
使用 LC/MS/MS 直接进样的方式,测定了饮用水中 9 种卤乙酸(HAAs)、溴酸盐和氯酸盐,该方法快速、简便、灵敏。
方案详情

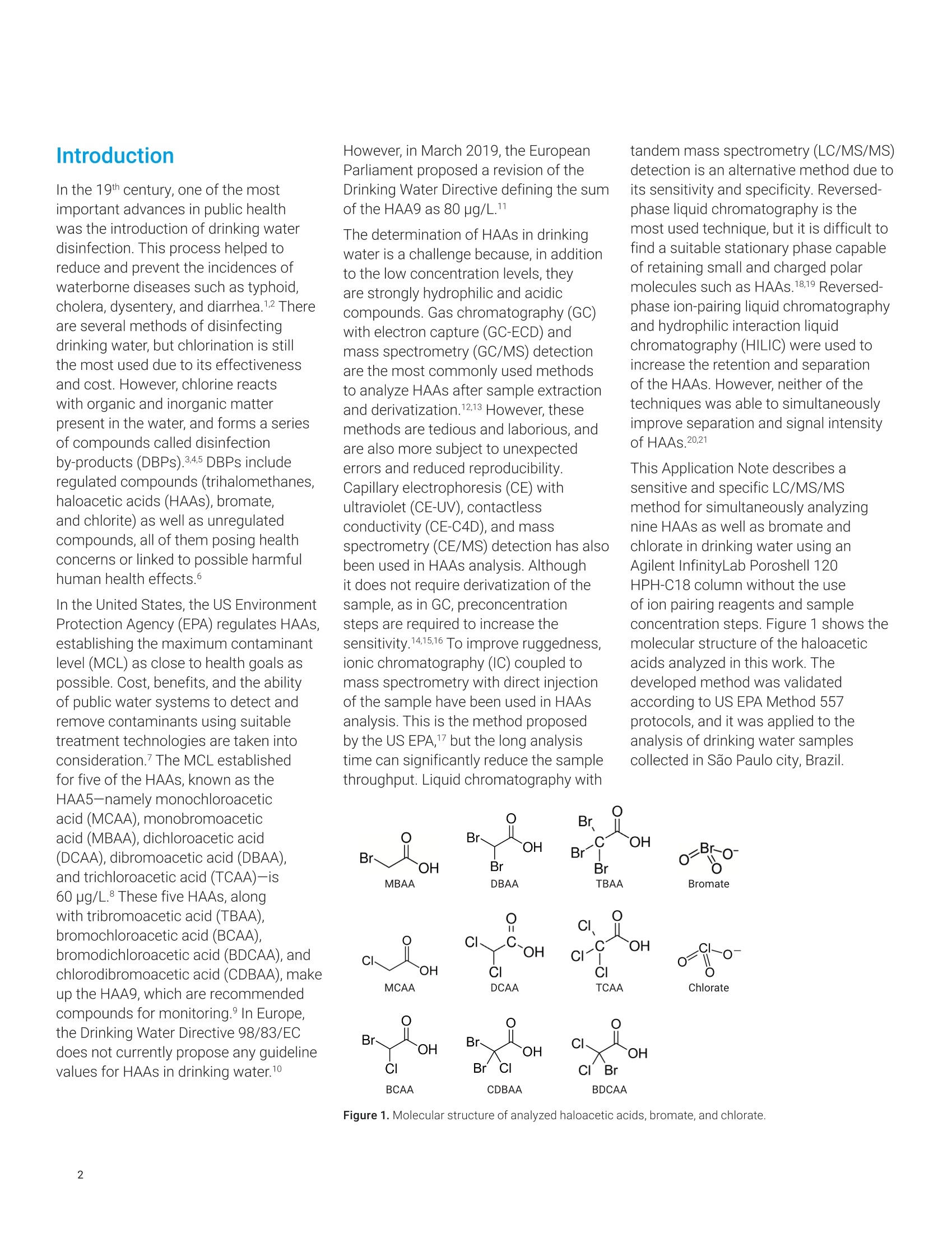
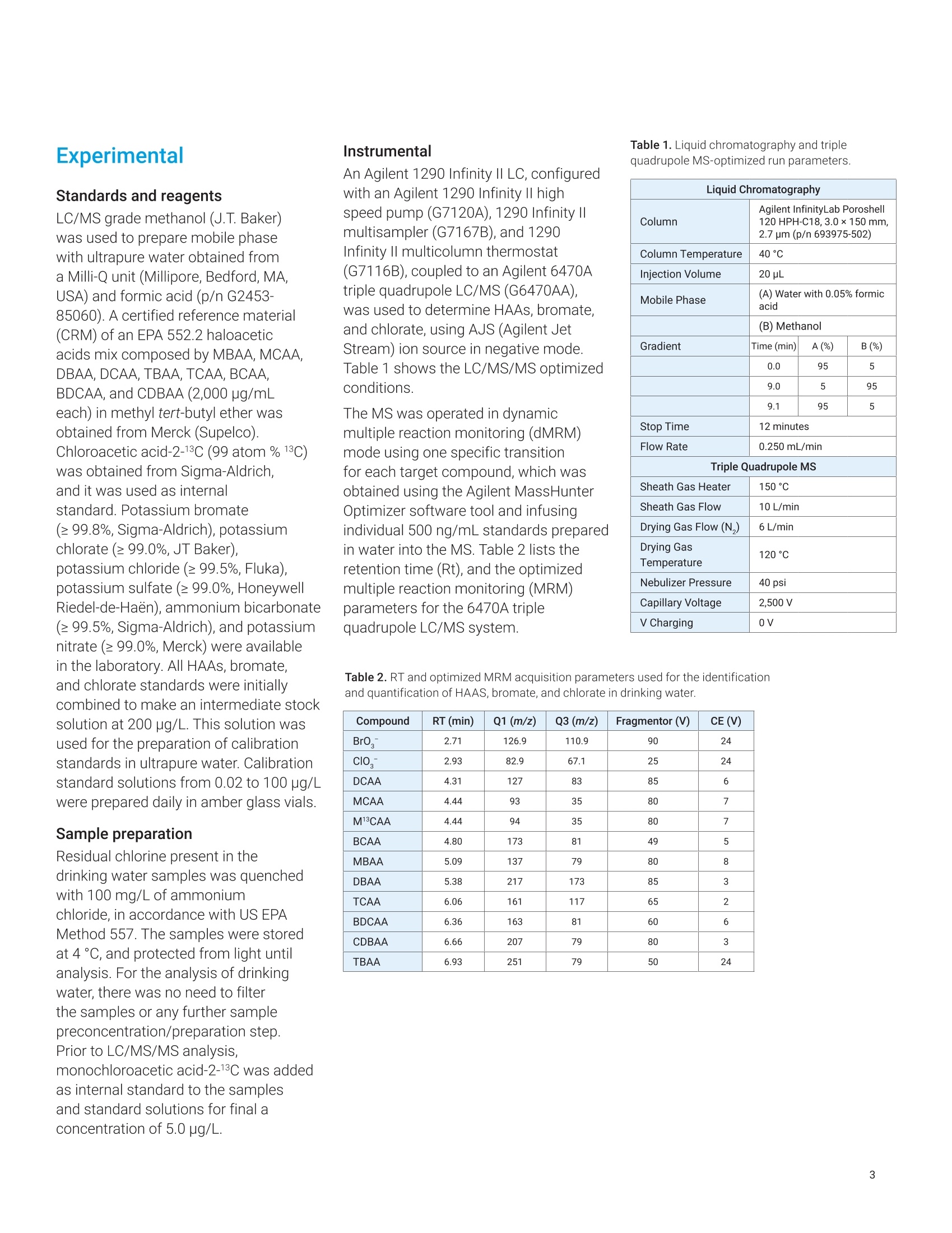
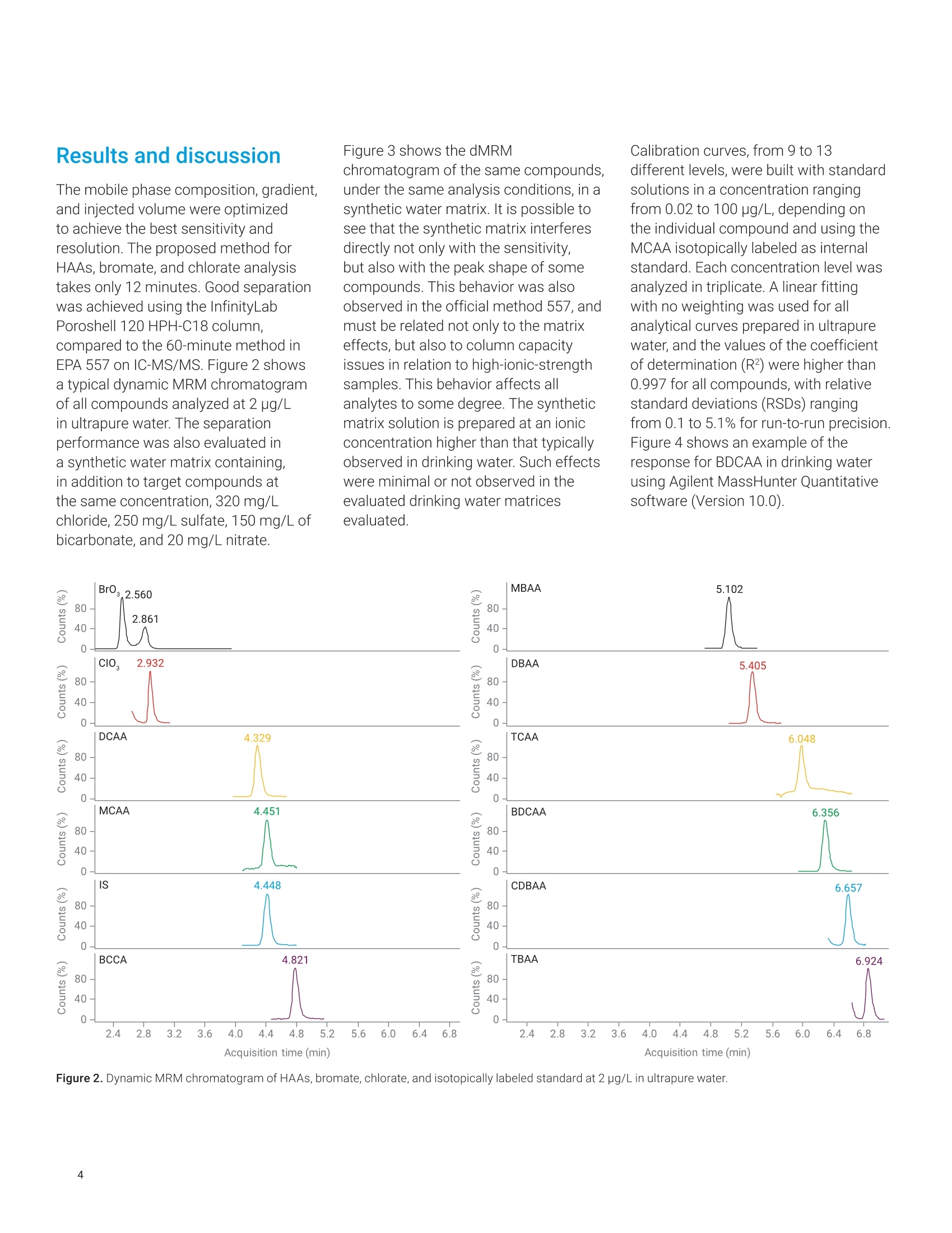
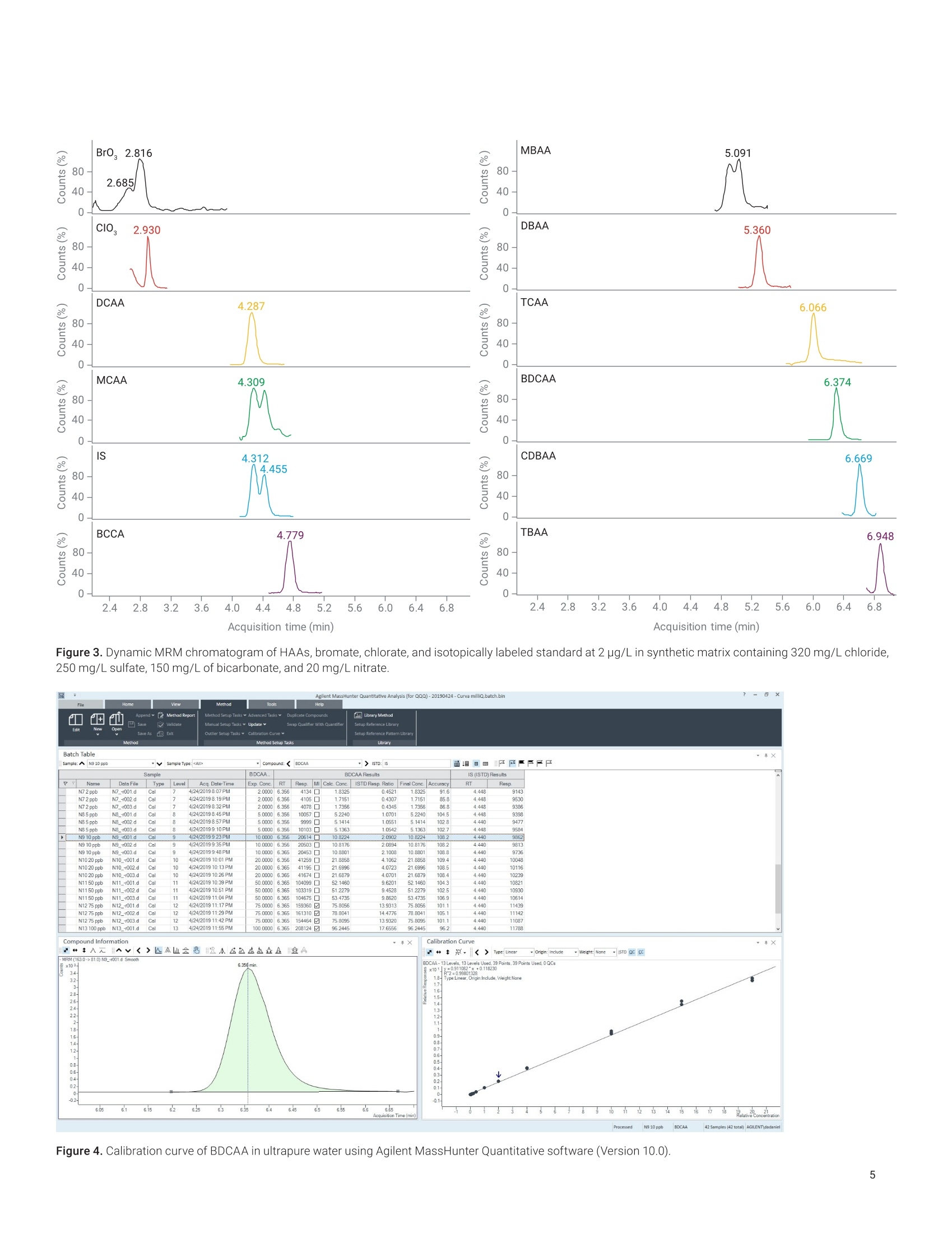
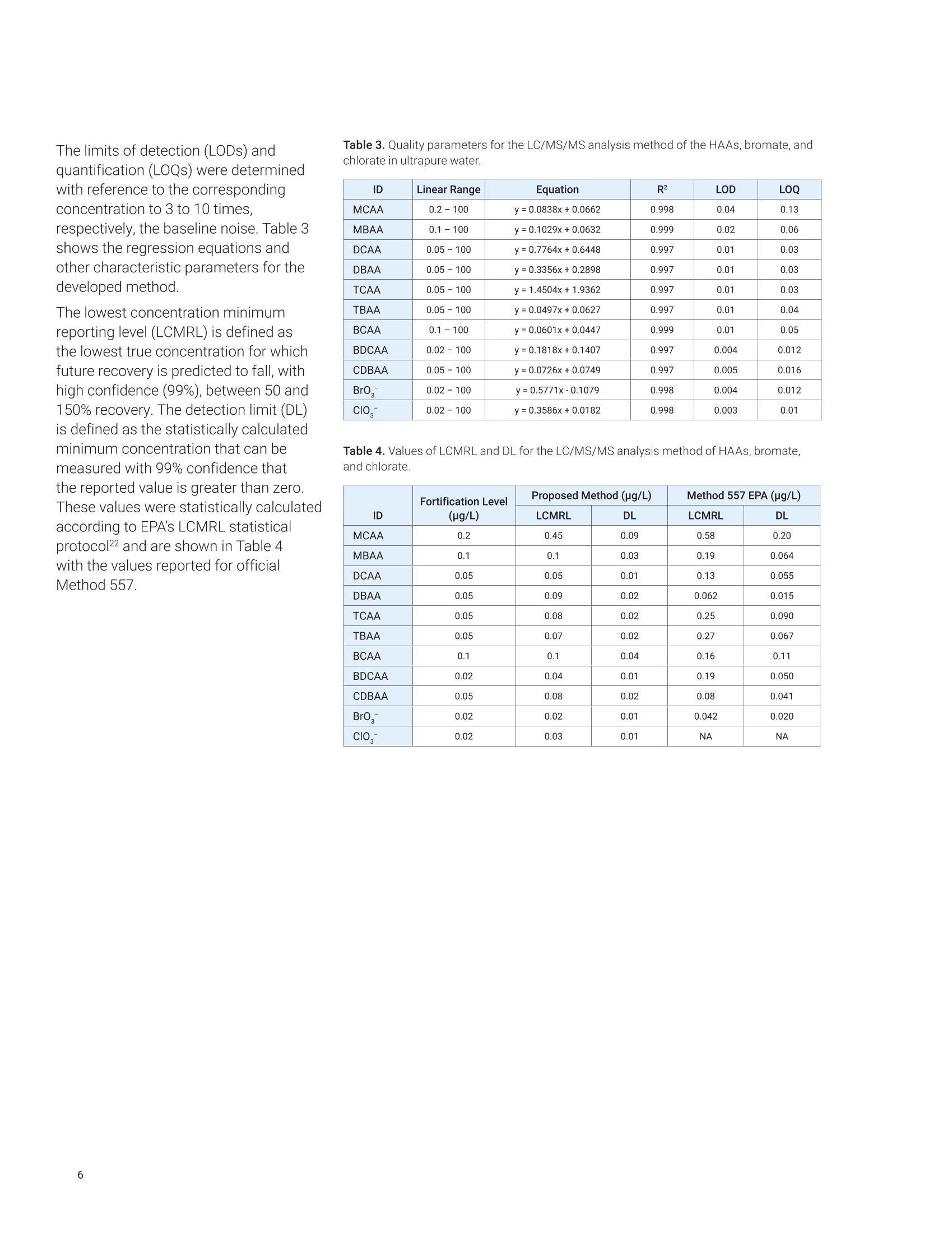
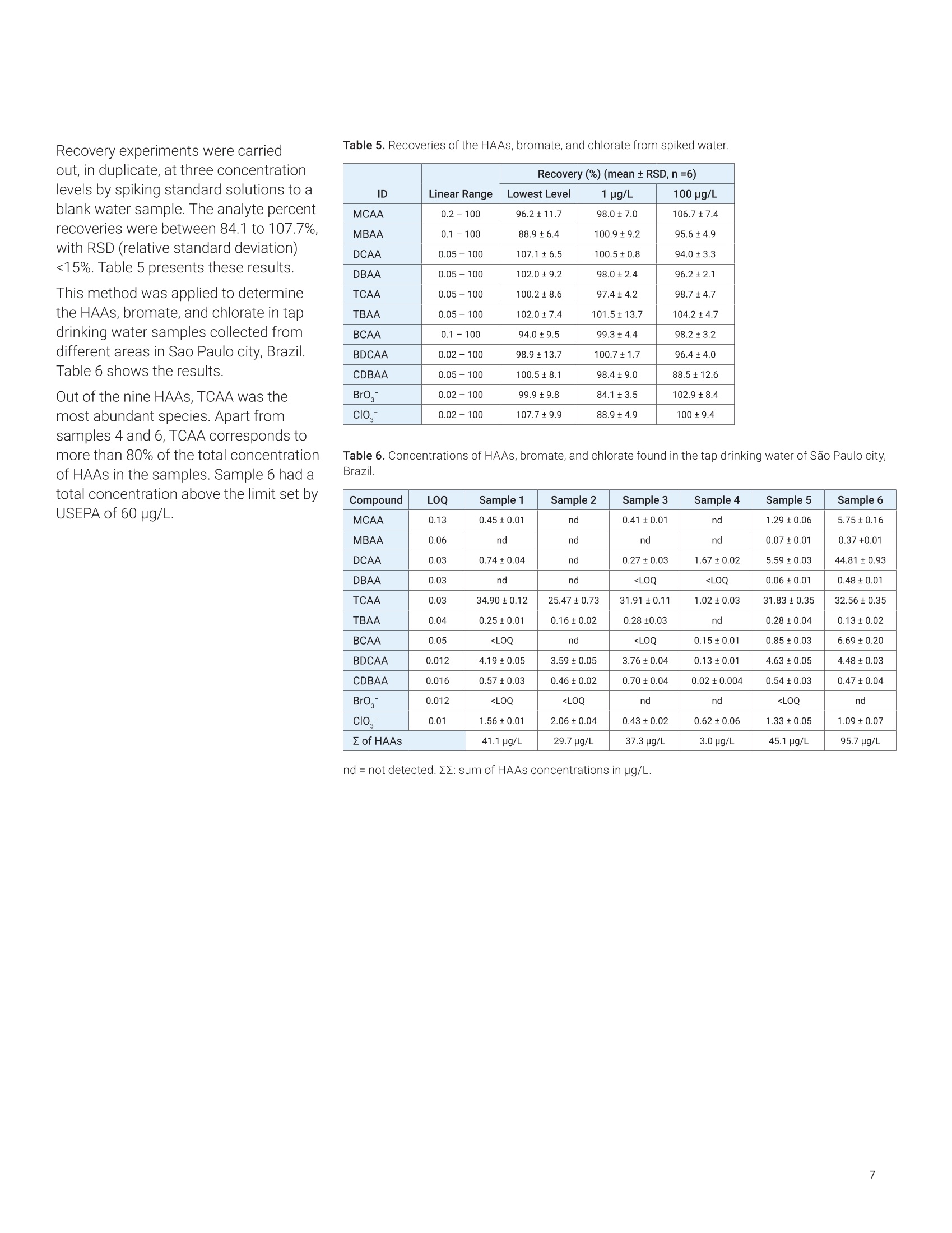
Claudimir Lucio do LagoDepartment of FundamentalChemistry,Institute of Chemistry, University of Sao Paulo, Brazil Daniela Daniel Agilent Technologies, Inc. Determination of Haloacetic Acids inDrinking Water by LC/MS/MS A fast, simple, and sensitive direct injection LC/MS/MS method has been developedfor the determination of nine haloacetic acids (HAAs), bromate, and chlorate indrinking water. The workflow uses an Agilent 1290 Infinity II LC coupled to anAgilent 6470A triple quadrupole LC/MS. Water samples were directly injectedwithout filtration and nine HAAs, bromate, and chlorate were separated in lessthan 8.0 minutes using an Agilent InfinityLab Poroshell 120 HPH-C18 column.The developed method is approximately five times faster than the current US EPAMethod 557, achieving limits of detection (LODs) from 0.003 to 0.04 pg/L. Theselimits are lower than required in the EU, US, and many other parts of the world.Linear calibration curves with determination coefficients (R2) greater than 0.997for all analytes in a range of 0.02 to 100 pg/L were achieved. The mean recoveriesof target compounds in spiked drinking water samples were from 85.2 to 107.7%,and no apparent signal suppression was observed in drinking water. Satisfactorymethod performance was also demonstrated in a synthetic matrix containing highionic concentration.Finally, the method was applied to determine HAAs, bromate,and chlorate in tap (drinking) water samples collected from different regions ofSao Paulo city, Brazil. In the 19th century,one of the mostimportant advances in public healthwas the introduction of drinking waterdisinfection. This process helped toreduce and prevent the incidences ofwaterborne diseases such as typhoid,cholera, dysentery, and diarrhea.12 Thereare several methods of disinfectingdrinking water, but chlorination is stillthe most used due to its effectivenessand cost. However, chlorine reactswith organic and inorganic matterpresent in the water, and forms a seriesof compounds called disinfectionby-products (DBPs).34.5 DBPs includeregulated compounds (trihalomethanes,haloacetic acids (HAAs), bromate,and chlorite) as well as unregulatedcompounds, all of them posing healthconcerns or linked to possible harmfulhuman health effects.6 In the United States, the US EnvironmentProtection Agency (EPA) regulates HAAs,establishing the maximum contaminantlevel (MCL) as close to health goals aspossible. Cost, benefits, and the abilityof public water systems to detect andremove contaminants using suitabletreatment technologies are taken intoconsideration.7 The MCL establishedfor five of the HAAs, known as theHAA5-namely monochloroaceticacid (MCAA), monobromoaceticacid (MBAA), dichloroacetic acid(DCAA), dibromoacetic acid (DBAA),and trichloroacetic acid (TCAA)-is60 pg/L. These five HAAs, alongwith tribromoacetic acid (TBAA),bromochloroacetic acid (BCAA),bromodichloroacetic acid (BDCAA), andchlorodibromoacetic acid (CDBAA), makeup the HAA9, which are recommendedcompounds for monitoring. In Europe,the Drinking Water Directive 98/83/ECdoes not currently propose any guidelinevalues for HAAs in drinking water.10 However, in March 2019, the EuropeanParliament proposed a revision of theDrinking Water Directive defining the sumof the HAA9 as 80 pg/L. The determination of HAAs in drinkingwater is a challenge because, in additionto the low concentration levels, theyare strongly hydrophilic and acidiccompounds. Gas chromatography (GC)with electron capture (GC-ECD) andmass spectrometry (GC/MS) detectionare the most commonly used methodsto analyze HAAs after sample extractionand derivatization.12,13 However, thesemethods are tedious and laborious, andare also more subject to unexpectederrors and reduced reproducibility.Capillary electrophoresis (CE) withultraviolet (CE-UV), contactlessconductivity (CE-C4D), and massspectrometry (CE/MS) detection has alsobeen used in HAAs analysis. Althoughit does not require derivatization of thesample, as in GC, preconcentrationsteps are required to increase thesensitivity.14,15,16 To improve ruggedness,ionic chromatography (IC) coupled tomass spectrometry with direct injectionof the sample have been used in HAAsanalysis. This is the method proposedby the US EPA,1 but the long analysistime can significantly reduce the samplethroughput. Liquid chromatography with tandem mass spectrometry (LC/MS/MS)detection is an alternative method due toits sensitivity and specificity. Reversed-phase liquid chromatography is themost used technique, but it is difficult tofind a suitable stationary phase capableof retaining small and charged polarmolecules such as HAAs.1819 Reversed-phase ion-pairing liquid chromatographyand hydrophilic interaction liquidchromatography (HILIC) were used toincrease the retention and separationof the HAAs. However, neither of thetechniques was able to simultaneouslyimprove separation and signal intensityof HAAs.20,21 This Application Note describes asensitive and specific LC/MS/MSmethod for simultaneously analyzingnine HAAs as well as bromate andchlorate in drinking water using anAgilent InfinityLab Poroshell 120HPH-C18 column without the useof ion pairing reagents and sampleconcentration steps. Figure 1 shows themolecular structure of the haloaceticacids analyzed in this work. Thedeveloped method was validatedaccording to US EPA Method 557protocols, and it was applied to theanalysis of drinking water samplescollected in Sao Paulo city, Brazil. Experimental Standards and reagents LC/MS grade methanol (J.T. Baker)was used to prepare mobile phasewith ultrapure water obtained froma Milli-Q unit (Millipore, Bedford, MA,USA) and formic acid (p/n G2453-85060). A certified reference material(CRM) of an EPA 552.2 haloaceticacids mix composed by MBAA, MCAA,DBAA, DCAA, TBAA, TCAA, BCAA,BDCAA, and CDBAA (2,000 pg/mLeach) in methyl tert-butyl ether wasobtained from Merck (Supelco).Chloroacetic acid-2-13C (99 atom %13C)was obtained from Sigma-Aldrich,and it was used as internalstandard. Potassium bromate(≥99.8%, Sigma-Aldrich), potassiumchlorate (≥99.0%, JT Baker),potassium chloride (≥99.5%,Fluka),potassium sulfate (≥99.0%, HoneywellRiedel-de-Haen), ammonium bicarbonate(≥99.5%, Sigma-Aldrich), and potassiumnitrate (≥99.0%, Merck) were availablein the laboratory. All HAAs, bromate,and chlorate standards were initiallycombined to make an intermediate stocksolution at 200 pg/L. This solution wasused for the preparation of calibrationstandards in ultrapure water. Calibrationstandard solutions from 0.02 to 100 pg/Lwere prepared daily in amber glass vials. Sample preparation Residual chlorine present in thedrinking water samples was quenchedwith 100 mg/L of ammoniumchloride, in accordance with US EPAMethod 557. The samples were storedat 4°C, and protected from light untilanalysis. For the analysis of drinkingwater, there was no need to filterthe samples or any further samplepreconcentration/preparation step.Prior to LC/MS/MS analysis,monochloroacetic acid-2-13C was addedas internal standard to the samplesand standard solutions for final aconcentration of 5.0 pg/L. Instrumental An Agilent 1290 Infinity II LC, configuredwith an Agilent 1290 Infinity Il highspeed pump (G7120A), 1290 Infinity IImultisampler (G7167B), and 1290Infinity Il multicolumn thermostat(G7116B), coupled to an Agilent 6470Atriple quadrupole LC/MS (G6470AA),was used to determine HAAs, bromate,and chlorate, using AJS (Agilent JetStream) ion source in negative mode.Table 1 shows the LC/MS/MS optimizedconditions. The MS was operated in dynamicmultiple reaction monitoring (dMRM)mode using one specific transitionfor each target compound, which wasobtained using the Agilent MassHunterOptimizer software tool and infusingindividual 500 ng/mL standards preparedin water into the MS. Table 2 lists theretention time (Rt), and the optimizedmultiple reaction monitoring (MRM)parameters for the 6470A triplequadrupole LC/MS system. Table 1. Liquid chromatography and triplequadrupole MS-optimized run parameters. Liquid Chromatography Column Agilent InfinityLab Poroshell120 HPH-C18,3.0×150 mm,2.7 pm (p/n 693975-502) Column Temperature 40°C Injection Volume 20 pL Mobile Phase (A) Water with 0.05% formicacid (B) Methanol Gradient Time (min) A(%) B (%) 0.0 95 5 9.0 5 95 9.1 95 5 Stop Time 12 minutes Flow Rate 0.250 mL/min Triple Quadrupole MS Sheath Gas Heater 150°C Sheath Gas Flow 10 L/min Drying Gas Flow (N,) 6 L/min Drying Gas Temperature 120°C Nebulizer Pressure 40 psi Capillary Voltage 2,500V VCharging 0V Table 2. RT and optimized MRM acquisition parameters used for the identificationand quantification of HAAS, bromate, and chlorate in drinking water. Compound RT (min) Q1 (m/z) Q3 (m/z) Fragmentor (V) CE(V) Br0, 2.71 126.9 110.9 90 24 Clo 2.93 82.9 67.1 25 24 DCAA 4.31 127 83 85 6 MCAA 4.44 93 35 80 7 M13CAA 4.44 94 35 80 7 BCAA 4.80 173 81 49 5 MBAA 5.09 137 79 80 8 DBAA 5.38 217 173 85 3 TCAA 6.06 161 117 65 2 BDCAA 6.36 163 81 60 6 CDBAA 6.66 207 79 80 3 TBAA 6.93 251 79 50 24 Results and discussion The mobile phase composition, gradient,and injected volume were optimizedto achieve the best sensitivity andresolution. The proposed method forHAAs, bromate, and chlorate analysistakes only 12 minutes. Good separationwas achieved using the InfinityLabPoroshell 120 HPH-C18 column.compared to the 60-minute method inEPA 557 on IC-MS/MS. Figure 2 showsa typical dynamic MRM chromatogramof all compounds analyzed at 2 pg/Lin ultrapure water. The separationperformance was also evaluated ina synthetic water matrix containing,in addition to target compounds atthe same concentration, 320 mg/Lchloride, 250 mg/L sulfate, 150 mg/L ofbicarbonate, and 20 mg/L nitrate. Figure 3 shows the dMRMchromatogram of the same compounds,under the same analysis conditions, in asynthetic water matrix. It is possible tosee that the synthetic matrix interferesdirectly not only with the sensitivity,but also with the peak shape of somecompounds.This behavior was alsoobserved in the official method 557, andmust be related not only to the matrixeffects, but also to column capacityissues in relation to high-ionic-strengthsamples.This behavior affects allanalytes to some degree. The syntheticmatrix solution is prepared at an ionicconcentration higher than that typicallyobserved in drinking water. Such effectswere minimal or not observed in theevaluated drinking water matricesevaluated. Calibration curves, from 9 to 13different levels, were built with standardsolutions in a concentration rangingfrom 0.02 to 100 pg/L, depending onthe individual compound and using theMCAA isotopically labeled as internalstandard.Each concentration level wasanalyzed in triplicate. A linear fittingwith no weighting was used for allanalytical curves prepared in ultrapurewater, and the values of the coefficientof determination (R?) were higher than0.997 forall compounds, with relativestandard deviations (RSDs) rangingfrom 0.1 to 5.1% for run-to-run precision.Figure 4 shows an example of theresponse for BDCAA in drinking waterusing Agilent MassHunter Quantitativesoftware (Version 10.0). Acquisition time (min) Acquisition time (min) Figure 3. Dynamic MRM chromatogram of HAAs, bromate, chlorate, and isotopically labeled standard at 2 pg/L in synthetic matrix containing 320 mg/L chloride,250 mg/L sulfate, 150 mg/L of bicarbonate, and 20 mg/L nitrate. ¥ Agilent MassH ntit alysis (for QQQ] bin 5 The limits of detection (LODs) andquantification (LOQs) were determinedwith reference to the correspondingconcentration to 3 to 10 times.respectively, the baseline noise. Table 3shows the regression equations andother characteristic parameters for thedeveloped method. The lowest concentration minimumreporting level (LCMRL) is defined asthe lowest true concentration for whichfuture recovery is predicted to fall, withhigh confidence (99%), between 50 and150% recovery. The detection limit (DL)is defined as the statistically calculatedminimum concentration that can bemeasured with 99% confidence thatthe reported value is greater than zero.These values were statistically calculatedaccording to EPA's LCMRL statisticalprotocol22 and are shown in Table 4with the values reported for officialMethod 557. Table 3. Quality parameters for the LC/MS/MS analysis method of the HAAs,bromate, andchlorate in ultrapure water. ID Linear Range Equation R2 LOD LOQ MCAA 0.2-100 y=0.0838x+0.0662 0.998 0.04 0.13 MBAA 0.1-100 y=0.1029x+0.0632 0.999 0.02 0.06 DCAA 0.05-100 y=0.7764x+0.6448 0.997 0.01 0.03 DBAA 0.05-100 y=0.3356x+0.2898 0.997 0.01 0.03 TCAA 0.05-100 y=1.4504x+1.9362 0.997 0.01 0.03 TBAA 0.05-100 y=0.0497x+0.0627 0.997 0.01 0.04 BCAA 0.1-100 y=0.0601x+0.0447 0.999 0.01 0.05 BDCAA 0.02-100 v=0.1818x+0.1407 0.997 0.004 0.012 CDBAA 0.05-100 y=0.0726x+0.0749 0.997 0.005 0.016 Br0, 0.02-100 y=0.5771x-0.1079 0.998 0.004 0.012 ClO, 0.02-100 y=0.3586x+0.0182 0.998 0.003 0.01 Table 4. Values of LCMRL and DL for the LC/MS/MS analysis method of HAAs, bromate,and chlorate. ID Fortification Level(ug/L) Proposed Method (pg/L) Method 557 EPA (ug/L) LCMRL DL LCMRL DL MCAA 0.2 0.45 0.09 0.58 0.20 MBAA 0.1 0.1 0.03 0.19 0.064 DCAA 0.05 0.05 0.01 0.13 0.055 DBAA 0.05 0.09 0.02 0.062 0.015 TCAA 0.05 0.08 0.02 0.25 0.090 TBAA 0.05 0.07 0.02 0.27 0.067 BCAA 0.1 0.1 0.04 0.16 0.11 BDCAA 0.02 0.04 0.01 0.19 0.050 CDBAA 0.05 0.08 0.02 0.08 0.041 BrO, 0.02 0.02 0.01 0.042 0.020 CIO, 0.02 0.03 0.01 NA NA Recovery experiments were carriedout, in duplicate, at three concentrationlevels by spiking standard solutions to ablank water sample. The analyte percentrecoveries were between 84.1 to 107.7%,with RSD (relative standard deviation)<15%. Table 5 presents these results. This method was applied to determinethe HAAs, bromate, and chlorate in tapdrinking water samples collected fromdifferent areas in Sao Paulo city, Brazil.Table 6 shows the results. Out of the nine HAAs,TCAA was themost abundant species. Apart fromsamples 4 and 6, TCAA corresponds tomore than 80% of the total concentrationof HAAs in the samples. Sample 6 had atotal concentration above the limit set byUSEPA of 60 pg/L. Recovery (%) (mean±RSD,n=6) ID Linear Range Lowest Level 1 pg/L 100 pg/L MCAA 0.2-100 96.2±11.7 98.0±7.0 106.7±7.4 MBAA 0.1-100 88.9±6.4 100.9±9.2 95.6±4.9 DCAA 0.05-100 107.1±6.5 100.5±0.8 94.0±3.3 DBAA 0.05-100 102.0±9.2 98.0±2.4 96.2±2.1 TCAA 0.05-100 100.2±8.6 97.4±4.2 98.7±4.7 TBAA 0.05-100 102.0±7.4 101.5±13.7 104.2±4.7 BCAA 0.1-100 94.0±9.5 99.3±4.4 98.2±3.2 BDCAA 0.02-100 98.9±13.7 100.7±1.7 96.4±4.0 CDBAA 0.05-100 100.5±8.1 98.4±9.0 88.5±12.6 Br0 0.02-100 99.9±9.8 84.1±3.5 102.9±8.4 clo 0.02-100 107.7±9.9 88.9±4.9 100±9.4 Table 6. Concentrations of HAAs, bromate, and chlorate found in the tap drinking water of Sao Paulo city,Brazil. Compound LOQ Sample 1 Sample 2 Sample 3 Sample 4 Sample 5 Sample 6 MCAA 0.13 0.45±0.01 nd 0.41±0.01 nd 1.29±0.06 5.75±0.16 MBAA 0.06 nd nd nd nd 0.07±0.01 0.37 +0.01 DCAA 0.03 0.74±0.04 nd 0.27±0.03 1.67±0.02 5.59±0.03 44.81±0.93 DBAA 0.03 nd nd
确定




还剩8页未读,是否继续阅读?
安捷伦科技(中国)有限公司为您提供《饮用水中卤乙酸检测方案(液相色谱仪)》,该方案主要用于饮用水中有机污染物 检测,参考标准--,《饮用水中卤乙酸检测方案(液相色谱仪)》用到的仪器有Agilent 1290 Infinity II 液相色谱系统、Agilent 6470 三重四极杆液质联用系统
推荐专场
相关方案
更多
该厂商其他方案
更多
















