
方案详情
文
This application note illustrates temperature-dependent CD and fluorescence measurements of lysozyme obtained simultaneously using the J-1500 CD spectrometer, FMO-522 Emission Monochromator accessory, and Temperature/Wavelength Scan Measurement program.
方案详情

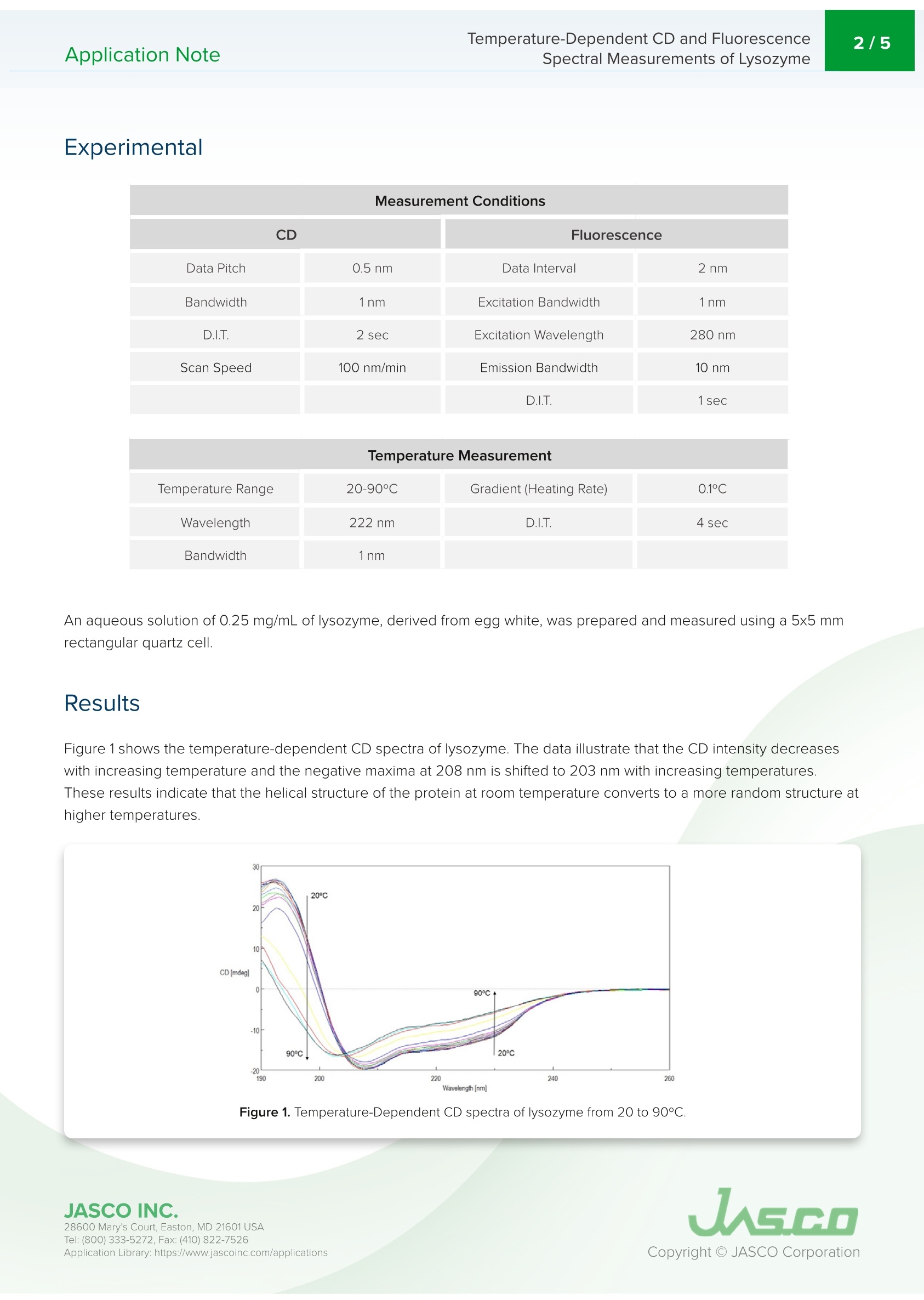
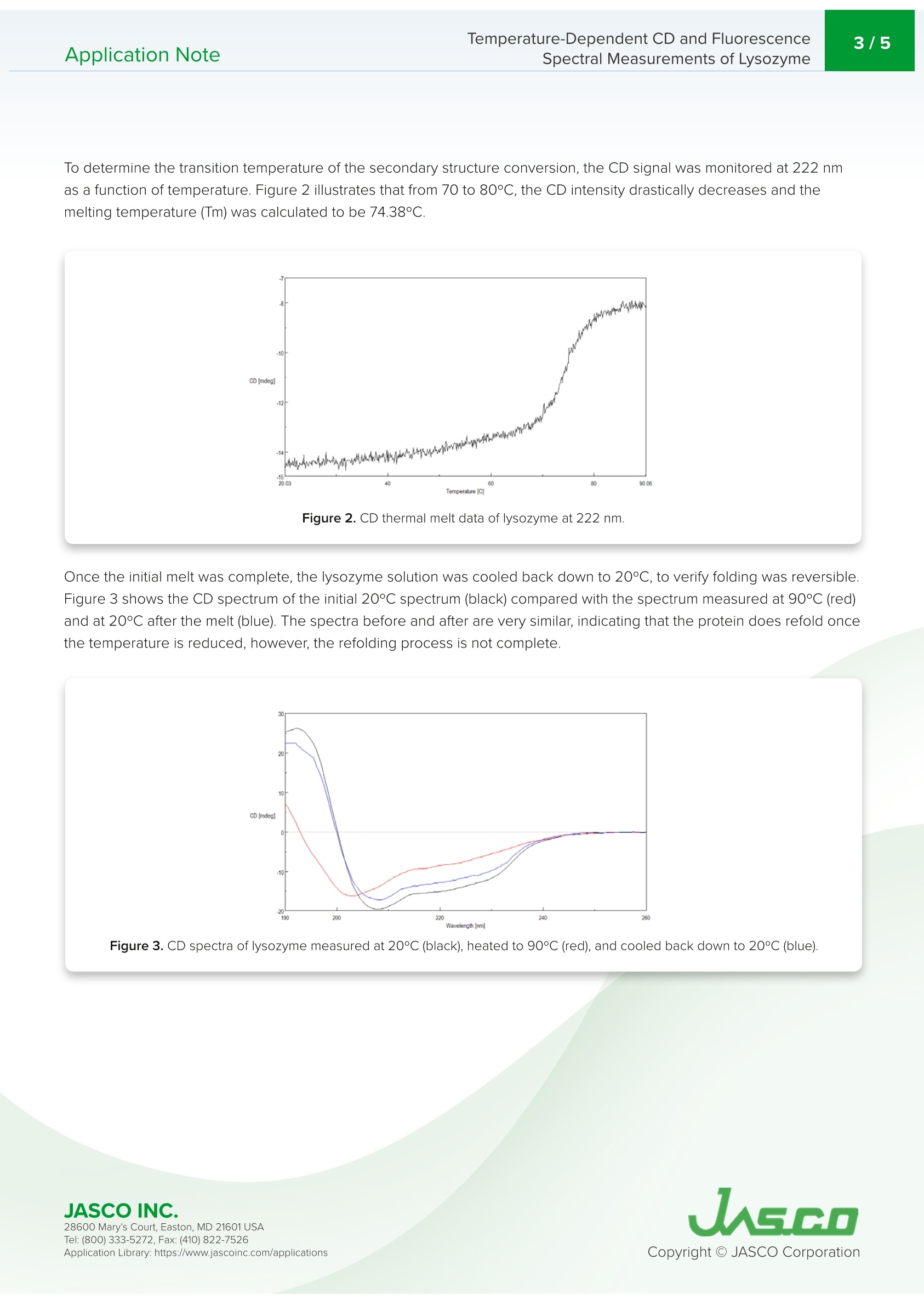
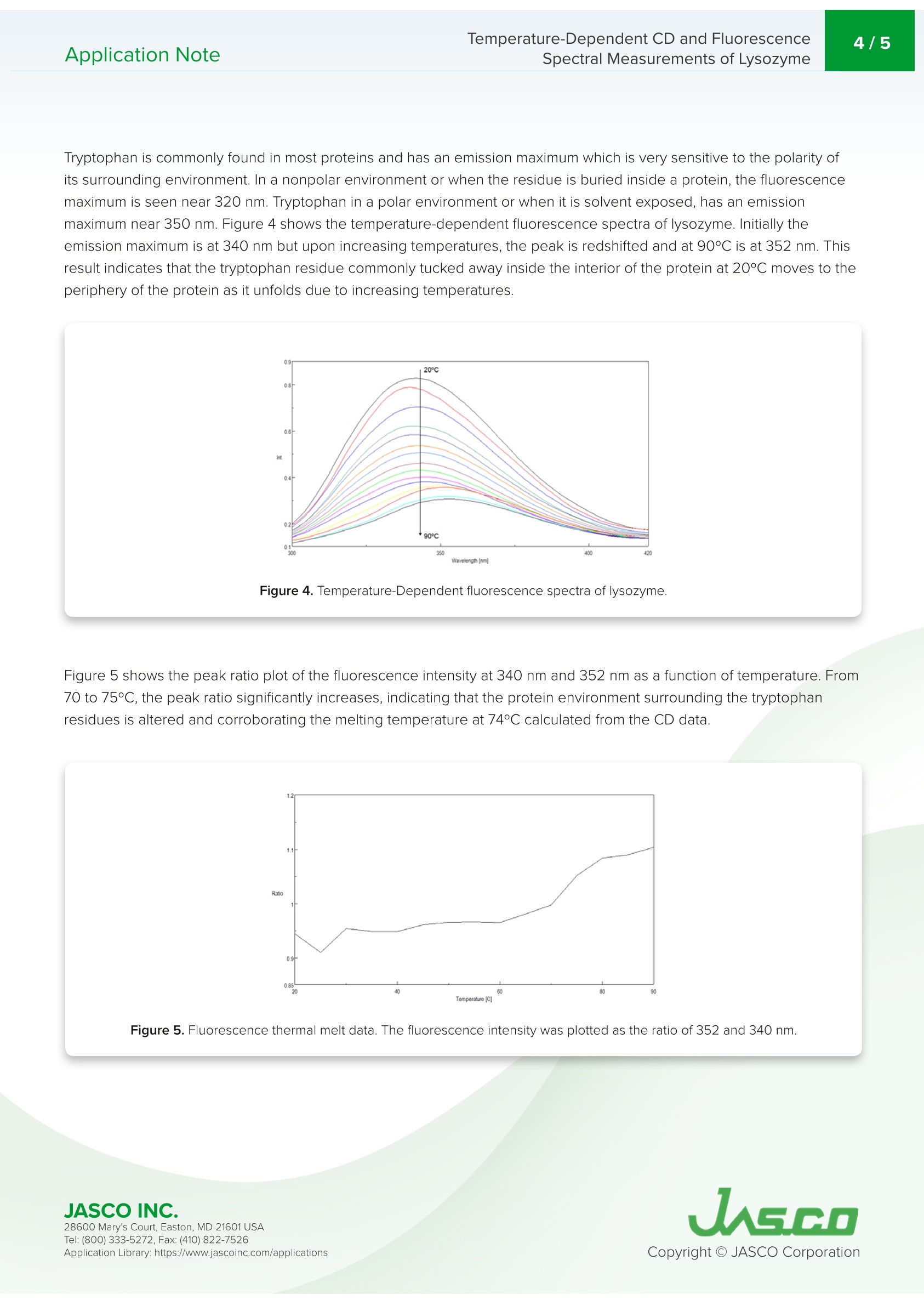
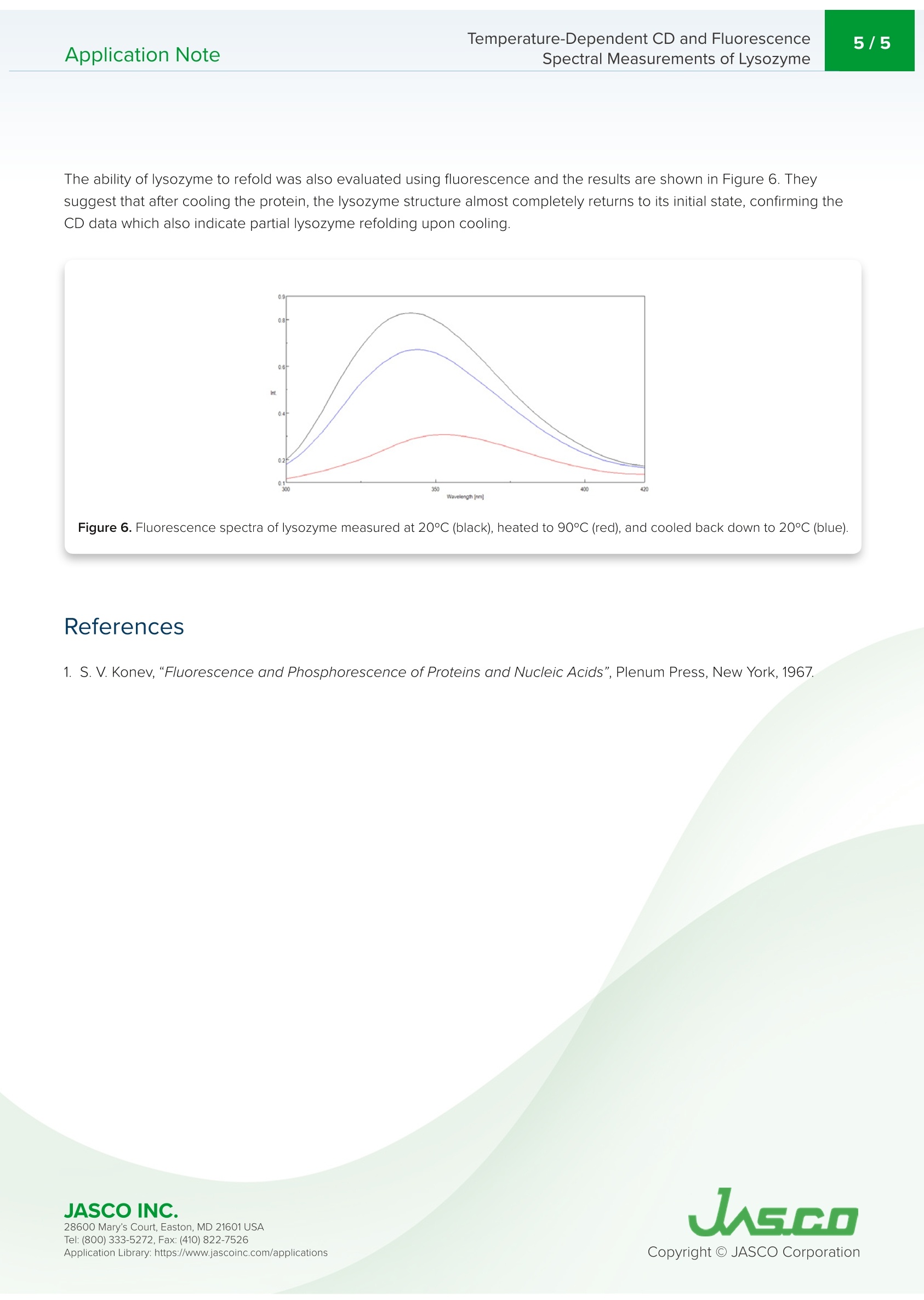
Application NoteCD-0024 2/5Temperature-Dependent CD and FluorescenceSpectral Measurements of LysozymeApplication Note Temperature-Dependent CD and FluorescenceSpectral Measurements of Lysozyme Introduction Recently, there has been a significant increase in the research andmanufacturing of biomedicines derived from proteins, which arebecoming more widely available in the biopharmaceutical industry.An important requirement in the manufacturing and quality controlof protein-based biopharmaceuticals is in the assessment of stabilityduring storage and the effects of storage conditions. The measurementofdenaturation and thermal stability are of considerable importance in J-1500 guaranteeing the efficacy of biopharmaceuticals. CD measurement offers significant Spectrometer advantage in the assessment of protein secondary structure due to its requirement for small amount of sample coupledwith high sensitivity measurement. Therefore,CD measurement is becoming one of the most popular techniquesused in the analysis of the thermal stability and changes in protein structure caused by ionic strength and pH. The useof fluorescence spectroscopy in the probing tryptophan residues also yields important information about the tertiarystructure of proteins. This application note illustrates temperature-dependent CD and fluorescence measurements of lysozyme obtainedsimultaneously using the J-1500 CD spectrometer, FMO-522 Emission Monochromator accessory, and Temperature/Wavelength Scan Measurement program. J-1500, FMO-522 Emission monochromator, Circular dichroism, Fluorescence,Secondary structure, Tertiary structure,Thermal stability, Biochemistry Measurement Conditions CD Fluorescence Data Pitch 0.5nm Data Interval 2 nm Bandwidth 1nm Excitation Bandwidth 1nm D.I.T. 2 sec Excitation Wavelength 280 nm Scan Speed 100 nm/min Emission Bandwidth 10 nm D.I.T. 1sec Temperature Range 20-90°C Gradient (Heating Rate) 0.1°C Wavelength 222 nm D.I.T. 4 sec Bandwidth 1nm An aqueous solution of 0.25 mg/mL of lysozyme,derived from egg white, was prepared and measured using a 5x5 mmrectangular quartz cell. Results Figure 1 shows the temperature-dependent CD spectra of lysozyme. The data illustrate that the CD intensity decreaseswith increasing temperature and the negative maxima at 208 nm is shifted to 203 nm with increasing temperatures.These results indicate that the helical structure of the protein at room temperature converts to a more random structure athigher temperatures. Figure 1. Temperature-Dependent CD spectra of lysozyme from 20 to 90°C. 28600 Mary's Court, Easton, MD 21601 USA Temperature-Dependent CD and Fluorescence . To determine the transition temperature of the secondary structure conversion, the CD signal was monitored at 222 nmas a function of temperature. Figure 2 illustrates that from 70 to 80°C, the CD intensity drastically decreases and themelting temperature (Tm) was calculated to be 74.38°C. Figure 2. CD thermal melt data of lysozyme at 222 nm. Once the initial melt was complete, the lysozyme solution was cooled back down to 20°C, to verify folding was reversibleFigure 3 shows the CD spectrum of the initial 20°C spectrum (black) compared with the spectrum measured at 90°℃ (red)and at 20°C after the melt (blue). The spectra before and after are very similar, indicating that the protein does refold oncethe temperature is reduced, however, the refolding process is not complete. Figure 3. CD spectra of lysozyme measured at 20°℃ (black), heated to 90°C (red), and cooled back down to 20°℃ (blue). 28600 Mary's Court, Easton, MD 21601USA Tryptophan is commonly found in most proteins and has an emission maximum which is very sensitive to the polarity ofits surrounding environment. In a nonpolar environment or when the residue is buried inside a protein, the fluorescencemaximum is seen near 320 nm. Tryptophan in a polar environment or when it is solvent exposed, has an emissionmaximum near 350 nm. Figure 4 shows the temperature-dependent fluorescence spectra of lysozyme. Initially theemission maximum is at 340 nm but upon increasing temperatures, the peak is redshifted and at 90°C is at 352 nm. Thisresult indicates that the tryptophan residue commonly tucked away inside the interior of the protein at 20°C moves to theperiphery of the protein as it unfolds due to increasing temperatures. Figure 4.Temperature-Dependent fluorescence spectra of lysozyme. Figure 5 shows the peak ratio plot of the fluorescence intensity at 340 nm and 352 nm as a function of temperature. From70 to 75°C, the peak ratio significantly increases, indicating that the protein environment surrounding the tryptophanresidues is altered and corroborating the melting temperature at 74°C calculated from the CD data. Figure 5. Fluorescence thermal melt data. The fluorescence intensity was plotted as the ratio of 352 and 340 nm. 28600 Mary's Court, Easton, MD 21601 USA Temperature-Dependent CD and FluorescenceApplication Note Spectral Measurements of Lysozyme The ability of lysozyme to refold was also evaluated using fluorescence and the results are shown in Figure 6. Theysuggest that after cooling the protein, the lysozyme structure almost completely returns to its initial state, confirming theCD data which also indicate partial lysozyme refolding upon cooling. Figure 6. Fluorescence spectra of lysozyme measured at 20°C (black), heated to 90°C (red), and cooled back down to 20°C (blue). References 28600 Mary's Court, Easton, MD 21601 USA JASCO INC.Mary's Court, Easton, MD USATel: ( Fax: ( pplication Library: https://www.jascoinc.com/applications JASCOINC.Tel: ( Fax: (opyright O JASCO CorporationApplication Library: https://www.jascoinc.com/applications This application note illustrates temperature-dependent CD and fluorescence measurements of lysozyme obtained simultaneously using the J-1500 CD spectrometer, FMO-522 Emission Monochromator accessory, and temperature/Wavelength Scan Measurement program.
确定

还剩3页未读,是否继续阅读?
JASCO 公司为您提供《蛋白质中溶解酶检测方案(圆二色光谱仪)》,该方案主要用于其他中溶解酶检测,参考标准--,《蛋白质中溶解酶检测方案(圆二色光谱仪)》用到的仪器有JASCO日本分光 圆二色光谱仪/圆二色谱 J-1500
推荐专场
相关方案
更多
该厂商其他方案
更多














