我们模拟了传统的黑灰咸蛋和盐泥涂层中所含的金属元素。采用K2CO3、CaCl2、MgCl2、ZnCl2和FeC6H5O7金属盐部分取代氯化钠(NaCl),研究了这些金属盐对鹌鹑蛋酸洗过程中理化性质、质地和微观结构的影响。筛选出几种合适的低钠替代品,为低钠盐鹌鹑蛋生产工艺优化提供理论依据。
方案详情

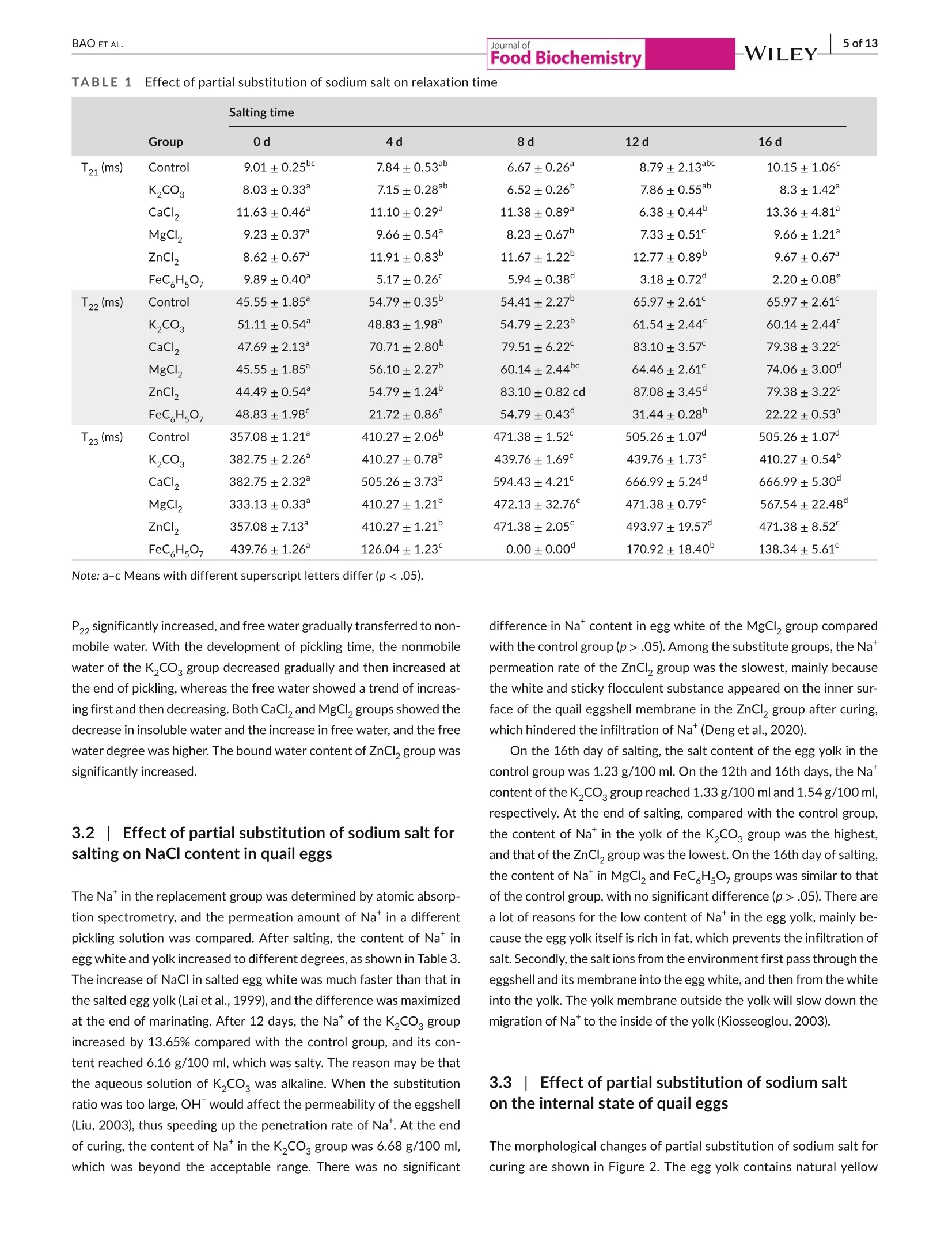
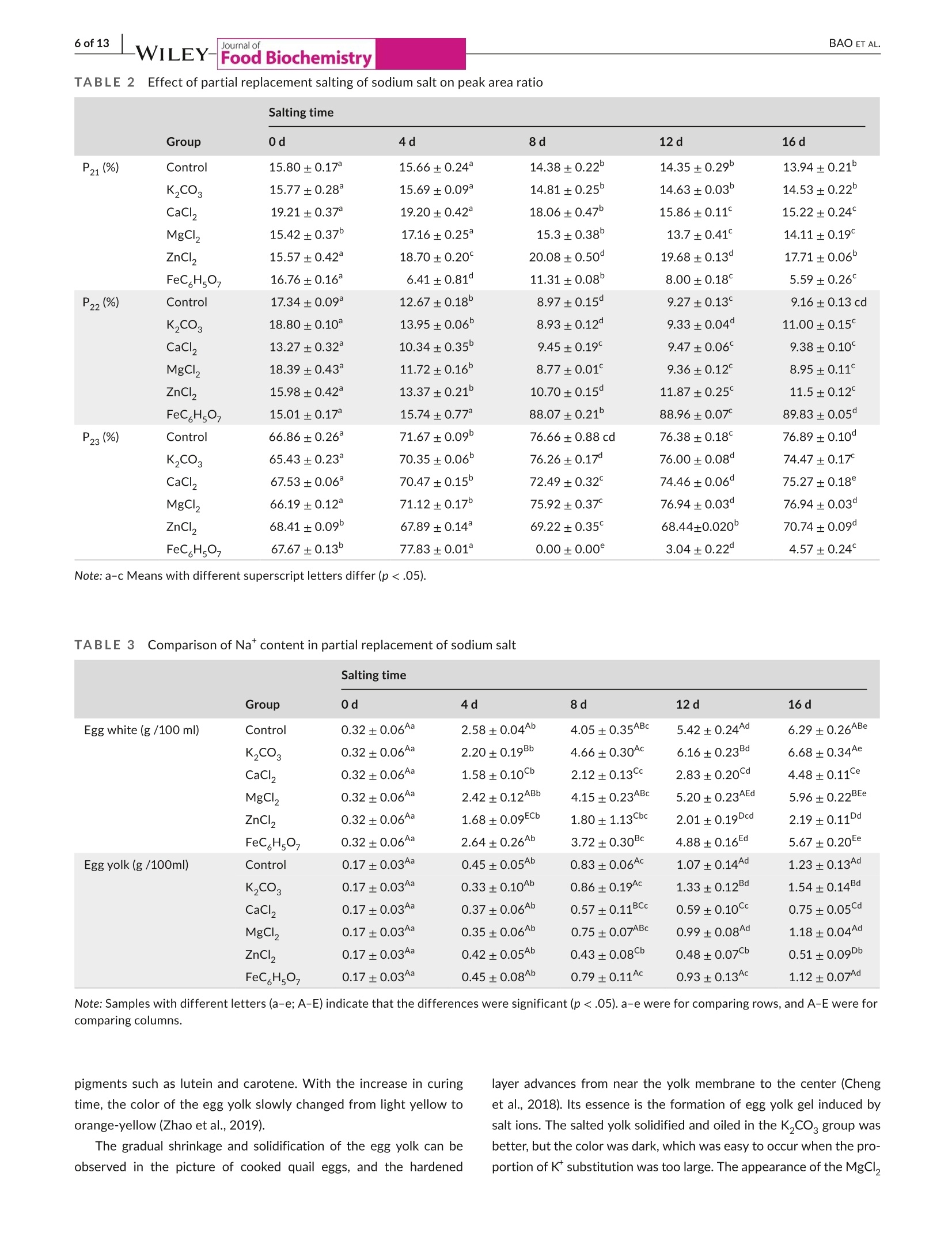
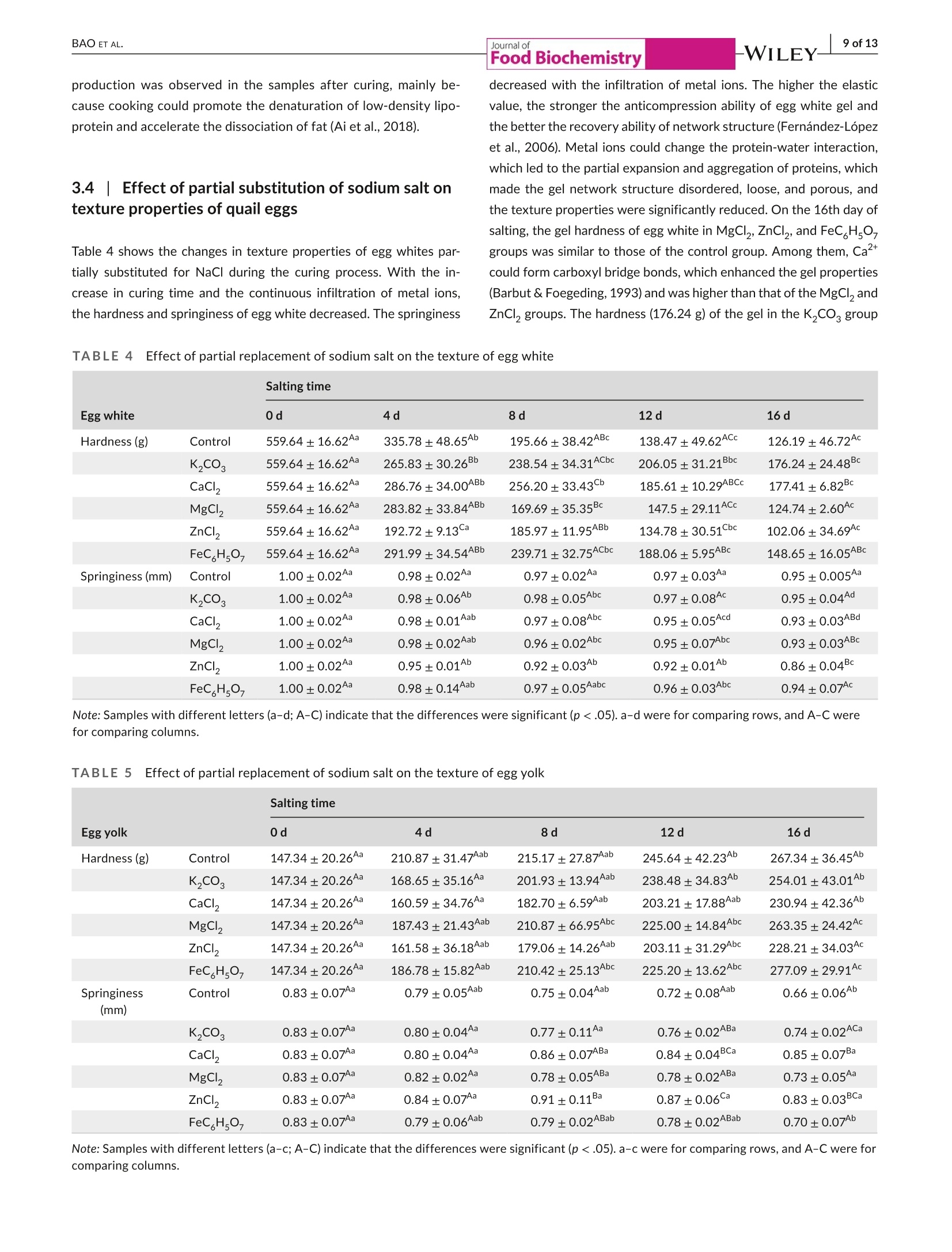
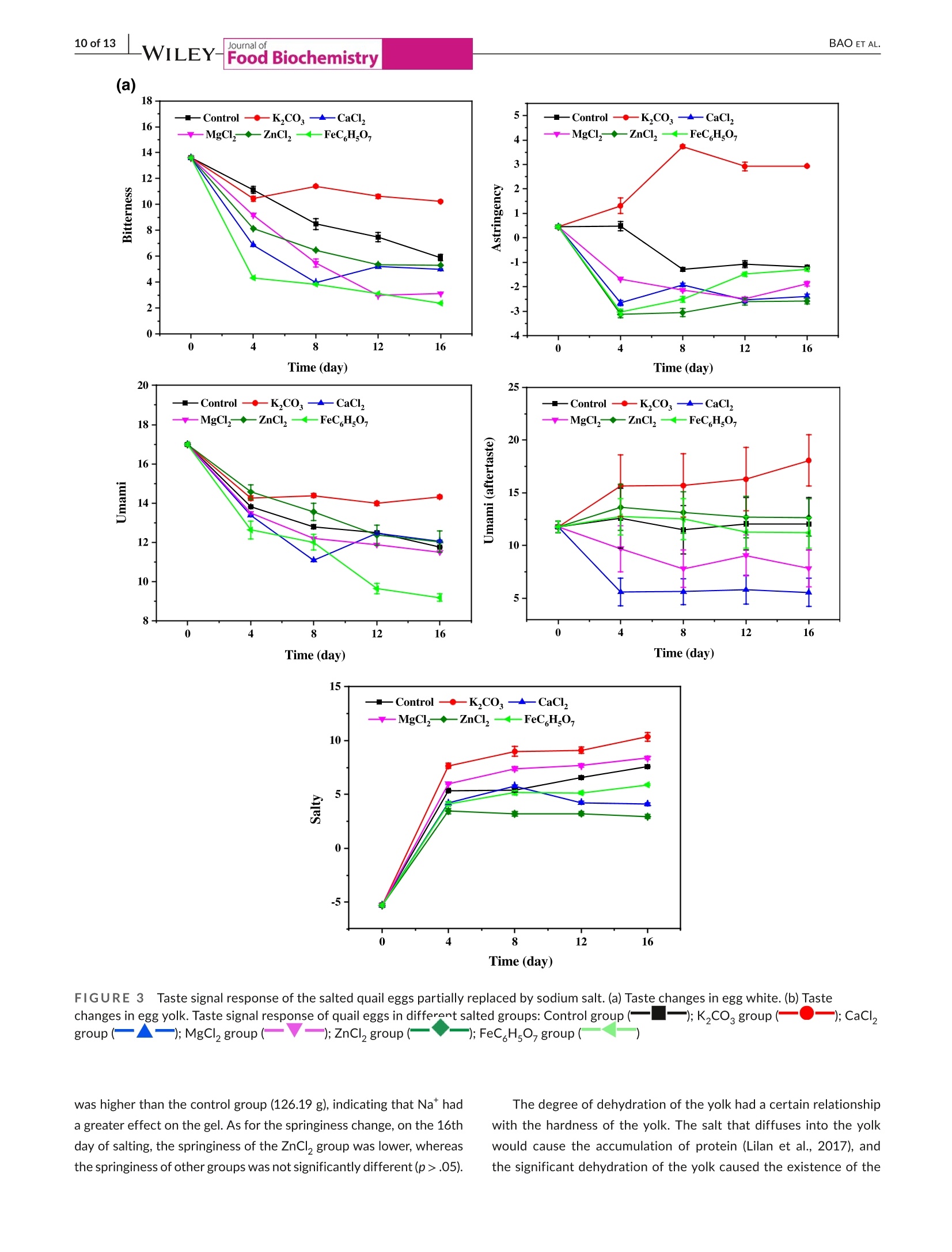
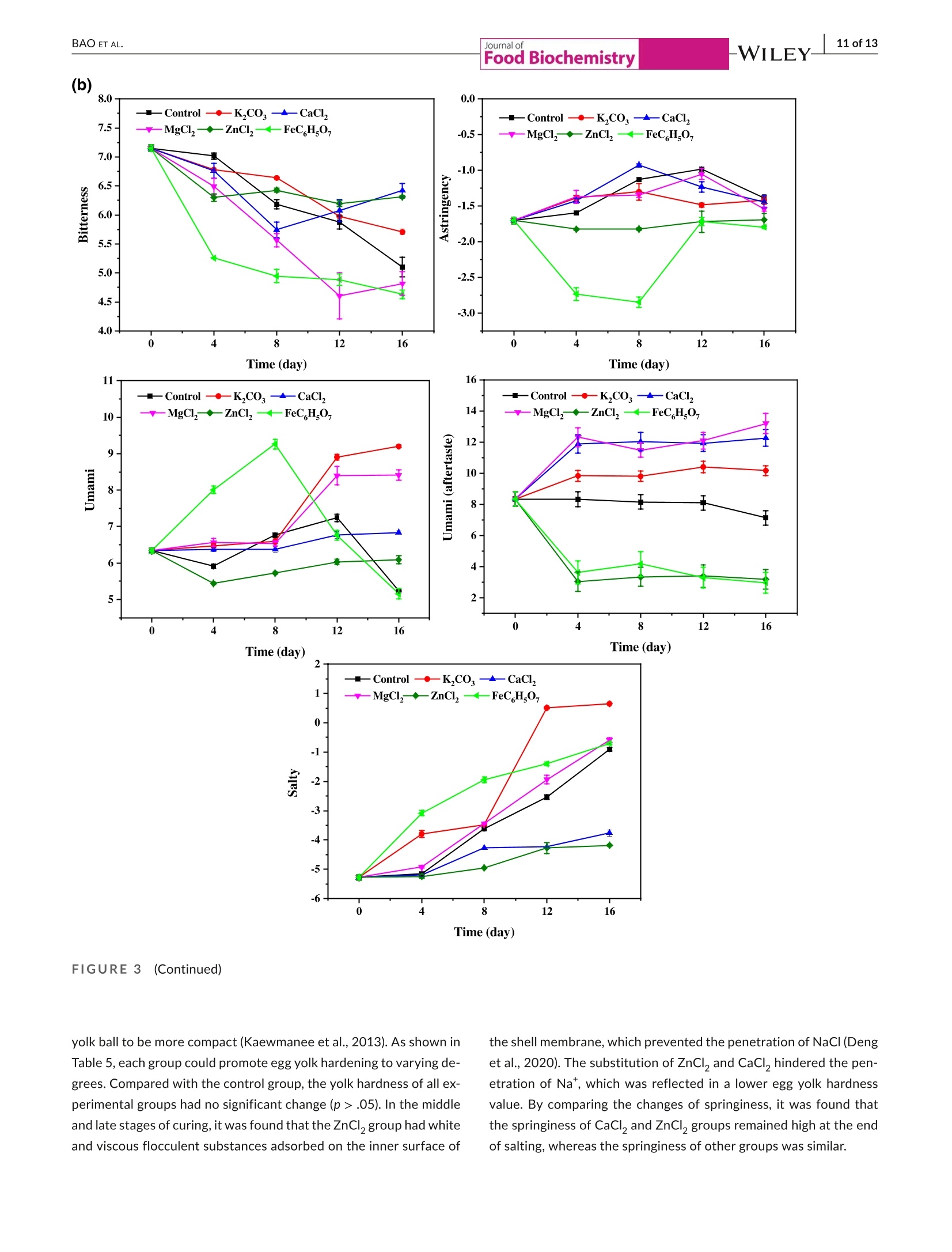
Check for updatesAccepted: 23 August 2021Revised: 19 August 2021Received: 31 May 2021DOI: 10.1111/jfbc.13941ORIGINALARTICL E BAO ET AL.2 of 13WILEY-Food Biochemistry wileyonlinelibrary.com/journal/jfbc@2021 Wiley Periodicals LLC. 1 of 13J Food Biochem. 2021;45:e13941.https://doi.org/10.1111/jfbc.13941 Journal ofFood Biochemistry WILEY Effect of partial substitution of sodium salt on the quality ofsalted quail eggs Abstract To improve the quality of salted quail eggs and solve the problem of excessive so-dium content in salted eggs, we selected substitutes (K,CO, CaCl, MgCl,, ZnCl,and FeC Hs0,) to partially replace NaCl and study its effect on water migration,physicochemical properties, and textural characteristics. The low-field nuclear mag-netic resonance technology (LF-NMR) was used to qualitatively analyze the moistureand proton content of quail eggs during the pickling process. The results showedthat the relaxation curves of ZnCl and FeCHs07 groups were significantly differentfrom those of other groups.The bound water content of the ZnCl, group increasedsignificantly, and FeC,Hs0, made the binding degree of water closer. The Na* ofdifferent substitute groups was determined by atomic absorption spectrometry; itwas found that the permeation rate of NaCl in the curing process was in the follow-ing order: K,COs> control group > MgCl> FeC Hs07> CaCl> ZnCl . Throughthe electronic tongue study and comparing the ripening period of salted quail eggs,it was found that the flavor and ripening time of salted quail eggs cured by ZnCl,and FeCHs0z were not suitable for low-sodium pickling preparation. At the sametime,CaCl, and MgCl were suitable for low-sodium pickling and could improve theproduct quality. When using K,CO, the substitution ratio can be reduced and two ormore compound-curing agents can be formed with CaCl, and MgCl2, thus reducingthe content of sodium salt in salted eggs. Practical applications We simulated the metallic elements contained in the traditional black ash-saltedeggs and salt mud coatings. By partial substitution of sodium chloride (NaCl) withdifferent metal salts (K,CO, CaCl, MgCl, ZnCl, and FeCHs), we studied theeffects of these metal salts on the physical and chemical properties, texture, andmicrostructure of quail eggs during the pickling process. Several suitable low-sodiumsubstitutes were screened out to provide a theoretical foundation for the processoptimization of low-sodium-salted quail eggs. KEY WORDS low-sodium salt substitute, microstructure, quail eggs, quality characteristics 1 INTRODUCTION In Asian countries, quail is an important resource of meat and eggsfor poultry (Bao et al., 2020; Baumgartner, 1994). In comparison,the volume of quail eggs is only one-fifth of the size of eggs (Singhet al.2007), but the content of nutrients such as protein, minerals,and vitamins is much larger than that of eggs (Abadi et al., 2018).Studies have shown that eating quail eggs regularly can improve thebody’s immunity (Liu et al.,2020), promote memory health (Toliket al., 2014), enhance brain activity (Tunsaringkarn et al., 2012), andstabilize the nervous system (Onyekwelu et al., 2018). Salted eggs, also known as pickled eggs and flavored eggs, are akind of traditional Chinese egg-processed products, mainly made frompoultry eggs and salted (Yuan et al., 2018). People are obsessed withthe taste of the salted egg yolk, and they not only eat it directly but alsomake it into moon cakes, dumplings, crisps, and so on (Kaewmaneeet al., 2010). Salted eggs are often pickled with sodium salt during pro-cessing, and the Na*contained in sodium salt helps maintain the nor-mal physiological function of the human body. Still, excessive intakefor a long time will increase the risk of diseases such as hypertension(Wang et al., 2015). In addition, the salt content of the yolk in the tradi-tional salted eggs is generally about 1%-3% (Bao et al.,2020), whereasthe salt content of most egg whites is significantly higher than that ofthe yolk, and the salinity value can reach 7%-10% (Lilan et al., 2017).This has caused deep trouble to consumers when they eat salted eggs.Therefore, in order to meet the healthy food concept pursued by mod-ern consumers, and without changing the expected characteristics ofthe salted egg yolk (such as tenderness, sand, and oil) and flavor duringthe salting process (Abdel Nour et al., 2011), it is very important to finda new method for salted eggs. In the traditional processing methods of salted eggs, the qualityof salted eggs processed by the plant ash method and the mud pack-ing method is better, and the salt content of egg white is lower. In themethod of plant ash, straw ash and salt water were used to make theash material, which was wrapped in fresh eggs and preserved. Themud packing method used mud and salt and mixed at a ratio of 3:1-5:1, which is evenly coated on the surface of fresh eggs for pickling(Huang et al., 2012). However, these two salting methods have somehealth and safety risks, which are not conducive to mass production.Therefore, metal salt can be used to partially replace sodium saltto improve the problem of high-sodium content in salted eggs. Toa certain extent, the risk of some chronic diseases caused by eatingsalted eggs can be reduced. In the current studies on sodium saltsubstitutes, Wu Ling et al. partially replaced NaCl with 6.5% KCI;the results showed that the NaCl content in the salted egg whitedecreased from 5.44% to 4.01%, and a higher proportion of substitu-tion would produce bitter taste (Ling et al., 2011). Alino et al partiallyreplaced NaCl with KCl, CaCl,and MgCl, in the processing of ham,and the results showed that KCl reduced the reduction degree ofwater activity, whereas CaCl, and MgCl, slowed down the rate ofsalt infiltration (Alino et al.,2010). In the early stage, we studied the changes of water migra-tion, physicochemical properties, and microstructure of quail eggs during the salting process, and determined the best curing time ofthe salted quail eggs (Bao et al., 2020). Therefore, in this study, wesimulated the metal elements contained in the traditional black ash-salted eggs and salt mud coatings. Through the partial substitutionof sodium salt (NaCl) by different metal salts (KCO, CaCl, MgCl2,ZnCl,,and FeC H,O,), we studied the effects of these metal salts onthe physical and chemical properties, texture, and microstructure ofquail eggs during the pickling process. Several suitable low-sodiumsubstitutes were screened out to provide a theoretical foundationfor the process optimization of low-sodium-salted quail eggs. 2 MATERIALS AND METHODS 2.1 Materials Sodium chloride (NaCl), potassium carbonate (K,CO ), calciumchloride (CaCl), magnesium chloride (MgCl), FeCHs0z, and Zincchloride (ZnCl,) were purchased from Hubei Tanggui Food Co., Ltd.(Hubei,China). Perchloric acid, nitric acid, and caesium chloride werebought from the Tianjin Fuyu Fine Chemical Co., Ltd.(Tianjin, China).Silver chloride, L (+)-tartaric acid, and potassium chloride were pur-chased from Wuxi Noble Metal LLC (Wuxi, China). 2.2 Salting of quail eggs Fresh quail eggs weighing about 10 g were selected from the market,cleaned with running water,then immersed in 6% salt water for den-sity selection, then washed and disinfected in food-grade disinfectant10 min, and washed and air-dried again with tap water. K CO , CaCl,MgCl, ZnCl, and FeC,Hs0, of 0.5 mol/L were mixed with 4 mol/LNaCl at 1:2, in which FeC,Hs0, was dissolved in hot water and thecontrol group was 4.5 mol/L NaCl. Finally, quail eggs were immersed indifferent pickling solutions at room temperature, and quail eggs werecollected and measured at each curing time (0,4,8, 12 and 16 days). 2.3 Determination of T2 relaxation time T, relaxation analysis was carried out with a low-field NMR analyzer(Suzhou Niuma Co., Ltd., Suzhou, China), referring to the method ofCheng et al.(2018), and the coil selection and instrument parameterswere slightly modified. The whole quail egg was placed on a radio-frequency coil with 40 mm for testing. Spin-spin imaging time T wasmeasured by CPMG (Carr-Purcell-Meiboom-Gill) sequence. We setthe parameters as follows: The r-value of T, (time between 90 pulsesand 180 pulses) was 200 us, and the length of the two pulses was12.0 and 24.0 us, respectively. The data from 8,000 echoes were ob-tained by 8 scan repetitions, and the repetition time was 3,500 ms.A multiexponential fitting curve was obtained by the SRIT algorithm,and the number of iterations was 1,000,000. The fitted peak posi-tion corresponds to the T, relaxation time of each proton, and the peak area corresponding to each proton was obtained by integrationand accumulation. 2.4| Nat content determination Nat content determination was carried out with flame atomic ab-sorption spectrometry (ZA3000, Hitachi, Tokyo,Japan). The sampleswere pretreated according to leggli et al. (2010). We added about 1 gof the sample into 10 ml of mixed acid (nitric acid: perchloric acid= 9:1) and digested it in the digestive tube. After the sample wasdigested to clarify, the volume was fixed to 50 ml after cooling. Theblank experimental group was used as a control. After the preparedsample solution was diluted 100x, the absorbance of the sample so-lution at 589.0 nm was directly measured by an atomic absorptionspectrophotometer. For each test solution, the measurement wasrepeated three times. We calculated the average absorbance valuesand subtracted the average absorbance value of the blank sample. 2.5Photographic imaging We performed sampling on 10 quail eggs every 4 days, 5 of whichwere cooked in a water bath at 90℃ for 20 min, cooled, dried andcut, and placed in a 60 mm disposable plastic Petri dish. The cross-section of the boiled quail eggs was photographed in a confinedspace with a specific light source. The other five quail eggs weredried and shelled, and their contents were poured into a 60 mm dis-posable plastic Petri dish and photographed in turn. 2.61| Texture profile analysis (TPA) TPA was measured on a TA-XT texture analyzer (Stable MicroSystems, Godalming, UK), as described by Ai et al. (2018). Wholequail eggs and 4 ml of egg white (placed in a 10 ml beaker) wereboiled at 90C for 20 min, and the yolk was taken from the cookedquail eggs and then cut into 1 cmcubes for TPA. A P0.5 probe wasselected for compression, and the remaining parameters were thespeed of 5 mm/s before test, 2 mm/s during test, and 2 mm/s aftertest, and the deformation rate was 50%. We selected P0.5 modelprobe compression, and the remaining parameters were measured.The primary indicators of hardness, springiness, and chewiness wereobtained through the TPA test results. Each sample was paralleledthree times and the average value was taken. 2.7|Determination of egg white and egg yolkelectronic tongue The electronic tongue system((TS-5000Z, INSENT, Japan)wasused to determine the flavor of salted eggs, mainly referring to themethod of Ma et al. (2015). First, the electrode was immersed in anion (cation) solution for 90 s, washed in the first two groups ofreference solution for 120 s, and immersed in reference solution 3for 30 s, and the potential of the reference solution was measured.The sample liquid potential was measured by immersion in the sam-ple solution for 30 s, and the potential difference was the responseof the basic taste. The electric potential was measured by washing3 s in reference solutions 4 and 5 and immersed in reference solu-tion 6 for 30 s, and the difference between the electric potential andthe reference liquid potential was aftertaste.AAE, CTO, CAO, AE1,and C00 correspond to the tests of fresh,salty, sour, astringent, andbitter taste, respectively. The sample was tested for 4 times, andthe test results were further analyzed after three times of selection. 2.881Statistics and analysis The data obtained from all experiments were analyzed using thecommercial SPSS 18.0 software (SPSS Inc., Chicago, Illinois, USA).One-way analysis of variance (ANOVA) was used to compare differ-ences between results. Data are reported as the mean ± standarddeviation (SD), and significance analysis by Duncan at 5%. The draw-ing was done by the Origin8.5 software. 3 RESULTS AND DISCUSSION 3.11| Effect of partial replacement of sodium salt onthe relaxation time and peak area of quail eggs Using low-field nuclear magnetic resonance (NMR), the moisturecontent of quail eggs and the distribution and migration of protonsrelated to oil and fat can be described systematically. As shown inFigure 1, after multiexponential fitting of the attenuation curve ob-tained from the CPMG pulse sequence, it is found that T,has threepeaks in the relaxation time distribution of 0.1~10,000 ms, in whichT2 represents water and lipid protons closely bound to proteins, T2represents water and lipid protons with greater degrees of freedom,and T23 represents free water (Yang et al.,2016). It was found thatthe relaxation curves of the ZnCl, and FeC H.O groups were significantly different from those of other groups. Among them, theboundary between the peak of immobile water (T) and free water(T) in the ZnCl, group became not obvious, which was related tothe flocculation of protein caused by Zn?+(Deng et al., 2020). Thebinding degree of water in the FeC H,O, group is closer, which ischaracterized by a sharp decrease of free water and a substantialincrease in water that is not easy to flow. Table 1 shows the effect of partial substitution of sodium salt onthe relaxation time. It is found that the degree of freedom of T, in thecontrol group decreases at first and then increases. The decrease of T,is related to the aggregation process of protein, and the fluidity of T,will increase with the increase in the destruction of lipoprotein in thelater stage of curing (Shao et al., 2016). Among them, T in the ZnClgroup migrates to a long relaxation time, whereas T21 in the FeC,HsO7 -WILEY-Journal of (b)18- -Control T23 16· · 4 days ·8 days 亿们86 12 days 16 days FIGURE 1 Low-field nuclear magnetic resonance spectra of the salted quail eggs with partial metal salt instead of sodium salt at differenttimes. (a)T, relaxation curves of control group.(b)Trelaxation curves ofK,CO, group.(c) T relaxation curves of CaCl, group.(d) T,relaxation curves of MgCl2 group. (e) T2 relaxation curves of ZnCl2 group.(f) T2 relaxation curves of FeCHs0group. T2 curves of quail eggsunder different curing times: Unsalted (_)-); Salted 4 days (_-); Salted 8 days(.); Salted 12 days (_-); Salted 16 days (_) group migrates to a short relaxation time most significantly. The de-gree of freedom of T2, in the control group increased gradually, whichwas related to the exudation process of oil. T in the FeC H0-groupdecreased significantly, which proved that the binding was closer. TheT2 degrees of freedom of the control group increased continuously,the T2 degrees of freedom of the FeC,Hsgroup was the smallest,and the T23 degrees of freedom of the CaCl, group was the largest. Table 2 shows the effect of partial substitution of sodium salt onthe ratio of peak area, in which the egg yolk gradually dehydrated togel and solidified, which led to a downward trend of P, in the controlgroup and an upward trend of Pdue to protein flocculation caused by Zn²*. In the FeCHs07group, P21 decreased most rapidly and P22increased significantly, which proved that the existence of Fe’+ledto a significant decrease in bound water and an obvious increase inthe content of nonmobile water. Except for the FeC H,O, group,there was a little difference in P among other groups. Comparedwith the control group, the P23 of the ZnCl,group changed a little,but the P2 of the FeC H,O, group decreased most rapidly and al-most disappeared in the later stage of curing. The LF-NMR study showed that the FeC H,O, group had a sig-nificant effect on the water content of quail eggs. The combinationdegree of T21, T22, and T2 was closer, P2 significantly decreased and Group 0d 4d 8d 12d 16d T21(ms) Control 9.01±0.25bc 7.84±0.53 6.67±0.26 8.79±2.13abc 10.15±1.06° K2CO 8.03±0.33 7.15±0.28 6.52±0.26° 7.86±0.55ab 8.3±1.42° CaCl 11.63±0.46° 11.10±0.29° 11.38±0.89° 6.38±0.44” 13.36±4.81 MgCI, 9.23±0.37° 9.66±0.54 8.23±0.67 7.33±0.51 9.66±1.21 ZnCl, 8.62±0.67 11.91±0.83 11.67±1.22° 12.77±0.89 9.67±0.67 FeC HsO, 9.89±0.40 5.17±0.26 5.94±0.38° 3.18±0.72° 2.20±0.08° T22 (ms) Control 45.55±1.85 54.79±0.35” 54.41±2.27° 65.97±2.61 65.97±2.61 K,CO3 51.11±0.54 48.83±1.98 54.79±2.23 61.54±2.44° 60.14±2.44 CaCl, 47.69±2.13 70.71±2.80° 79.51±6.22° 83.10±3.57 79.38±3.22 MgCl, 45.55±1.85° 56.10±2.27 60.14±2.44 64.46±2.61 74.06±3.00 ZnCl, 44.49±0.54 54.79±1.24° 83.10±0.82 cd 87.08±3.45° 79.38±3.22 FeCH507 48.83±1.98 21.72±0.86 54.79±0.43° 31.44±0.28 22.22±0.53 T23 (ms) Control 357.08±1.21 410.27±2.06° 471.38±1.52 505.26±1.07 505.26±1.07° K2CO3 382.75±2.26 410.27±0.78 439.76±1.69° 439.76±1.73 410.27±0.54 CaCl, 382.75±2.32° 505.26±3.73° 594.43±4.21 666.99±5.24° 666.99±5.30° MgCI, 333.13±0.33° 410.27±1.21 472.13±32.76 471.38±0.79° 567.54±22.48° ZnCl, 357.08±7.13° 410.27±1.21 471.38±2.05° 493.97±19.57° 471.38±8.52° FeC H,O, 439.76±1.26° 126.04±1.23 0.00±0.00° 170.92±18.40° 138.34±5.61 Note: a-c Means with different superscript letters differ (p <.05). P22 significantly increased, and free water gradually transferred to non-mobile water. With the development of pickling time, the nonmobilewater of the K,CO, group decreased gradually and then increased atthe end of pickling,whereas the free water showed a trend of increas-ing first and then decreasing. Both CaCl, andMgCl, groups showed thedecrease in insoluble water and the increase in free water, and the freewater degree was higher. The bound water content of ZnCl, group wassignificantly increased. 3.2Effect of partial substitution of sodium salt forsalting on NaCl content in quail eggs The Na* in the replacement group was determined by atomic absorp-tion spectrometry, and the permeation amount of Na* in a differentpickling solution was compared. After salting, the content of Na* inegg white and yolk increased to different degrees, as shown in Table 3.The increase of NaCl in salted egg white was much faster than that inthe salted egg yolk (Lai et al.,1999), and the difference was maximizedat the end of marinating. After 12 days, the Na*of the K,CO, groupincreased by 13.65% compared with the control group, and its con-tent reached 6.16 g/100 ml, which was salty. The reason may be thatthe aqueous solution of K,CO, was alkaline. When the substitutionratio was too large, OH would affect the permeability of the eggshell(Liu, 2003), thus speeding up the penetration rate of Na*. At the endof curing, the content of Na* in the K,CO, group was 6.68 g/100 ml,which was beyond the acceptable range. There was no significant difference in Na* content in egg white of the MgCl, group comparedwith the control group (p>.05). Among the substitute groups, the Na*permeation rate of the ZnCl, group was the slowest, mainly becausethe white and sticky flocculent substance appeared on the inner sur-face of the quail eggshell membrane in the ZnCl, group after curing,which hindered the infiltration of Na* (Deng et al., 2020). On the 16th day of salting, the salt content of the egg yolk in thecontrol group was 1.23 g/100 ml. On the 12th and 16th days,the Na content of the K,CO, group reached 1.33 g/100 ml and 1.54 g/100 ml,respectively. At the end of salting, compared with the control group,the content of Na* in the yolk of the K,CO, group was the highest,and that of the ZnCl, group was the lowest. On the 16th day of salting,the content of Na* in MgCl, and FeC H,O, groups was similar to thatof the control group, with no significant difference (p>.05). There area lot of reasons for the low content of Na* in the egg yolk, mainly be-cause the egg yolk itself is rich in fat, which prevents the infiltration ofsalt. Secondly, the salt ions from the environment first pass through theeggshell and its membrane into the egg white, and then from the whiteinto the yolk. The yolk membrane outside the yolk will slow down themigration of Na* to the inside of the yolk (Kiosseoglou, 2003). 3.3| Effect of partial substitution of sodium salton the internal state of quail eggs The morphological changes of partial substitution of sodium salt forcuring are shown in Figure 2. The egg yolk contains natural yellow Group 0d 4d 8d 12d 16d P21(%) Control 15.80±0.17° 15.66±0.24 14.38±0.22 14.35±0.29 13.94±0.21° K2CO, 15.77±0.28 15.69±0.09° 14.81±0.25 14.63±0.03 14.53±0.22° CaCl, 19.21±0.37 19.20±0.42° 18.06±0.47 15.86±0.11° 15.22±0.24° MgCl, 15.42±0.37° 17.16±0.25° 15.3±0.38” 13.7±0.41 14.11±0.19 ZnCl, 15.57±0.42° 18.70±0.20° 20.08±0.50° 19.68±0.13° 17.71±0.06° P22(%) FeC H507 16.76±0.16° 6.41±0.81° 11.31±0.08° 8.00±0.18° 5.59±0.26° Control 17.34±0.09° 12.67±0.18 8.97±0.15° 9.27±0.13° 9.16±0.13 cd K2CO3 18.80±0.10° 13.95±0.06° 8.93±0.12° 9.33±0.04 11.00±0.15 CaCl, 13.27±0.32 10.34±0.35° 9.45±0.19° 9.47±0.06° 9.38±0.10 MgCI, 18.39±0.43 11.72±0.16 8.77±0.01° 9.36±0.12 8.95±0.11° ZnCl, 15.98±0.42° 13.37±0.21 10.70±0.15° 11.87±0.25° 11.5±0.12 FeCH507 15.01±0.17° 15.74±0.77° 88.07±0.21 88.96±0.07 89.83±0.05° P23(%) Control 66.86±0.26° 71.67±0.09° 76.66±0.88 cd 76.38±0.18 76.89±0.10° K2CO 65.43±0.23° 70.35±0.06° 76.26±0.17° 76.00±0.08° 74.47±0.17 CaCl, 67.53±0.06° 70.47±0.15 72.49±0.32° 74.46±0.06° 75.27±0.18° MgCI, 66.19±0.12° 71.12±0.17 75.92±0.37° 76.94±0.03° 76.94±0.03° ZnCl, 68.41±0.09” 67.89±0.14 69.22±0.35° 68.44+0.020° 70.74±0.09° FeCH,O, 67.67±0.13 77.83±0.01 0.00±0.00° 3.04±0.22° 4.57±0.24 Note: a-c Means with different superscriptletters differ (p<.05). Salting time Group 0d 4d 8d 12d 16d Egg white (g /100 ml) Control 0.32±0.06^a 2.58±0.04^ 4.05±0.35ABc 5.42±0.24Ad 6.29±0.26ABe K2CO3 0.32±0.06Aa 2.20±0.19Bb 4.66±0.30Ac 6.16±0.23Bd 6.68±0.34^e CaCl, 0.32±0.06Aa 1.58±0.10C 2.12±0.13C 2.83±0.20Cd 4.48±0.11Ce MgCl, 0.32±0.06Aa 2.42±0.12ABb 4.15±0.23ABc 5.20±0.23AEd 5.96±0.22BEe ZnCl, 0.32±0.06Aa 1.68±0.09ECb 1.80±1.13Cbc 2.01±0.19Dcd 2.19±0.11Dd FeCH507 0.32±0.06Aa 2.64±0.26Ab 3.72±0.30Bc 4.88±0.16Ed 5.67±0.20Ee Egg yolk (g /100ml) Control 0.17±0.03Aa 0.45±0.05Ab 0.83±0.06AC 1.07±0.14Ad 1.23±0.13Ad K,CO, 0.17±0.03Aa 0.33±0.10^b 0.86±0.19Ac 1.33±0.12Bd 1.54±0.14Bd CaCl, 0.17±0.03Aa 0.37±0.06Ab 0.57±0.11BCc 0.59±0.10Cc 0.75±0.05Cd MgCI, 0.17±0.03Aa 0.35±0.06Ab 0.75±0.07ABc 0.99±0.08Ad 1.18±0.04Ad ZnCl, 0.17±0.03Aa 0.42±0.05Ab 0.43±0.08C 0.48+0.07Cb 0.51±0.09Db FeCH507 0.17±0.03Aa 0.45±0.08Ab 0.79±0.11^c 0.93±0.13^c 1.12±0.07Ad Note: Samples with different letters (a-e; A-E) indicate that the differences were significant (p<.05). a-e were for comparing rows, and A-E were forcomparing columns. pigments such as lutein and carotene. With the increase in curingtime, the color of the egg yolk slowly changed from light yellow toorange-yellow (Zhao et al., 2019). The gradual shrinkage and solidification of the egg yolk can beobserved in the picture of cooked quail eggs, and the hardened layer advances from near the yolk membrane to the center (Chenget al., 2018). Its essence is the formation of egg yolk gel induced bysalt ions. The salted yolk solidified and oiled in the K,CO, group wasbetter, but the color was dark, which was easy to occur when the pro-portion of K* substitution was too large. The appearance of the MgCl, Journal ofFood Biochemistry (a) (b) 16d (c) 0d 4d 8d 12d FIGURE 2The images of quail eggs pickled with metal salt partially replacing sodium salt at different pickling times. (a) The controlgroup.(b) K,COg partial replacement group.(c) CaCl, partial replacement group. (d) MgClpartial replacement group. (e)ZnCl partialreplacement group. (f) FeC,Hs9z partial replacement group ZnCl, delayed the mature period of the salted yolk and resulted in pooryolk shaping. The CaCl, group differed from other metal ions, whichwas more likely due to the calcium bridge with the greater screening (d) (e) 0d 4d 8d 12d 16 d (f) 0d 4d 8d 12d 16 d FIGURE 2((Continued) effect on the negatively charged carboxyl groups of proteins (Ganasenet al., 2011). In the ZnCl, group, white and sticky flocculent substanceswere found to adsorb on the inner surface of the shell membraneat the later stage of salting, hindering the infiltration of NaCl. ShaoYaoyao et al found that metal ions such as Zn?+ all promoted protein agglutination to varying degrees, resulting in the aggregation of pro-tein and metal ions, which resulted in increased turbidity and affectedthe further penetration of metal ions (Shao et al., 2016). A white and sticky flocculent substance appeared in the saltedegg yolk of the ZnCl, group. For cooked quail eggs, obvious oil production was observed in the samples after curing, mainly be-cause cooking could promote the denaturation of low-density lipo-protein and accelerate the dissociation of fat (Ai et al., 2018). 3.4 Effect of partial substitution of sodium salt ontexture properties of quail eggs Table 4 shows the changes in texture properties of egg whites par-tially substituted for NaCl during the curing process. With the in-crease in curing time and the continuous infiltration of metal ions,the hardness and springiness of egg white decreased. The springiness decreased with the infiltration of metal ions. The higher the elasticvalue, the stronger the anticompression ability of egg white gel andthe better the recovery ability of network structure (Fernandez-Lópezet al., 2006). Metal ions could change the protein-water interaction,which led to the partial expansion and aggregation of proteins, whichmade the gel network structure disordered, loose, and porous, andthe texture properties were significantly reduced. On the 16th day ofsalting, the gel hardness of egg white in MgCl,ZnCl , and FeC Hs07groups was similar to those of the control group. Among them, Cacould form carboxyl bridge bonds, which enhanced the gel properties(Barbut & Foegeding, 1993) and was higher than that of the MgCl, andZnCl, groups. The hardness (176.24 g) of the gel in the K,CO, group Salting time Egg white 0d 4d 8d 12d 16d Hardness (g) Control 559.64±16.62Aa 335.78±48.65Ab 195.66±38.42ABc 138.47±49.62ACc 126.19±46.72Ac K2CO, 559.64±16.62^a 265.83±30.26Bb 238.54±34.31ACbc 206.05±31.21Bbc 176.24±24.48Bc CaCl, 559.64±16.62^a 286.76±34.00ABb 256.20±33.43 185.61±10.29ABCc 177.41±6.82Bc MgC2 559.64±16.62Aa 283.82±33.84ABb 169.69±35.35Bc 147.5±29.11ACc 124.74±2.60Ac ZnCl, 559.64±16.62Aa 192.72±9.13 185.97±11.95ABb 134.78±30.51Cbc 102.06±34.69^C FeCH5O 559.64±16.62Aa 291.99±34.54ABb 239.71±32.75ACbc 188.06±5.95ABc 148.65±16.05ABc Springiness (mm) Control 1.00±0.02Aa 0.98±0.02^a 0.97±0.02^a 0.97±0.03^a 0.95±0.005Aa K2CO3 1.00±0.02Aa 0.98±0.06AD 0.98±0.05Abc 0.97±0.08Ac 0.95±0.04Ad CaCl, 1.00±0.028 0.98±0.01Aab 0.97±0.08Abc 0.95±0.05Acd 0.93±0.03ABd MgCl, 1.00±0.02Aa 0.98±0.02Aab 0.96±0.02Abc 0.95±0.07Abc 0.93±0.03ABc ZnCl, 1.00±0.02^a 0.95±0.01Ab 0.92±0.03^b 0.92±0.01^b 0.86±0.04 FeCH507 1.00±0.02Aa 0.98±0.14Aab 0.97±0.05Aabc 0.96±0.03Abc 0.94±0.07 Note:Samples with different letters (a-d; A-C) indicate that the differences were significant (p<.05). a-d were for comparing rows,and A-C werefor comparing columns. TABLE 5EEffect of partial replacement of sodium salt on the texture of egg yolk Salting time Egg yolk 0d 4d 8d 12d 16d Hardness (g) Control 147.34±20.26^ 210.87±31.47Aab 215.17±27.87Aab 245.64±42.23Ab 267.34±36.45AD K2CO3 147.34±20.26A 168.65±35.16^a 201.93±13.94Aab 238.48±34.83Ab 254.01±43.01Ab CaCl, 147.34±20.26Aa 160.59±34.76Aa 182.70±6.59Aab 203.21±17.88Aab 230.94±42.36A MgCI, 147.34±20.26Aa 187.43±21.43Aab 210.87±66.95Abc 225.00±14.84Abc 263.35±24.42AC ZnCl, 147.34+20.26 161.58±36.18Aab 179.06±14.26Aab 203.11±31.29Abc 228.21±34.03AC FeCH507 147.34±20.26Aa 186.78±15.82Aab 210.42±25.13Abc 225.20±13.62Abc 277.09±29.91^C Springiness Control 0.83±0.07Aa 0.79±0.05Aab 0.75±0.04Aab 0.72±0.08Aab 0.66±0.06Ab (mm) K2CO3 0.83±0.07Aa 0.80±0.04Aa 0.77±0.11^a 0.76±0.02ABa 0.74±0.02ACa CaCl, 0.83±0.07Aa 0.80±0.04^a 0.86±0.07ABa 0.84±0.04BCa 0.85±0.07Ba MgC, 0.83±0.07Aa 0.82±0.02Aa 0.78±0.05ABa 0.78±0.02ABa 0.73±0.05Aa ZnCl, 0.83±0.07Aa 0.84±0.07Aa 0.91±0.11Ba 0.87±0.06Ca 0.83±0.03BCa FeC,HsO 0.83±0.07Aa 0.79±0.06Aab 0.79±0.02ABab 0.78±0.02ABab 0.70±0.07Ab (a) 18 -—Control -→一K,CO -CaCl, 16 一MgCl→-ZnClz-FeC,H,0, 14· 12· 10 8 6· 4 2 0 0 4 8 12 16 Time (day) Time (day) Time (day) FIGURE3Taste signal response of the salted quail eggs partially replaced by sodium salt. (a) Taste changes in egg white. (b) Tastechanges in egg yolk. Taste signal response of quail eggs in differant salted groups: Control group (一一);K,CO, group ( ); CaCl,group ( ");MgCl,group(一 );ZnCl, group (一 ");FeCHs0group( was higher than the control group (126.19 g), indicating that Na* hada greater effect on the gel. As for the springiness change, on the 16thday of salting, the springiness of the ZnCl, group was lower, whereasthespringiness of other groups was not significantly different(p>.05). The degree of dehydration of the yolk had a certain relationshipwith the hardness of the yolk. The salt that diffuses into the yolkwould cause the accumulation of protein (Lilan et al., 2017), andthe significant dehydration of the yolk caused the existence of the (b) 8.0 0.0 一一Control· -K,CO--—CaCl, --Control 一K,COs --CaCl, 7.5· -MgCl,ZnCl,,-FeCH,0 -0.5- 一MgClZnCl,FeCH,07 7.0- -1.0- 6.5 -1.5 6.0 -2.0 5.5 5.0- -2.5· -3.0- 0 4 8 12 16 FIGURE 33(((Continued) yolk ball to be more compact (Kaewmanee et al., 2013). As shown inTable 5, each group could promote egg yolk hardening to varying de-grees. Compared with the control group, the yolk hardness of all ex-perimental groups had no significant change (p> .05). In the middleand late stages of curing, it was found that the ZnCl,group had whiteand viscous flocculent substances adsorbed on the inner surface of the shell membrane, which prevented the penetration of NaCI (Denget al., 2020). The substitution of ZnCl, and CaCl,hindered the pen-etration of Na, which was reflected in a lower egg yolk hardnessvalue. By comparing the changes of springiness, it was found thatthe springiness of CaCl, and ZnCl, groups remained high at the endof salting, whereas the springiness of other groups was similar. 3.5The effect of partial substitution of sodium salton the taste signal of quail eggs The taste signal response of egg white and the changes of taste indifferent substitution groups are shown in Figure 3a. Studies havefound that the saltiness of egg whites continues to increase withthe marinating time. Compared with the control group, 0.25 mol/Lof K,CO, partially replaced the egg white with the highest saltiness.The reason might be that the K,CO,aqueous solution was alkaline.When the ratio was too large, OH would affect the permeability ofthe eggshell (Liu, 2003) to accelerate the penetration rate of NaCl,resulting in a higher sense of saltiness. The saltiness of the egg whiteof the MgCl, group was closer to that of the control group. Zinc salthinders the penetration of NaCl, resulting in a lower salty taste inthe ZnCl,group. With the prolonging of salting time, the bitterness signal output ofegg white decreased gradually. The bitterness of the K,CO, group wasslightly higher than that of the control group, but lower than that ofthe egg white samples salted for 0 days. The astringency of the K,CO,group was relativelyhigh, and the value on the 16th day was 2.93±0.02.K,CO,can promote umami taste. The umami taste and umami after-taste of theK,CO, group were 21.67% and 49.96% higher than those ofthe control group. FeC H,O, hurts the umami taste. The umami tasteof the FeC H,O, group is 21.92% lower than that of the control group.CaCl, and MgCl, had different effects on the umami taste aftertaste,and the reduction ratios were 53.94% and 35.02%, respectively. The taste signal response of egg yolk and the changes of tastesin different pickling groups are shown in Figure 3b. According to theresults, the saltiness of the egg yolk continued to increase during thecuring process. The saltiness of egg yolk was much lower than theegg white, and the fat content in the egg yolk was the main influenc-ing factor. The order of saltiness of the egg yolk in each curing groupwas very similar to that of the egg white. On the 16th day of salting,the bitterness of egg yolk samples in K,CO,CaCl,and ZnCl, groupswas higher than that in the control group, but lower than that of theegg yolk samples salted for 0 days. The egg yolk umami aftertaste ofFeCHs0, and ZnCl, groups was similar and lower than that of thecontrol group. According to the research,both ZnCl, and FeCHs0zhad undesirable flavors on the salted quail eggs and were not suit-able as low-sodium substitutes. 4 (CONCLUSION The experimental results can exactly describe the changes in salt per-meability in quail eggs and the changes in the physical and chemicalproperties and microstructure of the egg white and yolk after partialsubstitution of NaCl with different metal salts. Studies have foundthat ZnCl, and FeC H.O, in various alternative salts have many ad-verse effects on quail eggs, and they are not suitable for low-sodiumpickling preparations. For K,CO, CaCl, and MgCl,two or more ofthem can be used. Salt is mixed and replaced to form a compoundpickling preparation. This research provides a theoretical basis for the process optimization of low-sodium-salted quail eggs, which will helpthe subsequent development of low-salt-salted quail eggs. ACKNOWLEDGMENTS We would like to acknowledge the financial support provided byNational Natural Science Foundation of China (32001725). CONFLICTOFINTEREST The author declares that there is no conflict of interest that could beperceived as prejudicing the impartiality of the research reported. AUTHOR CONTRIBUTIONS Zhijie Bao: Formal analysis; Investigation; Software. Yang Tian: Datacuration; Writing-original draft. Jie Gao: Data curation; Formal anal-ysis; Software. Da Kang: Data curation; Formal analysis. Songyi Lin:Conceptualization; Project administration. DATAAVAILABILITY STATEMENT The data that support the findings of this study are available fromthe corresponding author upon reasonable request. ORCIDSongyi Lin https://orcid.org/0000-0002-9204-948X REFERENCES ( Abadi,S., Huda, M., Azmi J a smi, K., Shakib Mohd.Noor, S.,Saf a r, J., Kilani Mohamed, A., H assan W an E m bong, W., Marzuki Mohamad, A.,Hehsan, A.,Basiron,B., Suhaila Ihwani, S.,Maseleno,A.,Muslihudin,M., S atria , F., Irawan, D., & Hartati, S. (2018). Determination o f the b est quail e ggs u s ing s i mple additive w e ighting. International Journal o f E ngineering and T e chnology, 7, 22 5 -230. https://doi. org/10.14419/ijet.v7i2.27.11967 ) ( Abdel N o ur, N., Ngadi, M., Pr a sher, S., & Karimi, Y. (2 0 11). Pr ed iction of egg f reshness and albumen quality using visible/near infrared spec-troscopy. F ood & Bioprocess Te c hnology, 4, 73 1 -736. https://doi.org/10.1007/s11947-009-0265-0 ) ( Ai, M.-M., Guo, S.-G., Zhou, Q .,Wu, W.-L., & Jiang, A.-M. (2018). The in- vestigation of the changes in physicochemical, texture and rheolog- i cal characteristics of salted duck egg yolk during salting . LWT-Food Science a nd T echnology, 88 , 1 1 9-125. https://doi.org/10.1016/j. lwt.2017.10.013 ) ( Alino, M., Grau,R., F uentes, A., & B arat, J. M .(2010). I n fluence of l o w-sodium mixtures of salts on the post-salting stage of dry-cured hamprocess. J ournal of Food E ngineering, 99(2), 198-205.https://doi.org/10.1016/j.jfoodeng.2010.02.020 ) ( Bao, Z., Kang, D., Li, C., Zhang, F. , & Lin,S. (2020). Effect of salting on thewater migration, physicochemical and textural characteristics, andmicrostructure of quail eggs.LWT-Food Science and Technology, 132,109847.https://doi.org/10.1016/j.lwt.2020.109847 ) ( Barbut, S., & Foegeding, E. A. (1993). Ca-induced g e lation o f p re- h eated whey protein is o late. J o urnal o f Food Science,58 ( 4), 867- 871. https://doi.org/10.1111/j.1365-2621.1993.tb09379.x ) ( B aumgartner,J. (1994). J a panese quail production, breeding and genet- i cs. World’s Poultry Science Journal,50,227-235. ) ( Cheng, S., Zhang, T., Wang,X., S o ng, Y., Wang, H.,Wang, H., Yang, P., & T an, M.( 2 018).Influence of salting p r ocesses on water and lip i d dy- namics, physicochemical and microstructure of duck egg.LWT-foodScience a nd T e chnology, 9 5 , 1 4 3-149. https://doi.org/10.1016/j. Iwt.2018.04.074 ) ( D eng, C . , Shao, Y . , X u, M ., Y ao, Y., Wu, N. A . , Hu, H . , Z h ao, Y . , & T u , Y. (2020). Effects of metal ions on the physico-chemical, mi c rostruc- tural and digestion characteristics of alkali-induced egg white gel.Food H ydrocolloids, 107,105956. https://doi.org/10.1016/j.foodhyd.2020.105956 ) ( F e rnandez-lopez,J.,Martinez,A., Fernandez-gines,J. M.,Sayas-barbera,E., Sendra, E. , & P erez-alvarez, J. A. (2006). Gelling and color properties of ostrich (Struthio camelus) egg white. Journal of Food Quality, 2 9(2), 171-183. https://doi.org/10.1111/j.1745-4557.2006.00065.x ) ( Ganasen, P. , & Benjakul, S. (2011). Ef f ect of three cations on the stabilityand microstructure of protein aggregate from duck egg white underalkaline condition. F o od Science & Technology International, 1 7 (4),342-349.https://doi.org/10.1177/1082013210382482 ) ( Huang, J . , Lin, J ., Zheng, H., Liu, L., Zheng, Y . Y . , & C h en, Y. S. (2 0 12).Effect of salting processing on composition and quality of duck yolkunder different method. Food Science & Technology, 3 7(4), 60-64.https://doi.org/10.13684/j.cnki.spkj.2012.04.045 ) ( leggli, C., Bohrer, D., do Nascimento,P. C. , de Carvalho, L. M ., & Gar c ia,S. C. (2010). Determination of sodium, potassium, calcium, mag-nesium, zinc, and iron in emulsified eg g samples by flame atomicabsorption spectrometry. Ta l anta, 80(3), 1282-1286. htt p s://doi.org/10.1016/j.talanta.2009.09.024 ) ( K aewmanee, T., Benjakul, S., & Visessanguan, W . (2010).Eff e ct of saltingprocesses on chemical c o mposition, textural properties and mi c ro-str u cture of duck egg. Journal of the Science of Food and Agriculture,89(4),625-633.https://doi.org/10.1002/jsfa.3492 ) ( K aewmanee, T., Be n jakul, S. , Visessanguan, W., & Gamonpilas, C. (2013). Effect of sodium chloride and osmotic dehydration on viscoelastic properties and thermal-induced transitions of duck egg y o lk. Food and Bioprocess Technology, 6(2), 3 67-376. https://doi.org/10.1007/ s11947-011-0667-7 ) ( Kiosseoglou, V. (2003). E g g yolk protein ge l s an d emulsions. Cur r ent Opinion i n Colloid & Interface Science, 8(4-5), 3 6 5-370. htt p s://doi.org/10.1016/S1359-0294(03)00094-3 ) ( Lai, K. M., Chi, S. P., & Ko,W. C.(1999). Changes in yolk states of duck egg during long-term b r ining. Journal o f Agricultural & Food Chemistry, 47(2),733-736.https://doi.org/10.1021/jf980486r ) ( Lilan, X., Yan, Z., Mingsheng, X., Yao, Y. , Xuliang , N. , Huaying, D. , Yong- Gang, T.,& Arda,Y. (2017). Effects of salting treatment on the phy s - icochemical properties, textural properties, and microstructures ofduck e ggs. P LoS O ne, 12(8), e0182912. https://doi.org/10.1371/ journal.pone.0182912 ) ( Ling, W. U ., S un, J ., Li-Qiang, L. E., & Mei-Hu, M . A. (2011). Ef f ect ofpotassium chloride as partial sodium ch l oride su b stitution in sa l tedduck eggs. F ood Science, 1 3, 14-19. ) ( Liu, L . Z. (2003).Reducing processing time of salted egg and improving its quality. Food Science & Technology, 1,36-37. ) ( L iu, L., Yang, R., Luo, X., Dong, K., Huang, X., S ong, H., Gao, H . , L i , S ., & H uang, Q . (2 0 20). Omics analysis of holoproteins and mo d ifiedproteins of quail egg. Food Chemistry, 326, 1 26983. h t tps://doi.org/10.1016/j.foodchem.2020.126983 ) ( Ma, M. H . , B i , Y. F ., Zhang, M. J . , & He, Y . (2015). Analysis o f e g g fl a - vor change during storage p e riod b y electronic s e nsory methods. Modern Food Science & Technology, 31(293-300),355. ) ( Onyekwelu, K. , I b egbu, M. D. , Eb e le, E. F., E b ele, I . J., & E jezie, C. S.(2018). Effects o f quail coturnix japonica egg diet on b o th the bloodsugar a nd the lipid profile of alloxan induced d iabetic albino rats.Biomedical Research,29(19), 3599-3604. ) ( Shao, J. H., Deng, Y. M . , S o ng, L. , B a tur, A . , Jia, N., & L i u, D. Y. (2016). Investigation the effects of protei n hydratio n states o n th e mo-bilit y water and fat in meat batter s by LF-NMR t echnique. L WT-Food S cience and Technology, 6 6, 1 -6. h ttps://doi.org/10.1016/j. lwt.2015.10.008 ) ( Singh, R. P., Panda, B., & Yadava, V. K. (2007). Effect of packaging andstorage o n the keeping q u ality o f pickled q u ail eggs. InternationalJournal of Food Science & Technology, 24(3), 2 83-290. https://doi.org/10.1111/j.1365-2621.1989.tb00646.x ) ( T olik, D. , Poawska, E ., Charuta, A.,Nowaczewski, S., & Cooper, R . (2014). Characteristics of egg p arts, chemical c omposition and nu t ritivevalue o f Japanese q u ail eggs-A rev i ew. Fol i a Biologica, 62(4), 287-292.https://doi.org/10.3409/fb62_4.287 ) ( T unsaringkarn, T., Tungjaroenchai, W., & S i riwong, W. (2 0 12). Nu t rient benefits o f quail (Coturnix coturnix japonica) eggs. Annals Food Science and Technology, 13(2), 122-131. ) ( Wang, G . , Z h ang, N. , We i , Y.-F., Jin, Y . -M., Z h ang, S. - Y., C he ng, X.,Ma, Z.- L .,Zhao, S . - Z ., C hen,Y.-P . , Chuai,M., Hocher, B., & Ya ng,X. (2015). The impact of high-salt exposure on cardiovasculardevelopment in the early c h ick e m bryo. Journal of ExperimentalBiology, 218, 3468-3477.https://doi.org/10.1242/jeb.129486 ) ( Yang, H., Zhang, W. , Li, T.,Zheng, H., Khan,M. A., Xu,X.,Sun, J .,& Zhou, G.(2016). Effect of protein structure o n w ater and fat distributionduring meat gelling. Food C hemistry, 204, 2 39-245. https://doi.org/10.1016/j.foodchem.2016.01.053 ) ( Yuan, L., Zhang,J., Wu, J., Gao, Z., Xie, X., Wang, Z., & Wang, X. ( 20 1 8). The e ffect o n quality of pickled salted duck e ggs using the novelmethod of pulsed pressure osmotic dehydration. Journal of Food Processing and P reservation, 42(4), 1-9. https://doi.org/10.1111/ jfpp.13581 ) ( Zhao, Y ., Xu, M., Yao, Y., Wu, N., Du, H., & T u, Y.(2019). Changes in physico-chemical properties, microstructure, protein s tructuresand intermolecular force o f egg yolk, plasma and granule gels duringsalting. Food Chemistry, 2 75,6 0 0-609. htt p s://doi.org/10.1016/j. foodchem.2018.09.078 ) 我们模拟了传统的黑灰咸蛋和盐泥涂层中所含的金属元素。采用K2CO3、CaCl2、MgCl2、ZnCl2和FeC6H5O7金属盐部分取代氯化钠(NaCl),研究了这些金属盐对鹌鹑蛋酸洗过程中理化性质、质地和微观结构的影响。筛选出几种合适的低钠替代品,为低钠盐鹌鹑蛋生产工艺优化提供理论依据。检测仪器:电子舌 TS-5000Z 日本INSENT公司文献信息:
确定








还剩11页未读,是否继续阅读?
产品配置单
北京盈盛恒泰科技有限责任公司为您提供《鹌鹑蛋中理化性质、质地和微观结构检测方案(感官智能分析)》,该方案主要用于蛋制品中理化分析检测,参考标准--,《鹌鹑蛋中理化性质、质地和微观结构检测方案(感官智能分析)》用到的仪器有电子舌、日本INSENT味觉分析系统(电子舌)
推荐专场
感官智能分析系统(电子鼻/电子舌)
相关方案
更多
该厂商其他方案
更多
















