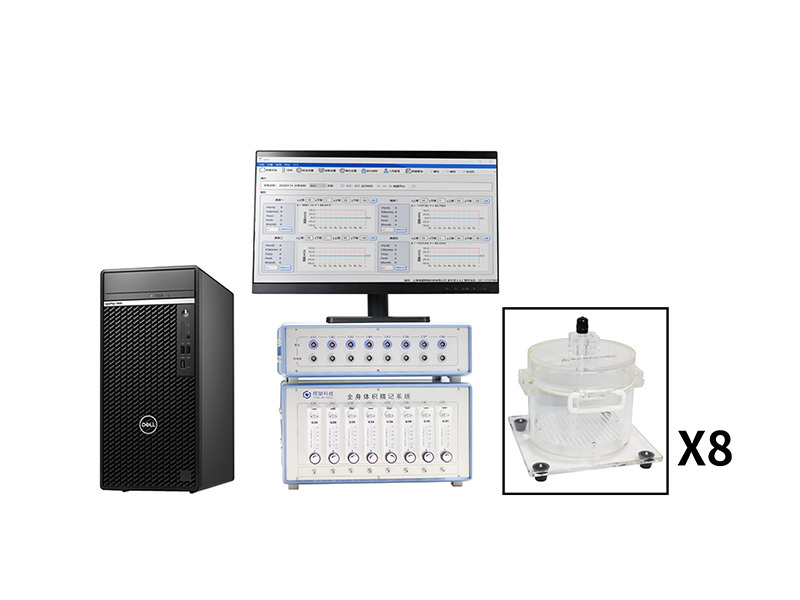
方案详情
文
近日北京中医药大学的周玉美老师及其团队研究了TMDCT在调节Treg/Th17细胞免疫平衡中的作用和其相关的潜在代谢和肠道生物标志物。相关成果发布在英文学术期刊《Frontiers》上。
方案详情

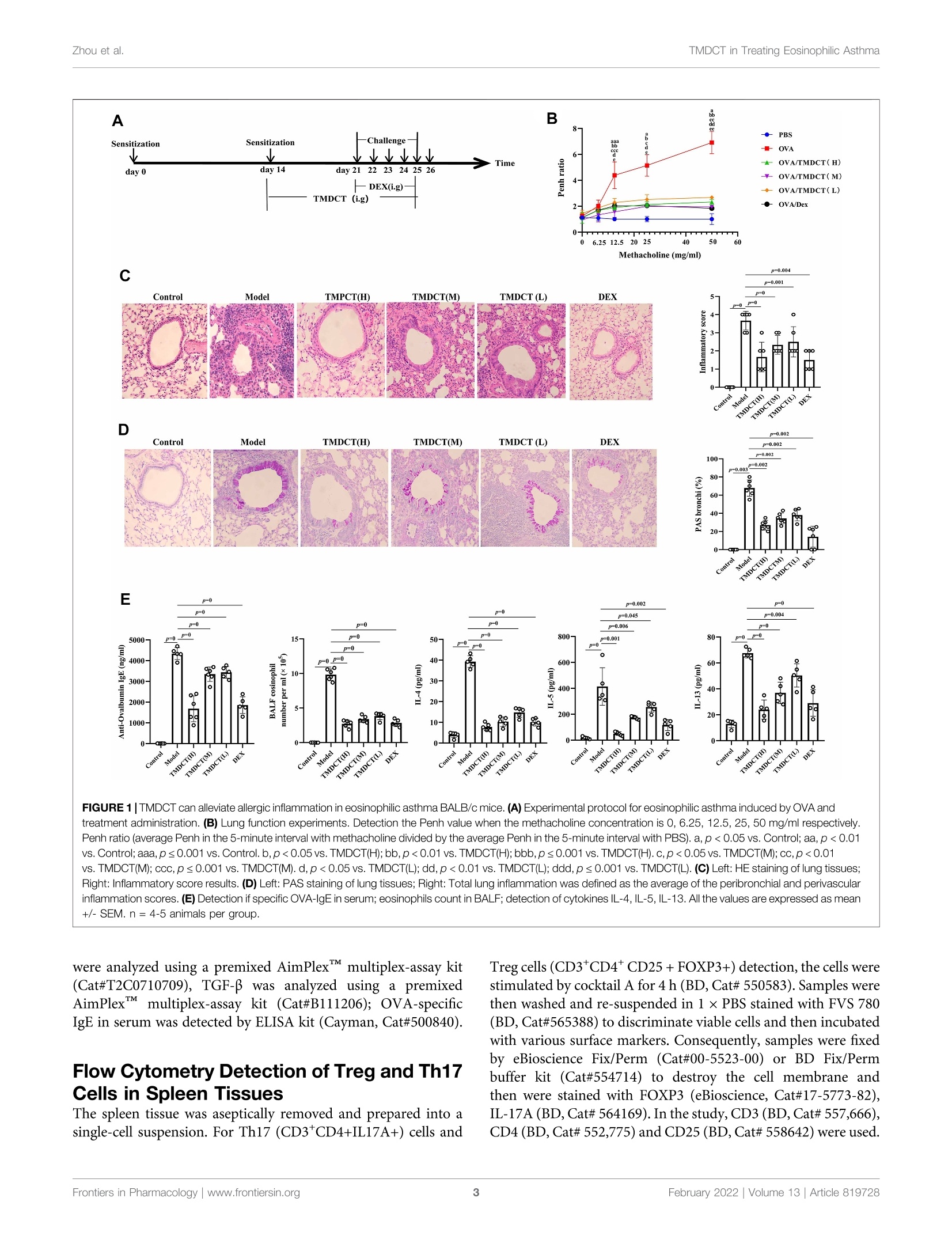
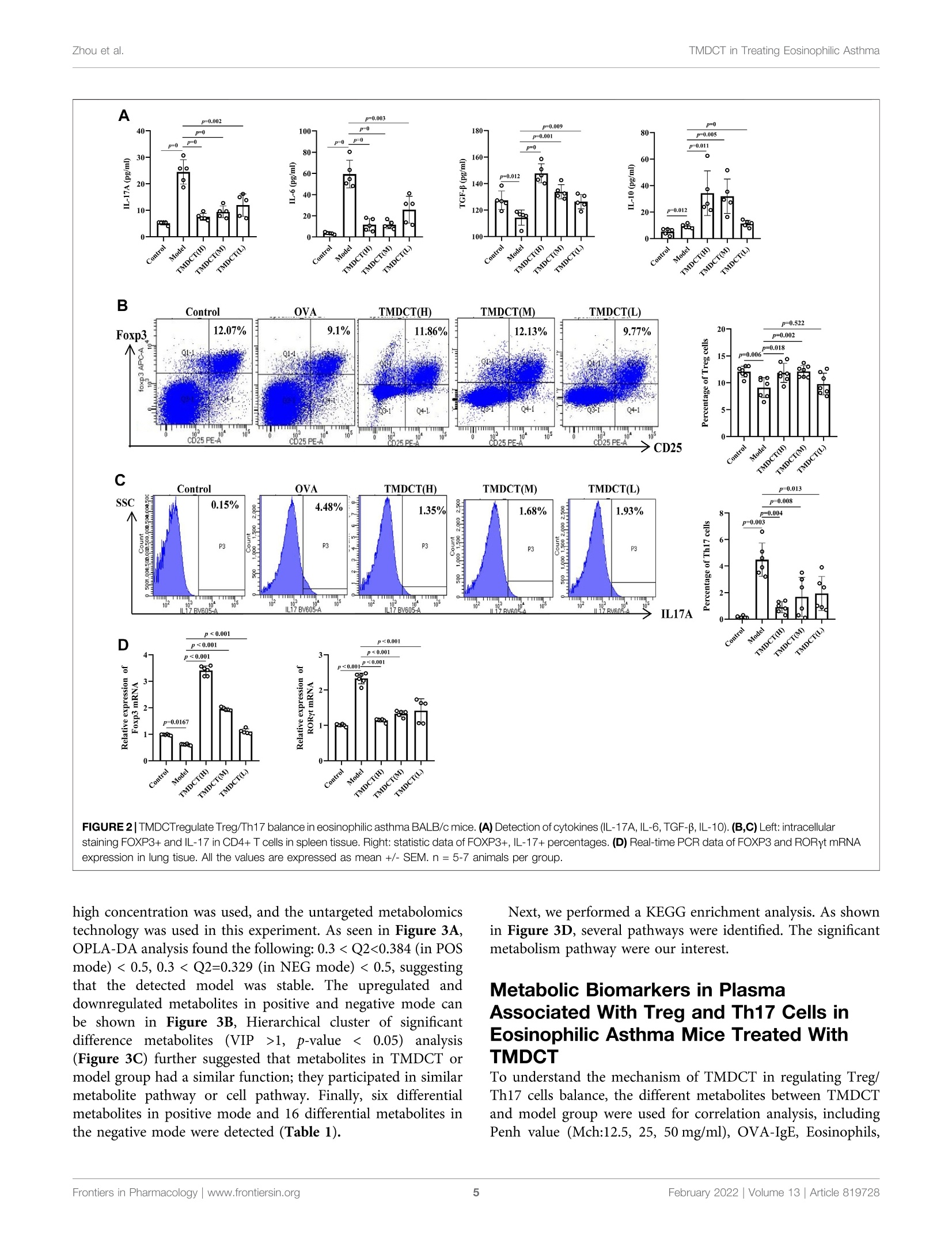
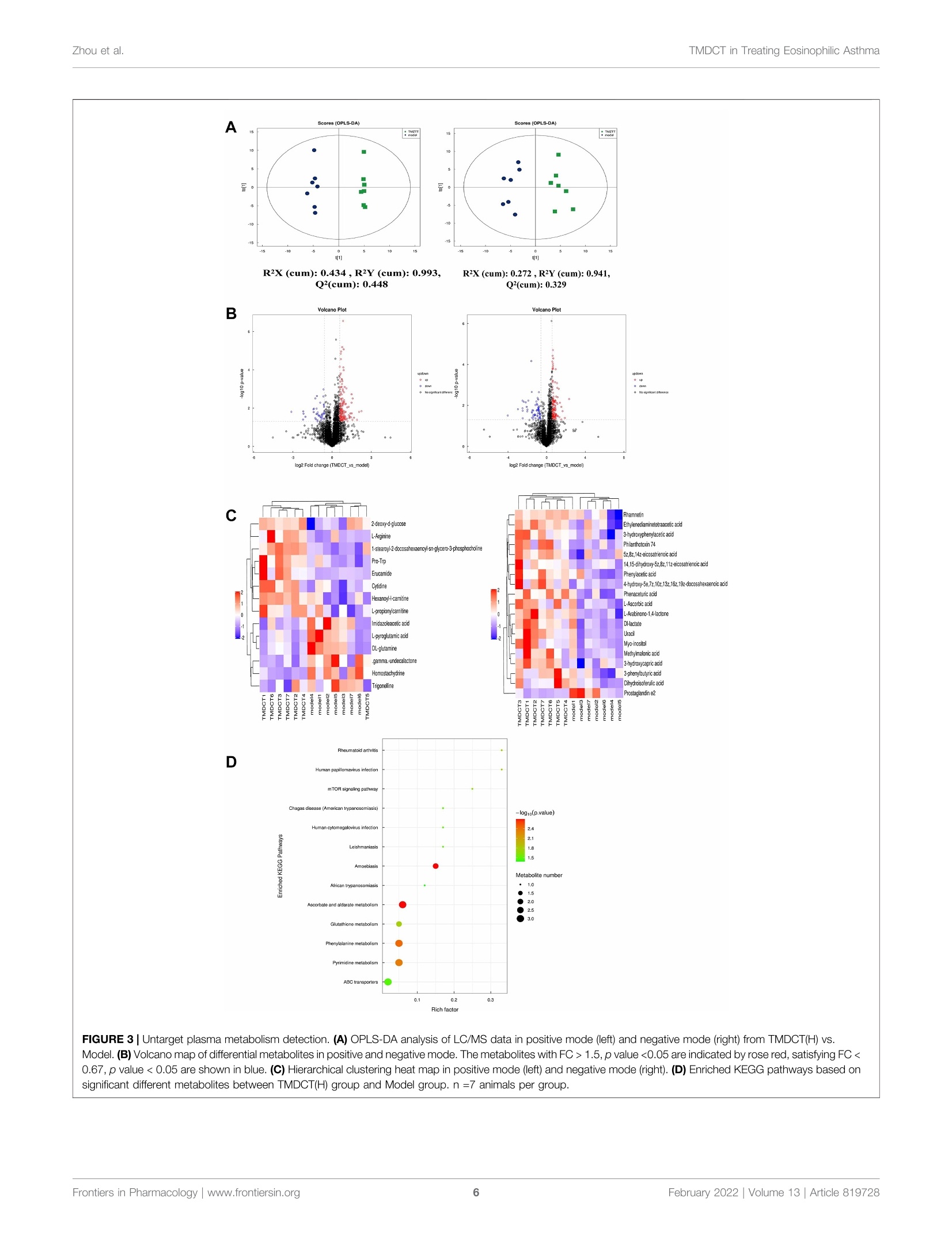
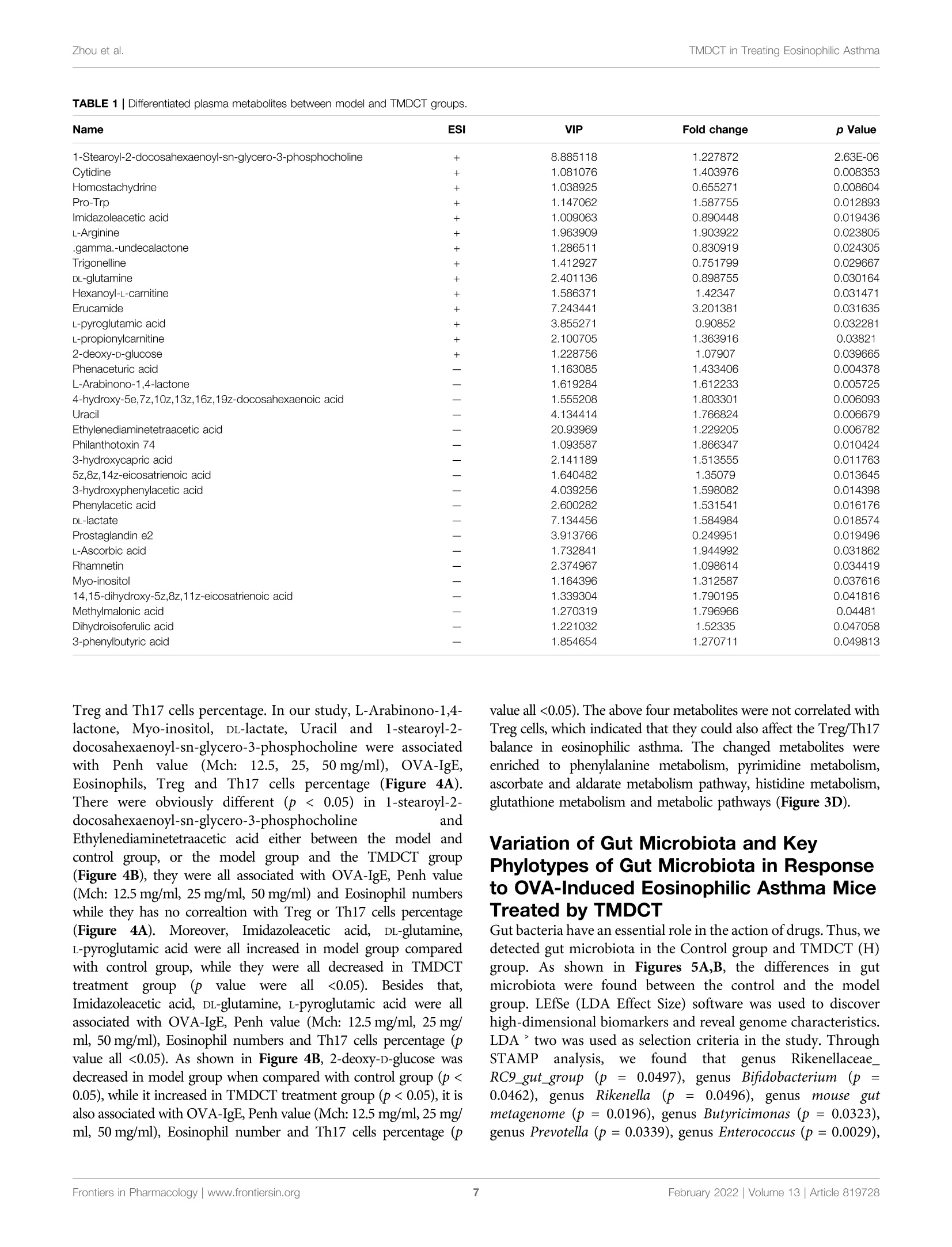
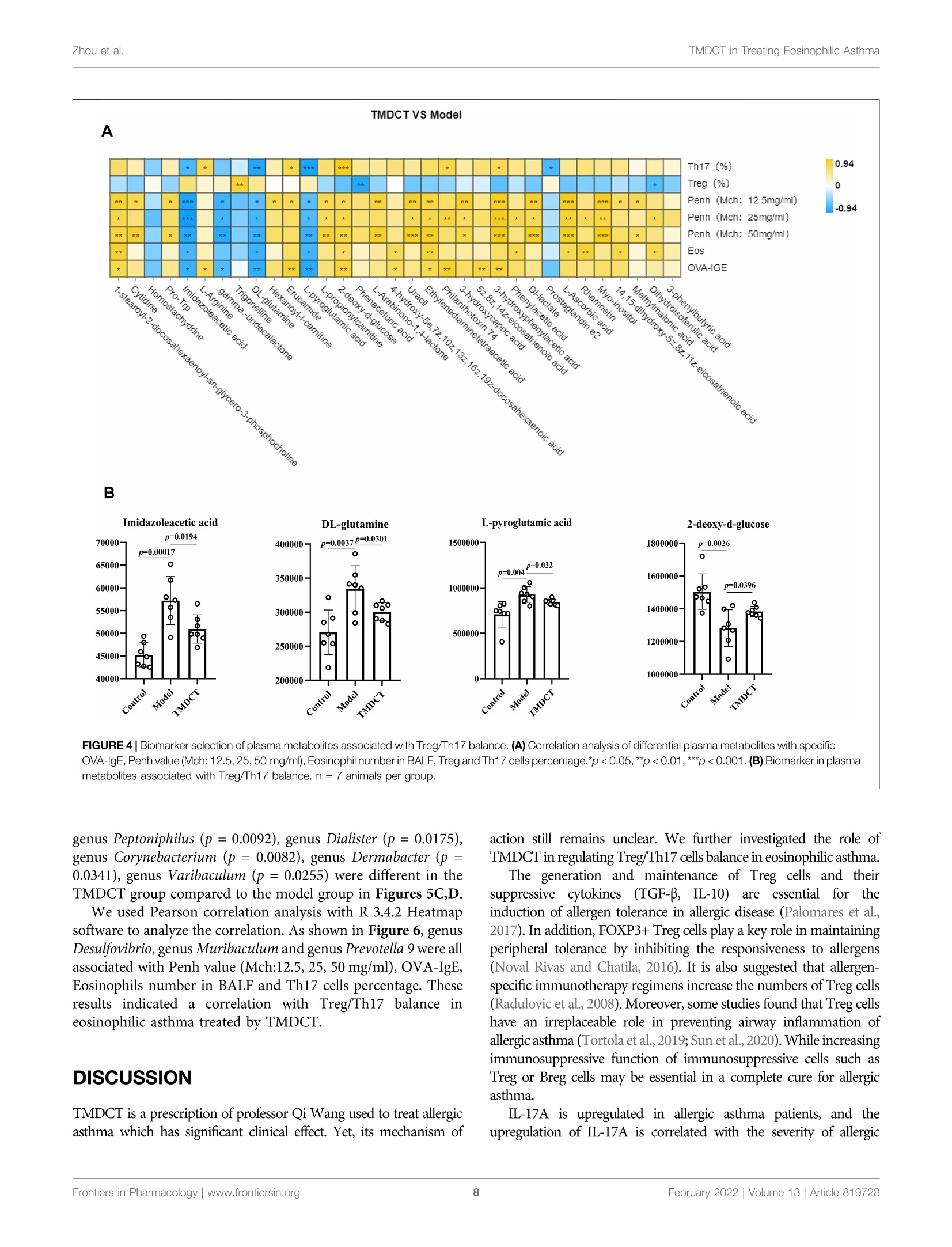
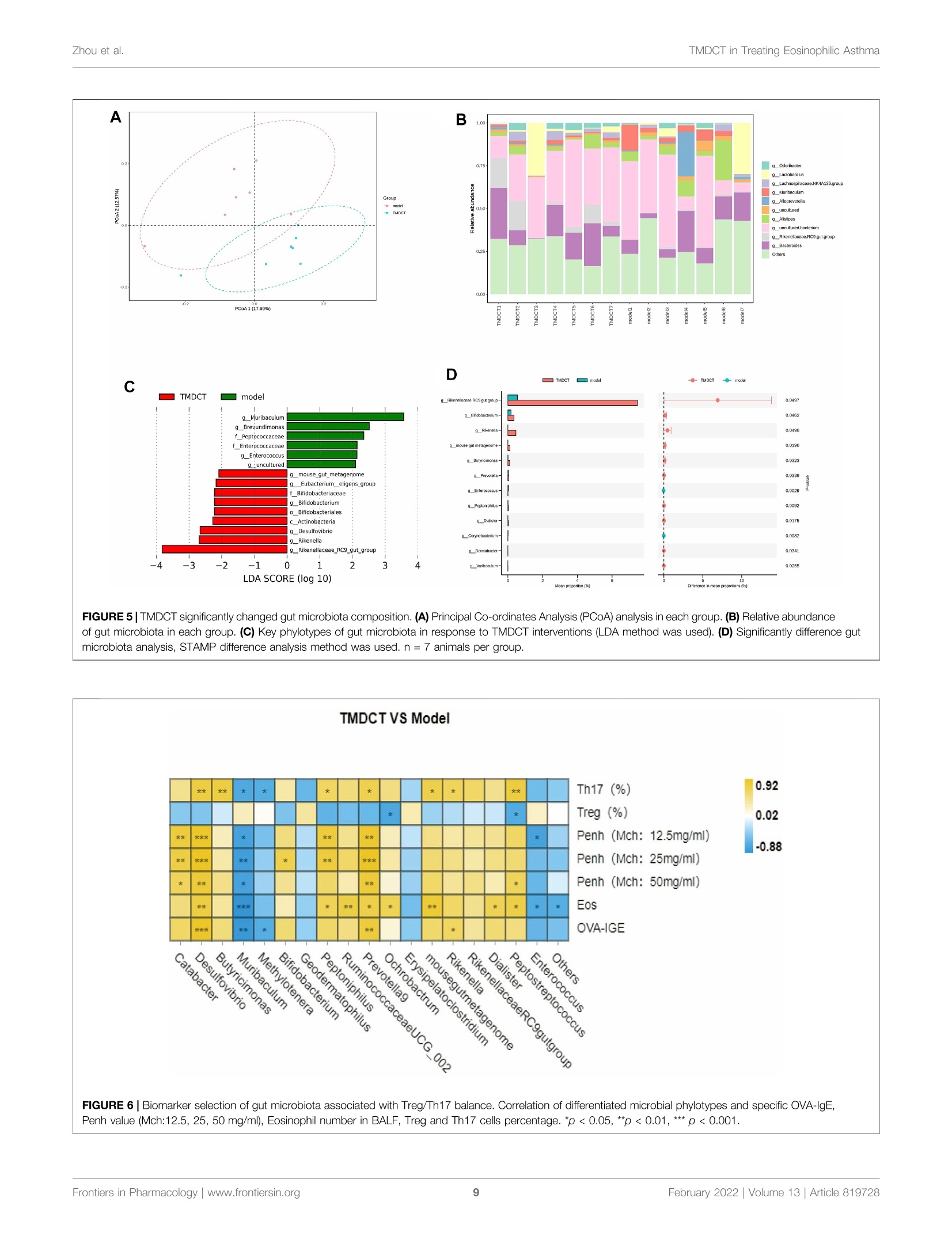
frontiersin PharmacologyORIGINAL RESEARCHpublished: 08 February 2022doi: 10.3389/fphar.2022.819728 Zhou et al.TMDCT in Treating Eosinophilic Asthma OPEN ACCESS Edited by: Songwen Tan, Central South University, China Reviewed by: Wenhu Zhou, Central South University, ChinaMuhammad Shahzad,University of Health Sciences, Pakistan *Correspondence: Ji Wang doctorwang2009@126.comQi Wangwangqi710@126.com Specialty section:This article was submitted toTranslational Pharmacology,a section of the journalFrontiers in Pharmacology Received: 22 November 2021Accepted: 24 January 2022Published: 08 February 2022 Citation:Zhou Y, Zhao H, Wang T, Zhao X,Wang J and Wang Q (2022) Anti-Inflammatory and Anti-asthmaticEffects of TMDCT Decoction inEosinophilic Asthma Through Treg/Th17 Balance.Front. Pharmacol.13:819728. doi: 10.3389/fphar.2022.819728 Anti-Inflammatory and Anti-asthmaticEffects of TMDCT Decoction inEosinophilic Asthma Through Treg/Th17 Balance Yumei Zhou', Haihong Zhao, Tieshan Wang, Xiaoshan Zhao', Ji Wang*and Qi Wang * 'National Institute of TCM Constitution and Preventive Medicine, School of Chinese Medicine, Beijing University of ChineseMedicine, Beijing, China, Beijing Research Institute of Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China Tuo-Min-Ding-Chuan decoction (TMDCT) is a Traditional Chinese Medicine (TCM) formulaconsisting of twelve herbs that can relieve the symptoms and treat allergic asthma. Yet, theunderlying mechanism of action is still unclear. In this study, we investigated the effect ofTMDCT in regulating Treg/Th17 cells immune balance and explored potential metabolicand gut biomarkers associated with Treg and Th17 cells in eosinophilic asthma micetreated by TMDCT. We found that TMDCT increases Treg cells percentage and decreasesTh17 cells percentage in the ovalbumin (OVA) -induced eosinophilic asthma mice model.Furthermore, Imidazoleacetic acid, DL-glutamine,L-pyroglutamic acid, 2-deoxy-D-glucosewere preliminary identified as biomarkers in plasma metabolites treated by TMDCT,meanwhile genus Desulfovibrio, genus Butyricimonas and genus Prevotella 9 werepreliminary identified as gut microbiota biomarkers after TMDCT treatment. Theseresults provide an experimental foundation for the treatment of allergic asthma withChinese herbal compounds. Keywords: Tuo-Min-Ding-Chuan decoction, Treg/Th17, eosinophilic asthma, anti-inflammatory, anti-asthmatic INTRODUCTION Asthma is a chronic inflammatory disease which is difficult to control affects more than 300 millionpeople worldwide. With current rising trends, it is expected that this number will reach 400 millionpeople by 2025. According to some reports, there are nearly 250,000 asthma-related deaths each year,many of which are avoidable (Christiansen and Zuraw, 2019). Inflammatory disorder in allergic asthma is characterized by broncho-construction, bronchial hyper-1A一responsiveness, and even tissue damage and the most common type of asthma (National AsthmaEducation and Prevention Program, 2007). If not properly treated, allergic asthma can progress to chronicobstructive lung disease or other disease associated with airways and lung tissue. The burden of allergicdiseases has increased over recent years, as evidenced by a high incidence of patients suffering from thesediseases and incurring high financial costs (Bousquet et al., 2016). Over the years, anti-inflammatory drugshave been developed, such as inhaled steroids and bronchodilators that can relieve symptoms. Yet, existingdrugs cannot completely cure the patient, and have an elevated recurrence rate. Moreover, conventionaldrugs have been associated with certain side effects. E.g., an inhaled corticosteroid (ICS), the dominanttreatment in type 2-high asthmatic inflammation, can suppress the endocrine system, which assists body'simmune system fight against infection, while long-term steroid treatments can lead to anxiety anddepression. Allergic asthma is induced by an abnormal type 2 immuneresponse to inhaled allergens, such as house dust mites (HDM),grass pollen, animal dander, and mold (Caminati et al., 2018). Inthe airway, the inflammatory response is mainly caused by Th2type inflammation, inducing cytokines IL-4, IL-5, and IL-13overexpression, which consequently activate the expression ofIgE and the infiltration of eosinophils and mast cells in the airway. Previous studies have shown that the imbalance of Th1/Th2cells may be involved in the pathogenesis of airway inflammationin asthma (Berker et al.,2017). Besides that, some recent studiessuggested that insufficient Th1 cell differentiation is not the onlycause leading to over-differentiation and activation of Th2 cells. Ithas been discovered that Treg cells have an essential role inregulating the body’s immune balance. Dysfunctional Treg cells,unable to effectively suppress excessive Th2 response, may lead toasthma and other allergic diseases. Th2-type inflammation is involved in all types of asthma(mild, moderate, and severe). Also, inflammation caused byTh17 cells has been associated with a progression of asthma(Israel and Reddel, 2017). The imbalance between Treg cells andTh cells such as Th1, Th2 and Th17 cell responses in allergicasthma is associated with the development of allergic asthma(Noval Rivas and Chatila, 2016). Genetic and immunologicalevidence suggested that Treg cells can promote tolerance toallergens and prevent allergic disorders (Palomares et al,2014). Thus, it is believed that the inflammation of allergicasthma inflammation and increasing Treg cells number orstimulating the proliferation of immunosuppressive cells suchas Treg cells is a new strategy for the treatment of allergic asthma. Recently, some new drugs with the ability to exert Treg cells inthe body’s immune system or stimulate differentiation of Tregcells have been tested. Although great development has beenachieved using allergen immunotherapy((AIT) and otherimmunotherapy, treatmentit of allergiccaasthmaand itsrecurrence remains challenging. New treatment options thatcan regulate the immune balance of Treg/Th17 are currentlyemerging. TMDCT is a TCM formula consisting of twelve herbs that canrelieve the symptoms and treat allergic asthma. Nevertheless, theunderlying mechanism of action is still unclear. TMDCT contain 12kinds of traditional Chinese medicine, it can suppresses inflammationof allergic asthma and inhibit the degranulation of mast cell (Qinet al., 2021). In our study, we investigated the effect of TMDCT inregulating Treg/Th17 cells balance and explored potential metabolicand gut biomarkers associated with Treg and Th17 cells ineosinophilic asthma mice treated with TMDCT using non-targeted metabolome and 16S rDNA technology. MATERIALS AND METHODS Mice BALB/c mice (female, 6-8weeks old, 17-20 g) were obtainedfrom Beijing Vital River Laboratory Animal Technology Co. Ltdin China. Keeping mice in specific pathogen-free conditions inBeijing University of Chinese Medicine. All mice were kept at acontrolled room (25±1℃,45-60% humidity). All animal studies were conducted in accordance with the institutional animal careregulations of Beijing University of Chinese Medicine and wereconducted in accordance with AAALAC and IACUC guidelines. OVA-Induced Allergic Asthma Mice Modeland Grouping The OVA-induced eosinophilic asthma BALB/c mice model wasconstructed following a previously described approach (Dumaset al., 2018). Briefly, mice were intraperitoneally injected with2 mg of OVA (Sigma-Aldrich, Cat#A5503) mixed with 2 mgImject M Alum Adjuvant (Invitrogen, Cat#77161) and PBS onday 0 and day 14. From the 21st to 25th days post-injection, in thechallenge phase, mice are continuously nebulized with 1% OVAfor 30 min as shown in Figure 1A. OVA-induced allergic asthma mice were then divided in thefollowing groups: High-dose TMDCT group (40.56g/kg/dTMDCT (H group)), Middle-dose TMDCT treatment group(20.28 g/kg/d TTNMDCT (Migroup)), 1Low-doseeTMDCTtreatmentgroupp(10.14 g/kg/dTMDCT (L group)), andpositive group (dexamethasone 1 mg/kg/d). TMDCT,containing 12 kinds of Chinese medicine, was prepared aspreviously described(Qineetal..2021). lTMDCT anddexamethasone (1 mg/kg/d, a positive drug for suppressingeosinophilic asthma) were given daily at the same time fromthe 21st to 25th days 1 hour before challenge. Detection of Measured AirwayResponsiveness We used 0, 6.25, 12.5, 25, and 50 mg/ml methacholine (mch)(Sigma, Cat#A2251) to detect enhanced pause (Penh), whichreflected lung function according to noninvasive measurement ofairway hyperresponsiveness by whole-body phelthysmography(WBP-4MR, TOW, China) (Sun et al.,2021). Histological Sections and PathologyScoring Lungs were fixed with 4% paraformaldehyde (PFA) at 4℃, afterparaffin-embedded sections and stained them with hematoxylinand eosin (HE) (to examine cell infiltration detection) or periodicacid-schiff stain (PAS) (to examine mucus production) in lungtissues. Scoring of inflammatory cells and goblet cells wasperformed in at least three different fields for each lungsection as described (Duan et al.,2004). The mean scores werecalculated using five animals. Bronchoalveolar Lavage Fluid, SerumCytokines, and the Culture Supernatant ofTreg and Th17 Cells Cytokines Analysis Twenty-four hours afterrtthe last aerosolchallenge,bronchoalveolar lavage (BAL) fluid was collected by syringethree times with 1 ml PBS containing 1% BSA. Eosinophils inBAL fluid were counted by cell sorting and counting instrument.Cytokine levels of IL-4, IL-5, IL-13,IL-10, IL-17A, IL-6 in BALF FIGURE 1|TMDCT can alleviate allergic inflammation in eosinophilic asthma BALB/c mice. (A)Experimental protocol for eosinophilic asthma induced by OVA andtreatment administration. (B) Lung function experiments. Detection the Penh value when the methacholine concentration is 0, 6.25, 12.5, 25, 50 mg/ml respectively.Penh ratio (average Penh in the5-minute interval with methacholine divided by the average Penh in the 5-minute interval with PBS). a,p<0.05 vs. Control; aa, p<0.01vs. Control; aaa, p≤0.001 vs. Control. b, p<0.05 vs. TMDCT(H); bb, p<0.01 vs.TMDCT(H); bbb,p ≤ 0.001 vs.TMDCT(H). c, p<0.05 vs.TMDCT(M); cc,p<0.01vS. TMDCT(M); ccc, p≤0.001 vs. TMDCT(M). d, p<0.05 vs. TMDCT(L); dd, p <0.01 vs. TMDCT(L); ddd, p ≤ 0.001 vs. TMDCT(L). (C) Left: HE staining of lung tissues;Right: Inflammatory score results. (D) Left: PAS staining of lung tissues; Right: Total lung inflammation was defined as the average of the peribronchial and perivascularinflammation scores. (E) Detection if specific OVA-IgE in serum; eosinophils count in BALF; detection of cytokines IL-4, IL-5, IL-13. All the values are expressed as mean+/-SEM. n = 4-5 animals per group. were analyzed using a premixed AimPlex" multiplex-assay kit(Cat#T2C0710709), TGF-Bwas analyzed using a premixedAimPlex_multiplex-assay kit (Cat#B111206); OVA-specificIgE in serum was detected by ELISA kit (Cayman, Cat#500840). Flow Cytometry Detection of Treg and Th17Cells in Spleen Tissues The spleen tissue was aseptically removed and prepared into asingle-cell suspension. For Th17 (CD3*CD4+IL17A+) cells and Treg cells (CD3*CD4*CD25+FOXP3+) detection, the cells werestimulated by cocktail A for 4 h (BD, Cat# 550583). Samples werethen washed and re-suspended in 1 ×PBS stained with FVS 780(BD,Cat#565388) to discriminate viable cells and then incubatedwith various surface markers. Consequently, samples were fixedby eBioscience Fix/Perm (Cat#00-5523-00) or BD Fix/Permbuffer kit (Cat#554714) to destroy the cell membrane andthen were stained with FOXP3 (eBioscience, Cat#17-5773-82),IL-17A (BD, Cat# 564169). In the study, CD3 (BD, Cat# 557,666),CD4 (BD, Cat# 552,775) and CD25 (BD, Cat# 558642) were used. Finally, samples were analyzed with the LSR Fortessa cell analyzer(BD) and BD FACSDiva 8.0.3 software. Real-Time PCR Detection of Foxp3 andRORyt mRNA Foxp3 and RORyt mRNA in the lung tissues were detected. RNAextraction kit (Tiangen Biotech (Beijing) co, LTD, Cat#DP419)was used. RNA reverse transcription into cDNA using a cDNASynthesis Kit (ThermoFisher, Cat#K1622). The primers sequencewas the following: B-actin (FP: GACCCAGATCATGTTTGAGACCT; RP: TCCAGGGAGGAAGAGGATGC); RORy (FP:CGCACCAACCT CTTTTCACG; RP: TGGCAAACTCCACCACATACTG); Foxp3 (FP: CTTCAAGTACCACAATATGCGACC; RP: GCGAACATGCGAGTAAACCAA). Untargeted Metabolomics Detection ofPlasma Plasma was collected with 1.5 ml Eppendorf Tubes containingEDTA((ethylene diamineetetraaceticc acid),whichwerecentrifuged at 4℃ by 1,500 g, 15 min. Rremove the protein byMethanol/acetonitrile (1:1, v/v) and centrifuged at 14000g, 4℃for 15 min. All LC-MS analyses were performed at ShanghaiApplied Protein Technology Co., Ltd. R package (ropls) was used to analyzed the processed data.Unsupervisedprincipallcomponentanalysis (PCA) andsupervisedorthogonalilPpartial leastsquares discriminantanalysis (OPLS-DA) were used to evaluate sample stability.The VIP (variables in the projection) value of the OPLS-DAmodel was calculated, metabolites with VIP’1, p-value 0.05 isconsidered to be the significant changed metabolites. After multivariate and univariable analysis, searched thesignificant metabolites in the Human Metabolome database(http://www.hmdb.ca), METLIN (https://metlin. scripps.edu),KEGG (http://www.kegg.com) and Chemspider (http://www.chemspider.com/). Cytoscape software was used to perform+enrichment analysis and visualization. Pearson correlationanalysis was used to perform and determine the correlationbetween twoo variables. KEGG enrichment analysiswasperformed using MetaboAnalyst (www.metaboanalyst.ca). Thevolcano plot and clustering analysis were performed using R. Gut Microbiota Analysis Using CTAB/SDS method to extracte the total genome DNA.Then, the V3-V4 regions in 16S rDNA were amplified using aspecific primer included in the barcode. Next, an Illumina Miseq/HiSeq2500 platform was used to build a library. We used PCoA(Principal Co-ordinates Analysis) to study the similarity ordifference of sample community composition, and LEfSe (LDAEffect Size) to quantify the biomarkers in different groups. Statistical Analysis In this study, t-test, one-way ANOVA test (Turkey or Dunnett),Wilcoxon rank-sum test, Kruskal-Wallis test or Wilcoxon rank-sum test was used. pvalue 0.05 was considered as statistically significant. Pearson analysis with R3.4.2 Heatmap was used toanalyze the correlation between metabolites and other index, thecorrelation between gut microbiota and other index. RESULTS TMDCT Alleviates Airway Inflammation inBALB/C Mice With Eosinophilic Asthma To evaluate the treatment effect of TMDCT in treatingeosinophilic asthma, an OVA-induced eosinophilic asthmamodel was constructed as described in Figure 1A. The mostsignificant effect ofTMDCT(H) group in reducing cell infiltration(Figure 1C) and decreasing goblet cell hyperplasia was observedin the TMDCT(H) group (Figure 1D), further indicating thatTMDCT can alleviate airway inflammation. Moreover, TMDCTreduced airway resistance (Penh ratio), which indicated that lungfunction in TMDCT with a high concentration group had the besteffect (Figure1B). To investigate the role of TMDCT in allergic airwayinflammation, we detected OVA-specific IgE levels in serum.TMDCT significantly decreased OVA-specific IgE concentrationin serum (Figure 1E). Additionally, the eosinophils number inBALF was reduced, and IL-5 levels were decreased in BALF of theTMDCT group, especially in the H group (Figure 1E). Also,TMDCT significantly reduced IL-4 and IL-13 cytokines in BALF.These data suggested that TMDCT could alleviate allergic airwayinflammation. TMDCT can Increase Treg Cells Percentageand Decrease Th17 Cells Percentage in theSpleen Th17 and Treg cells have important role in driving and restrainingairway inflammation in patients with asthma (Seumois et al., 2020).To further investigate whether TMDCT can regulate immuneresponse, we detected Treg and Th17 cells percentage in spleentissues. Surprisingly, TMDCT increased Treg cells percentage anddecreased Th17 cells percentage; the most significant effect was seenin the TMDCT(H) group (Figures 2B,C). Next, we analyzed TGFB, IL-10, IL-6, and IL17A cytokines inBALF. As shown in Figure 2A, TMDCT stimulated TGF and IL-10 and suppressed IL-6 and IL17A (p value were all <0.05), whichis consistent with the Treg and Th17 percentage detection. At the mRNA level, TMDCT increased the Foxp3 (transcriptionfactor of Treg cells) and decreased RORyt (transcription factor ofTh17 cells) relative expression (Figure 2D). These data suggested thatTMDCT could decrease Th17 cells percentage, and increase Tregcells percentage in spleen tissues, among them TMDCT(H) has thebest effect. Identification Plasma Metabolites ofTMDCT To further explore the mechanism of TMDCT in regulating theimmune balance in eosinophilic asthma, we examined potentialplasma metabolite biomarkers of TMDCT. The TMDCT with a staining FOXP3+ and IL-17 in CD4+ T cells in spleen tissue. Right: statistic data of FOXP3+,IL-17+ percentages.(D) Real-time PCR data of FOXP3 and RORyt mRNAexpression in lung tisue. All the values are expressed as mean +/- SEM. n =5-7 animals per group. high concentration was used, and the untargeted metabolomicstechnology was used in this experiment. As seen in Figure 3A,OPLA-DA analysis found the following: 0.31, p-value <0.05) analysis(Figure 3C) further suggested that metabolites in TMDCT ormodel group had a similar function; they participated in similarmetabolite pathway or cell pathway. Finally, six differentialmetabolites in positive mode and 16 differential metabolites inthe negative mode were detected (Table 1). Next, we performed a KEGG enrichment analysis. As shownin Figure 3D, several pathways were identified. The significantmetabolism pathway were our interest. Metabolic Biomarkers in PlasmaAssociated With Treg and Th17 Cells inEosinophilic Asthma Mice Treated With TMDCT To understand the mechanism of TMDCT in regulating Treg/Th17 cells balance, the different metabolites between TMDCTand model group were used for correlation analysis, includingPenh value (Mch:12.5, 25, 50 mg/ml), OVA-IgE, Eosinophils, FIGURE 3|Untarget plasma metabolism detection. (A) OPLS-DA analysis of LC/MS data in positive mode (left) and negative mode (right) from TMDCT(H) vs.Model.(B) Volcano map of differential metabolites in positive and negative mode. The metabolites with FC >1.5, p value<0.05are indicated by rose red, satisfying FC<0.67, p value <0.05 are shown in blue. (C) Hierarchical clustering heat map in positive mode (left) and negative mode (right). (D) Enriched KEGG pathways based onsignificant different metabolites between TMDCT(H) group and Model group. n =7 animals per group. TABLE 1|Differentiated plasma metabolites between model and TMDCT groups. Treg and Th17 cells percentage. In our study, L-Arabinono-1,4- lactone, Myo-inositol, DL-lactate, Uracil and 1-stearoyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine were associatedwithPenhvalue (Mch:i:12.5,25, 50 mg/ml),, OVA-IgE,Eosinophils,Treg and Th17 cells percentage (Figure 4A).There were obviously different (p < 0.05) in 1-stearoyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine andEthylenediaminetetraacetic acid either between the model andcontrol group, or the model group and the TMDCT group(Figure 4B), they were all associated with OVA-IgE, Penh value(Mch: 12.5 mg/ml, 25 mg/ml, 50 mg/ml) and Eosinophil numberswhile they has no correalPerceItion with Treg or Th17 cells percentage(Figure4A)..Moreover, Imidazoleacetic acid, DL-glutamine,L-pyroglutamic acid were all increased in model group comparedwith control group, while they were all decreased in TMDCTtreatment group (p uee werere aall <0.05). IBesidestthat,Imidazoleacetic acid, DL-glutamine, L-pyroglutamic acid were allassociated with OVA-IgE, Penh value (Mch: 12.5mg/ml, 25 mg/ml, 50 mg/ml), Eosinophil numbers and Th17 cells percentage (pvalue all <0.05). As shown in Figure 4B, 2-deoxy-D-glucose wasdecreased in model group when compared with control group (p<0.05), while it increased in TMDCT treatment group (p<0.05), it isalso associated with OVA-IgE, Penh value (Mch: 12.5 mg/ml, 25 mg/ml, 50 mg/ml), Eosinophil number and Th17 cells percentage (p value all <0.05). The above four metabolites were not correlated withTreg cells, which indicated that they could also affect the Treg/Th17balance in eosinophilic asthma. The changed metabolites wereenriched to phenylalanine metabolism, pyrimidine metabolism,ascorbate and aldarate metabolism pathway, histidine metabolism,glutathione metabolism and metabolic pathways (Figure 3D). Variation of Gut Microbiota and KevPhylotypes of Gut Microbiota in Responseto OVA-Induced Eosinophilic Asthma MiceTreated by TMDCT Gut bacteria have an essential role in the action of drugs. Thus, wedetected gut microbiota in the Control group and TMDCT (H)group. As shown in Figures 5A,B, the differences in gutmicrobiota were found between the control and the modelgroup. LEfSe (LDA Effect Size) software was used to discoverhigh-dimensional biomarkers and reveal genome characteristics.LDAtwo was used as selection criteria in the study. ThroughSTAMpaanalysis, Weefound that genus RikenellaceaeRC9_gut_group(p=0.0497),genusBifidobacteriumi((p =0.0462), genusRikenella(P=0.0496),),genusmouseegutmetagenome (p=0.0196), genus Butyricimonas (p= 0.0323),genus Prevotella (p= 0.0339), genus Enterococcus (p=0.0029), FIGURE 4| Biomarker selection of plasma metabolites associated with Treg/Th17 balance. (A) Correlation analysis of differential plasma metabolites with specificOVA-IgE, Penh value (Mch: 12.5,25,50 mg/ml), Eosinophil number in BALF,Treg and Th17 cells percentage.*p<0.05,**p<0.01,***p<0.001. (B)Biomarker in plasmametabolites associated with Treg/Th17 balance. n=7 animals per group. genus Peptoniphilus (p=0.0092), genus Dialister (p=0.0175),genus Corynebacterium (p= 0.0082), genus Dermabacter (p=0.0341), genus Varibaculum (p= 0.0255) were different in theTMDCT group compared to the model group in Figures 5C,D. We used Pearson correlation analysis with R 3.4.2 Heatmapsoftware to analyze the correlation. As shown in Figure 6, genusDesulfovibrio, genus Muribaculum and genus Prevotella 9 were allassociated with Penh value (Mch:12.5, 25,50 mg/ml), OVA-IgE,Eosinophils number in BALF and Th17 cells percentage. Theseresults indicated a correlation with Treg/Th17 balance ineosinophilic asthma treated by TMDCT. DISCUSSION TMDCT is a prescription of professor Qi Wang used to treat allergicasthma which has significant clinical effect. Yet, its mechanism of action still remains unclear. We further investigated the role ofTMDCT in regulating Treg/Th17 cells balance in eosinophilic asthma. The generation and maintenance of Treg cells and theirsuppressive cytokines (TGF-B, IL-10)are essentialfor theinduction of allergen tolerance in allergic disease (Palomares et al.,2017). In addition, FOXP3+ Treg cells play a key role in maintainingperipheral tolerance by inhibiting the responsiveness to allergens(Noval Rivas and Chatila, 2016). It is also suggested that allergen-specific immunotherapy regimens increase the numbers of Treg cells(Radulovic et al., 2008). Moreover, some studies found that Treg cellshave an irreplaceable role in preventing airway inflammation ofallergic asthma (Tortola et al., 2019; Sun et al.,2020). While increasingimmunosuppressive function of immunosuppressive cells such asTreg or Breg cells may be essential in a complete cure for allergicasthma. IL-17A is upregulated in allergic asthma patients, and theupregulation of IL-17A is correlated with the severity of allergic FIGURE 5|TMDCT significantly changed gut microbiota composition. (A) Principal Co-ordinates Analysis (PCoA) analysis in each group. (B) Relative abundanceof gut microbiota in each group. (C) Key phylotypes of gut microbiota in response to TMDCT interventions (LDA method was used). (D) Significantly difference gutmicrobiota analysis, STAMP difference analysis method was used. n= 7 animals per group. FIGURE 6| Biomarker selection of gut microbiota associated with Treg/Th17 balance. Correlation of differentiated microbial phylotypes and specific OVA-IgE,Penh value (Mch:12.5, 25, 50 mg/ml), Eosinophil number in BALF, Treg and Th17 cells percentage. *p<0.05, **p<0.01, *** p <0.001. asthma. TGF-B induces the synthesis of the transcription factor ofRORyt, which is specific for the Th17 cells (Ivanov et al., 2006). In thisstudy, IL17A and TGF-B, transcription factor RORyt of Th17 cells,and transcription factor FOXP3 of Treg cells were used to analyze thebalance of Treg/Th17 cells after TMDCT treatment. Our dataggested thatTMDCT canSsuppresstheSsymptomsofeosinophilic asthma and regulate the balance of Treg/Th17 cells.Interestingly, dexamethasone has similar anti-inflammatory and anti-asthma effects when compared with TMDCT in our study.TMDCTcan also treat eosinophilic asthma by regulating Treg/Th17 immunebalance the same as dexamethasone does (Kianmehr et al, 2017).While there are too many drawbacks of dexamethasone, a new drugshould be explored. Next, we used non-targeted metabolome to explore potentialmetabolic biomarkers in the plasma that were associated withTreg and Th17 cells in eosinophilic asthma mice treated withTMDCT. Imidazoleacetic acid, DL-glutamine, 2-deoxy-D-glucose,L-pyroglutamic acid were identified as plasma biomarkers ofTMDCT and were all associated with Treg/Th17 cells balance.They were enriched in phenylalanine metabolism, pyrimidinemetabolism, ascorbate and aldarate metabolism pathway, histidinemetabolism, glutathione metabolism and metabolic pathways, etc. Allof the plasma metabolites preliminary proved that TMDCT couldregulate Treg/Th17 balance in eosinophilic asthma, which is also theadvantage of multi-target effects of traditional Chinese medicineTMDCT. Previous studies found that glutamine can distinctregulate Th17 differentiation (Johnson et al., 2018), which canbetter explain the reasons for the change in Th17 cells percentagewhich is consistent with our results. While, until now there are nostudy found imidazoleacetic acid, 2-deoxy-D-glucose,I-pyroglutamicacid neithor can regulate Treg, Th17 cells nor regulate thedifferentiation of Treg or Th17 cells, which are not foundassociated with Treg/Th17 balance in allergic asthma. Gut, lung, and skin microbiome exposures can also influence theoccurrence and the development of allergy disease (Kemter andNagler, 2019). The gut microbiota is essential in systemic immuneregulatory network of allergic disease (Sommer and Backhed, 2013)and can regulate drug pharmacokinetics, such as absorption anddistribution (Sousa et al., 2008; Tremaroli and Backhed, 2012; El Aidyet al., 2015; Zhang et al, 2018). Besides that, the gut-lung axis transfersmetabolites and immunomodulatory signals between the gut andlungs. Many studies have shown an increase in the number ofrespiratory diseases due to deviations in gut ecology (Reddy et al,2012; Budden et al, 2017). Omics technologies, includingmetabolomics, can be used to begin to understand relevantmolecular changes. In this study, we used omics technology todetect the effect on of TMDCT in regulating Treg/Th17 cells, andthen used Non-targeted metabolome and 16S rRNA technology toanalyze the biomarkers after TMDCT treatment and relative factorscorrelated with Treg/Th17 balance. We found that gut microbiotagenus Rikenellaceae_ RC9_gut_group, genus Bifidobacterium, genusRikenella, genus mouse gut metagenome, genus Butyricimonas, genusPrevotella, genus Enterococcus, genus Peptoniphilus, genus Dialister,genus Corynebacterium, genus Dermabacter, genus Varibaculumwere significantly different after TMDCT treatment. Bifidobacterium is anti-inflammatory bacteria that can relieveallergic asthma in mice by regulating Th1/Th2 balance (Wang et al., 2020). Allergic patients with chronic asthma usually havelow levels of Bifidobacterium (Hevia et al., 2016). Oral administration of Enterococcus faecalis can suppressallergic asthmatic responseassociatedlvwith attenuation1 ofTh17celldevelopment (Zhanget al., 2012). Fecaltransplantation containing gut Rikenellaceae bbacterialcanalleviate acute liver injury in mice through regulating Treg/Th17 balance(Liu et al., 2021), while it cannot alleviateeosinophilic asthma. Corynebacterium may regulate Th17/Tregcells in the intestinal mucosal immunity (Zhang et al., 2019);However, there are still no studies in allergic asthma. So far, wehave not find relevant research about genus Desulfovibrio, genusMuribaculum or genus Prevotella 9 are related with Th17 cellspercentage in eosinophilic asthma disease, they were the gutmicrobiota biomarker after TMDCT treatment. This study has some limitations. First, only the BALB/ceosinophilic asthma model was used in this study. The effectof TMDCTshould be further examined in other asthma models.Also, clinical research and other allergic asthma models arerequired to prove the mechanism of TMDCT in treatingallergic asthma. Much more study of the biomarkers ofTMDCT treatment should be developed to further explain themechanism of TMDCT in treating allergic asthma, which will bea new strategy to treat allergic asthma. DATA AVAILABILITY STATEMENT The datasets presented in this study can be found in onlinerepositories.S.TThenamessof the repository/repositoriesandaccession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA799688. .ETHICS STATEMENT The animal study was reviewed and approved by BeijingUniversity of Chinese Medicine AUTHOR CONTRIBUTIONS YZ, JW, XZ and QW conceived and designed the experiments;YZ, HZ and TW performed the experiments; YZ analyzed thedata and wrote the paper.All authors have read and agreed to thepublished version of the manuscript. FUNDING This work was supported by the General program of NationalNatural Science Foundation of China (No.81973715,82174243),the General project of Beijing Natural Science Foundation(No.7202110), China Postdoctoral Science Foundation (No.2021M690476)andttheInnovation TeamnaanddTTalentsCultivation Program of National Administration of TraditionalChinese Medicine (No: ZYYCXTD-C-202001). REFERENCES ( Berker, M., F ra nk, L . J . , Gefner, A. L . , Grassl, N., Holtermann, A. V., Hop p ner, S.,et al. (2017). Allergies- A T Cells Perspective in the Era beyond the T H 1/TH2 P aradigm. C l in. Immunol. 17 4 , 73-83. doi:10.1016/j.clim.2016.11.001 ) ( Bousquet,J., Hellings, P. W .,Agache, I, Be d brook, A., Bachert, C., Bergmann, K.C., et al . (2016) . ARIA 2016 : Care Pathways Implementing Emerging Technologies for P redictive Medicine in R hinitis and Asthma a c ross the Life Cycle. Clin. Transl Allergy 6, 47 . doi:10.1186/s13601-016-0137-4 ) ( Budden, K. F . , Gellatly, S. L., Wood, D . L., C ooper, M. A., M o rrison, M., Hugenholtz, l P., e ta l al. (2017). Emerging P athogenic Links b etween M icrobiota a n d th e Gut-Lung axis. Nat . Rev. Microbiol. 15, 55-6 3 . doi:10. 1038/nrmicro.2016.142 ) ( Caminati, M., Pham, D. L., Bagnasco, D., and Canonica, G. W.(2018). Type 2 Immunityin Asthma. World Allergy Organ. J. 1 1, 13. doi:10. 1 186/s40413-018-0192-5 ) ( Christiansen, S. C., and Zuraw, B . L. ( 2019). Treatment of Hy p ertension in Patients with Asthma. R eply. N. E n gl. J. Me d . 381, 227 9 -1057. doi: 1 0.1056/ NEJMra180034510.1056/NEJMc1913646 ) ( Duan, W., Chan, J . H ., Wong, C. H., Leung, B. P ., and Wong, W.S. (20041950). Anti-inflammatory E ffects of M itogen-Activated Protein Kinase K inase Inhibitor U0126 in an Asthma Mouse Model. J. Immunol. 17 2 , 70 5 3-7059. doi:10.4049/jimmunol.172.11.7053 ) ( D umas, A., Bernard, L . , Poquet, Y., Lugo-Villarino, G., and Neyrolles, O.(2018). The R ole of the L u ng Mi c robiota an d the Gut-Lung axis in Res p iratory I nfectious Diseases. Cell Microbiol 20, e12966. doi:10.11 1 1/cmi.12966 ) ( El Aidy, S ., van den Bogert, B., and Kleerebezem,M. (2 0 15). The Small Intestine Microbiota, N utritional Modulation a n d Relevance fo r He a lth. Cu r r. Op i n. Biotechnol. 3 2, 1 4-20. doi:10.1016/j.copbio.2014. 0 9.005 ) ( Hevia, A., Milani, C . , L o pez, P., D o nado, C. D ., Cuervo, A., Gonzalez, S., et al . (2016). Allergi c Patient s wit h Long-Term A sthma D i splay Low Levels of Bifidobacterium Ad olescentis. P l oS one 11, e0 1 47809. doi :1 0.1371/journal. pone.0147809 ) ( I srael, E. , and Reddel, H. K . ( 2017). Severe and D i fficult-To-Treat Asthma inAdults. N. Engl. J . Med. 377, 965-976. doi:10.1056/NEJMra1608969 ) ( I vanovII, McKenzie, B. S.,Zh o u,L., T adokoro, C. E., Lepe l ley, A., Lafa i lle, J.J., et a l .(2006). The Orphan N uclear Receptor R ORgammat Directs the Differentiation P rogram o f Proinflammatory I L -17+ T Helper Ce l ls. Cell 1 26, 1 121-1133. doi:10.1016/j.cell.2006.07.035 ) ( J ohnson,M. O., Wolf, M. M.,Madden, M. Z., Andrejeva, G., Sugiura, A., Contreras,D. C., et al. (2018). Distinct Regulation of Th17 a n d Th1 Cell Differentiation by Glutaminase-dependent Met a bolism. Cell 175, 1780- e 19. doi:10 . 1016/j.cell. 2018.10.001 ) ( K emter, A. M., a n d Nagler, C. R. (2 0 19). In f luences on Al l ergic Mechanismsthrough G ut, Lung, a n d Sk i n Mi c robiome Expo s ures. J. Clin . Invest. 12 9 , 1483- 1 492. doi:10.1172/jci.12461010.1172/JCI124610 ) ( Kianmehr,M., Haghmorad, D., Nosratabadi, R. , Rezaei, A., Alavinezhad, A., andBoskabady, M. H . (2017). T he Effect of Zataria Multiflora o n Th1/Th2 and Th17/T Regulatory in a Mouse Model of Allergic A sthma. Front. Pharmacol. 8,458. doi:10.3389/fphar.2017.00458 ) ( Liu, Y ., Fan, L ., Cheng, Z., Y u, L ., C ong, S., H u, Y . , et a l . ( 2021). F ecal T ransplantation A lleviates Acute L iver I njury in M ice t h rough Re g ulating Treg/Th17 C y tokines B a lance. S c i. R ep. 1 1, 1 611. d oi:10.1038/s41598-021- 81263-y ) ( NationalAsthmaEducationandPreventionProgram (2007). Expert Panel Report 3(EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J . A llergy Clin. Immunol. 12 0 , S9 4 -S138. d oi:10.1016/ j .jaci.2007. 09.043 ) ( Noval Rivas, M., and C h atila, T. A. ( 2016). R egulatory T C e lls in Allergic D iseases. J . Allergy Clin. Immunol. 1 38, 639- 6 52. doi:10.1016/j.jaci.2016.06.003 ) ( Palomares, O., Akdis, M., Martin-Fontecha, M., a nd A kdis, C . A. (2017). Mechanisms o f I m mune Reg u lation in A ll e rgic Di s eases: t h e Ro l e of Regulatory T and B Cells. Immunol. Rev. 27 8 , 219-236. doi : 10.1111/imr . 12555 ) ( P alomares, O.,Martin-Fontecha,M.,Lau e ner, R., Tr a idl-Hoffmann, C., Cavkaytar, O., Akdis, M., et al . (2014) . Regulatory T Cells and Immune Regulation o f Allergic Diseases: Roles of IL-10 and TGF-B. Genes Immun. 1 5,511- 5 20. doi:10. 1038/gene.2014.45 ) ( Q in, J., Lv, M., Jiang, Z, Meng, X . , Wang, Y., Cui, J., e t al.(2021).Tuo-Min-Ding-Chuan Decoction A l leviate Ovalbumin-Induced Allergic Asthma by InhibitingMast Cell D egranulation a n d Do w n-Regulating the Diff e rential Exp r ession P roteins. Front. Pharmacol. 12, 725953. doi:10 . 3389/fphar.2021.725953 ) ( R adulovic, S., Jacobson, M. R., Durham,S. R . , a nd Nouri-Aria, K . T.(2008).Grass P ollen Immunotherapy Induces F o xp3-Expressing CD4+ CD25+ Cell s in the Nasal M ucosa. J. Allergy Clin. I mmunol. 121, 14 6 7-e1.doi:10.1016/j.jaci.2008.03.013 ) ( R eddy, A. T. , Lakshmi, S. P., and Reddy, R . C. (2012). Murine Model of Allergen I nduced A sthma. J o VE, 1 4 ( 6 3). e 3771. d o i:10.3791/3771 ) ( Seumois, G., Ramirez-Suastegui, C., S c hmiedel, B. J.,L i ang, S.,P e ters, B., Sette A., et al. (2020). Single-cell Transcriptomic Analysis of Allergen-specific T Cells in Allergy and Asthma. S ci. Immunol. 5 . e aba6087. d oi:10.1126/sciimmunol. aba6087 ) ( Sommer, F.,and Backhed, F. (2013). The G ut Microbiota-Mmasters o f H ost D evelopment and Ph y siology. Nat. Re v . Microbiol. 1 1 , 2 2 7-238. doi:10.1038/nrmicro2974 ) ( S ousa,T., Paterson,R., Moore, V., Carlsson, A., A b rahamsson, B., and Bas i t, A. W. (2008). The Gastrointestinal Microbiota as a Site f o r the Biotransformation of D rugs. I nt. J . Pharm. 3 63,1 - 25. doi:10.1016/j.ijpharm.2008.07.009 ) ( S un, L., Fan , M ., Huang, D. , Li, B . , Xu, R., Gao, F . , e t al. (2021). Clodronate-loaded Liposomal and F ibroblast-Derived Exosomal Hybrid System for Enhanced D rug Delivery to Pulmonary Fi b rosis. Biomaterials271,120761.doi:10.1016/j. biomaterials.2021.120761 ) ( Sun, L., Fu,J., Lin, S. H., Sun,J. L., X ia, L., Lin, C. H., et al. (2020). Particulate Matterof 2.5 um or Less in Diameter Disturbs the Balance of TH17/regulatory T Cellsby Targeting Glutamate Oxaloacetate Transaminase 1 and Hypoxia-Inducible F actor la i n an Asthma Model.J. Allergy Clin. I m munol. 145, 402-414. doi:10. 1 016/j.jaci.2019.10.008 ) ( Tortola, L ., Pawelski, H., Sonar, S. S. , Ampenberger, F., Kurrer, M., a nd Kopf,M.(2019). IL-21 Promotes Allergic Airway Inflammation by Driving Apoptosis of F oxP3+ Regulatory T Cells. J. Allergy Clin. Immunol. 143, 2178-e5.doi:10.1016/ j.jaci.2018.11.047 ) ( T remaroli, V., a nd Backhed, F. ( 2 012). F u nctional In t eractions be t ween the GutMicrobiota and 1Host M etabolism. Nature 489,242-249. d o i:10.1038/ nature11552 ) ( Wang, W . , L u o, X . , Z h ang, Q., H e , X., Zhang, Z. , a n d Wa n g, X. (20 2 0). Bifidobacterium I nfantis R elieves Allergic Asthma i n M i ce by Reg u lating Th1/Th2. M ed. S ci. Monit.26, e920583. doi:10.12659/msm.920583 ) ( Zhang, B ., An, J , S himada, T., L iu, S., and Maeyama, K. ( 2 012). Oral Administratio n of E nterococcus faecalis FK-23 Su ppresses T h 17 Ce ll D evelopment an d A t tenuates A l lergic A irway Responses in M i ce. Int. J. Mol. Med. 30, 248-254. doi:10.3892/ijmm.2012.1010 ) ( Zhang, J , Z hang, J., and W ang, R . ( 2 018). G u t Microbiota Mo d ulates DrugPharmacokinetics. Drug Metab. R ev.5 0 , 3 5 7-368. d o i:10.1080/03602532. 2018.1497647 ) ( Z hang, L. , S o ng, P. , Zhang, X., Metea, C., Sc h l eisman, M., K a rstens, L., et al. ( 2 019). Alpha-Glucosidase Inhibitors Alter Gut Microbiota and Am e liorate Collagen- Induced Arthritis. Front.Pharmacol. 10, 1 684. d oi:10.3389/fphar.2019.01684 ) ( Conflict o f I nterest: The authors declare that the research was conducted in theabsence of a ny commercial or financial relationships that could be construed as a p otential conflict of interest. ) Publisher’s Note: All claims expressed in this article are solely those of the authorsand do not necessarily represent those of their affiliated organizations, or those ofthe publisher, the editors and the reviewers. Any product that may be evaluated inthis article, or claim that may be made by its manufacturer, is not guaranteed orendorsed by the publisher. Copyright @ 2022 Zhou, Zhao, Wang, Zhao, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons AttributionLicense (CC BY). The use, distribution or reproduction in other forums is permitted,provided the original author(s) and the copyright owner(s) are credited and that theoriginal publication in this journal is cited, in accordance with accepted academicpractice. No use, distribution or reproduction is permitted which does not complywith these terms. ebruary Volume Article rontiers in Pharmacology|www.frontiersin.org rontiers in Pharmacology|www.frontiersin.orgFebruary Volume Article 近日北京中医药大学的周玉美老师及其团队研究了TMDCT在调节Treg/Th17细胞免疫平衡中的作用和其相关的潜在代谢和肠道生物标志物。相关成果发布在英文学术期刊《Frontiers》上。Yumei Zhou, Haihong Zhao, Tieshan Wang, Xiaoshan Zhao, Ji Wang and Qi Wang,Anti-Inflammatory and Anti-asthmatic Effects of TMDCT Decoction in Eosinophilic Asthma Through Treg/ Th17 Balance. Frontiers, 2022.819728:哮喘——是一种难以控制的慢性炎症性疾病,影响着全球3亿多人。随着目前的上升趋势,预计到2025年这一数字将达到4亿人.根据权威报告报到,每年有250,000例与哮喘相关的死亡病例,其中许多是可以避免的(Christiansen Zuraw,2019年)。TMDCT是一种中药配方,由十二种草药组成,可以缓解症状和治疗过敏性哮喘。然而,潜在的作用机制仍不清楚。TMDCT含有12种中药,可抑制过敏性哮喘炎症,抑制肥大细胞脱颗粒(Qin et al, 2021)。在我们的研究中,我们研究TMDCT在调节Treg/Th17细胞平衡过程中起到了积极的作用,并在使用非靶向代谢组和16S rDNA技术接受TMDCT治疗的嗜酸性哮喘小鼠中探索了与Treg和Th17细胞相关的潜在代谢和肠道生物标志物。OVA诱导的过敏性哮喘小鼠模型和分组OVA诱导的嗜酸性哮喘BALB/C小鼠模型按照先前描述的方法构建(Dumas et al., 2018)。简而言之,在第1天和第14天,将2 mg OVA (Sigma-Aldrich,Cat#A5503)与 2mg ImjectTM Alum Adjuvant (Invitrogen ,Cat#77161)和PBS混合后腹膜内注射小鼠。从第21天到第25天注射后,在挑战阶段, 小鼠用1% OVA连续雾化30分钟,如图1A所示。Treg细胞及其抑制性细胞因子(TGF-B、IL-10)的产生和维持对于在过敏性疾病中诱导过敏原耐受起到至关重要的作用(Palomares 等,2017)。此外,FOXP3+ Treg细胞通过抑制对过敏原的反应,在维持外周耐受性方面发挥关键性作用(Noval Rivas和Chatila ,2016)。还建议过敏原特异性免疫治疗方案增加Treg细胞的数量(Radulovic 等,2008)。此外,一些研究发现Treg细胞在预防过敏性哮喘气道炎症方面具有不可替代的作用(Tortola et al., 2019; Sun et al, 2020)。同时增加Treg或Breg细胞等免疫抑制细胞的免疫抑制功能可能对彻底治愈过敏性哮喘至关重要。塔望科技研发生产的4通道小鼠全身体积描记系统(WBP-4R)可对清醒自由活动动物呼吸参数进行测量,如呼吸频率,潮气量,气道高反应性测试(Airway hyperresponsiveness,AHR)等。Sun, L., Fan, M., Huang, D., Li, B., Xu, R., Gao, F., et al. (2021). Clodronate-loaded Liposomal and Fibroblast-Derived Exosomal Hybrid System for Enhanced Drug Delivery to Pulmonary Fibrosis. Biomaterials 271, 120761. doi:10.1016/j.biomaterials.2021.120761
确定





还剩9页未读,是否继续阅读?
上海塔望智能科技有限公司为您提供《小鼠中哮喘治疗研究检测方案(其它常用设备)》,该方案主要用于其他中其他检测,参考标准--,《小鼠中哮喘治疗研究检测方案(其它常用设备)》用到的仪器有塔望全身体积描记系统WBP-8MR、塔望全身体积描记系统WBP
相关方案
更多
该厂商其他方案
更多










