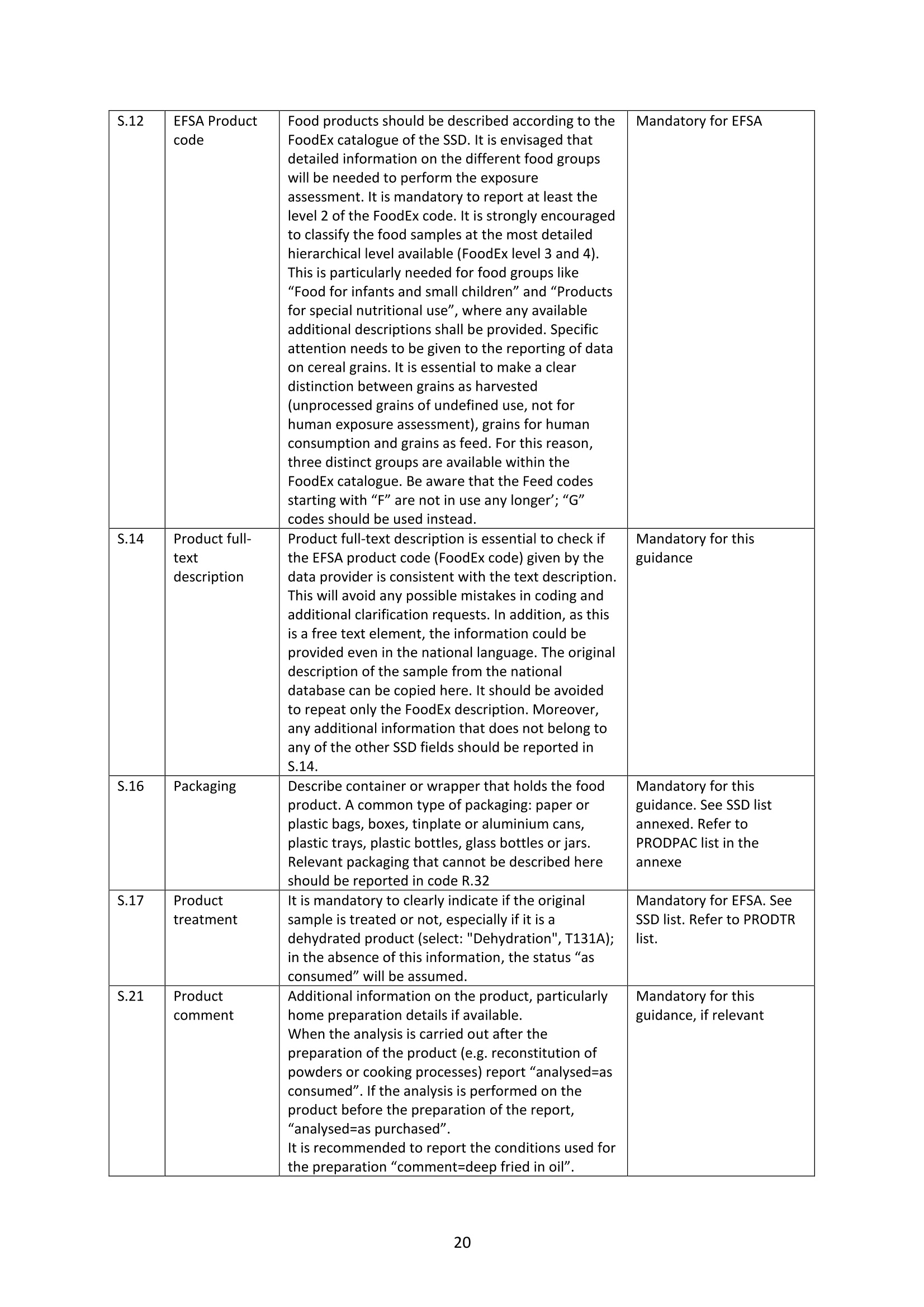
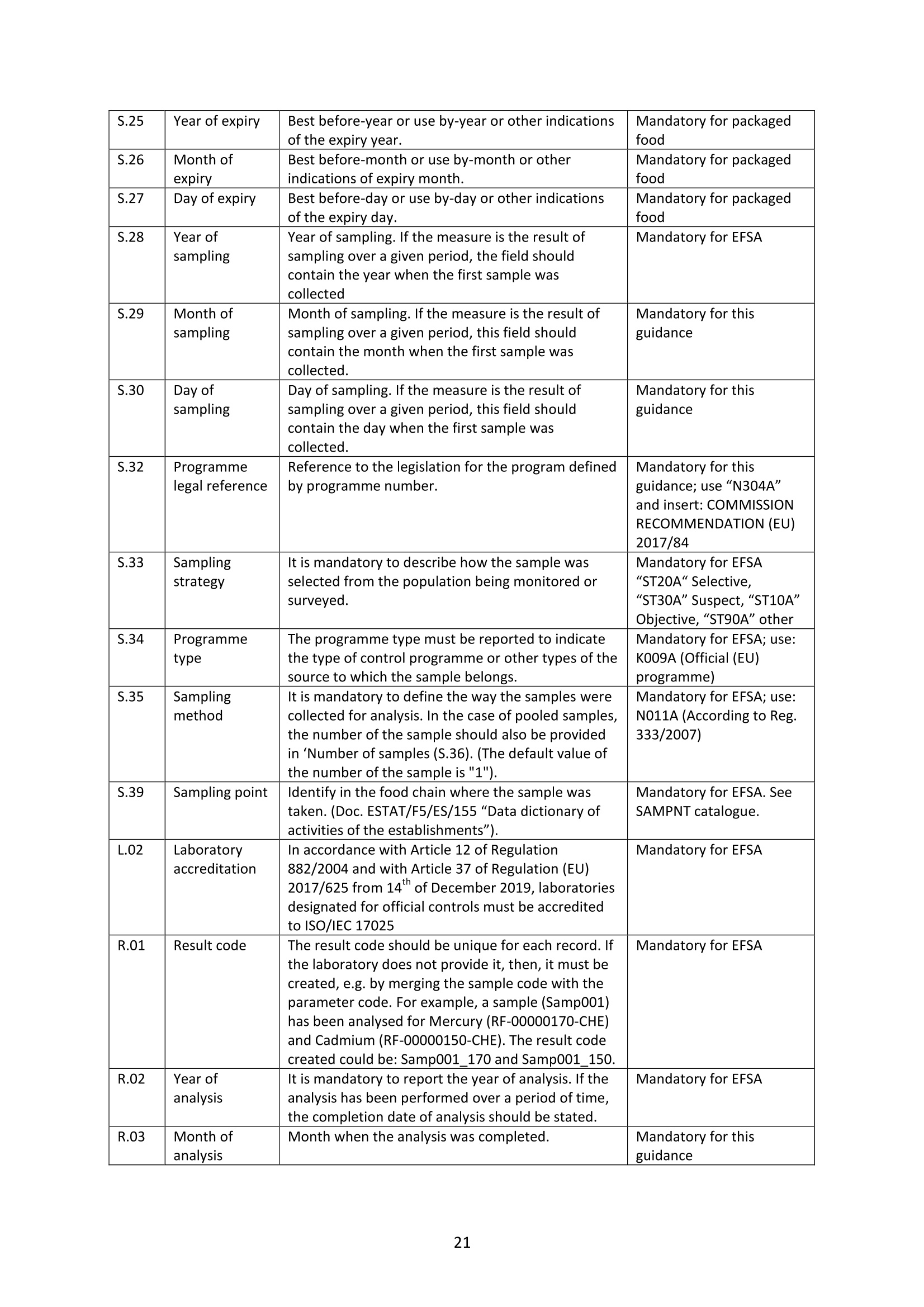
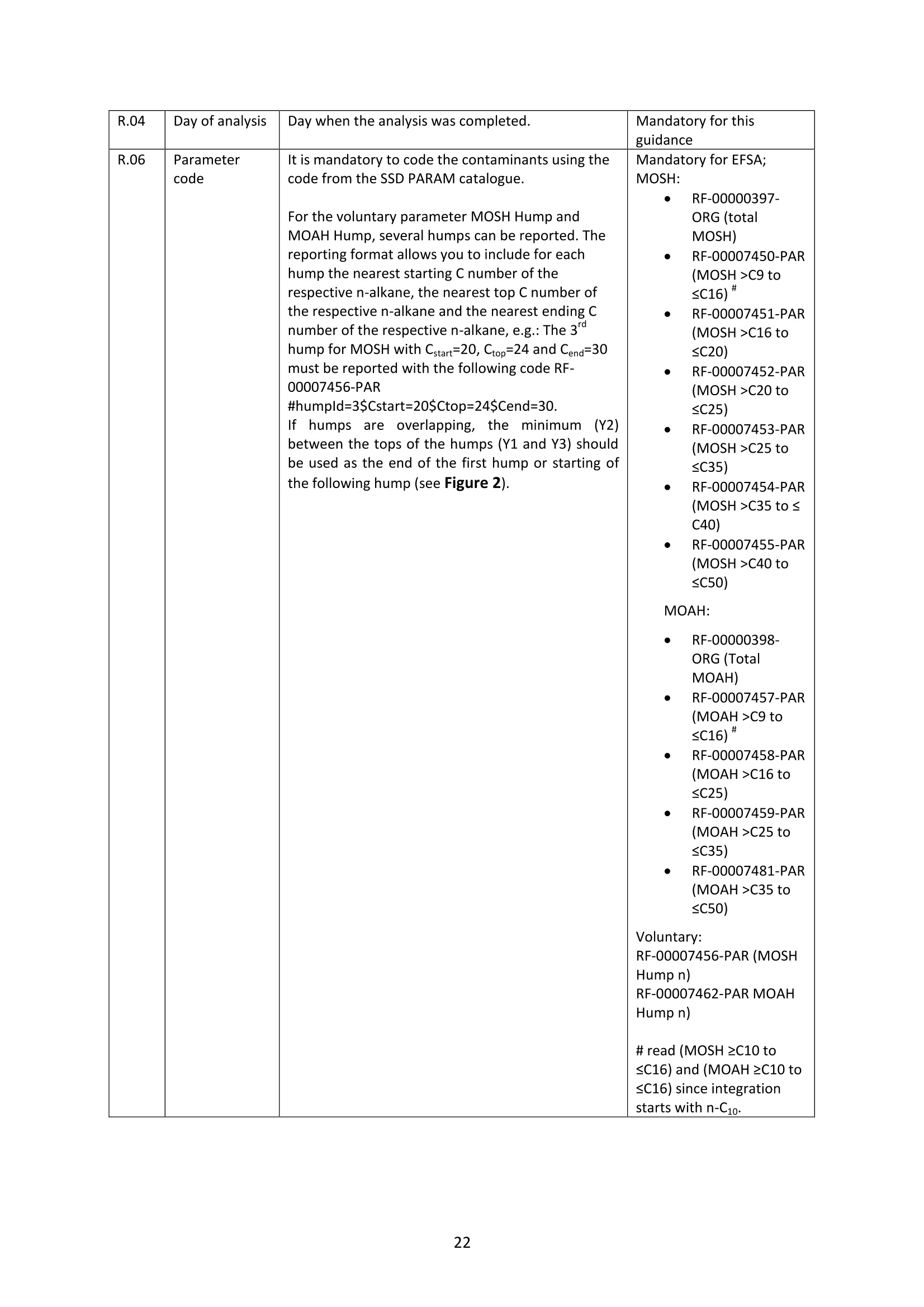
GERSTEL的HPLC-GC-FID系统可在线全自动检测MOSH和MOAH矿物油污染物方案完全符合欧洲标准DIN EN 16695: 2017-08要求。使用双通道GC进样,双FID检测器,30分钟完成MOSH和MOAH的分析,实时显示LC和GC的色谱图。所有部件自行研发制造,无须额外的控制盒来控制样品的分析参数和载气的压力,系统更稳定,控制更容易,并且节省空间。哲斯泰的MAESTRO软件,完全嵌入安捷伦菜单,可以控制LC泵,LC检测器,以及“溶剂蒸发出口”的电磁阀开关时间,以及整个分析序列。自行研发制造的样品制备模块,由MAESTRO软件统一控制,以实现各种样品的自动化制备步骤。如“全自动环氧化”,“馏分收集”以及“油脂中固醇和PAH的分析”等。自带图谱分析软件,自动积分计算MOSH和MOAH的含量,轻松获取测试报告
方案详情

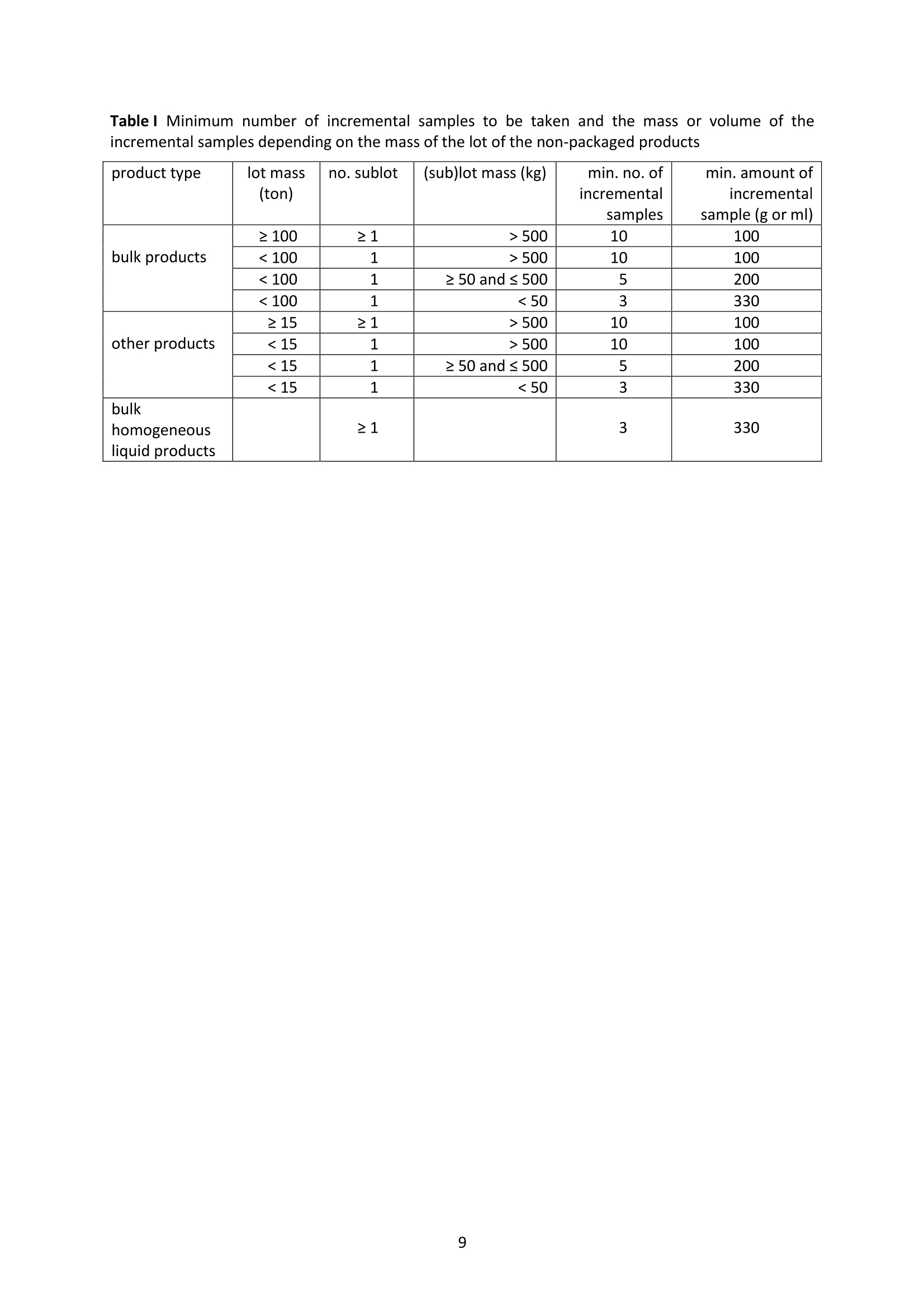
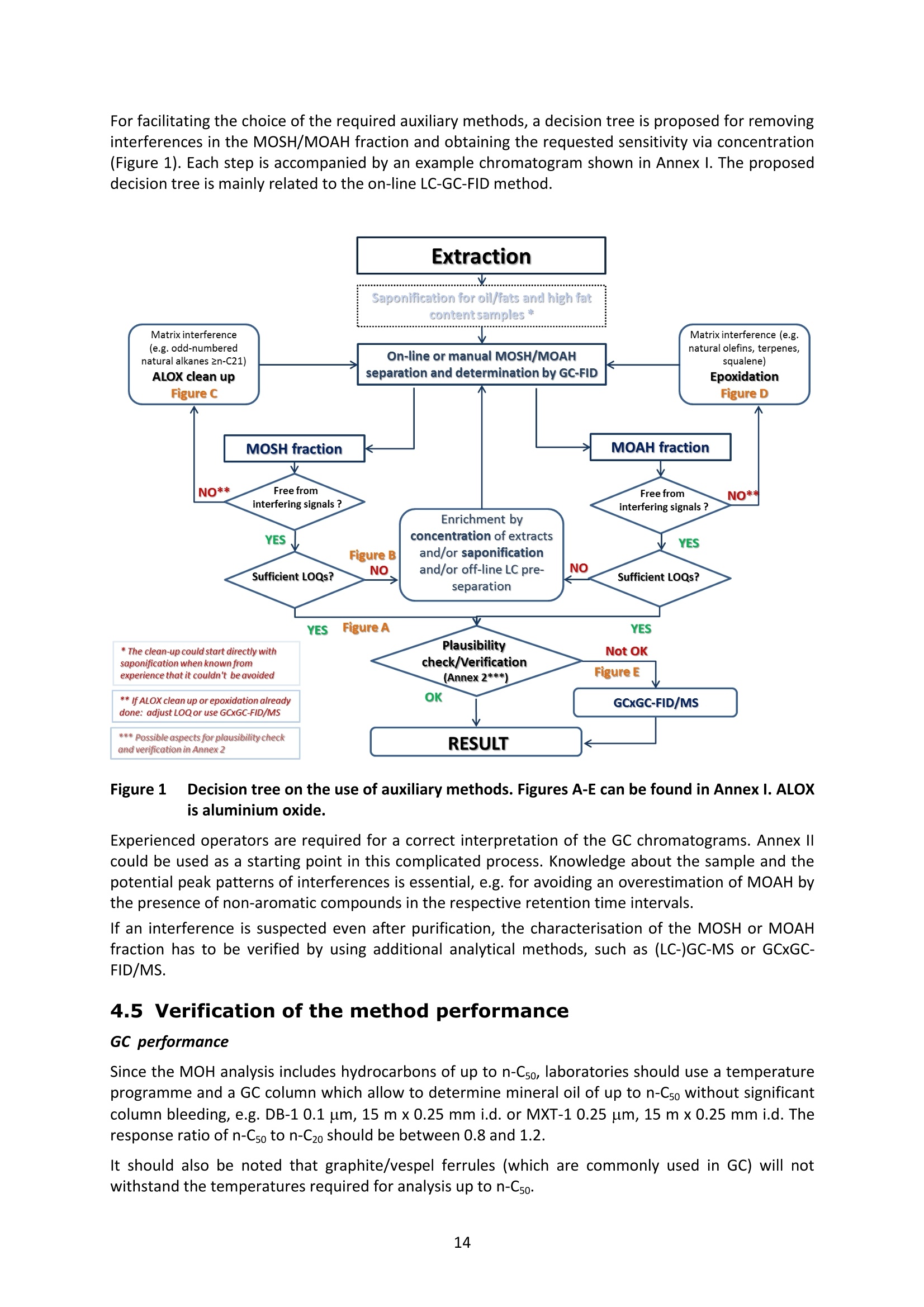
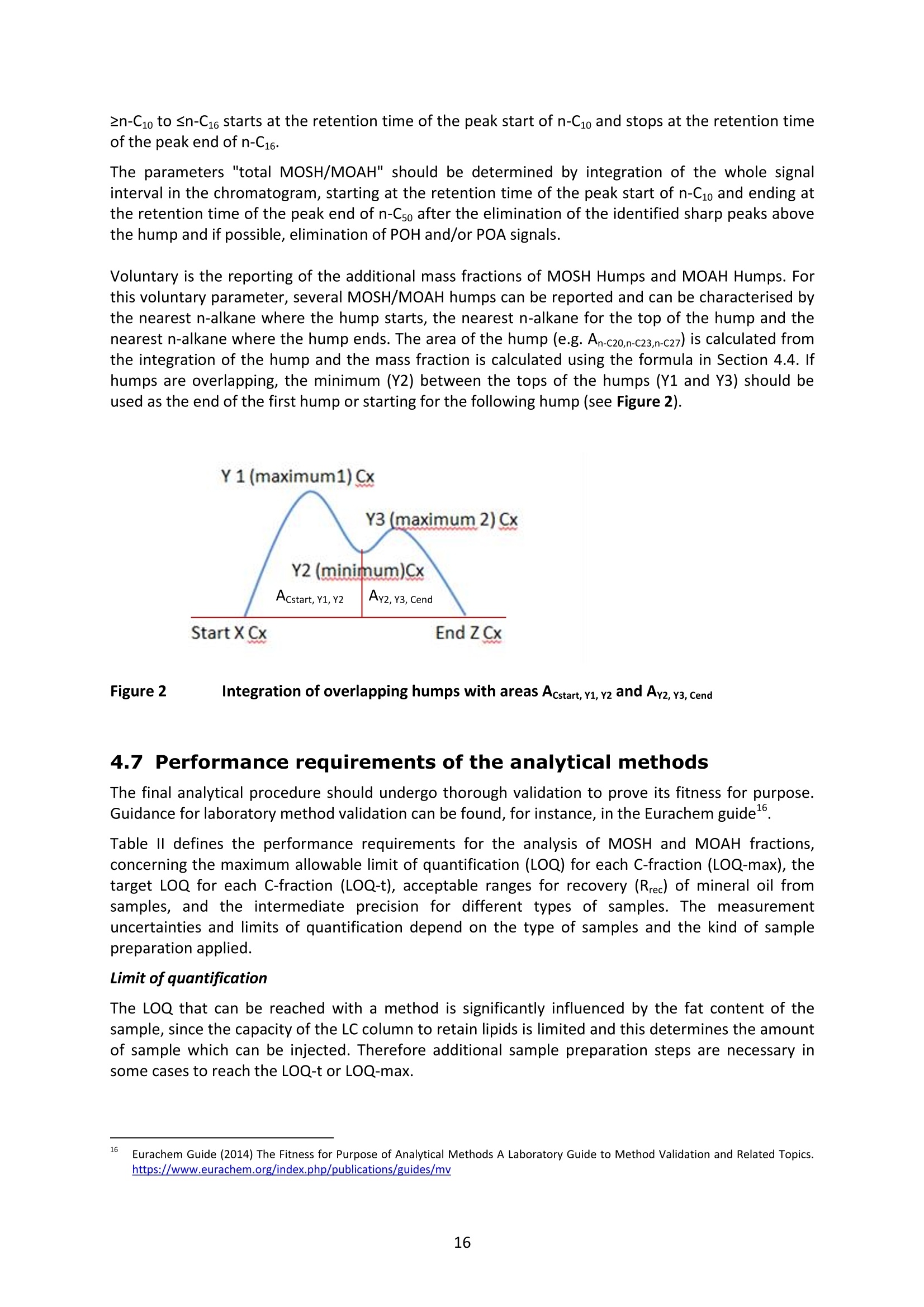
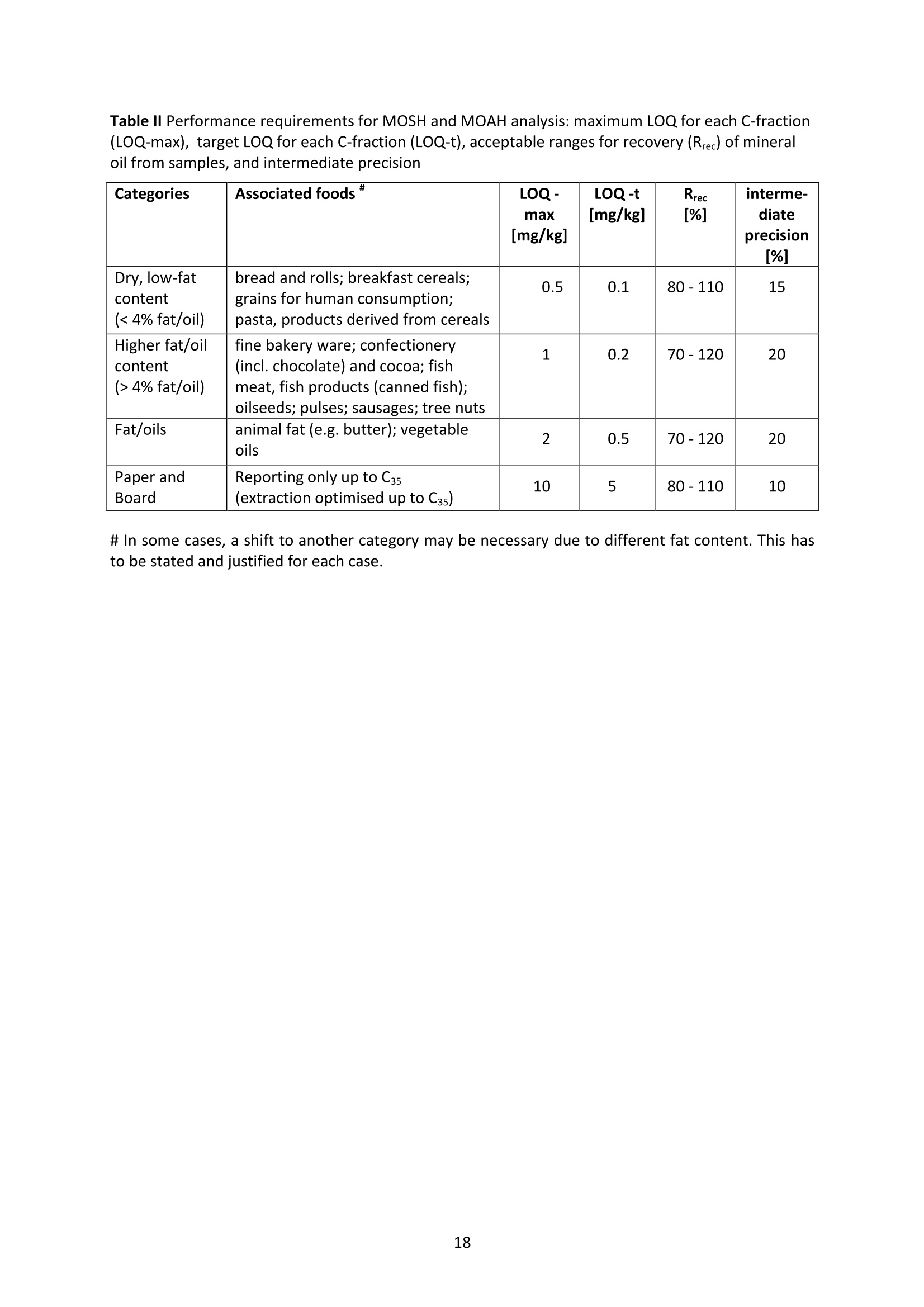
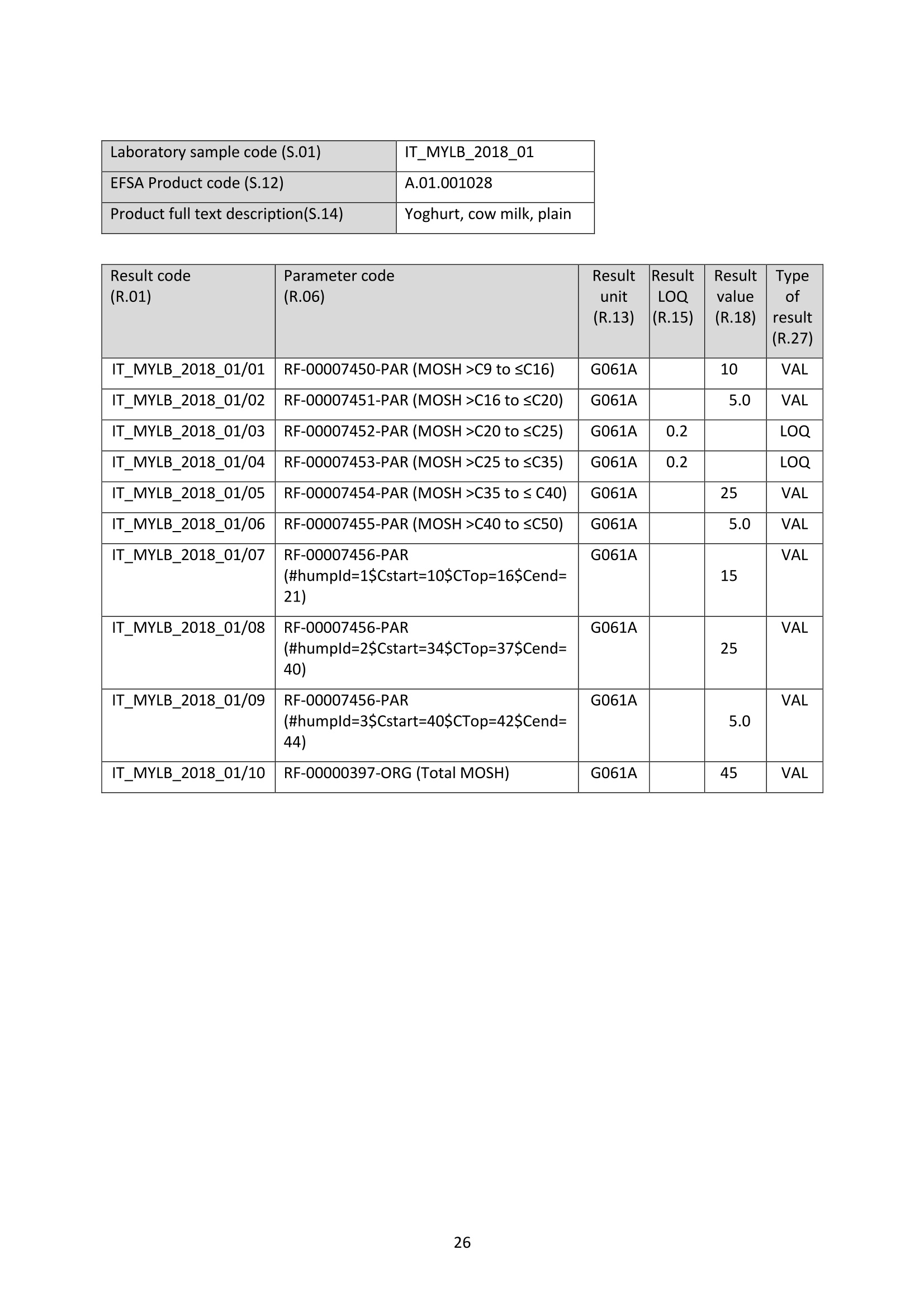
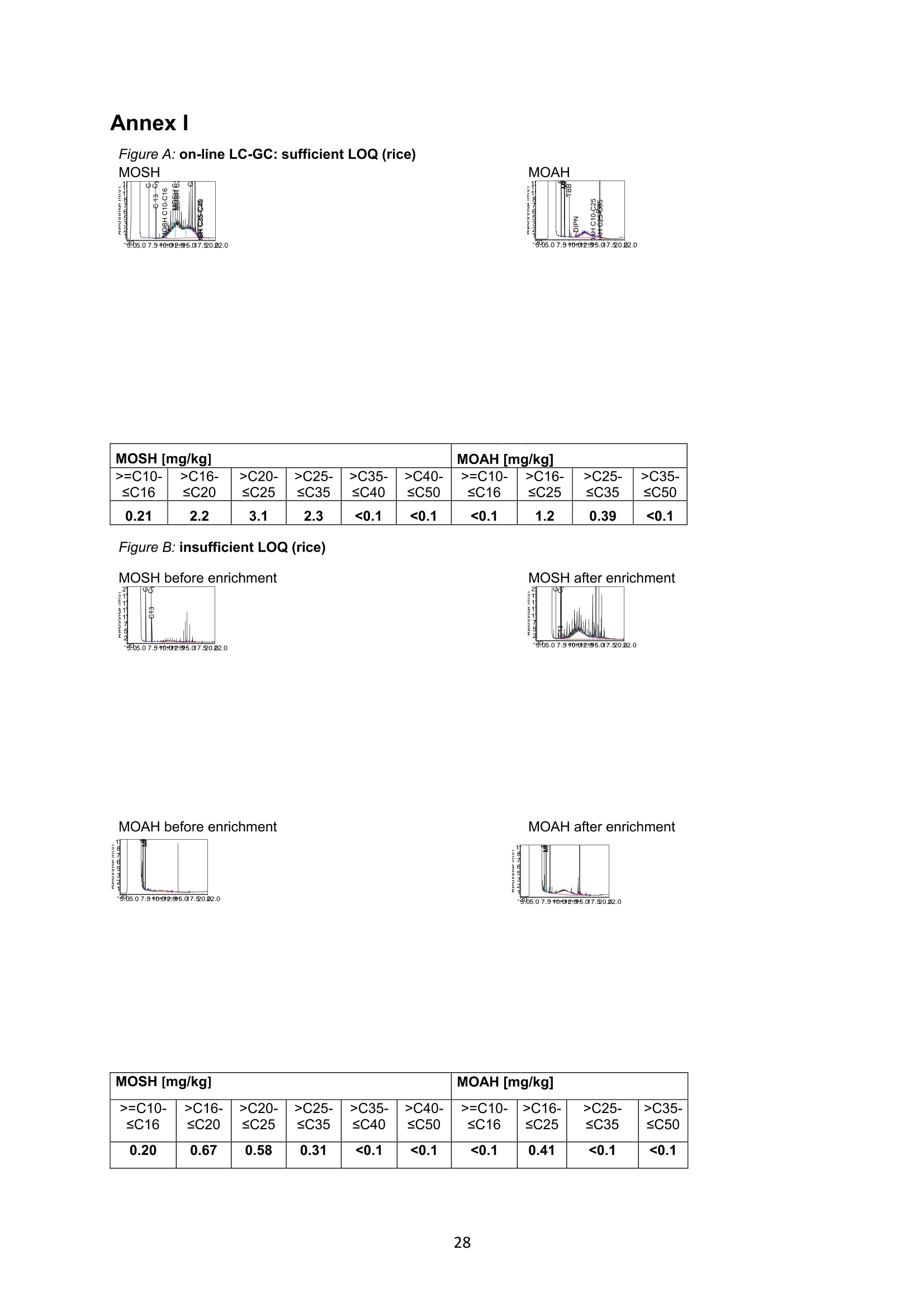
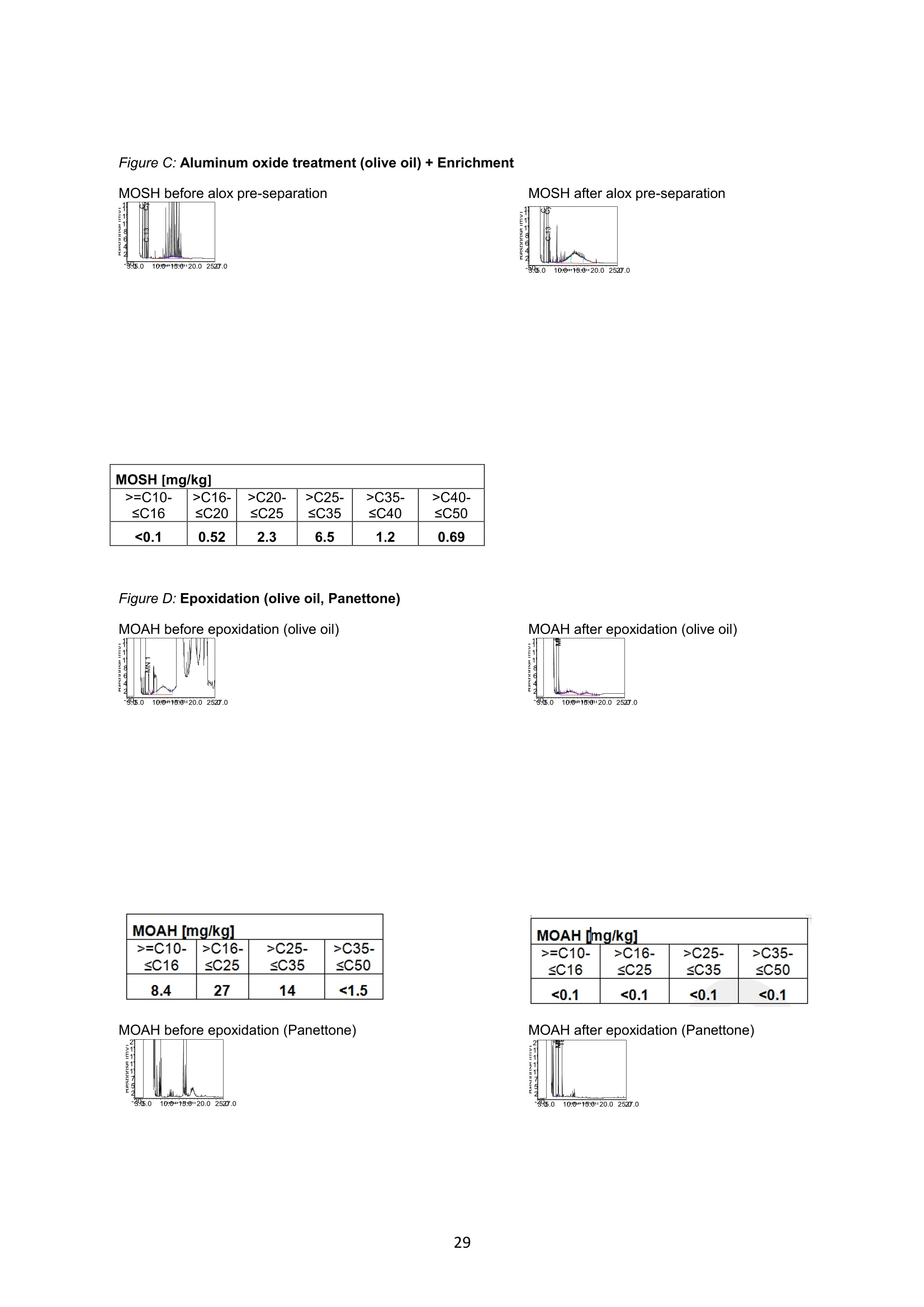
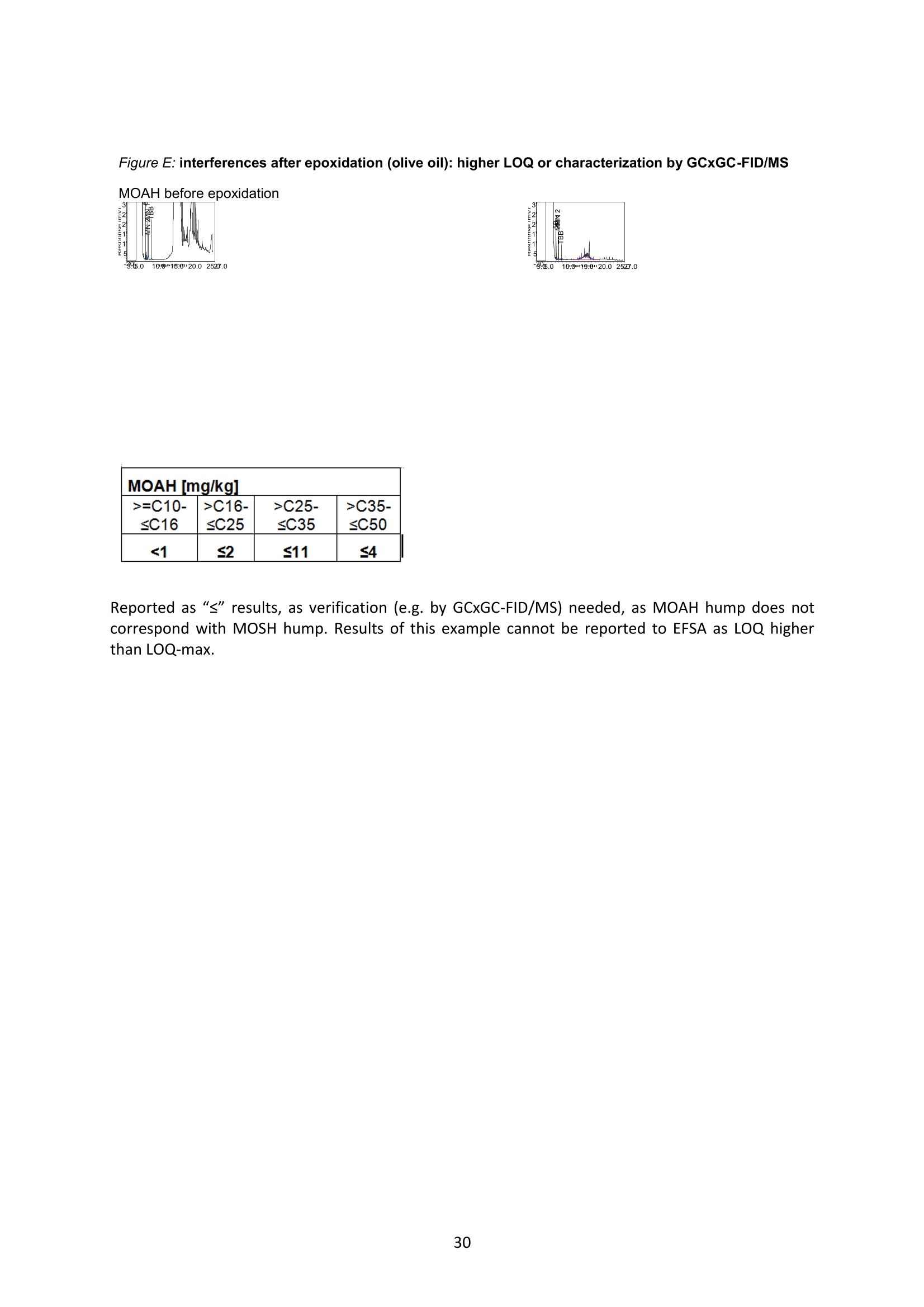
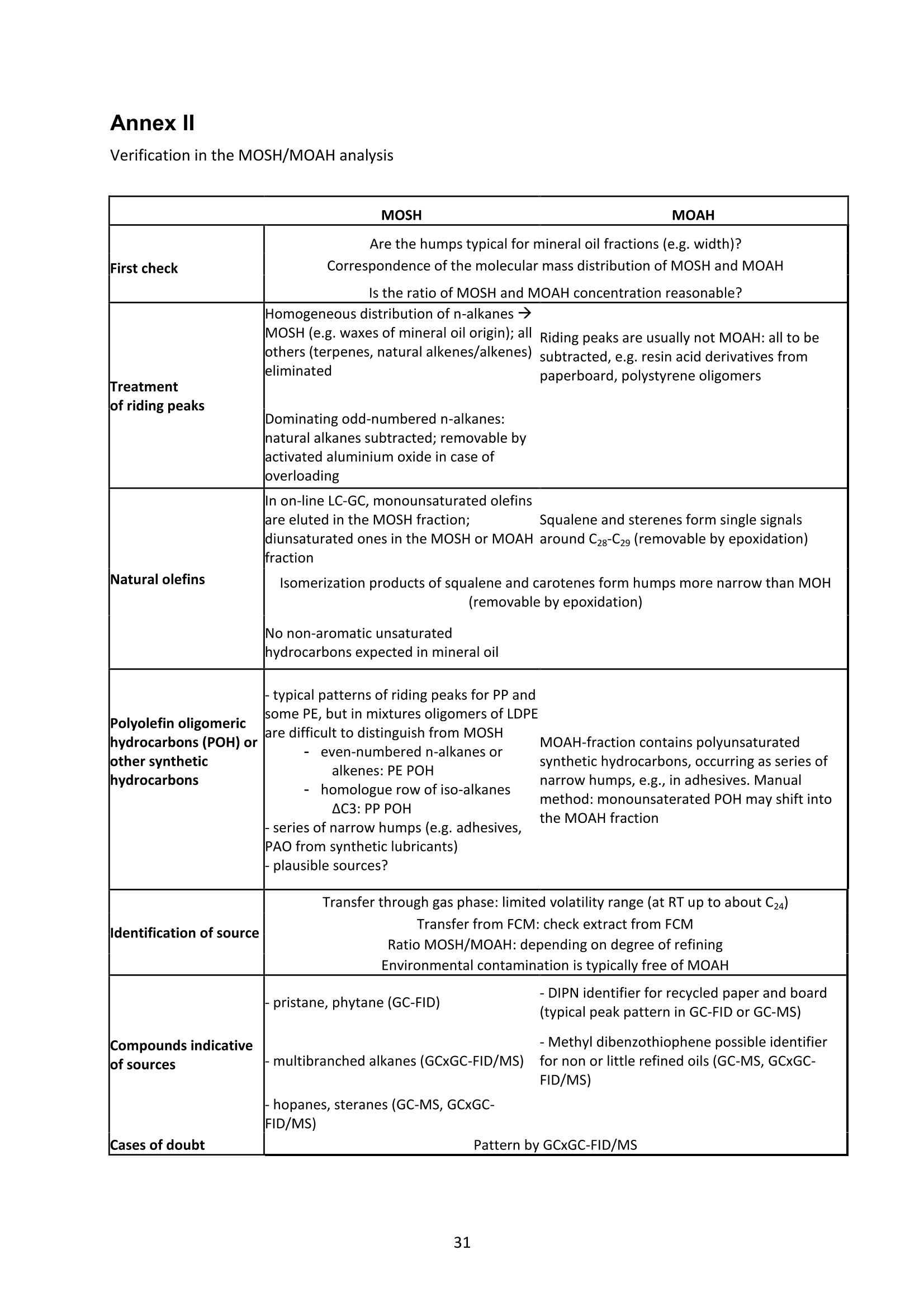
JRC TECHNICAL REPORTS Guidance on sampling, analysis and datareporting for the monitoring of mineral oilhydrocarbons in food and food contactmaterials In the frame of CommissionRecommendation (EU)2017/84 S. Bratinova, E. Hoekstra (Editors) 2019 This publication is a Technical report by the Joint Research Centre (JRC), the European Commission’s science and knowledge service. It aimsto provide evidence-based scientific support to the European policymaking process. The scientific output expressed does not imply a policyposition of the European Commission. Neither the European Commission nor any person acting on behalf of the Commission is responsiblefor the use that might be made of this publication. Contact informationStefanka BratinovaEuropean Commission, Joint Research Centre (JRC)European Union Reference Laboratory for Food Contact MaterialsVia Enrico Fermi, 2749, TP260, 21027 Ispra (VA), Italy JRC-FCM@ec.europa.eu JRC Science Hub https://ec.europa.eu/jrc JRC115694 EUR 29666 EN PDF ISBN 978-92-76-00172-0 ISSN 1831-9424 doi:10.2760/208879 Luxembourg: Publications Office of the European Union, 2019 C European Union, 2019 Reuse is authorised provided the source is acknowledged. The reuse policy of European Commission documents is regulated byDecision 2011/833/EU (OJ L 330,14.12.2011, p.39). For any use or reproduction of photos or other material that is not under the EU copyright, permission must be sought directly from thecopyright holders. How to cite this report: S. Bratinova, E. Hoekstra (Editors) Guidance on sampling, analysis and data reporting for the monitoring of mineraloil hydrocarbons in food and food contact materials, Luxembourg: Publications Office of the European Union, 2019 ISBN 978-92-76-00172-0, doi:10.2760/208879, JRC115694 All images C European Union 2019 Contents Acknowledgements..... ..2 Executive summary....... ..3 1Introduction ...... ..4 2SScope.......... ..5 3. Sampling....... ..6 4Analysis....... .10 4.1Description of the mineral oil hydrocarbons.... .10 4.2 Definition of the measurands........ .11 4.3Background of MOH analysis......... . .m .11 4.4Outline of the analytical approach.. .12 4.5 Verification of the method performance....... .14 4.6 Quantification...... .15 4.77IPerformance requirements of the analytical methods....... .16 5Reporting of results.......... .19......................m ...............................................i................................... .List of abbreviations ....... .27 Annexl...... .28 Annexll......31 Acknowledgements This Guidance has been prepared by the Task Force on Mineral Oil established by the EuropeanUnion Reference Laboratory for Food Contact Materials (EURL-FCM). Members of the Task Force are experts from National Reference Laboratories (NRL) for Food ContactMaterials, experts of food analysis and representatives from DG SANTE and the EURL-FCM: Maurus Biederman and Gregor McCombie (Kantonales Labor Zurich,NRL-CH) Dimitrios Chrysafidis (General Chemical State Laboratory of Greece, EL) Isabelle Deyris and Ronan Jaouannet (Service commun des laboratoires DGDDI et DGCCRF,NRL-FR) Liam Dolan (Health Service Executive,NRL-IE) Thomas Funke and Christophe Goldbeck (Chemisches und VeterinaruntersuchungsamtMunsterland-Emscher-Lippe, DE) Carmen Igualda and Yovana Sanchis (La Fundacion para el Fomento de la InvestigacionSanitaria y Biomedica de la Comunitat Valenciana (Fisabio), ES) Jurg Daniel (Amt fur Verbraucherschutz und Veterinarwesen AVSV St. Gallen, CH) ● Oliver Kappenstein (Bundesinstitut fur Risikobewertung, NRL-DE) Verena Koospal (Chemisches und Veterinaruntersuchungsamt Stuttgart, DE) Wenceslao Moreda (Instituto de la Grasa (IG), Centro de Investigacion de la Agencia EstatalConsejo Superior de Investigaciones Cientificas (CSIC), ES) Els van Hoeck (Scientific Institute of Public Health (WIV-ISP), NRL-BE) Bastiaan Schupp, Veerle Vanheusden and Frans Verstraete (European Commission, DGSANTE) Stefanka Bratinova, Eddo Hoekstra, Giorgia Beldi, Natalia Jakubowska, Lubomir Karasek, PiotrRobouch, Sandro Valzacchi (EURL-FCM,European Commission, Joint Research Centre (JRC)) Furthermore, the following EFSA colleagues are acknowledged for their input to the reporting sectionof this guidance: Marco Binaglia, Stefano Cappe, Valentina Bocca, Davide Gibin, Monica Giulivo andGiulio Di Piazza. Executive summary This guidance document covers specific directions for sampling and analysis of mineral oil saturatedhydrocarbons (MOSH) and mineral oil aromatic hydrocarbons (MOAH) in food and FCM in the frameof Recommendation (EU) 2017/84 for the monitoring of mineral oils. It provides guidance on the minimum performance requirements of the analytical methods fit forMOSH/MOAH monitoring. The guidance should be used by all stakeholders involved in thedetermination of mineral oil hydrocarbons in food and FCM, i.e. food inspectors, official controllaboratories, laboratories in industry and laboratories of non-governmental organisations. This guidance aims to support the generation of reliable data for the occurrence of both fractions -MOSH and MOAH- and to enable reporting by laboratories that are already familiar with theanalytical approaches and have proven their analytical performance in relevant proficiency testing(PT) schemes. For laboratories that are not familiar with MOSH/MOAH analysis, this guidance gives the minimumperformance requirements and references to current analytical approaches described in thescientific literature. It does not provide standard operating procedures. 1.Introduction consumers are exposed to a range of mineral oil hydrocarbons (MOH) via food. Major sources ofMOH in food are food packaging and additives, processing aids, and lubricants. Technical grade MOHcontains up to about 50 % mineral oil aromatic hydrocarbons (MOAH); approved food grade mineraloil saturated hydrocarbons (MOSH) (white oils) are reported to contain less than 1 % of MOAH.Estimated MOSH exposure ranges from 0.03 to 0.3 mg/kg b.w. per day, with higher exposure inchildren. Except for white oils, exposure to MOAH is about 20 % of that of MOSH. In 2012, the Scientific Panel on Contaminants in the Food Chain (CONTAM Panel) of the EuropeanFood Safety Authority (EFSA), on request by the Commission, issued an Opinion' concluding that thepotential human health impact of groups of substances among the MOH vary widely. Occurrence data were available only for a limited number of food groups and only from a fewcountries. At that time MOAH measurements did not exist for the majority of the samples. Until2009, only the MOSH fraction was routinely analysed. Then it was possible to extend the method tothe MOAH fraction. MOAH, however, may act as genotoxic carcinogens, while some mineral oilsaturated hydrocarbons (MOSH) can accumulate in human tissue and may cause adverse effects inthe liver. As some MOAH are considered mutagenic and carcinogenic, it is important to organisemonitoring of MOH to better understand the relative presence of MOSH and MOAH in foodcommodities that are major contributors to dietary exposure. Commission Recommendation (EU) 2017/84 requests the Member States to monitor animal fat,bread and rolls (including fine bakery ware), breakfast cereals, confectionery (including chocolateand cocoa), fish meat, fish products (canned fish), grains for human consumption, ices and desserts,oilseeds, pasta, sausages, tree nuts, vegetable oil. It is required to sample food and pre-packagedfood and to analyse the food and, if relevant, food contact materials (FCM) as well for the presenceof mineral oil and to report the results to EFSA. The analysis of MOH in food and FCM, especially in food with high fat content, is very demanding interms of methodology and interpretation. It requires harmonisation amongst laboratories in terms ofdefinitions, performance characteristics and data reporting to EFSA. According to the Recommendation (EU) 2017/84 "to ensure the reliability of the obtained analyticaldata, Member States should ensure the availability of suitable analytical equipment and gainsufficient experience in the analysis of MOH both in food and in FCM before generating analyticalresults. To ensure the uniform application of this recommendation, the European Union ReferenceLaboratory for FCM (EU-RL) should provide further guidance to the competent authorities of theMember States and other interested parties, including guidance on information that could becollected during investigations, as well as methods of sampling and analysis ... the Member Statesshould collaborate with the EU-RL to jointly develop that guidance in accordance with their needs fordeveloping analytical capabilities." Following the Recommendation, this guidance document aims at giving directions to the MemberStates with respect to sampling, describing the performance characteristics of the analyticalapproaches,as well as defining the way the results should be reported to EFSA. ( E FSA (2012) Scientific Opinion on Mineral Oil Hydrocarbons in Food. EFSA Journal 1 0 (6), 2704. ) ( 2 B iedermann M, Fiselier K, Grob K. (2009)J Agric Food Chem 57, 8711. ) 2Scope This guidance document has been developed for supporting the implementation of CommissionRecommendation (EU) 2017/84. It provides guidance on sampling, analysis and reporting of theresults for the content of total MOSH and MOAH and various carbon fractions of MOSH and MOAH.This guidance shall be used by food inspectors, official control laboratories, commercial laboratories(independent and industry) and laboratories of non-governmental organisations that monitormineral oil in food and FCM. This guidance aims to 1facilitate harmonised sampling of food and FCM for MOSH and MOAH analysis;2 facilitate harmonised reporting to EFSA by laboratories that are already familiar with theanalytical approaches and have proven their analytical performance in relevant proficiencytesting (PT) schemes; 3. give the essential performance requirements for the methods to be applied in MOSH/MOAHanalysis; 4. give references to current analytical approaches described in the scientific literature forlaboratories that are not familiar with the analytical methodology This guidance does not aim to provide standard operating procedures. If other guidance or standard operating procedures are necessary, these would be developed in theframe of future planned hands-on training courses. This guidance should enable stakeholders to sample, analyse and report mineral oils in food and, ifrelevant, in FCM in a harmonised manner. 33Sampling Recommendation (EU) 2017/84 refers explicitly to Regulation (EC) No 333/2007 laying down themethods of sampling and analysis for sampling food, and relating to the control of lead, cadmium,mercury, inorganic tin, inorganic arsenic, 3-MCPD and polycyclic aromatic hydrocarbons in food. Only the following sections of this Regulation are relevant for sampling procedures for mineral oil infood: Annex Part A; Annex Part B: sections B.1.1 (just for official control); B.1.2 to B.1.6, B.1.7 (firstparagraph); B.1.8; B.2 (not the last paragraph related to tin B.2.2); and B.3. Since Recommendation (EU) 2017/84 does not concern official controls, strict adherence toRegulation (EC) No 333/2007 is not obligatory in the context of mineral oil monitoring. In the following paragraph the guidance applies to sampling, where the main focus is on collectingfrom different suppliers as many products as possible in a given food category as listed inRecommendation (EU) 2017/84. Guidance for sampling As the focus of the Guideline is on monitoring and not official control, it is not obligatory to followsections B.1.2, B.1.4 and B.1.5 for the sampling of mineral oil in lots and sub-lots. Moreover, it is not obligatory to adopt section B.1.6, particularly in taking samples for enforcement,defence and referee purposes. If unused packaging material from the same batch that was used to package the food is still availableat the food business operator, then it should also be sampled as it may provide useful information toidentify the source of any contamination that is found in the packaged food. The person performing sampling should take all necessary precautions to avoid contamination of thesample. For example, the use of cosmetics such as hand creams should be avoided. The sample collection tools should be free from mineral oil contamination. Unpackaged food should be sampled in containers which are inert for mineral oil. Only containersthat are impermeable to MOH, do not release interfering substances and do not adsorb MOH, shouldbe used. Glass or polyethylene terephthalate (PET) containers have the identified properties and aremostpreferred. Each new batch of sample containers should be checked for mineral oilcontamination. If a mineral oil contamination is detected, the containers should be washed beforeuse with purified n-hexane and dried at the highest temperature possible. Glass sample containerscould also be annealed, preferably at 400 ℃. Mineral oil contamination of sample containers needsto be checked for each new batch after such treatment. NOTES: Polyolefin sample containers, made of, e.g. polyethylene or polypropylene, may release polyolefinoligomeric hydrocarbons (POH). These containers are not suitable unless appropriate precautionssuch as lining with aluminium foil are taken to prevent contamination of the samples. Metal sample containers and aluminium foil may have a mineral oil film on their surface due totheir production. These containers would only be suitable upon ensuring that they are free ofmineral oil residues. The mineral oil residues could be removed by rinsing with purified n-hexane. Paperboard boxes are generally not suitable even for the secondary packaging of the samples. After collecting the sample, the sample container should be closed with a Polytetrafluoroethylene(PTFE)-layered lid or a glass stopper. Otherwise, the sample container must be covered first withaluminium foil before being sealed with its cap or stopper. The aluminium foil also needs to be checked for residual mineral oil contamination on its surface. No rubber rings should be used to closethe container. Pre-packaged food or FCM should be wrapped in aluminium foil at the point of sampling and keptwrapped until being analysed to prevent cross-contamination. If the pre-packaged food sample isbrought into the laboratory without being wrapped in aluminium foil,this should be documented. All contamination of the sample, e.g. by the use of tape or adhesives (paper/plastic labels) or contactwith paper or paperboard, should be prevented. However, the sample must remain properlyidentifiable, e.g. by using a permanent marker. If glass sample containers cleaned in a washing machine are reused, then the efficiency of thewashing procedure should be checked. Reused sample containers do not need to be checked forresidual mineral oil contamination if the washing procedure was effective in removing such residues. No tape or adhesives (paper/plastic labels) should be used to fix the aluminium foil that covers thepre-packaged food. The sample identification number should be written on the aluminium foil using a permanentmarker. Recording of information during sampling The following information on the food sample should be recorded. The relevant EFSA reporting dataelements are mentioned in brackets. EFSA requirements 3laid down in the Standard Sample Description on Food and Feed (SSD1) are: Laboratory sample code (S.01) - expressed by a unique sample identification number, not longerthan 20 characters. Country of sampling (S.04) - This is the country where the food was selected for laboratorytesting. Country of origin of the product (S.06)- This is the country where the food originated. Area of origin for fisheries or aquaculture activities code (S.08)- FAO Fisheries areas. EFSA Product code (S.12)- Food products should be described according to the FoodExcatalogue of the Standard Sample Description (SSD). It is mandatory to report, at least, level 2 ofthe FoodEx code. It is strongly encouraged to classify the food samples at the most detailedhierarchical level available (FoodEx level 3 and 4). This is particularly needed for food groups like"Food for infants and small children” and “Products for special nutritional use”, where anyavailable additional descriptions shall be provided. Specific attention needs to be given to the reporting of data on cereal grains. It is essential tomake a clear distinction between grains as harvested (unprocessed grains of undefined use, notfor human exposure assessment), grains for human consumption, and grains as feed. Product full-text description (S.14) - This is essential to check if the EFSA product code (FoodExcode) given by the data provider is consistent with the text description. This will avoid anypossible mistakes in coding and additional clarification requests. Also, as this is a free textelement, the information could be provided even in the national language. The originaldescription of the sample from the national database can be copied here. It should be avoidedto repeat just the FoodEx description. Moreover, any additional information that does notbelong to any of the other SSD fields should be reported in S.14. ( EFSA (2010) Standard sa m ple description for food an d feed. EF S A Journal 8(1), 1457 ) ● Packaging (S.16)- Describe the container or wrapper that holds the product, e.g. multi-layermaterial or inner bag incl., further information on the material of layers; the presence of abarrier and assembled packaging material. Product treatment (S.17) - It is mandatory to indicate explicitly if the original sample is treatedor not, especially if it is a dehydrated product. Product comment (S.21)-Additional information on the product, particularly preparation detailsif available. Year, month and day of expiry (S.25, S.26, S.27) - Best-before date or use by year or otherindications of the expiry date. ● Year, month and day of sampling (S.28, S.29, S.30)- If the sample is the result of sampling over agiven period, this field should contain the year, month or day when the first sample wascollected. Sampling strategy (S.33)- It is mandatory to describe how the sample was selected from thepopulation being monitored or surveyed. . Programme type (S.34)-The sampling programme type must be reported to indicate the type ofcontrol programme or other types of the source to which the sample belongs. Sampling method (S.35)- It is mandatory to define the way the samples were collected foranalysis. In the case of aggregated samples, the number of the incremental samples should bereported. Sampling point (S.39)- Point in the food chain where the sample was taken. History of the food or pre-packaged sample- e.g. about possible contamination sources duringfood processing or contact with secondary packaging, transport boxes, jute bags, batching oils"(R.32). Additional information: Article number. European Article Numbering (EAN) code . Batch or lot number. ● Total mass of aggregated food sample. ● Labels (physically or photocopy)- In the context of pre-packaged food in paper and board, themass of the food and the packaging needs to be determined. Mass of packaged food sample (if possible). Mass of packaging material (if possible). Table I summarises the minimum number of incremental samples to be taken and the mass of theincremental samples depending on the mass of the (sub) lot of the non-packaged products. ( T oolbox for Preventing t he T ransfer of Undesired M ineral Oil H ydrocarbons i n to Food (2017) G e rman Fed e ration of Food Law and Food Science (B l l e. V ). ht t p s: //www.bll.de/download/to o lb o x-for-preven t ing-t h e - transfer-of-undesired-mineral - oi l -hydrocarbons- into-food 5 ) ( https://en.wikipedia.org/wiki/International Ar t icle Number ) Tablel Minimum number of incremental samples to be taken and the mass or volume of theincremental samples depending on the mass of the lot of the non-packaged products product type lot mass(ton) no. sublot (sub)lot mass (kg) min. no. of incremental samples min. amount of incremental sample (g or ml) bulk products ≥100 ≥1 >500 10 100 <100 1 >500 10 100 <100 1 ≥50 and≤500 5 200 <100 1 <50 3 330 other products ≥15 ≥1 > 500 10 100 <15 1 >500 10 100 <15 1 ≥50 and ≤ 500 5 200 <15 1 <50 3 330 bulk homogeneousliquid products ≥1 3 330 4 Analysis 4.1L Description of the mineral oil hydrocarbons Mineral oil hydrocarbons (MOH) are a complex mixture of hydrocarbons, which originate from crudemineral oils or which are produced from coal, natural gas or biomass through Fischer-Tropschsynthesis. MOH does not include hydrocarbons: - naturally occurring in food: such as n-alkanes of odd numbered carbons (from C21 to C35) ornatural olefins of terpenic origin (such as squalene, sterene or carotenoids ). - such as POH (polyolefin oligomeric hydrocarbons) potentially migrating from plastic packaging(e.g. polyethylene or polypropylene packaging) or synthetic isoparaffins with short and long sidechains used e.g. in synthetic lubricants and adhesives. MOH are divided into two main types-MOSH and MOAH. Mineral oil saturated hydrocarbons (MOSH)° MOSH comprise paraffins (open chain hydrocarbons) and naphthenes (cyclic hydrocarbons), whichare mostly highly alkylated and originate either directly from mineral oil or are formed duringrefining by hydrogenation of aromatic compounds or other conversion processes. Paraffins (open chain hydrocarbons) are distinguished from naphthenes (hydrocarbons with at leastone saturated ring). Paraffins can be grouped into the linear n-alkanes (those with at least about 20carbons are forming waxes) and the branched hydrocarbons, usually being liquids. Naphthenes tendto be highly alkylated and originate either from mineral oil or from hydrogenation of aromatics. Mineral oil aromatic hydrocarbons (MOAH)° MOAH contain at least one aromatic ring. They include polyaromatic compounds, but should bedistinguished from the compounds commonly termed polyaromatic hydrocarbons (PAH), such asbenzopyrenes, which are formed at high temperatures. PAH are only slightly alkylated and can beanalysed as individual substances, whereas MOAH are usually alkylated to more than 98 %, consistof large numbers of compounds and form broad chromatographic signals (humps) with hardly anysharp peak signal on top. Polyolefin oligomeric hydrocarbons (POH) POH are oligomers of polyolefins, such as polyethylene, polypropylene and polybutylenes. FCM usescomprise plastic bags, containers or films, sealable heat layers (e.g. in aluminium bags) and otherlamination as well as adhesives and plasticisers. The POH that are potentially relevant for humanhealth and determined by the described method are at the low end of the molecular mass range ofthe oligomers. They largely consist of branched hydrocarbons, sometimes mono-unsaturatedhydrocarbons and are eluted from the HPLC column in the MOSH fraction. POH may sometimes bedistinguishable from MOSH by their chromatographic pattern, but it is difficult (and for some POHimpossible) to differentiate and chromatographically separate them from the MOSH if both arepresent. Poly Alpha Olefins (PAO)° Similar to POH, PAO are isoparaffins with short main hydrocarbon chains and long side chains ofsynthetic origin.Usually they can be identified by their characteristic chromatographic pattern. ( Biedermann M., Grob K. (2012). Journal of Chrom. A 1255,56 ) ( ' G rob K ., B iedermann M., Caramaschi A., P acciarelli B. (1 9 91) J. High Resolut. Ch r omatogr. 14, 33 ) ( °Biedermann M., GrobK. (2012) Journal of Chrom. A 1255, 76 ) 4.2 Definition of the measurands For the analytical determination of the MOSH/MOAH content in food and FCM measurands have tobe defined, which reflect the MOSH/MOAH descriptions above. MOSH The total MOSH measurand is defined as the total mass fraction of MOSH - expressed inmg MOSH / kg sample -after separation from MOAH and removal of all possible interferences in theextract, as quantified by integration of the whole signal interval in the GC/FID chromatogrambetween the retention times of the peak start of n-C10 and the peak end of n-Cso after subtracting theidentified sharp peaks not belonging to MOSH and using cyclohexylcyclohexane (CyCy) as internalstandard (IS). Another hydrocarbon could be used as IS, provided its response factor is identical. Other detectiontechniques are acceptable, provided that equivalent results are demonstrated. The MOSH fraction may include polyolefin oligomeric hydrocarbons and hydrocarbons from polyalpha olefins (PAOs) in case where their separation/substraction is impossible. The presence of POHand/or POA should be clearly reported. MOAH The total MOAH measurand is defined as the total mass fraction of MOAH - expressed inmg MOAH/ kg sample - after separation from MOSH and removal of all possible interferences in theextract, as quantified by integration of the whole signal interval in the GC/FID chromatogrambetween the retention times of the peak start of n-Ci0 and the peak end of n-Cso after subtracting theidentified sharp peaks not belonging to MOAH and using 1-or 2-methylnaphthalene as IS. Another hydrocarbon could be used as IS, provided its response factor is identical. Other detectiontechniques are acceptable, provided that equivalent results are demonstrated. 4.3 Background of MOH analysis Over the past decade several approaches have been suggested for the determination of MOSH andMOAH in FCM and food, all of them having certain advantages and drawbacks. MOSH/MOAHseparation and its subsequent determination could be achieved by applying on-line LC-GC-FID, off-line HPLC followed by GC-FID or manual off-line separation of MOSH/MOAH followed by GC-FID. It is not possible to separate the mineral oils into single components because they typically contain acomplex mixture of alkanes and other compounds. The combination of LC, which separates MOSHfrom MOAH, and GC-FID for quantification allows for an appropriate determination of the MOSH andMOAH content. In the GC-FID chromatograms of the MOSH and MOAH fractions, further fractionscan be defined based on the retention time of the corresponding n-alkanes under the samechromatographic conditions. It has been decided in agreement with EFSA to collect data for mineraloils up to n-Cso atoms in their molecules in order to reflect the composition of some lubricant oil withheavier oil fractions. Until recently, all the reported data included only hydrocarbons up to n-C40. On-line LC-GC has the advantages of high separation efficiency, high sample throughput, reducedsolvent consumption and sample manipulation, thus enhancing the reproducibility of the method.On-line LC-GC-FID analysis enables the re-use of the same LC column. Solvent consumption is lowerthan with most conventional liquid chromatographic sample preparation methods, including solid ( Biedermann-Brem, Kas p rick N., Simat T., G r ob K. ( 2 012) Food Addit.Contam. A 29, 449. ) phase extraction (SPE). On-line coupling to GC is integrating sample preparation into the final analysisand is fully automating rather complex procedures. As it is a closed system, it also avoidscontamination during sample preparation, which is of particular importance for analytes that arewidely present in laboratories, such as mineral oil hydrocarbons. On the other hand, the sensitivity islimited by the capacity of the LC column. Dedicated instrumentation and skilled operators arerequired. An LC column could also be applied for a pre-separation in the off-line mode by collecting the MOSHand MOAH fractions using automated fraction collectors. LC separating columns with a largerinternal diameter (4.6 mm instead of 2 mm) could be used for this approach. A larger sample couldbe injected into the LC column compared to on-line coupling. In order to achieve similar detectionlimits as in on-line coupling, a fifth of the fraction should be injected into the GC. Nevertheless itrequires larger volume injection in the GC system even if the fractions are significantly enrichedbeforehand. This is the greatest challenge of this technique, in addition to contamination-relatedproblems that could occur during the collection of the MOSH and MOAH fractions. The third possibility is to follow a pure "off-line" method, using a glass column filled with silica/AgNOgto separate the MOSH and MOAH fractions1. The method is very time consuming and requires strictmeasures to prevent contamination of the sample from the consumables and the environment. Flame ionisation detection (FID) is neither sensitive nor selective. It provides almost identicalresponses to all hydrocarbons, making it a preferred detector for MOSH/MOAH quantifications.However, due to the lack of selectivity, additional sample preparation techniques to eliminateinterferences and to enrich both MOSH and MOAH’fractions may need to be applied. Until now, most of the data on the MOSH and MOAH content in foods and FCM were produced afterapplying chromatographic separation using automated on-line coupled LC-GC and quantification byFID. Today, the LC-GC-FID method is referred to as the method of choice for the quantification of mineraloils in routine analysis111. Many private laboratories apply this on-line method since it has clearadvantages over the "manual"off-line methods, despite the need for a sophisticated instrument. With difficult samples and matrices, further characterisation of the MOSH/MOAH fractions can beperformedby using additional analytical techniques, e.g. GC-MS, LC-GC-FID/MS or GCxGC-FID/Ms13,14,15. However the need for further characterisation must be decided on a case by case basisby an experienced analyst. 4.4 Outline of the analytical approach As a general recommendation, the methods published by Kantonales Labor Zurich and BfR6,9,11 can befollowed for determination of the MOSH/MOAH content in food and FCM. Also other approaches,complying with the performance requirements as defined in section 4.6 could be applied. In short MOSH and MOAH are extracted from the sample matrix using an organic solvent after theaddition of internal and verification standards. The extract is submitted to isolation and separation ofthe MOSH and MOAH fractions. MOSH and MOAH fractions are separated on a HPLC silica gelcolumn or a glass column filled with silica/AgNOs using e.g. a n-hexane/dichloromethane gradient. ( Th e compendium of the Federal Inst i tute for Risk Assessment (Bf R ) and the Cantonal Lab o ratory of Zurich (KLZH) (2012)“Determination of mineral oil II h I ydrocarbons SB in foo da ndpackagin g m a terial". ht t ps:/ /www. b fr.b u nd.de/c m / 3 43/ me s s ung- von - mineral oel-kohlenwasserstoffen-in -l ebensmit t eln-u n d-ve r packungsm a teri a l i en . pdf ) ( "Biedermann M, Munoz C, Grob K. (2017) J Chrom.A 1521, 140 ) ( Spack L., Leszczyk G . , Varela J., Simian H., Gude T., Stadle r R. (2017) Food Additive s & Contaminants: Part A 34(6), 1052 ) ( Po p ulin T., Biedermann M., Gr o b K., Moret S., Conte L. (2004) Food Additives and Con t aminants 21(9), 893 ) Each fraction is transferred in large volume either on-line or off-line to a GC pre-column. Solventvapours are discharged via a solvent vapour exit located between the uncoated pre-column and theGC separation column or by using a solvent vent in a PTV injector. Volatile components are retainedby solvent trapping applying a partially concurrent eluent evaporation. High boiling components,spread over the entire length of the flooded zone, are refocused by the retention gap technique. The signal area in the FID chromatogram attributed to MOSH/MOAH is calculated by integration ofthe chromatogram covering the range of en-C10 to C2s n-alkanes. Epoxidation is a purification step that may be necessary for the quantification of MOAH. Thispurification step allows the elimination of olefins like squalene, which elute within the MOAHfraction and interfere with its quantification (e.g. olive oil, palm oil). Epoxidation also removes certainolefins co-eluting with the MOSH fraction. Therefore, epoxidation may also be used as a purificationstep for the MOSH fraction as well. So far, the epoxidation step is the best compromise to removeolefins even though it is in some cases not fully quantitative and the efficiency may be sampledependent. Depending on the sample, this reaction may induce the epoxidation of a part of theMOAH or the incomplete removal of interfering olefins. Saponification followed by extraction could also be applied to difficult samples with a high fatcontent. Saponification has the advantage that it can be applied to all food types (dry and wet, witheither a low or a high fat content), avoiding the need to remove water before the extraction step.Furthermore, it efficiently removes high amounts of fat, but it can be very solvent- and time-consuming. Further lowering of the quantification limits by enrichment of the MOAH and/or MOSH fractionscould be necessary for some samples. For facilitating the choice of the required auxiliary methods, a decision tree is proposed for removinginterferences in the MOSH/MOAH fraction and obtaining the requested sensitivity via concentration(Figure 1). Each step is accompanied by an example chromatogram shown in Annex l. The proposeddecision tree is mainly related to the on-line LC-GC-FID method. Figure 1 Decision tree on the use of auxiliary methods. Figures A-E can be found in Annex l. ALOXis aluminium oxide. Experienced operators are required for a correct interpretation of the GC chromatograms. Annex Ilcould be used as a starting point in this complicated process. Knowledge about the sample and thepotential peak patterns of interferences is essential, e.g. for avoiding an overestimation of MOAH bythe presence of non-aromatic compounds in the respective retention time intervals. If an interference is suspected even after purification, the characterisation of the MOSH or MOAHfraction has to be verified by using additional analytical methods, such as (LC-)GC-MS or GCxGC-FID/MS. 4.5 Verification of the method performance GC performance Since the MOH analysis includes hydrocarbons of up to n-Cso, laboratories should use a temperatureprogramme and a GC column which allow to determine mineral oil of up to n-Cso without significantcolumn bleeding, e.g. DB-1 0.1 um, 15 m x 0.25 mm i.d. or MXT-1 0.25 um, 15 m x 0.25 mm i.d. Theresponse ratio of n-Cso to n-C20 should be between 0.8 and 1.2. It should also be noted that graphite/vespel ferrules (which are commonly used in GC) will notwithstand the temperatures required for analysis up to n-Cso. selection ofinternal and verification standards9 In principle, many hydrocarbons are suitable as internal standards for the quantification of the MOSHand MOAH fraction by FID since the response factors are very similar. The same holds for verificationstandards, which control whether the analytical procedure or the LC or GC column are performingwell. MOSH Cyclohexylcyclohexane (Cycy) is a suitable internal standard. It is not found in relevant quantities inmineral oils and packaging. Cycy is eluted just before n-C13 from apolar GC columns (coated withdimethylpolysiloxanes). This separation is usually incomplete on dimethylpolysiloxanes with 5%phenyl substitution. To keep the loss of internal standard under control, a verification standard of n-C11 could be added inthe same amount as Cycy. In case of losses of volatiles, n-Ci1 is likely to be lost to a greater extentthan Cycy. Caution need to be applied when the n-Ci1 area is smaller than Cycy: it may indicate lossof Cycy by evaporation or that Cycy was co-eluted with a sample component. A second verification standard, n-C13, could be added at half of the amount of Cycy. It is elutedclosely after Cycy, and it creates a typical pair of peaks that is easily recognised. Furthermore, itenables the verification of the chromatographic separation of CyCy and n-C13 for each analysis. MOAH The standards for MOAH analysis might be selected analogously. The closely eluting pair of 1- and 2-methylnaphthalene (MN) is easily recognised as internal standards.The two peaks should be of thesame area: a difference could indicate co-elution of the larger peak with a sample component. n-Pentyl benzene (5B) can be used to monitor the losses of volatiles. 1,3,5-Tri-tert-butyl benzene (TBB) was historically introduced as a marker for the start of the MOAHfraction, however it turned out in the context of MOH analysis in cosmetic, that di(2-ethylhexyl)benzene (DEHB) is more suitable as it elutes together with the first MOAH. It is proposed as a markerfor the start of the MOAH fraction in a recent publication. (TBB)/DEHB and perylene (Per) could verify the start and the end of the MOAH fraction. 4.6;Quantification MOSH and MOAH are quantified according to the equation mentioned in Section 4.4. Sub-fractions of MOSH and MOAH (so-called C-fractions) in the chromatograms are defined by theposition of the elution signals of n-alkanes from the GC column. The following MOSH and MOAH C-fractions are defined: MOSH: MOAH: total MOSH Total MOAH MOSH≥n-C10 to sn-C16 MOAH ≥n-C10 to ≤n-C16 MOSH>n-C16 to n-C16 to ≤n-C25 MOSH>n-C20 to sn-C25 MOAH >n-C25 to ≤n-C35 MOSH>n-C25 to sn-C35 MOAH >n-C35 to n-C5o MOSH>n-C35 to n-C40 to sn-C50 Each C-fraction starts at the retention time of the peak end of the first n-alkane of the range andstops at the retention time of the peak end of the second n-alkane of the range. Only the C-fraction ≥n-C10 to sn-C16 starts at the retention time of the peak start of n-C1o and stops at the retention timeof the peak end of n-C16. The parameters "total MOSH/MOAH" should be determined by integration of the whole signalinterval in the chromatogram, starting at the retention time of the peak start of n-Cio and ending atthe retention time of the peak end of n-Cso after the elimination of the identified sharp peaks abovethe hump and if possible, elimination of POH and/or POA signals. Voluntary is the reporting of the additional mass fractions of MOSH Humps and MOAH Humps. Forthis voluntary parameter, several MOSH/MOAH humps can be reported and can be characterised bythe nearest n-alkane where the hump starts, the nearest n-alkane for the top of the hump and thenearest n-alkane where the hump ends. The area of the hump (e.g. An-c20,n-C23,n-C27) is calculated fromthe integration of the hump and the mass fraction is calculated using the formula in Section 4.4. Ifhumps are overlapping, the minimum (Y2) between the tops of the humps (Y1 and Y3) should beused as the end of the first hump or starting for the following hump (see Figure 2). Figure 2 Integration of overlapping humps with areas Acstart, Y1, Y2 and Ay2, Y3, Cend 4.7Performance requirements of the analytical methods The final analytical procedure should undergo thorough validation to prove its fitness for purpose.Guidance for laboratory method validation can be found, for instance, in the Eurachem guide. Table Il defines the performance requirements for the analysis of MOSH and MOAH fractions,concerning the maximum allowable limit of quantification (LOQ) for each C-fraction (LOQ-max), thetarget LOQ for each C-fraction (LOQ-t), acceptable ranges for recovery (Rrec) of mineral oil fromsamples, and the intermediate precision for different types of samples. The measurementuncertainties and limits of quantification depend on the type of samples and the kind of samplepreparation applied. Limit of quantification The LOQ that can be reached with a method is significantly influenced by the fat content of thesample, since the capacity of the LC column to retain lipids is limited and this determines the amountof sample which can be injected. Therefore additional sample preparation steps are necessary insome cases to reach the LOQ-t or LOQ-max. ( 16 Eurachem Guide (2014) The Fit n ess for Pu r pose of A nalytical Me t hods A Labo r atory Guide to Method Validation and Related Topics. https : //www.eurachem.o r g/index.php/publications/gu i des/mv ) The LOQ derived from the chromatographic signal of MOSH or MOAH can be influenced by the signalshape. As a rule, ca. 50-100 ng MOSH or MOAH should be injected on the GC separation column. TheLOQ can be estimated taking into account the sample preparation steps, including the enrichmentstep and the mass of the sample. For different types of food matrices, there are two LOQs defined: a ‘target-LOQ'(LOQ-t) and a‘maximum-LOQ'(LOQ-max). The LOQ-t is the analytically achievable quantification limit for themajority of matrices of each food category, whereas the LOQ-max should not be exceeded for anyanalytical methods used in MOSH or MOAH analysis. The LOQ is differentiated for the fat content ofthe food and for paper and board. The laboratory should aim to achieve the LOQ-t mentioned inTable ll. Recovery The method applied for the determination of the MOSH and MOAH in food and FCM shall ensurethat the recovery (Rrec) is within the acceptable range as indicated in Table II. In the chromatographic methods used for the determination of MOSH and MOAH, mineral oilconcentrations are automatically corrected for recovery through the use of internal standards (IS) forquantification. The ratio (signal (analyte) / signal (IS)) is assumed to be constant throughout theanalytical process, which compensates for eventual losses of the analyte. For certain foods (solid foods and packaging materials), inclusions could severely hamper theextraction process of mineral oil or render it virtually impossible. In this case,the internal standarddoes not represent the mineral oil sufficiently as it will not have been penetrated into the inclusions. The following matrices are critical: Foods with crystalline components, such as products with high sugar content (e.g. powdereddrinks,infant formula) and pasta. Paperboard: Ethanol improves the extraction of small molecules by swelling. Plastics: readily permeable plastics (e.g. polyolefins) are extracted much faster than effectivebarrier materials. A check can be carried out by rinsing the sample extracted under normal conditions (removal ofresidual extract) and carrying out subsequent extractions under harsher conditions (several times,longer extraction times, higher temperature). Intermediate precision The requirements on intermediate precision, also called within-laboratory or in-house precision, ofanalytical results of MOSH and MOAH for foods with different fat content are indicated in Table Il. The intermediate precision should be calculated following the Eurachem guide. Table Il Performance requirements for MOSH and MOAH analysis: maximum LOQ for each C-fraction(LOQ-max), target LOQ for each C-fraction (LOQ-t), acceptable ranges for recovery (Rrec) of mineraloil from samples, and intermediate precision Categories Associated foods ‘# LOQ-max[mg/kg] LOQ -t[mg/kg] Rrec[%] interme-diateprecision [%1 Dry,low-fatcontent(<4% fat/oil) bread and rolls; breakfast cereals; grains for human consumption;pasta, products derived from cereals 0.5 0.1 80-110 15 Higher fat/oilcontent (> 4% fat/oil) fine bakery ware; confectionery(incl. chocolate) and cocoa; fish meat, fish products (canned fish);oilseeds; pulses; sausages; tree nuts 1 0.2 70-120 20 Fat/oils animal fat (e.g. butter); vegetableoils 2 0.5 70-120 20 Paper andBoard Reporting only up to C3s(extraction optimised up to C35) 10 5 80-110 10 # In some cases, a shift to another category may be necessary due to different fat content. This hasto be stated and justified for each case. 55IReporting of results Results shall be reported: in mg/kg;with two significant figures (e.g. 150, 15, 1.5 or 0.15 mg/kg); and rounded using the rules in section B.2 of ISO 80000-1:2009. For each report's result, a short description of the analytical steps in the applied procedure shall bereported. The analyst should specify how the reported expanded measurement uncertainty was estimated. Data has to be reported in the EFSA database. The following data elements are relevant, eithermandatory or recommended, and required to report to EFSA". For the datasets generated in thepast and fulfilling the requirements laid down in the chapters before, a symbol "0" could be enteredwhen there are no data for some of the mandatory elements, in order to be able to proceed. Code Data elements Description/requirement Comment S.01 Laboratory sample code Laboratory sample code must be identified by aunique sample identification number, not longerthan 20 characters. Mandatory for EFSA S.03 Language The language used to complete the free text fieldsof the table must be specified. Mandatory for EFSA S.04 Country ofsampling The country of sampling is the country where thecommodity was selected for laboratory testing. Mandatory for EFSA S.06 Country oforigin of theproduct The country of origin is the country where thecommodity originates. It is particularly important toavoid the code "unknown (XX)", especially whenreporting data from raw agricultural commodities. Ifit is still unknown, provide info in S.14 Mandatory for EFSA S.08 Area of originfor fisheries oraquacultureactivities code Fisheries or aquaculture area specifying the origin ofthe sample (FAO Fisheries areas). Mandatory for this guidance, for relevantfood ( 17 EFSA (2017) S pecific reporting requirements for contaminants a nd food additives o ccurrence data s u bmission. E F SA supporting publication EN-1262. and its annex"Specific_Requirements_2016_data_Annexes.xlsx" ) 18 ( EFSA(2013) S t andard Sa m ple Description ver. 2. 0 . EFSA Journal 11(10), 3424 ) S.12 EFSA Productcode Food products should be described according to theFoodEx catalogue of the SSD. It is envisaged thatdetailed information on the different food groups will be needed to perform the exposure assessment. It is mandatory to report at least the level 2 of the FoodEx code.It is strongly encouragedto classify the food samples at the most detailedhierarchical level available (FoodEx level 3 and 4).This is particularly needed for food groups like“Food for infants and small children"and“Productsfor special nutritional use", where any availableadditional descriptions shall be provided.Specificattention needs to be given to the reporting of dataon cereal grains. It is essential to make a cleardistinction between grains as harvested(unprocessed grains of undefined use, not forhuman exposure assessment), grains for humanconsumption and grains as feed. For this reason, three distinct groups are available within the FoodEx catalogue. Be aware that the Feed codes starting with "F" are not in use any longer';“G" codes should be used instead. Mandatory for EFSA S.14 Product full-text description Product full-text description is essential to check if the EFSA product code (FoodEx code) given by thedata provider is consistent with the text description.This will avoid any possible mistakes in coding andadditional clarification requests. In addition, as thisis a free text element, the information could beprovided even in the national language. The originaldescription of the sample from the nationaldatabase can be copied here. It should be avoidedto repeat only the FoodEx description. Moreover,any additional information that does not belong toany of the other SSD fields should be reported ins.14. Mandatory for this guidance S.16 Packaging Describe container or wrapper that holds the foodproduct. A common type of packaging: paper orplastic bags,boxes, tinplate or aluminium cans,plastic trays, plastic bottles, glass bottles or jars.Relevant packaging that cannot be described hereshould be reported in code R.32 Mandatory for this guidance. See SSD listannexed. Refer to PRODPAC list in the annexe S.17 Product treatment It is mandatory to clearly indicate if the original sample is treated or not, especially if it is adehydrated product (select: "Dehydration", T131A);in the absence of this information, the status "asconsumed"will be assumed. Mandatory for EFSA. SeeSSD list. Refer to PRODTRlist. S.21 Product comment Additional information on the product,particularly home preparation details if available.When the analysis is carried out after the preparation of the product (e.g. reconstitution ofpowders or cooking processes) report“analysed=asconsumed". If the analysis is performed on theproduct before the preparation of the report, “analysed=as purchased". It is recommended to report the conditions used forthe preparation“comment=deep fried in oil". Mandatory for this guidance, if relevant S.25 Year of expiry Best before-year or use by-year or other indicationsof the expiry year. Mandatory for packagedfood S.26 Month ofexpiry Best before-month or use by-month or otherindications of expiry month. Mandatory for packagedfood S.27 Day of expiry Best before-day or use by-day or other indicationsof the expiry day. Mandatory for packagedfood s.28 Year of sampling Year of sampling. If the measure is the result ofsampling over a given period, the field shouldcontain the year when the first sample was collected Mandatory for EFSA S.29 Month of sampling Month of sampling. If the measure is the result ofsampling over a given period, this field shouldcontain the month when the first sample wascollected. Mandatory for thisguidance S.30 Day of sampling Day of sampling. If the measure is the result ofsampling over a given period,this field shouldcontain the day when the first sample wascollected. Mandatory for thisguidance S.32 Programmelegal reference Reference to the legislation for the program definedby programme number. Mandatory for this guidance; use"N304A"and insert: COMMISSIONRECOMMENDATION (EU)2017/84 S.33 Samplingstrategy It is mandatory to describe how the sample wasselected from the population being monitored orsurveyed. Mandatory for EFSA“ST20A"Selective, “ST30A” Suspect,“ST10A"Objective,“ST90A"other S.34 Programmetype The programme type must be reported to indicatethe type of control programme or other types of thesource to which the sample belongs. Mandatory for EFSA; use:K009A (Official (EU) programme) S.35 Samplingmethod It is mandatory to define the way the samples werecollected for analysis. In the case of pooled samples,the number of the sample should also be providedin 'Number of samples (S.36). (The default value ofthe number of the sample is"1"). Mandatory for EFSA; use:N011A (According to Reg.333/2007) S.39 Sampling point Identify in the food chain where the sample wastaken. (Doc.ESTAT/F5/ES/155"Data dictionary ofactivities of the establishments"). Mandatory for EFSA. SeeSAMPNT catalogue. L.02 Laboratoryaccreditation In accordance with Article 12 of Regulation 882/2004 and with Article 37 of Regulation (EU)2017/625 from 14"of December 2019, laboratoriesdesignated for official controls must be accreditedto ISO/IEC 17025 Mandatory for EFSA R.01 Result code The result code should be unique for each record. If the laboratory does not provide it, then, it must becreated, e.g. by merging the sample code with theparameter code. For example, a sample (Samp001)has been analysed for Mercury (RF-00000170-CHE)and Cadmium (RF-00000150-CHE). The result codecreated could be: Samp001_170 and Samp001_150. Mandatory for EFSA R.02 Year ofanalysis It is mandatory to report the year of analysis. If theanalysis has been performed over a period of time,the completion date of analysis should be stated. Mandatory for EFSA R.03 Month ofanalysis Month when the analysis was completed. Mandatory for thisguidance R.04 Day of analysis Day when the analysis was completed. Mandatory for this guidance R.06 Parametercode It is mandatory to code the contaminants using thecode from the SSD PARAM catalogue. For the voluntary parameter MOSH Hump andMOAH Hump, several humps can be reported. Thereporting format allows you to include for eachhump the nearest starting C number of the respective n-alkane, the nearest top C number ofthe respective n-alkane and the nearest ending Cnumber of the respective n-alkane, e.g.: The 3"hump for MOSH with Cstart=20, Ctop=24 and Cend=30must be reported with the following code RF-00007456-PAR #humpld=3$Cstart=20SCtop=24$Cend=30.If humps are overlapping, the minimum (Y2)between the tops of the humps (Y1 and Y3) shouldbe used as the end of the first hump or starting ofthe following hump (see Figure 2). Mandatory for EFSA;MOSH: . RF-00000397-ORG (totalMOSH) ● RF-00007450-PAR(MOSH>C9 toC16 to≤C20) RF-00007452-PAR(MOSH>C20to≤C25) RF-00007453-PAR(MOSH>C25 to≤C35) RF-00007454-PAR(MOSH>C35 to ≤C40) ● RF-00007455-PAR(MOSH>C40 to≤C50) MOAH: ● RF-00000398-ORG (TotalMOAH) . RF-00007457-PAR(MOAH>C9 to ≤C16))" RF-00007458-PAR(MOAH>C16 toC25 toC35 to ≤C50) Voluntary: RF-00007456-PAR (MOSH Humpn) RF-00007462-PAR MOAHHump n) # read (MOSH ≥C10 to C40 to C9 to ≤C16) G061A 10 VAL IT_MYLB_2018_01/02 RF-00007451-PAR (MOSH>C16 to ≤C20) G061A 5.0 VAL IT_MYLB_2018_01/03 RF-00007452-PAR(MOSH>C20 to ≤C25) G061A 0.2 LOQ IT_MYLB_2018_01/04 RF-00007453-PAR (MOSH>C25 to ≤C35) G061A 0.2 LOQ IT_MYLB_2018_01/05 RF-00007454-PAR (MOSH>C35 to≤C40) G061A 25 VAL IT_MYLB_2018_01/06 RF-00007455-PAR (MOSH>C40 to ≤C50) G061A 5.0 VAL IT_MYLB_2018_01/07 RF-00007456-PAR (#humpld=1$Cstart=10$CTop=16$Cend=21) G061A 15 VAL IT_MYLB_2018_01/08 RF-00007456-PAR (#humpld=2$Cstart=34SCTop=37$Cend=40) G061A 25 VAL IT_MYLB_2018_01/09 RF-00007456-PAR (#humpld=3$Cstart=40$CTop=42$Cend=44) G061A 5.0 VAL IT_MYLB_2018_01/10 RF-00000397-ORG (Total MOSH) G061A 45 VAL List of abbreviations DIPN diisopropylnaphthalene EAN European Article Numbering EURL European Union Reference Laboratory FID flame ionisation detector GC gas chromatography GCxGC comprehensive two-dimensional gas chromatography LC liquid chromatography LOQ limit of quantification MOAH mineral oil saturated hydrocarbons MOSH mineral oil aromatic hydrocarbons MS mass spectrometry NMR nuclear magnetic resonance NRL National Reference Laboratorv OCL Official Control Laboratorv PAO poly alpha olefins POH polyolefin oligomeric hydrocarbons PT proficiency testing SPE solid phase extraction SSD Standard Sample Description AnnexI Figure A: on-line LC-GC: sufficient LOQ (rice) MOSH [mg/kg] MOAH [mg/kg] >=C10- ≤C16 >C16- ≤C20 >C20-≤C25 >C25- ≤C35 >C35-≤C40 >C40-≤C50 >=C10-≤C16 >C16- ≤C25 >C25- ≤C35 >C35-≤C50 0.21 2.2 3.1 2.3 <0.1 <0.1 <0.1 1.2 0.39 <0.1 Figure B:insufficient LOQ (rice) MOSH before enrichment MOAH before enrichment MOAH after enrichment -3905.07.5 40:02:55.017.52022.0 -3.55.07.510.02:515.017.520.22.0 MOSH [mg/kg] MOAH [mg/kg] >=C10-≤C16 >C16-≤C20 >C20-≤C25 >C25-≤C35 >C35-≤C40 >C40-≤C50 >=C10-≤C16 >C16-≤C25 >C25-≤C35 >C35-≤C50 0.20 0.67 0.58 0.31 <0.1 <0.1 <0.1 0.41 <0.1 <0.1 Figure C: Aluminum oxide treatment (olive oil)+ Enrichment MOSH before alox pre-separation MOSH after alox pre-separation MOSH [mg/kg] >=C10-≤C16 >C16- ≤C20 >C20-≤C25 >C25-≤C35 >C35-≤C40 >C40-≤C50 <0.1 0.52 2.3 6.5 1.2 0.69 Figure D: Epoxidation (olive oil, Panettone) MOAH before epoxidation (olive oil) MOAH after epoxidation (olive oil) MOAH [mg/kgl >=C10-sC16 >C16-sC25 >C25-sC35 >C35-sC50 8.4 27 14 <1.5 MOAH img/kg] >=C10-sC16 >C16-sC25 >C25-sC35 >C35-sC50 <0.1 <0.1 <0.1 <0.1 MOAH before epoxidation (Panettone) MOAH after epoxidation (Panettone) 3.65.010.0-15:020.0 2527.0 cc3.5.010016:020.02527.0 Figure E: interferences after epoxidation (olive oil): higher LOQ or characterization by GCxGC-FID/MS MOAH before epoxidation MOAH [mg/kg] >=C10-sC16 >C16-C25 >C25-sC35 >C35-sC50 <1 2 ≤11 54 Reported as “s"results, as verification (e.g. by GCxGC-FID/MS) needed, as MOAH hump does notcorrespond with MOSH hump. Results of this example cannot be reported to EFSA as LOQ higherthan LOQ-max. Annex Verification in the MOSH/MOAH analysis MOSH MOAH First check Are the humps typical for mineral oil fractions (e.g. width)?Correspondence of the molecular mass distribution of MOSH and MOAH Is the ratio of MOSH and MOAH concentration reasonable? Treatment of riding peaks Homogeneous distribution of n-alkanes →MOSH (e.g. waxes of mineral oil origin); allRiding peaks are usually not MOAH: all to beothers (terpenes, natural alkenes/alkenes)subtracted, e.g. resin acid derivatives fromeliminated paperboard, polystyrene oligomers Dominating odd-numbered n-alkanes: natural alkanes subtracted; removable by activated aluminium oxide in case of overloading Natural olefins In on-line LC-GC, monounsaturated olefins are eluted in the MOSH fraction; Squalene and sterenes form single signalsdiunsaturated ones in the MOSH or MOAH around C28-C29(removable by epoxidation) fraction Isomerization products of squalene and carotenes form humps more narrow than MOH(removableby epoxidation) No non-aromatic unsaturated hydrocarbons expected in mineral oil Polyolefin oligomerichydrocarbons (POH) orother synthetichydrocarbons -typical patterns of riding peaks for PP and some PE, but in mixtures oligomers of LDPEare difficult to distinguish from MOSH MOAH-fraction contains polyunsaturated -even-numbered n-alkanes orsynthetic hydrocarbons, occurring as series ofalkenes: PE POHnarrow humps, e.g., in adhesives. Manual- homologue row of iso-alkanesmethod: monounsaterated POH may shift intoAC3: PP POHthe MOAH fraction-series of narrow humps (e.g. adhesives, PAO from synthetic lubricants) -plausible sources? Identification of source Transfer through gas phase: limited volatility range (at RT up to about C24) Transfer from FCM: check extract from FCM Ratio MOSH/MOAH: depending on degree of refining Environmental contamination is typically free of MOAH Compounds indicativeof sources Cases of doubt -DIPN identifier for recycled paper and board-pristane, phytane (GC-FID)(typical peak pattern in GC-FID or GC-MS) -Methyl dibenzothiophene possible identifier-multibranched alkanes (GCxGC-FID/MS) for non or little refined oils (GC-MS, GCxGC-FID/MS) -hopanes, steranes (GC-MS, GCxGC- FID/MS) Pattern by GCxGC-FID/MS As the science and knowledgeservice of the European Commission,the Joint Research Centre's missionis to support EU policies withindependent evidence throughoutthe whole policy cycle. EU Science Hubec.europa.eu/jrcy@EU_ScienceHubfEU Science Hub -Joint Research CentremJoint Research CentreEU Science Hub ISBN 978-92-76-00172-0 EUR EN 哲斯泰的HPLC-GC-FID在线全自动 MOSH & MOAH 检测方案,严格符合欧洲标准DIN EN 16995:2017-08中的要求。 今天让我们一起来解读一下这个标准,并且来探究一下哲斯泰解决方案的特点。DIN EN 16995:2017-08内容框架1.范围2.规范参考3.术语和定义4.原理5.试剂6.仪器7.样品储备8.测试样品的制备9.分析样品的制备10.液相和气相色谱11.精确度12.测试报告1. 范围欧洲标准DIN EN 16995:2017-08,是“基于植物油和以植物油为基础的食品的在线HPLC-GC-FID分析测定矿物油饱和烃(MOSH)和矿物油芳烃(MOAH)”的标准方法。 是“通过在线HPLC-GC-FID对植物油脂中的饱和烃和芳香烃(从C10到C50)进行测定。检测的样品类型是植物油,蛋黄酱,人造黄油等以植物油为基础的食品。注意,此标准无意应用于其他样品基质。”其实,我们分析化学中,抛开样品基质说最低检测下限的,都是“不严谨”的,是“耍流氓”,所以此标准中的检测下限,是根据样品的类型而言,而不是对所有样品都一样的,也并不代表测试系统的能力上限。根据实验室间研究的结果,该方法已被证明适用于各自高于10 mg / kg的MOSH和MOAH质量浓度。“该方法可用于分析矿物油饱和烃(MOSH)和/或矿物油芳烃(MOAH)。”就是说,对于其他样品基质,如奶粉,巧克力,大米,食品包装等等,此检测方法也是适用的。但是由于基质不同,样品的前处理过程不一,最低检测下限也不同,所以不能归到此标准中。这也再一次说明样品种类的重要性和此标准的严谨性。3. 术语和定义MOSH 代表矿物油饱和烃:是Mineral Oil Saturated Hydrocarbons的缩写。指链烷烃(开链的,通常是支链的)和环烷烃(环状的,烷基化的)烃。MOAH 代表矿物油芳香烃:是Mineral Oil Aromatic Hydrocarbons的缩写。主要为烷基的芳烃4. 原理“通过HPLC-GC-FID系统分离 MOSH 和 MOAH 的馏分。MOSH 和 MOAH馏分在硅胶柱上使用正己烷/二氯甲烷梯度分离,并分别通过Y界面以450µL馏分形式转移到GC,而甘油三酸酯则保留在HPLC柱上。溶剂蒸气通过位于未涂覆的前色谱柱和GC分离柱之间的溶剂出口排出。挥发性成分通过溶剂捕集得以保留。高沸点成分遍布整个区域,并通过保留间隙技术重新聚焦。”这段是最重要的方法原理介绍,目前市面上的 MOSH & MOAH 系统,凡是使用HPLC-GC-FID方法检测的,都是应用的这个原理。当然,系统和系统之间的区别在于细节,包括这里提到的使用Y界面分别转移 MOSH 和 MOAH 的馏分,现代化的仪器已经可以使用双通道,双FID,并行检测 MOSH 和 MOAH 的馏分,使样品通量加倍。可以在30分钟内,同时检测 MOSH 和 MOAH。哲斯泰的全自动在线HPLC-GC-FID系统也是一样的原理和性能。哲斯泰的全自动在线HPLC-GC-FID系统的双通道示意图哲斯泰的全自动在线HPLC-GC-FID系统的GC双前色谱柱及双色谱柱示意图,满足标准中“溶剂蒸气通过位于未涂覆的前色谱柱和GC分离柱之间的溶剂出口排出。”平行的色谱柱放置设计,便于色谱柱的更换和系统的维护。接下来是介绍此标准需要使用“验证标准试剂”的原因“归因于矿物油的面积是通过减去正链烷烃(天然存在的烃),萜烯,角鲨烯及其异构化产物,甾类和具有类胡萝卜素结构的烯烃而引起的尖峰而计算出的。添加验证标准,以监控适当的HPLC分馏和GC转移条件。”以及使用纯化技术“氧化铝”的原因:“一些植物油的C21-C33范围内含有奇数正构烷烃,其数量严重使MOSH馏分的色谱图超载,并可能与矿物油峰重叠。在这种情况下,建议使用其他清理技术。活化的氧化铝强烈保留长链正构烷烃。污染食用油的矿物油几乎全部由支链和环状成分组成,这些成分未被活性氧化铝保留。因此,使用活性氧化铝能够去除植物石蜡。”值得注意的是,在此标准中,“9.3 用氧化铝净化MOSH馏分”是“可选”的技术,并不是必须的。而下面的“环氧化”技术,则是标准的必备功能。和使用纯化技术“环氧化”的原因:“环氧化是MOAH定量所必需的纯化步骤。该纯化步骤可消除烯烃(如角鲨烯),它们在MOAH馏分中洗脱并干扰定量(例如橄榄油,棕榈油)。环氧化还去除了与MOSH馏分共同洗脱的某些烯烃,因此环氧化也可用作MOSH馏分的纯化步骤。目前,环氧化步骤是去除烯烃的最佳折衷方案,尽管它不是完全定量的,而且效率可能取决于样品。根据样品的不同,该反应可能会引起部分MOAH的环氧化或干扰烯烃的去除不完全。”10. 液相和气相色谱我们跳过“试剂,样品储存,样品制备”部分,直接来到液相和气相色谱部分,这部分介绍了液相和气相色谱的装备(setup)和工作条件参数。把在“原理”部分的内容做了具体的细节介绍。其中一个在线HPLC-GC-FID重要的系统组成部分是需要实现“10.6 溶剂蒸汽出口”。其原因是:“溶剂蒸发形成大量被载气稀释的蒸气,需要将其丢弃。因此,使用溶剂蒸汽出口,该出口位于未涂覆的预柱和GC分离柱之间。”,而要求是“关闭蒸气出口的时间取决于目标馏分与打开的溶剂出口之间的转移......以便将包含再浓缩的挥发性溶质的溶剂的最后部分引导到分离柱中。时间根据保留间隙的长度而变化。" 也就是说,关闭溶剂出口的时间需要精确,从而保证溶剂被排出,而被测的目标馏分组分被传输到GC分离柱中”哲斯泰实现的“溶剂蒸汽出口”的装置是“Early Vapor Exit”,是通过设置电磁阀的开关时间以及载气压力控制,来精确控制溶剂蒸汽的排出时间和体积450µL。(这里的450µL是标准的要求,并不代表设备的极限。客户可以根据其他方法或标准的需求,自行调节时间和排出的体积。)系统和系统之间的区别在于细节,哲斯泰的“溶剂蒸汽出口”装置,完全是自行开发,所以不需要使用额外的的LC-GC控制样品分析的方法参数以及载气压力。哲斯泰的系统可以直接通过嵌入安捷伦主菜单的方法菜单,一起控制所有的方法参数。载气压力通过安捷伦系统控制。系统更稳定,操作更简单。更重要的是,客户可以自行改变方法参数,这对方法的开发十分重要。哲斯泰实现的“溶剂蒸汽出口”的装置“Early Vapor Exit”非常小巧精致,无须额外实验室空间,可以完美的与GC整合。完全嵌入安捷伦Open Lab CDS的哲斯泰Maestro软件,一个界面同时编辑LC的参数,如泵和检测器。在Maestro软件可以通过编辑指令,控制“溶剂蒸发出口”的电磁阀的开关时间和反冲洗阀的时间。灵活的更具客户需求,设定时间,以此来控制溶剂排出的体积。系统额外的功能与其说是额外的功能,不如说是潜在的功能。其实就是在我们的全自动多功能的样品制备平台 MPS robotic 上去实现一系列手动样品制备的步骤和最后的进样。哲斯泰拥有众多的样品制备模块,可以实现包括振荡,离心,蒸发,称量,过滤,馏分收集等等的制备工作。 并统一由Maestro软件控制,编辑方法和序列易如反掌。这里针对我们的样品,系统可以实现“全自动环氧化”,“馏分收集”,当然也可以实现 “油脂中固醇和PAH的分析”。这些例子仅仅是MPS robotic平台结合模块和软件可以实现的自动化样品前处理的具体的例子,其潜力可以由客户的需求而被不断激发。哲斯泰的全自动在线HPLC-GC-FID系统(包括全自动环氧化的配置)系统和系统之间的区别在于细节,哲斯泰系统自带图谱分析软件,轻松得到MOSH和MOAH的定量数据,使分析工作更轻松,更精确。带图谱分析软件,自动积分计算峰面积,图示MOAH的色谱图和软件界面总结1、哲斯泰HPLC-GC-FID系统可在线全自动检测MOSH和MOAH矿物油污染物2、完全符合欧洲标准DIN EN 16695: 2017-08要求3、双通道GC进样,双FID检测器,30分钟完成MOSH和MOAH的分析,实时显示LC和GC的色谱图4、所有部件自行研发制造,无须额外的控制盒来控制样品的分析参数和载气的压力,系统更稳定,控制更容易,并且节省空间5、哲斯泰的MAESTRO软件,完全嵌入安捷伦菜单,可以控制LC泵,LC检测器,以及“溶剂蒸发出口”的电磁阀开关时间,以及整个分析序列。6、哲斯泰自行研发制造的样品制备模块,由MAESTRO软件统一控制,以实现各种样品的自动化制备步骤。如“全自动环氧化”,“馏分收集”以及“油脂中固醇和PAH的分析”等7、自带图谱分析软件,自动积分计算MOSH和MOAH的含量,轻松获取测试报告拓展阅读对食品和食品接触材料中矿物油烃的检测感兴趣的朋友,可以参考欧盟总部联合研究中心JRC发表的《食品和食品接触材料检测器中监测矿物油烃的采样,分析和数据报告指南》,里面推荐的HPLC-GC-FID方法,就是在哲斯泰的仪器上研发的。
确定





















还剩34页未读,是否继续阅读?
GERSTEL(哲斯泰)为您提供《食品和食品包装中矿物油污染( MOSH & MOAH )检测方案(自动进样器)》,该方案主要用于其他食品中环境污染物检测,参考标准--,《食品和食品包装中矿物油污染( MOSH & MOAH )检测方案(自动进样器)》用到的仪器有GERSTEL LabWorks 平台
推荐专场
相关方案
更多
该厂商其他方案
更多















