方案详情
文
采用LaVision公司的IRO和CCD相机构成的增强型CCD相机系统,对CH*的自发荧光图像进行了测量,研究了N2和CO2稀释对C3H8/O2混合气在涡流管式火焰燃烧器中的燃烧特性的影响。
方案详情

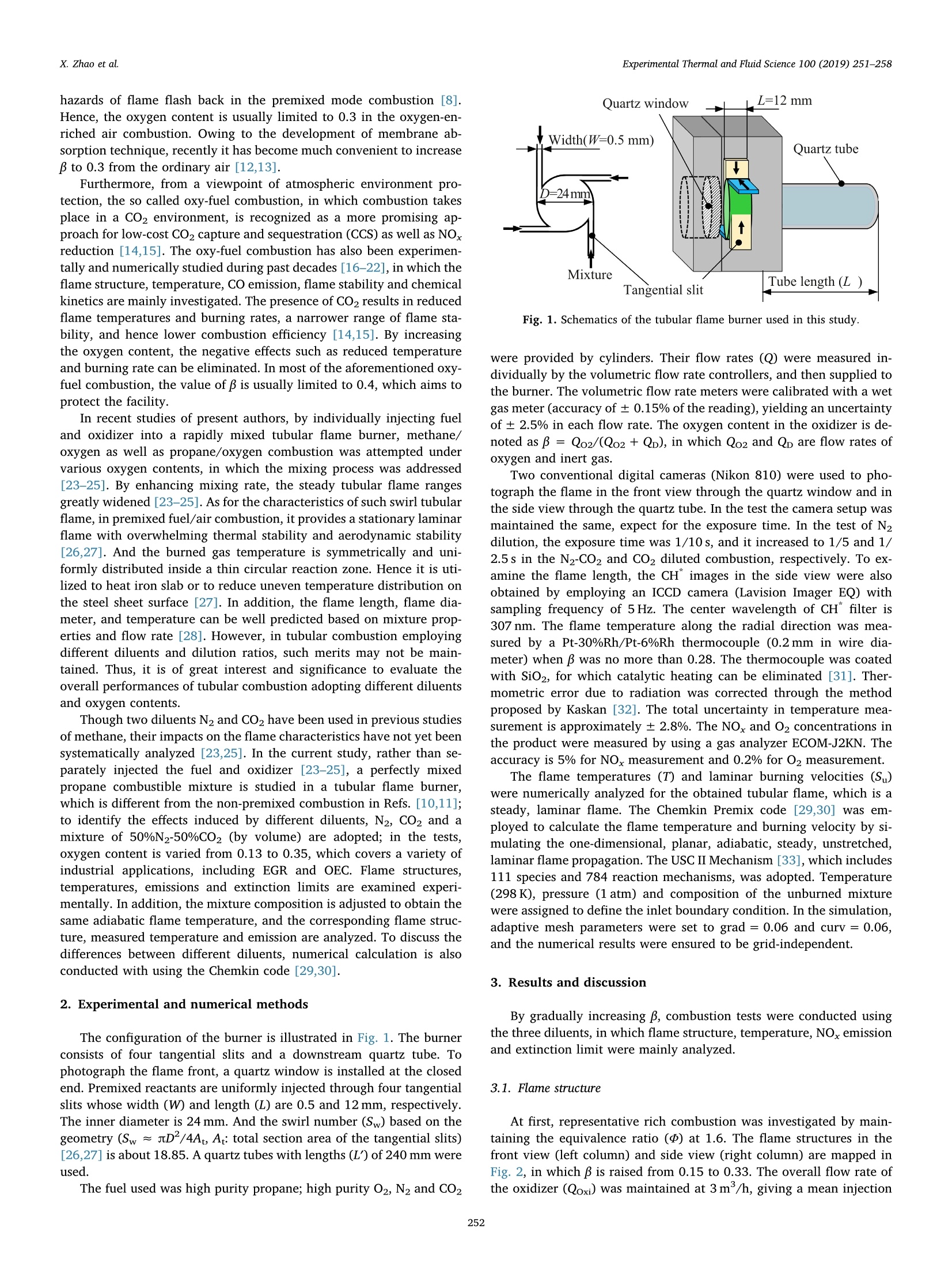
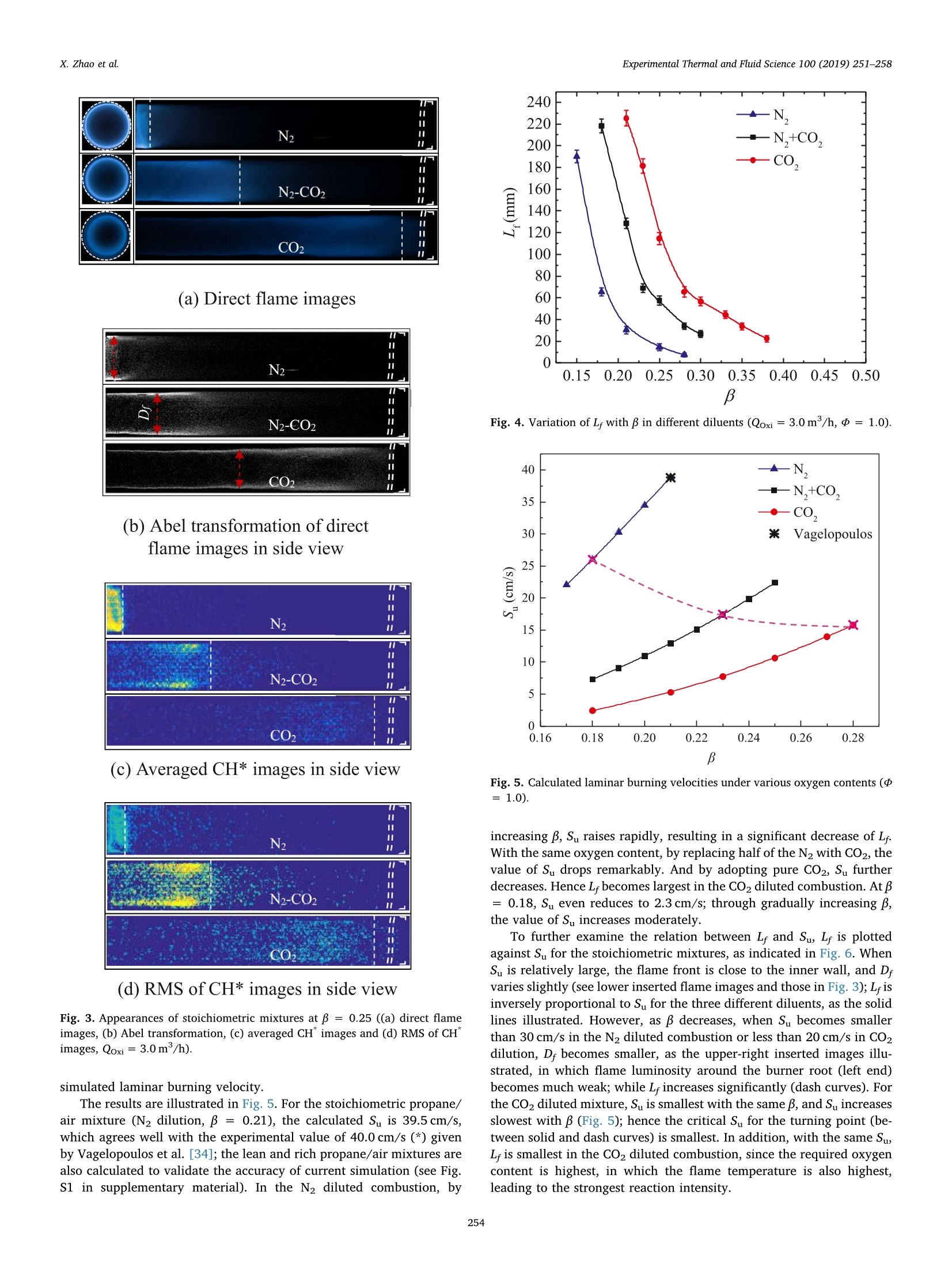
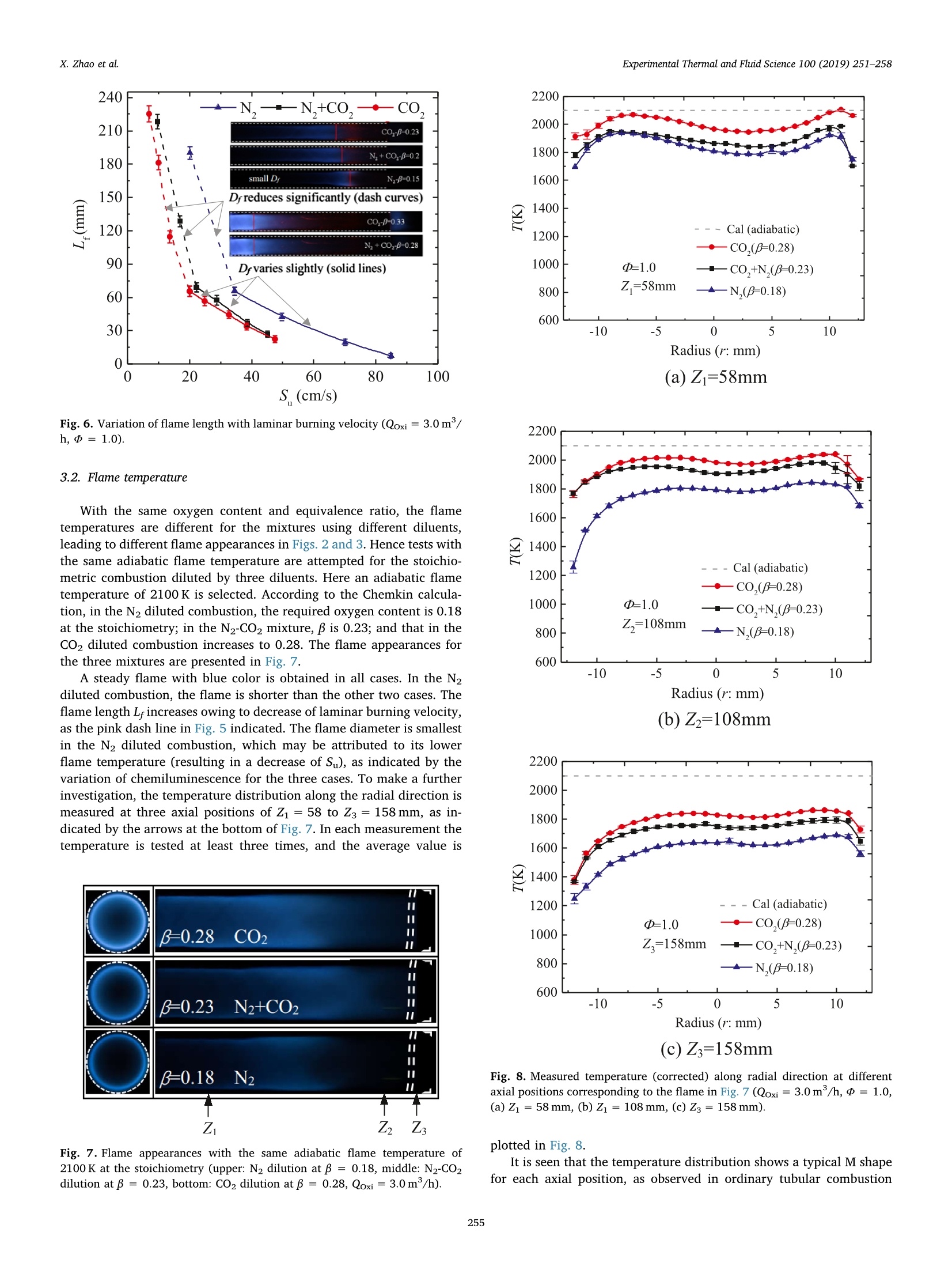
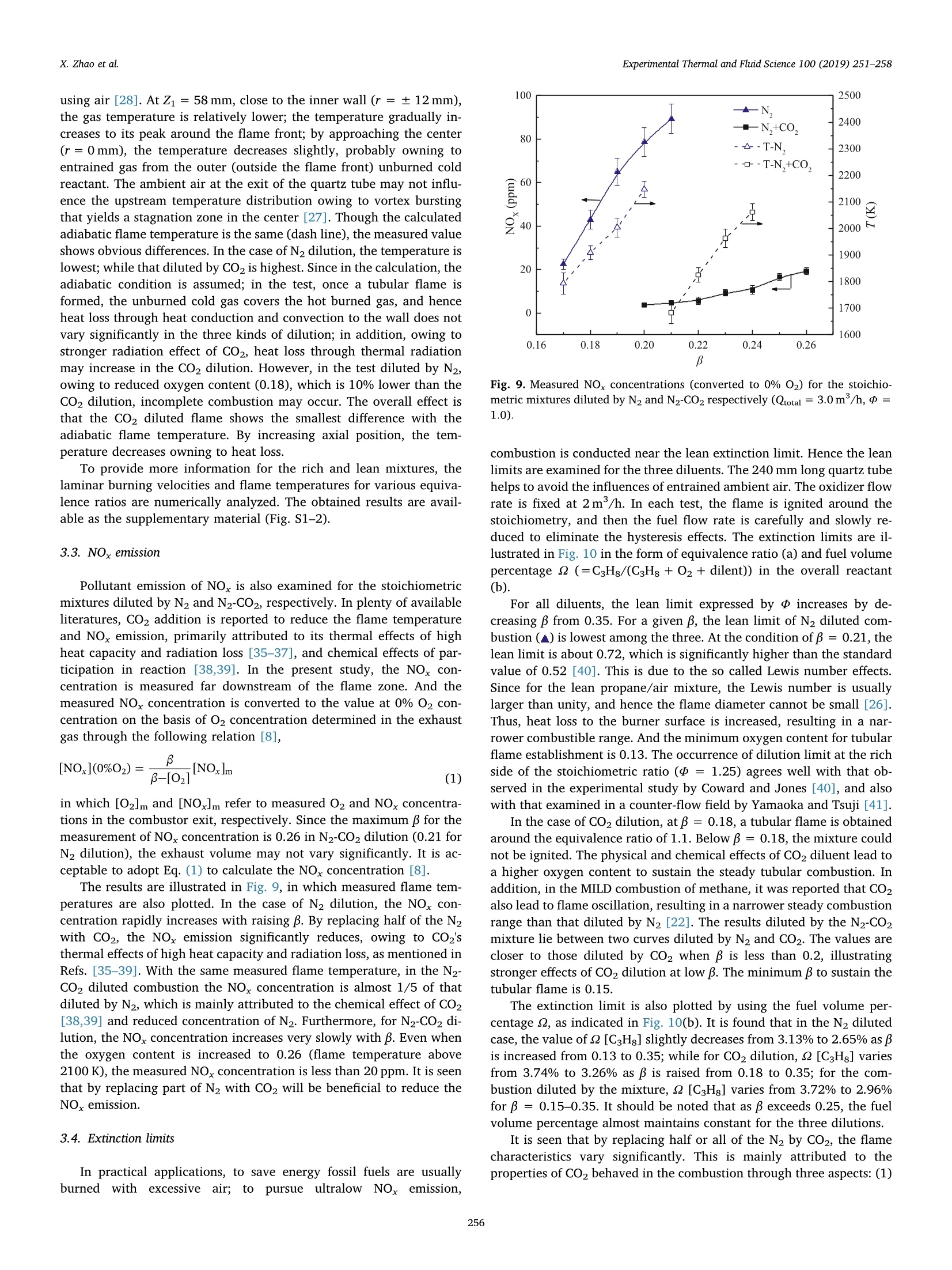
Experimental Thermal and Fluid Science 100 (2019) 251-258Contents lists available at ScienceDirect Experimental Thermal and Fluid Science 100 (2019) 251-258X. Zhao et al. Experimental Thermal and Fluid Science journal homepage: www.elsevier.com/locate/etfs Effects of N2 and CO2 dilution on the combustion characteristics of C3Hg/O2mixture in a swirl tubular flame burner Xiaoyao Zhao, Baolu Shi *, Weikang Peng, Qing Cao, Dingjiang Xie, Wei Dong,Ningfei Wang School of Aerospace Engineering, Beijing Institute of Technology, No.5 ZhongGuanCun South Street, HaiDian, Beijing 100081, ChinaAECC Sichuan Gas Turbine Establishment, No. 6 Xinjun Street, Xindu District, Chengdu, Sichuan 610500, China ARTICLEINFO ABSTRACT Keywords:Dilution effectOxygen contentTubular flameFlame structureEmission Premixed C3Hg/O2 combustion has been investigated by adopting N2, CO2 and their mixture (50%N2-50% CO2by volume) as the diluents, respectively. The oxygen content (mole fraction) in the oxidizer is varied from 0.13to 0.35, which covers the operating conditions of EGR and OEC. The flame structures, temperatures, burningvelocities and NO,emissions are examined under various oxygen contents. Detailed observations show that asteady, uniform tubular flame can be obtained over a wide range from lean to rich. By increasing oxygen content,the flame diameter increases slightly; the flame length decreases significantly, which is almost inversely pro-portional to laminar burning velocity. During decreasing the burning velocity to less than about 20 cm/s, aturning point appears and the flame length becomes extensively large. With the same oxygen content andequivalence ratio, by replacing half or all of the N2 diluent with CO2, the flame temperature and burning velocitydecrease significantly. To obtain the same adiabatic flame temperature, the CO2 diluted mixture requires thehighest oxygen content among the three diluents, however, its measured flame temperature is also highest. In theN2-CO2 diluted combustion, owing to thermal and chemical effects of CO2, the NOx emission reduces remarkablycomparing with N2 dilution; under the same flame temperature, the NO, concentration reduces to almost 1/5 ofthat diluted by N2. The lean extinction limit raises by increasing CO2 content in the diluent, while the limitdecreases with increasing oxygen content. 1. Introduction In combustion systems air is the most frequently used oxidizer,which adopts N2 as the diluent. Recently oxidizers using different di-luents such as CO2[1] and H2O [2] are also commonly adopted; inaddition, the oxygen content (mole fraction, B) in the oxidizer is alsovaried to satisfy specific applications. For instance, to reduce pollutantemissions such as NOx and soot, a single diluent or a mixture of N2,H2Oand Co has been added into the air/fuel mixture [1,3,4], which isusually adopted to mimic the exhaust gas recirculation (EGR) in enginesystems. The nitrogen dilution has proved to significantly influence theburning velocity, flame stability [5] and soot formation [6]. It is alsostated that CO2 has a stronger inhibiting effect on laminar burningvelocity than N2 in the syngas fuel combustion at atmospheric condition[7]. In a diesel engine and a co-flow ethylene diffusion flame, thethermal effect of CO2 is recognized as the most important factor gov-erning the soot reactivity, followed by the dilution and chemical effects[1]. On the other hand, oxidizer with elevated oxygen concentration isalso adopted, known as the oxygen-enhanced combustion (OEC) [8]. Ina variety of industrial heating applications, oxygen has been added toreplace part of the air, known as the oxygen-enriched air combustion,toadvance the combustion processes. By increasing oxygen content in thethermal process, plenty of benefits such as increased energy efficiency,productivity, flame stability and reduced exhaust gas volume can berealized [8,9]. During the past decades, extensive studies have beenconducted on the oxygen-enriched air combustion through variousburner systems. Zhen et al. examined variations of flame appearances,temperatures, overall pollutant emissions and heating behaviors withoxygen concentration in a swirling inverse diffusion flame [10]; in aturbulent non-premixed swirling burner, characteristics such as flamestability, emissions and flow field dynamics of methane-oxygen en-riched air combustion were systematically examined [11]. It is foundthat by increasing oxygen content, the flame temperature raises sig-nificantly, which leads to severe NO,formation through the thermal NOroute; in addition, the increased burning velocity may cause potential E-mail address: shibaolu@bit.edu.cn (B. Shi). ( h t t p s :// doi . or g/ 1 0.1016/ j .expthe rm f l usc i. 201 8 .09.009 ) ( Received 15 November 2017; R e ceived in revised form 10 September 2018; Accepted 1 0 September 2018 ) hazards of flame flash back in the premixed mode combustion [8].Hence, the oxygen content is usually limited to 0.3 in the oxygen-en-riched air combustion. Owing to the development of membrane ab-sorption technique, recently it has become much convenient to increaseBto 0.3 from the ordinary air [12,13]. Furthermore, from a viewpoint of atmospheric environment pro-tection, the so called oxy-fuel combustion, in which combustion takesplace in a CO2 environment, is recognized as a more promising ap-proach for low-cost CO2 capture and sequestration (CCS) as well as NOxreduction [14,15]. The oxy-fuel combustion has also been experimen-tally and numerically studied during past decades [16-22], in which theflame structure, temperature, CO emission, flame stability and chemicalkinetics are mainly investigated. The presence of CO2 results in reducedflame temperatures and burning rates, a narrower range of flame sta-bility, and hence lower combustion efficiency [14,15]. By increasingthe oxygen content, the negative effects such as reduced temperatureand burning rate can be eliminated. In most of the aforementioned oxy-fuel combustion, the value of B is usually limited to 0.4, which aims toprotect the facility. In recent studies of present authors, by individually injecting fueland oxidizer into a rapidly mixed tubular flame burner, methane/oxygen as well as propane/oxygen combustion was attempted undervarious oxygen contents, in which the mixing process was addressed[23-25]. By enhancing mixing rate, the steady tubular flame rangesgreatly widened [23-25]. As for the characteristics of such swirl tubularflame, in premixed fuel/air combustion, it provides a stationary laminarflame with overwhelming thermal stability and aerodynamic stability[26,27]. And the burned gas temperature is symmetrically and uni-formly distributed inside a thin circular reaction zone. Hence it is uti-lized to heat iron slab or to reduce uneven temperature distribution onthe steel sheet surface [27]. In addition, the flame length, flame dia-meter, and temperature can be well predicted based on mixture prop-erties and flow rate [28]. However, in tubular combustion employingdifferent diluents and dilution ratios, such merits may not be main-tained. Thus, it is of great interest and significance to evaluate theoverall performances of tubular combustion adopting different diluentsand oxygen contents. Though two diluents N2 and CO2 have been used in previous studiesof methane, their impacts on the flame characteristics have not yet beensystematically analyzed [23,25]. In the current study, rather than se-parately injected the fuel and oxidizer [23-25], a perfectly mixedpropane combustible mixture is studied in a tubular flame burner,which is different from the non-premixed combustion in Refs.[10,11];to identify the effects induced by different diluents, N2, CO2 and amixture of 50%N2-50%CO2 (by volume) are adopted; in the tests,oxygen content is varied from 0.13 to 0.35, which covers a variety ofindustrial applications, including EGR and OEC. Flame structures,temperatures, emissions and extinction limits are examined experi-mentally. In addition, the mixture composition is adjusted to obtain thesame adiabatic flame temperature, and the corresponding flame struc-ture, measured temperature and emission are analyzed. To discuss thedifferences between different diluents, numerical calculation is alsoconducted with using the Chemkin code [29,30]. 2. Experimental and numerical methods The configuration of the burner is illustrated in Fig. 1. The burnerconsists of four tangential slits and a downstream quartz tube. Tophotograph the flame front, a quartz window is installed at the closedend. Premixed reactants are uniformly injected through four tangentialslits whose width (W) and length (L) are 0.5 and 12 mm, respectively.The inner diameter is 24 mm. And the swirl number(Sw) based on thegeometry (Sw≈D/4A, A: total section area of the tangential slits)[26,27] is about 18.85. A quartz tubes with lengths (L) of 240 mm wereused. The fuel used was high purity propane; high purity 02, N2 and CO2 Fig. 1. Schematics of the tubular flame burner used in this study. were provided by cylinders. Their flow rates (Q) were measured in-dividually by the volumetric flow rate controllers, and then supplied tothe burner. The volumetric flow rate meters were calibrated with a wetgas meter (accuracy of ±0.15% of the reading), yielding an uncertaintyof ± 2.5% in each flow rate. The oxygen content in the oxidizer is de-noted as β=Qo2/(Qo2 +Qp), in which Qo and Qp are flow rates ofoxygen and inert gas. Two conventional digital cameras (Nikon 810) were used to pho-tograph the flame in the front view through the quartz window and inthe side view through the quartz tube. In the test the camera setup wasmaintained the same, expect for the exposure time. In the test of N2dilution, the exposure time was 1/10 s, and it increased to 1/5 and 1/2.5 s in the N2-CO2 and CO2 diluted combustion, respectively. To ex-amine the flame length, the CH images in the side view were alsoobtained by employing an ICCD camera (Lavision Imager EQ) withsampling frequency of 5 Hz. The center wavelength of CH" filter is307 nm. The flame temperature along the radial direction was mea-sured by a Pt-30%Rh/Pt-6%Rh thermocouple (0.2mm in wire dia-meter) when B was no more than 0.28. The thermocouple was coatedwith SiO, for which catalytic heating can be eliminated [31]. Ther-mometric error due to radiation was corrected through the methodproposed by Kaskan [32]. The total uncertainty in temperature mea-surement is approximately± 2.8%. The NOx and O2 concentrations inthe product were measured by using a gas analyzer ECOM-J2KN.Theaccuracy is 5% for NOx measurement and 0.2% for O2 measurement. The flame temperatures (T) and laminar burning velocities (Su)were numerically analyzed for the obtained tubular flame, which is asteady, laminar flame. The Chemkin Premix code [29,30] was em-ployed to calculate the flame temperature and burning velocity by si-mulating the one-dimensional, planar, adiabatic, steady, unstretched,laminar flame propagation. The USC II Mechanism [33], which includes111 species and 784 reaction mechanisms, was adopted. Temperature(298 K), pressure (1 atm) and composition of the unburned mixturewere assigned to define the inlet boundary condition. In the simulation,adaptive mesh parameters were set to grad=0.06 and curv = 0.06,and the numerical results were ensured to be grid-independent. 3. Results and discussion By gradually increasing B, combustion tests were conducted usingthe three diluents, in which flame structure, temperature, NOx emissionand extinction limit were mainly analyzed. 3.1. Flame structure At first, representative rich combustion was investigated by main-taining the equivalence ratio (d) at 1.6. The flame structures in thefront view (left column) and side view (right column) are mapped inFig. 2, in which B is raised from 0.15 to 0.33. The overall flow rate ofthe oxidizer (Qoxi) was maintained at 3m/h, giving a mean injection (a) N, dilution (b)N,-CO, dilution (c) CO, dilution Fig. 2. Variation of rich flame structure (三=1.6) with oxygen content ((a) N2dilution, (b) N2-CO2 dilution, and (c) CO2 dilution, Qoxi=3.0 m/h). velocity of 34.72 m/s. In Fig. 2(a), pure N2 is used as the diluents; inFig. 2(b), half of the N2 is replaced by CO2; in Fig. 2(c), pure CO2 isadopted. The dash circles show the positions of the inner wall. The exit(end) of the quartz tube is also illustrated in the side view. In the case of pure N2 dilution (Fig. 2(a)), a steady and uniform tubular flame has been obtained. At a low value of β = 0.15, a blue-green flame with very thin flame front is observed. Part of the fuel isburned outside the quartz tube; by increasing β to 0.18, the flamediameter(D ) slightly increases in the front view, while in the side view,the flame presents green color; by further raising B to 0.21and0.28, theflame chemiluminescence intensifies and the flame shrinks in length. By replacing half of the N2 with CO2 (Fig. 2(b)), a tubular flamefailed to be established at β=0.15.The flame appearances for mixturesof β= 0.18-0.28 are presented. At B= 0.18, a blue flame with weakluminosity is observed. Comparing with that diluted by pure N2, theflame diameter decreases. By increasing B, the flame diameter graduallyincreases in the front view, and in the side view, the flame length (L)decreases. Here the flame length means the length of a tubular flame inwhich a complete circular reaction zone with the same diameter ismaintained. In these tests only a blue-color flame is observed, which isdifferent from that in Fig. 2(a). In Fig. 2(c), a steady and uniformtubular flame with blue color is also observed between β= 0.18-0.33.Comparing with that in Fig. 2(b), the flame diameter decreases andflame length increases for the same B. Owing to reduced flame tem-perature and weakened C2"emission caused by chemical effects ofCO2,only a blue color flame is obtained in the N2-CO2 and CO2 dilutedcombustion. Next, the stoichiometric combustion was attempted. Fig.3 showsthe results of three diluents with fixed oxygen content of β= 0.25. Inthe direct flame images (Fig. 3(a)), a blue flame is obtained. Differentfrom those of = 1.6, the reaction zone is limited inside the quartztube. By replacing half of the N2 with CO2 (middle row), D. becomessmaller comparing with that of N2 dilution, while L increases. In thecase of pure CO2 dilution, D. decreases distinctly while L increasessignificantly. The flame image in the side view is a line-of-sight result. Hence,Abel transformation for such axisymmetric flame is carried out toquantitatively discuss D (as indicated by the arrow). The results aremapped in Fig. 3(b), in which the flame outline can be identified. Thevalue of D decreases with increasing the CO2 content in the oxidizer. Tomake a further analysis, the averaged CH" images obtained from 50instantaneous images are mapped in Fig. 3(c), and the correspondingroot mean square (RMS) images are also presented (Fig.3(d)). Here theRMS image can assist to determine the end of flame when the CHsignal is week. Thus, in the present study L is determined by the CHimage. In the tests, L is observed to decrease as B increases for the re-presentative rich, stoichiometric and lean mixtures. Here the length isplotted against the oxygen content for the stoichiometric combustion,as illustrated in Fig. 4. The oxidizer flow rate is maintained at 3 m/h.In the N2 diluted combustion (▲), the flame length is much smaller thanthe other two, and by increasing B, L decrease significantly. In the CO2diluted combustion (O), L is largest, and it also decreases with B. Themixture diluted by N2-CO2(■) show the same variation trend, and itscorresponding L lies between those diluted by CO2 and N2. It is inter-esting to note that as L exceeds about 70 mm during decreasing B, aturning point appears for the three diluents, beyond which L increasesmore significantly. The observed phenomena are strongly correlated with the laminarburning velocity. On one hand, the flame front of the tubular flame isalways stabilized at a radial position where the radial velocity balancesthe burning velocity. On the other hand, in the obtained laminar tub-ular flame, most reactants are consumed at the flame surface (area).Accordingly, the volume burning rate (Qburning) can be calculated asQburning=DLSu. In the fuel/air combustion, Ishizuka et al. reportedthat the overall flow rate of reactant is in proportion to the volumeburning rate, i.e., Qtotal o D LSu [28]. To examine such relation incombustion using different diluents under various oxygen contents, atfirst, Su is examined. Here the stoichiometric mixture is selected as anexample, and Su is calculated by Chemkin. Since the obtained flame is alaminar, stretched, premixed flame, it is acceptable to adopt the (a) Direct flame images (b) Abel transformation of directflame images in side view (C) Averaged CH* images in side view (d) RMS of CH* images in side view Fig. 3. Appearances of stoichiometric mixtures atβ=0.25 ((a) direct flameimages, (b) Abel transformation, (c) averaged CH"images and (d) RMS of CH"images,Qoxi=3.0m/h). simulated laminar burning velocity. The results are illustrated in Fig. 5. For the stoichiometric propane/air mixture (N2 dilution, β=0.21), the calculated Su is 39.5 cm/s,which agrees well with the experimental value of 40.0 cm/s (*) givenby Vagelopoulos et al. [34]; the lean and rich propane/air mixtures arealso calculated to validate the accuracy of current simulation (see Fig.S1 in supplementary material). In the N2 diluted combustion by Fig. 4. Variation of L with in different diluents (Qoxi=3.0m/h,d=1.0). Fig.5. Calculated laminar burning velocities under various oxygen contents (=1.0). increasing B, Su raises rapidly, resulting in a significant decrease of L.With the same oxygen content, by replacing half of the N2 with CO2, thevalue of Su drops remarkably. And by adopting pure CO2, Su furtherdecreases. Hence L becomes largest in the CO2 diluted combustion. At B=0.18, Su even reduces to 2.3 cm/s; through gradually increasing B,the value of Su increases moderately. To further examine the relation between L and Su, L is plottedagainst S for the stoichiometric mixtures, as indicated in Fig. 6. WhenSu is relatively large, the flame front is close to the inner wall, and Dvaries slightly (see lower inserted flame images and those in Fig. 3); Lisinversely proportional to Su for the three different diluents, as the solidlines illustrated. However, as B decreases, when S becomes smallerthan 30 cm/s in the N2 diluted combustion or less than 20 cm/s in CO2dilution, Dr becomes smaller, as the upper-right inserted images illu-strated, in which flame luminosity around the burner root (left end)becomes much weak; while L increases significantly (dash curves). Forthe CO2 diluted mixture, Su is smallest with the same B,and Su increasesslowest with B (Fig.5); hence the critical Su for the turning point (be-tween solid and dash curves) is smallest. In addition, with the same S,L is smallest in the CO diluted combustion, since the required oxygencontent is highest, in which the flame temperature is also highest,leading to the strongest reaction intensity. Fig.6. Variation of flame length with laminar burning velocity (Qoxi=3.0m/h,p=1.0). 3.2. Flame temperature With the same oxygen content and equivalence ratio, the flametemperatures are different for the mixtures using different diluents,leading to different flame appearances in Figs. 2 and 3. Hence tests withthe same adiabatic flame temperature are attempted for the stoichio-metric combustion diluted by three diluents. Here an adiabatic flametemperature of 2100 K is selected. According to the Chemkin calcula-tion, in the N2 diluted combustion, the required oxygen content is 0.18at the stoichiometry; in the N2-CO2 mixture, B is 0.23; and that in theCO2 diluted combustion increases to 0.28. The flame appearances forthe three mixtures are presented in Fig. 7. A steady flame with blue color is obtained in all cases. In the N2diluted combustion, the flame is shorter than the other two cases. Theflame length L increases owing to decrease of laminar burning velocity,as the pink dash line in Fig. 5 indicated. The flame diameter is smallestin the N2 diluted combustion, which may be attributed to its lowerflame temperature (resulting in a decrease of S), as indicated by thevariation of chemiluminescence for the three cases. To make a furtherinvestigation, the temperature distribution along the radial direction ismeasured at three axial positions of Z1 = 58 to Z3= 158 mm, as in-dicated by the arrows at the bottom of Fig. 7. In each measurement thetemperature is tested at least three times, and the average value is Fig. 7. Flame appearances with the same adiabatic flame temperature of2100 K at the stoichiometry (upper: N2 dilution at β= 0.18, middle: N2-CO2dilution atβ=0.23, bottom: CO2 dilution at B=0.28, Qoxi=3.0 m/h). Radius (r: mm) (a)Zi=58mm (b)Zz=108mm (c) Z3=158mm Fig. 8. Measured temperature (corrected) along radial direction at differentaxial positions corresponding to the flame in Fig. 7 (Qoxi =3.0m/h, p=1.0,(a) Z1 = 58 mm, (b) Z1 =108 mm, (c) Z3=158mm). plotted in Fig. 8. It is seen that the temperature distribution shows a typical M shapefor each axial position, as observed in ordinary tubular combustion using air [28]. At Z1 = 58 mm, close to the inner wall (r = ±12 mm),the gas temperature is relatively lower; the temperature gradually in-creases to its peak around the flame front; by approaching the center(r=0 mm), the temperature decreases slightly, probably owning toentrained gas from the outer (outside the flame front) unburned coldreactant. The ambient air at the exit of the quartz tube may not influ-ence the upstream temperature distribution owing to vortex burstingthat yields a stagnation zone in the center [27]. Though the calculatedadiabatic flame temperature is the same (dash line), the measured valueshows obvious differences. In the case of N2 dilution, the temperature islowest; while that diluted by CO2 is highest. Since in the calculation, theadiabatic condition is assumed; in the test, once a tubular flame isformed, the unburned cold gas covers the hot burned gas, and henceheat loss through heat conduction and convection to the wall does notvary significantly in the three kinds of dilution; in addition, owing tostronger radiation effect of CO2, heat loss through thermal radiationmay increase in the CO2 dilution. However, in the test diluted by N2,owing to reduced oxygen content (0.18), which is 10% lower than theCO2 dilution, incomplete combustion may occur. The overall effect isthat the CO2 diluted flame shows the smallest difference with theadiabatic flame temperature. By increasing axial position, the tem-perature decreases owning to heat loss. To provide more information for the rich and lean mixtures, thelaminar burning velocities and flame temperatures for various equiva-lence ratios are numerically analyzed. The obtained results are avail-able as the supplementary material (Fig. S1-2). 3.3. NOx emission Pollutant emission of NOx is also examined for the stoichiometricmixtures diluted by N2 and N2-CO2, respectively. In plenty of availableliteratures, CO2 addition is reported to reduce the flame temperatureand NO, emission, primarily attributed to its thermal effects of highheat capacity and radiation loss [35-37], and chemical effects of par-ticipation in reaction [38,39]. In the present study, the NOx con-centration is measured far downstream of the flame zone. And themeasured NO, concentration is converted to the value at 0% 02 con-centration on the basis of O2 concentration determined in the exhaustgas through the following relation [8], in which [O2]m and [NOx]m refer to measured O2 and NOx concentra-tions in the combustor exit, respectively. Since the maximum B for themeasurement of NOx concentration is 0.26 in N2-CO2 dilution (0.21 forN2 dilution), the exhaust volume may not vary significantly. It is ac-ceptable to adopt Eq.(1) to calculate the NOx concentration [8]. The results are illustrated in Fig. 9,in which measured flame tem-peratures are also plotted. In the case of N2 dilution, the NOx con-centration rapidly increases with raising B. By replacing half of the N2with CO2, the NOx emission significantly reduces, owing to CO2'sthermal effects of high heat capacity and radiation loss, as mentioned inRefs. [35-39]. With the same measured flame temperature, in the N2-CO2 diluted combustion the NOx concentration is almost 1/5 of thatdiluted by N2, which is mainly attributed to the chemical effect of CO2[38,39] and reduced concentration of N2. Furthermore, for N2-CO2 di-lution, the NOx concentration increases very slowly with B. Even whenthe oxygen content is increased to 0.26 (flame temperature above2100K), the measured NOx concentration is less than 20 ppm. It is seenthat by replacing part of N2 with CO2 will be beneficial to reduce theNOx emission. 3.4. Extinction limits In practical applications, to save energy fossil fuels are usuallyburneddwith excessive air; to pursue ultralow NOx emission, Fig. 9. Measured NO, concentrations (converted to 0% 02) for the stoichio-metric mixtures diluted by N2 and N2-CO2 respectively (Qtotal =3.0m/h,d=1.0). combustion is conducted near the lean extinction limit. Hence the leanlimits are examined for the three diluents. The 240 mm long quartz tubehelps to avoid the influences of entrained ambient air. The oxidizer flowrate is fixed at 2 m/h. In each test, the flame is ignited around thestoichiometry, and then the fuel flow rate is carefully and slowly re-duced to eliminate the hysteresis effects. The extinction limits are il-lustrated in Fig. 10 in the form of equivalence ratio (a) and fuel volumepercentage Q (=C3Hg/(C3Hg+02 +dilent)) in the overall reactant(b). For all diluents, the lean limit expressed by increases by de-creasing B from 0.35. For a givenB, the lean limit of N2 diluted com-bustion (▲) is lowest among the three. At the condition of β=0.21, thelean limit is about 0.72, which is significantly higher than the standardvalue of 0.52 [40]. This is due to the so called Lewis number effects.Since for the lean propane/air mixture, the Lewis number is usuallylarger than unity, and hence the flame diameter cannot be small [26].Thus, heat loss to the burner surface is increased, resulting in a nar-rower combustible range. And the minimum oxygen content for tubularflame establishment is 0.13. The occurrence of dilution limit at the richside of the stoichiometric ratio (= 1.25) agrees well with that ob-served in the experimental study by Coward and Jones [40], and alsowith that examined in a counter-flow field by Yamaoka and Tsuji [41]. In the case of CO2 dilution,at β=0.18, a tubular flame is obtainedaround the equivalence ratio of 1.1. Below β=0.18, the mixture couldnot be ignited. The physical and chemical effects of CO2 diluent lead toa higher oxygen content to sustain the steady tubular combustion. Inaddition, in the MILD combustion of methane, it was reported that CO2also lead to flame oscillation, resulting in a narrower steady combustionrange than that diluted by N2[22]. The results diluted by the N2-CO2mixture lie between two curves diluted by N2 and CO2. The values arecloser to those diluted by CO2 when B is less than 0.2, illustratingstronger effects of CO2 dilution at low B. The minimum β to sustain thetubular flame is 0.15. The extinction limit is also plotted by using the fuel volume per-centage , as indicated in Fig. 10(b). It is found that in the N2 dilutedcase, the value of Q [CsHg] slightly decreases from 3.13% to 2.65% as pis increased from 0.13 to 0.35; while for CO2 dilution, Q [C3Hg] variesfrom 3.74% to 3.26% as B is raised from 0.18 to 0.35; for the com-bustion diluted by the mixture, 2 [C3Hg] varies from 3.72% to 2.96%for β=0.15-0.35. It should be noted that as B exceeds 0.25, the fuelvolume percentage almost maintains constant for the three dilutions. It is seen that by replacing half or all of the N2 by CO2, the flamecharacteristics vary significantly. This is mainly attributed to theproperties of CO2 behaved in the combustion through three aspects: (1) (a)Equivalence ratio (b) Propane volume percentage Fig. 10. Measured lean extinction limits by equivalence ratio (a) and propane volume percentage (b) under different oxygen contents by using the 240 mm com-bustion tube (Qoxi =2.0 m/h). Physical property of CO2. The molar heat capacity of CO2 is larger thanthat of N2, and hence, the flame temperature becomes lower for a fixedoxygen content. This leads to a narrow flammable range for CO2 dilu-tion (Fig. 10). (2) Thermodynamic property. CO2 reacts and convertsinto CO according to the chemical equilibrium C02→CO +0. Ac-cording to our Chemkin calculation, the mole fraction of CO2 in theburned gas becomes smaller than the total mole fraction of the CO2given as a diluent and the CO2 formed from propane through completereaction, and the ratio of the difference to the latter, namely,n=[{(CO2)diluent + (CO2)complete reaction} -(CO2)calculated]/{(CO2)diluent+(CO2)complete reaction} increases with B. For instance, the value of n is6.5% for the flame temperature of 2004 K at β=0.25 and=1.0,whereas n becomes 12.6% for the flame temperature of 2442 K at β=0.4 andd=1.0. (3) Chemical kinetics in elementary reactions. Forexample, the third-body efficiency of CO2 is about three times as big asthat of N2 in the important chain termination elementary reaction ofH+02+M→HO2+ M [42]. In addition, as illustrated by Liu et al.[43], CO2 affects the flame properties through CO +OH→CO2 + H.They have pointed out that in the CO2 diluted methane/oxygen flame,the chemical effect of CO2 significantly reduces the burning velocitywhen the CO2 concentration is high enough. In a recent study of oxy-fuel combustion of methane, the chemical effects of CO2 on the heatrelease rate for specific oxygen contents have been examined in detail,in which the key reactions to the combustion temperature were listed[44]. In the premixed syngas combustion [45], CO2 dilution was re-ported to significantly reduce the laminar flame speed and extinctionstrain rate, dominated by CO2 thermal effect followed by the chemicaleffect. The diffusivity change effect was considered negligible. Thus, theburning velocity of CO2 diluted mixture is lower than that of O2/N2mixture, resulting in a small diameter and a large flame length (Figs. 3and 4). 4. Conclusions Inert gas is added into the combustible mixture to suppress pollutantemissions such as NOx and soot, which is usually adopted in EGR sys-tems; on the other hand, in thermal industries oxygen is added to in-crease flame temperature and thermal efficiency, particularly in thefuel/oxygen combustion diluted by CO2, known as oxy-fuel combus-tion. In this study premixed propane/oxygen combustion is examined ina swirl tubular flame burner by adopting N2,N2-C02 mixture (50%-50%by volume) and Co2 as the diluents, respectively. In tests the oxygencontent (mole fractions,B) is raised up to 0.35 from 0.13. A steady anduniform tubular flame is established from lean to rich conditions underelevated oxygenconcentrations.The flames showsignificant differences in appearance by varying the diluent type, equivalence ratioand oxygen content: in the N2 diluted combustion, at the rich condition(equivalence ratio of 1.6), a green-color flame is observed when β ex-ceeds 0.18, while in that diluted by N2-CO2 mixture and CO2, only ablue-color flame is observed even when B is raised to 0.33; by in-creasing B, the flame diameter increases slightly and the flame lumin-osity intensifies; the flame length reduces remarkably. The variation offlame length is strongly correlated with the laminar burning velocity.When the burning velocity is larger than 20 cm/s, where the flamediameter is slightly smaller than the burner diameter, the flame lengthis inversely proportional to the laminar burning velocity. By decreasingto reduce the burning velocity, a turning point appears and the flamelength increases more significantly. Owing to physical and chemical effects of CO2, the flame tem-perature and laminar burning velocity decrease with increasing CO2content in the diluent, which directly leads to a relative narrowercombustible range. In the N2 diluted combustion, the minimum oxygencontent to sustain the steady combustion is 0.13, while that diluted byCO2 is 0.18. And in the N2-CO2 diluted combustion, the minimum B is0.15. To achieve the same adiabatic flame temperature, the oxygencontent should be much increased for CO2 dilution, however, themeasured temperature is highest in the CO2 diluted combustion owningto increased fuel consumption. By replacing half of the N2 by CO2, theNOx emission can be significantly reduced even under the elevatedoxygen concentration; with the same flame temperature, the NO,concentration becomes only 1/5 of that diluted by pure N2. On thewhole, the current study provides a useful guide to the tubular com-bustion that adopts EGR or OEC, particularly for the thermal processwhich needs a deterministic flame with uniform temperature. Acknowledgment This study is supported by National Natural Science Foundation ofChina (No. 51676016 and 91641204). Appendix A. Supplementary material Supplementary data to this article can be found online at https://doi.org/10.1016/j.expthermflusci.2018.09.009. References [1] K. Al-Qurashi, A.D. Lueking, A.L. Boehman, The deconvolution of the thermal, di-lution, and chemical effects of exhaust gas recirculation (EGR) on the reactivity ofengine and flame soot, Combust. Flame 158 (2011) 1696-1704. [2] J. Santner, F.L. Dryer,Y. Ju, The effects of water dilution on hydrogen, syngas, and ( e th y lene f l a mes a t e l e va ted pr e s sur e, Proc . Co m b u s t. I n s t . 34 (201 3 ) 7 19 - 7 2 6. ) ( [3] 1 F . S . L i u , H. S . G u o , G . J . S mal l wood, O. L . G u l de r , Th e ch emi cal e f f ect s o f c a r b o n d io xide as an add i tiv e i n a n ethy l e n e d if fu sio n fla m e: i mpl i c atio n s f o r s o ot a nd N O f ormation , Combu s t . F l ame 125 (201 1 ) 778- 7 87. ) ( [ 4 ] S . L i, Y. Zha n g, X . Q iu, B. L i , H. Z ha n g , E f f ec ts o f i ne r t d ilu t ion a n d preh e atin g t e m pera t ure o n l e a n f l a mmabi l i t y l i mit o f s yng as , E n e rg y F ue l s 2 8 (2 0 1 4 ) 3 44 2 - 3 4 52 . ) ( [5] H . K o b a yas h i, H . H a g i war a , H . K a ne k o, Y. O g a mi, Ef f ects of C O 2 d i l u t io n on tur- b ul e nt prem i xed flames at h i g h pr e s sur e an d hig h t e m p era tu re, Pro c . Com b u s t. Ins t . 3 1 (20 07) 1 451 -1 458. ) ( [6] Q . W an g, G . L e g r os, J . B on n et y , C . M o r in, Ex p er ime n t al c h a r a cte r iz a t io n of the d if f eren t n itrogen d i l u t i o n e f f ects o n s o o t f o rmation in e thy l ene d i f f usion f l a m e s , P r o c. C o m b ust. I n s t. 3 6 (201 7 ) 3 227 - 3 2 3 5. ) ( [7] C . P ra t h ap , A. R ay , M . R. R a vi, E f f e cts o f dilution w i t h car b on d i o x ide on the la m i n a r b u rn i n g v e l o c i ty a n d f l a m e s t a b i l i ty o f H2 - CO m i x tur e s a t a t mo sp heric c on d i ti o n , C ombust. F lame 1 5 9 ( 2 012 ) 482- 49 2. ) C.E. Baukal, Oxygen-Enhanced Combustion, CRC Press, FL, 1998. ( 8 C .E . Bau k al , O xyg en- E n h anced C o mb us t i o n, se c o nd e d ., C R C P re s s, Bo ca R a t on L o n don N ew Yo rk, 2 013. ) ( [10 ] H . S . Z h e n , C. W . Le un g , C .S . C h eu ng, C o mbus t ion charact e ristics o f a swirl i ng i n - v ers e d iff us i o n fla me upon o x yg e n c o n t e n t v a r iat i on, A p p l . E n e rg y 88 ( 2 01 1) 2925-2933. ) ( [11 ]N . M er l o,T. B o u shaki, C . Ch a u v e a u , S . D e P e rsis, L . P i l l i e r, B . S ar h, I . G o k al p , C ombusti on ch ara c te r i sti c s o f m e t h a n e - ox y g e n e n han c ed ai r t u r bu l e n t n o n - p re- m ixed s w irlin g flames , E x p. T he rm a l F l u id S c i . 5 6 ( 2 0 1 4 ) 53- 60. ) ( [12] S . G . K imu r a, W .R . B r o w all, M e mbrane oxygen en ri c h m e nt: I. D e m o ns t r a t io n of m embr a n e oxy g e n e n ri c h men t for n a t u ra l ga s c ombus t io n , J. M em b r . Sc i . 29 (198 6 ) 69-77. ) ( [13] H . Lin , M . Z hou, J. L y, J . Vu , J .G . Wij m ans, T. C . M e rk e l , J . Ji n , A . H a ldem a n, E. H. Wa ge ner, D . Rue , Me m br a n e- bas ed o x yg e n - enric h e d c ombus t io n , I nd . E n g. C h em. R es . 52 ( 201 3) 1 082 0 -108 3 4 . ) ( [14] M . B . T o f t e g a ard, J . B rix, P .A . J e n s e n, P. Glarborg , A.D. J e ns e n, Oxy- f uel c omb u s- t i o n of s olid fue l s , P ro g . E n e rg y Combust . S c i . 36 ( 2 0 1 0 ) 581-625. ) ( [15] ] T . Fu j i mor i , T . Y a m ada , Re ali z at i on of o xy - fu el co mb ustion fo r n e a r z e r o emi s sio n p ower ge nera ti on, Pr o c. C ombust. I n st. 3 4 (2013 ) 2 1 1 1 -2 1 3 0. ) ( [16] I . S a a n u m, M . D i ta ran to, Expe r i men t al s t udy o f o xy- f u e l c o mbu s tion u n d e r gas t u r b i ne c on d i t i ons , E n e r gy F u e l s 3 1 ( 2 0 1 7) 4 44 5 - 4 45 1 . ) ( [17] A . A m a t o, B . H u dak , P . D. S ouza,P . D . C arl o, D . R . Nob l e , D.E. S car b o r o u gh , J . Se i tzman, J . L i e uwe n , M ea s urem e nt s a nd a n a l y s is o f CO and O 2 e m i s sions i n C H 4 / C O , / O, fla m es, P r oc . C o m bus t . I n st . 3 3 ( 2 0 1 1 ) 3 3 9 9-3 4 0 5. ) ( [18] R . M ars h , J. R un y on, A . Gi l es, A . M orr is , D. Pu g h , A. V a ler a- Me d ina , P . Bo w e n , P r e m ixe d m et ha n e o x y- c ombu s tion in n i t r o g e n a nd c a r b on d i o xide a tmosph e res: m ea s urement of op era t i ng lim i t s, f lame l o ca t io n a n d e mi s s i ons, P r oc . Co m bust . I ns t. 3 6 ( 2 016 ) 39 4 9 - 3 958. ) ( [19 ] H . K . K i m , Y . Kim, S tudi e s o n combu s tion charac t e r i s tic s a n d f l ame l e n gth o f t ur- b u l e nt o xy-fu e l f l a m e s, E n ergy F u e l s 21 ( 2 0 07) 1 4 59 - 1467 . ) ( [20] | P. He il, D . T o p o rov, M . F o r st e r , R . K neer, E xpe r i m e n t a l i nve s ti gatio n on the e f f e ct of 0 2 an d CO 2 o n bu r n i n g r at es d uring ox y -f ue l com bu stion o f m et h a n e , Proc. C ombu s t.I n s t . 3 3 ( 2 01 1 ) 3 407-3 4 1 3. ) ( [21] T . M en d iara, P . G la r b o r g , R ebu r n c h e m istr y i n o x y- fu e l co m b u st i o n of m e tha ne , E ner gy F uels 2 3 ( 2 0 0 9) 3 565 - 35 72. ) ( [22 ] P . S a bi a, G . S o r re n t i n o, A . C hin n ici , A . C a v a l i e r e , R . R a gu cc i , D yn a mi c be h a v i ors i n m eth a ne m i l d and o x y-f u el c om bu sti on, E n erg y F uels 29 ( 2 015) 1 9 78- 1 9 86. ) ( [23] ] B . Shi, D . S h imokur i , S . I shi z u k a, Meth a ne / ox y g en c o mb u stio n i n a ra p idl y m ix e d t y p e tu b u l a r f l a me b ur ne r, P r oc. C o mbust. I nst. 3 4 ( 2 013) 3 3 6 9 -3377 . ) ( [24 ] ] B . S hi , W. Pe n g, B. L i, J . Hu , N . W a ng, S . I s h i zu ka , C O2 d ilu te d prop a ne /ox yg e n c ombu s tion in a r a p idly m i xed t y p e tu b u l a r fl a me b u r ner, Pr o c . C o m bust . I ns t . 3 6 ) ( ( 2017) 4 261 -4 268. ) ( [25] B . L i, B. Shi , X . Z ha o , K . Ma, D . X i e , D . Z ha o , J . L i , O x y- fu el combu s tion of m e than e i n a s wir l t u b u l a r fl a m e bur n er under var i o us oxyge n con t ent s : o p e r a t i on lim i t s a n d c om bu st i on i n s tabil i t y, E x p. T hermal F lu id Sci. 90 ( 2 018) 1 15-1 2 4 . ) ( [26] S . I shiz u ka , C h ar a ct e r i s ti cs o f tu b ul ar fl a mes , P rog. E ner g y C o mbust . Sc i. 19 ( 1 9 9 3 ) . 18 7- 226 ) ( [27] : S . I sh i zuka, D. Dun n -Rankin, R . W . P i tz, R . J . K ee, Y . Z han g , H . Z hu, T . T a k en o , M . N i s hio ka , D. Shi m oku r i , Tu bu la r combust i on , Moment um P ress , N e w Yor k, 2013. ) ( [ 2 8] S . I shiz uk a , R . H a g iwar a , M . Su z uki , A . N a k a mura , O . Ha m ag u chi , Combustion c ha r a c te r ist i c s o f a t ubu l a r f l ame bu rn er , J a p an Soc . M ec h . Eng rs . 65 ( 1 9 99 ) 3 845- 3 852. ) ( [ 29] R .J.K e e, J . F. Grcar, M.D. Smooke, J. A . Miller, A Fortran program for mo d elingsteady laminar one-dimensional p remixed f l ames, Sandia National L aboratories, oco Livermore, Report No. SAND85-8240, 1985. ) ( [ 30] R . J. Kee, G. Dixon-Lewis, J. Warnatz, M.E. Co l trin, J.A. M i ller, A Fortran computer code package for the evaluation of gas-phase,multicomponent transport properties, S andia N ational Laborator i es, Livermore , Repor t No . SAND86-8246B, 1 998. ) ( [31] . J . M . M adson, E . A . T h eby , S iO2 c o a te d t hermoco u ples, C o mbust. S ci . T echnol . 3 6 ( 1 9 84 ) 205 -2 09. ) ( [32] W .E . K a s kan, T he de p e nd en ce of f l a me t emperatu r e o n m ass b u r n ing v el o cit y, Sy m p. ( I n t . ) Co m b u st . 6 (1957) 134-14 3 . ) ( [ 33] H . Wang, X. Y o u, A.V. J o shi, S.G. D avis, A. Laskin, F. Egolfopoulos , C.K . Law , USC Mech Version I. High-temperature combustion r eaction model of H2/CO/C1-4 compounds May 2007. ) ( [34] C .M. V a ge l o p o u lo s , F . N. Eg ol fopoulos , C . K . L a w, F u r the r con sider a t i o n s o n the d e te rm i n a tion of l a m i n a r f l a me s p ee d s wi t h t he c ou n te r flow t wi n - fl a me t echniqu e , Sym p . ( I n t . ) C o m b u s t . 25 ( 199 4 ) 13 41-1 3 47. ) ( [35] | K . Lee , H. K i m , P . P a r k, S . Y a n g, Y . K o , C O2 r a d i ati o n h e a t l o s s eff e ct s o n NO e m is si o n s a n d c o m b us ti on i n s ta b il iti es in l e a n pr emix e d f l ame s, F ue l 106 (2 013) 6 8 2-6 8 9. ) ( [ 3 6] S . W ang , C . J i , B . Zhang, X . Cong, X. Liu, Eff ec t of CO 2 dilutio n o n combustion and e m is si o n s c ha r ac t eri s t ics of the h yd r o gen- e nr ic h e d g a soline e ng i ne , E n er gy 9 6 (20 1 6 ) 1 1 8-1 2 6 . ) ( [37] N . G a s co i n, Q . Y a n g , K. C het e houna, T h er ma l ef f e c ts o f C O2 o n t h e N O , f o rmat io n b e hav ior i n t he CH 4 d iffu s i on co mb u stio n s y st e m , Ap pl. T her m al E n g . 1 1 0 (2 0 1 7) 1 44-149 ) ( [38] 1D . L iu, C h emica l eff e c t s of carbon di o xide addi t ion o n dime t hy l ether a n d et hanol f lame s : a co m pa r a tiv e s t u d y , Ener gy F u els 2 9 ( 5 ) (2015 ) 3385 - 3 39 3 . ) ( [39 ] D . S h im o kuri, S . F u kuba , S. I sh i z uk a, F u nda m ent a l i n v es ti g a t io n o n t h e F u e l-NO , e missio n o f t h e o x y -fu el c o m bu stio n w i t h a t u b u lar f l a m e b u r ne r , Pr oc. C o m bu st. I n st . 3 5 (20 1 5)3 5 73- 3 580. ) [40]( ( C .F. Coward, G.W. Jones, US B u reau of Mines Bull 5 0 3, 1 9 52. [41] I 1 . Y amao ka , H . T suj i, E x t in ct ion o f n e ar-s to ich i ome t r ic f l a m es di l u t e d w i t h nitrogen ) in a stagnation flow, Symp. (Int.) Combust. 22 (1988)1565-1572. ( [ 4 2] J . A . M i l l e r , C . T . B owm a n , M echa n is m a n d m o d eli ng o f n itr og en c he m is try in c omb u s t io n , P ro g. E n er gy C o mb u s t . S ci 1 5 ( 1 989) 287- 3 3 8 . ) ( [43] F . L i u, H. G u o, G. J . S m al lw o o d , T h e c h emic a l e f fect o f CO2 r e p l a c em en t of N 2 i n a ir o n t he b ur n ing v e l oc it y o f C H4 and H 2 p rem i xed fl a m es , C ombust . Fla me 1 33 (2 00 3)4 95-497 . ) ( [44] Y . S o ng, C . Z ou, Y . H e, C. Zh eng, T he c hem i c a l m e cha n i s m o f t he ef f e ct of CO 2 on t he t emper a t u r e i n m et hane ox y - fue l c ombu st i o n , Int . J . H e at M a ss Tr a ns f e r 86 (20 1 5 )6 2 2-628. ) ( [45] Y . Z hang , W. S he n, H. Z h an g, Y. Wu, J. L u , E ff e c ts of iner t d i l u tion on t he p ro p a- g a t ion a n d e x ti nc t i on o f l e a n premi x ed s y n ga s / ai r f l a m e s , F u e l 1 57 ( 2 015) . 1 1 5- 12 1. ) Available online September Premixed C3H8/O2 combustion has been investigated by adopting N2, CO2 and their mixture (50% N2-50% CO2 by volume) as the diluents, respectively. The oxygen content (mole fraction) in the oxidizer is varied from 0.13 to 0.35, which covers the operating conditions of EGR and OEC. The flame structures, temperatures, burning velocities and NOx emissions are examined under various oxygen contents. Detailed observations show that asteady, uniform tubular flame can be obtained over a wide range from lean to rich. By increasing oxygen content, the flame diameter increases slightly; the flame length decreases significantly, which is almost inversely proportional to laminar burning velocity. During decreasing the burning velocity to less than about 20 cm/s, a turning point appears and the flame length becomes extensively large. With the same oxygen content and equivalence ratio, by replacing half or all of the N2 diluent with CO2, the flame temperature and burning velocity decrease significantly. To obtain the same adiabatic flame temperature, the CO2 diluted mixture requires the highest oxygen content among the three diluents, however, its measured flame temperature is also highest. In the N2-CO2 diluted combustion, owing to thermal and chemical effects of CO2, the NOx emission reduces remarkably comparing with N2 dilution; under the same flame temperature, the NOx concentration reduces to almost 1/5 of that diluted by N2. The lean extinction limit raises by increasing CO2 content in the diluent, while the limit decreases with increasing oxygen content.
确定




还剩6页未读,是否继续阅读?
北京欧兰科技发展有限公司为您提供《涡流管式火焰燃烧器中CH自发荧光检测方案(粒子图像测速)》,该方案主要用于其他中CH自发荧光检测,参考标准--,《涡流管式火焰燃烧器中CH自发荧光检测方案(粒子图像测速)》用到的仪器有德国LaVision PIV/PLIF粒子成像测速场仪、LaVision IRO 图像增强器、PLIF平面激光诱导荧光火焰燃烧检测系统、LaVision DaVis 智能成像软件平台
推荐专场
相关方案
更多
该厂商其他方案
更多