5 3.2 17 18 4. Conclusions 19 2D MXene was prepared by the etching of TisAlC2 and hybridized with carboxylated MWCNTs 20 byyeelectrostaticCself-assemblyto0(obtain MXene-MWCNTsScomposite. Electrochemical 21 characterizations of MXene-MWCNTs/SPE were carried out with the redox responses greatly 22 enhanced, which revealed that the cooperation between MXene and MWCNTs on the electrode23 surface improved the electrochemical performances of the modified electrode. The addition of24 MWCNTs effectively inhibits the disordered accumulation of MXene nanosheets with a stable 25 composite formed. MXene-MWCNTs/SPE based electrochemical sensor was applied to IAA 26 detection with a low detection limit of 16.7 nmol/L and a wide linear range of 0.05~125.0 umol/L. Furthermore, this electrochemical sensor was employed to detect IAA in plant samples, which also 2 enlightened the applications of hand-held wireless portable electrochemical sensing. 3 4 Acknowledgments 5 This work is supported by Hainan Provincial Natural Science Foundation (2019RC190), Science 6 and Technology project of Hainan province (ZDYF2020204), the Open Foundation of Key 7 Laboratory of Laser Technology and Optoelectronic Functional Materials of Hainan Province 8 (2022LTOM02). 9 10 Declaration of conflict interest 11 The authors declare that they have no known conflict of interests or personal relationships that 12 could have appeared to influence the work reported in this paper. 13 14 References 15 [1] P. Hernandez, M. Dabrio-Ramos, F. Paton, Y. Ballesteros, L. Hernandez, Talanta 1997,44,1783. 16 [2] M. Lebuhn, A. Hartmann,J. Chromatogr. A 1993, 629,255. 17 [3] E. A. Schneider, F. Wightman, Ann. Rev. Plant Physiol. 1974,25,487. 18 [4] Z. Xi, Z. Zhang, Y. Sun, Z. Shi, W. Tian, Talanta 2009,79,216. 19 [5] Z. Ma, L. Ge, A. S. Y. Lee, J. W. H. Yong, S. N. Tan, E. S. Ong, Anal. Chim. Acta 2008, 610,274. 20 [6] F. Mattivi, U. Vrhovsek, G. Versini, J. Chromatogr. A 1999, 855,227. 21 [7] T. T. Duong, T. T. L. Nguyen, T. H. V. Dinh, T. Q. Hoang, T. N. Vu, T. O. Doan, T. M. A. Dang, T. P. 22 Q. Le, D. T. Tran, V. N. Le, Q. T. Nguyen, P. T. Le, T. K. Nguyen, T. D. Pham, H. M. Bui, Chemosphere23 2021,284,131242. ( 24 [8]X. B. Yin, D. Y. Liu, J. Chromatogr A 2008, 1212,130. ) ( 25 [9] A. Segura Carretero, C. Cruces-Blanco, M. Soriano Pena, S. Cortacero Ramirez, A. Fernandez Gutierrez, ) 26 J. Agric. Food Chem. 2004, 52,1419. ( 27 [10] N. Ilic, I . Habus, L. S. Barkawi, S. Park, Z. Stefanic, B. Kojic-Prodic, J. D. Cohen, V. Magnus, Bioorg. 28 Med. Chem. 2005,13,3229. ) 29 [11] M. Y. Emran, M. A. Shenashen, H. Morita, S. A. El-Safty, Biosens. Bioelectron. 2018,109,237. ( [12] H. Teymourian, M. Parrilla, J. R. Sempionatto, N. F. Montiel, A. Barfidokht, R. Van Echelpoel, K. De 2 Wael, J. Wang, ACS Sens. 2020,5,2679. ) ( 3 [13] E. De Rycke, C. Stove, P. Dubruel, S. De Saeger, N. Beloglazova, Biosens. Bioelectron. 2020, 169, 4 112579. ) ( 5 [14] M. Parrilla, N. Felipe Montiel, F. Van Durme, K. De Wael, Sens. Actuators B Chem. 2021,337,129819. ) ( 6 [15]L. Rao, Y. Zhu, Z. Duan, T. Xue, X. Duan, Y. Wen, A. S. Kumar, W. Zhang, J . Xu, A. Hojjati-Najafabadi, 7 Chemosphere 2022,301,134595. ) ( 8 [16]Y. Sun, B. Wang, X. He, Y. Wang, L. Chen, Y. Zhu, G. Li, W. Sun, New J. Chem. 2021, 45, 20396. ) ( 9 [17] H. Cheng, J. Liu, Y . Sun, T. Zhou, Q. Yang, S. Zhang, X. Zhang, G. Li, W. Sun, RSC Adv. 2020, 10, 10 42340. ) ( 11 [18]Y. J. Ai, J . Liu, L. J. Yan, G. J. Li, X. H. Wang, W. Sun, J. Chin. Chem. Soc. 2022,69,359. ) ( 12 [19] G. Luo, Y. Deng, X. Zhang, R. Zou, W. Sun, B. Li, B. Sun, Y. Wang, G. Li, New J. Chem. 2019,43, 13 16819. ) ( 14 [20] F. Cai, Q. Wang, X. Chen, W. Qiu, F. Zhan, F. Gao, Q. Wang, Biosens. Bioelectron. 2017, 98,310. ) ( 15 [21]Y. Ai , N. Gao,Q. Wang, F. Gao, D. B. Hibbert, C. Zhao,J. Electroanal. Chem. 2020,872,114161. ) ( 16 [22] V. Myndrul, E. Coy, N. Babayevska, V. Zahorodna, V. Balitskyi, I . Baginskiy, O. Gogotsi, M. Bechelany, 17 M. T . Giardi, I . Iatsunskyi, Biosens. Bioelectron. 2022,207,114141. ) ( 18 [23]Y. Zhang, L. Zhang, C. Li, J. Han, W. Huang, J. Zhou, Y. Yang, Microchem. J. 2022,181,107713. ) ( 19 [24 ] S. Taj i k, Z. Dourandish, F. G. Nejad, A. Aghaei Afshar, H. Beitollahi, Micromachines (Basel) 2022,13, 20 369. ) ( 21 [25] H. Beitollahi, F. Garkani Nejad, Z. Dourandish, S. Tajik, Environ. Res. 2022, 214,113725. ) ( 22 [26] L. Hernandez-Saravia, T. Martinez, J. Llanos, M. Bertotti, Microchem. J. 2021,160,105629. ) ( 23 [27] A. M. Santos, A. Wong, T. M. Prado, E. L. Fava, O. Fatibello-Filho, M. D. P. T. Sotomayor, F. C. ) c 24 Moraes, Talanta 2021, 224, 121804. ( 25 [28] J. Schram, M. Parrilla, A. Slosse, F. Durme, J.Aberg, K. Bjork, S. Bijvoets, S. Sap, M. Heerschop, K. 26 Wael, Microchem.J. 2022,179,107518. ) ( 27 [29] A. Raziq , A. Kidakova, R. Boroznjak, J. Reut , A. Opik, V. Syritski, Biosens. Bioelectron. 2021,178, 28 113029. ) ( [30] K. Pungjununa, S.Nantaphola, N.Praphairaksita, W. Siangprohb, S. Chaiyoa, O. Chailapakul, Sens. 2 Actuators, B 2020,318,128241. ) ( 3 [31 ] D. Cao, M. Ren, J. Xiong, L. Pan, Y. Wang, X. Ji, T. Qiu, J. Yang, C. J. Zhang, Electrochim. Acta 2020, 4 348,136211. ) ( 5 [32] X. Wang, S. Kajiyama, H. Iinuma, E. Hosono, S. Oro, I. Moriguchi , M. Okubo, A. Yamada, Nat. 6 Commun. 2015,6,6544. ) ( 7 [33] Q. X. Xia, J. Fu, J. M. Yun, R. S. Mane, K. H. Kim, RSC Adv. 2017, 7,11000. ) ( 8 [34 ] X. Ma, X. Tu, F. Gao, Y . Xie, X. Huang, C. Fernandez, F. Qu, G. Liu, L. Lu, Y. Yu, Sens. Actuators B 9 Chem. 2020, 309,127815. ) ( 10 [35] R.G. Compton, C.E. Banks, Understanding Voltammetry, 2nd ed., Place Imperial College Press, London, 11 2010. ) ( 12 [36]Y. Hu, X. Wang, C. Wang, P. Hou, H. Dong, B. Luo, A. Li, RSC Adv. 2020, 10, 3115 . ) ( 13 [37] L. Sun, X. Liu, L. Gao, Y. Lu, Y. Li , z. Pan, N. Bao, H. Gu, Anal. Lett . 2015,48,1578. ) ( 14 [38] T. Gan, C. Hu, Z. Chen, S. Hu, Talanta 2011,85,310. ) ( 15 [39] Z. L. Feng, Y. Yao, J. K. Xu, L. Zhang, Z. F. Wang, Y. P. Wen, Chin Chem Lett 2014,25,511. ) ( 16 [40] M. Li, Y. Kuang, Z. Fan, X. Qin, S. Hu, Z. Liang, Q. Liu, W. Zhang, B. Wang, Z. Su, Sensors 2022, 22, 17 2222. ) ( 18 [41 ] F. Liu, J. Tang, J . Xu, Y. Shu, Q. Xu, H. Wang, X. Hu, Biosens. Bioelectron. 2016,86,871. ) ( 19 [42 ] K. Qu, S. Ming, H. Jia, B. Guo, X. Liu, N. Jian, G. Niu, B. Lu, J . Xu, Int. J. Electrochem. Sci. 2018, 13, 20 11663. ) ( 21 [43]Y. J. Yang, X. Xiong, K. Hou, S. Hu, Russ. J. Electrochem. 2011,47,47. ) ( 22 [44]Y. Yardim, M. E. Erez, Electroanalysis 2011,23,667. ) ( 23 [45] Z. F. Jiang, D. D. Liu, T. Q. Wang, X. L. Liang, Y. H. Cui, Z. H. Liu, W. B. Li, J Integr Agric 2020, 19, 24 953. ) 25 [46] C. S. Buer, G. K. Muday, Plant Cell 2004, 16, 1191. 26 [47] Z. Ma, Z. Lu, T. Jiang, Y. Wang, Analysis and Testing Technology ofInstruments 2005, 11,174. Color graphic for the Table of Contents 2 3 Preparation of MXene-MWCNTs/SPE for electrochemical detection ofIAA. This article is protected by copyright. All rights reserved.
确定














还剩16页未读,是否继续阅读?
雷迪美特中国有限公司为您提供《【EmStat3Blue电化学应用】吲哚-3-乙酸便携式电化学传感器,基于自组装MXene和多壁碳纳米管复合修饰丝网印刷电极》,该方案主要用于林产品中含量分析检测,参考标准--,《【EmStat3Blue电化学应用】吲哚-3-乙酸便携式电化学传感器,基于自组装MXene和多壁碳纳米管复合修饰丝网印刷电极》用到的仪器有EmStat4X便携式电化学分析仪
推荐专场
















