搅拌棒吸附萃取(SBSE)成功地应用于各种样品基质中用以检测和分析低至超痕量气味活性化学物质。极性吸附材料聚乙二醇-二甲基硅氧烷(EG-Silicone)Twister®显著增强了这项技术的实用性,使带氢键供体的极性化合物(如酚类和羧酸—重要的气味和风味化合物)的有效萃取成为可能。本文采用PDMS和EG-Silicone Twister® (磁力搅拌棒)的不同组合,采用不同的萃取技术对样品进行了分析。SBSE技术与热解析-气相色谱/质谱和峰反卷积相结合,构成了一个重要的多功能分析工具包,可帮助香料化学家进行异味和恶臭的分析。同时使用PDMS和EG-Silicone磁力搅拌棒萃取样品,可以同时检测非极性和极性分析物,覆盖广泛的挥发性和不同的浓度。
方案详情

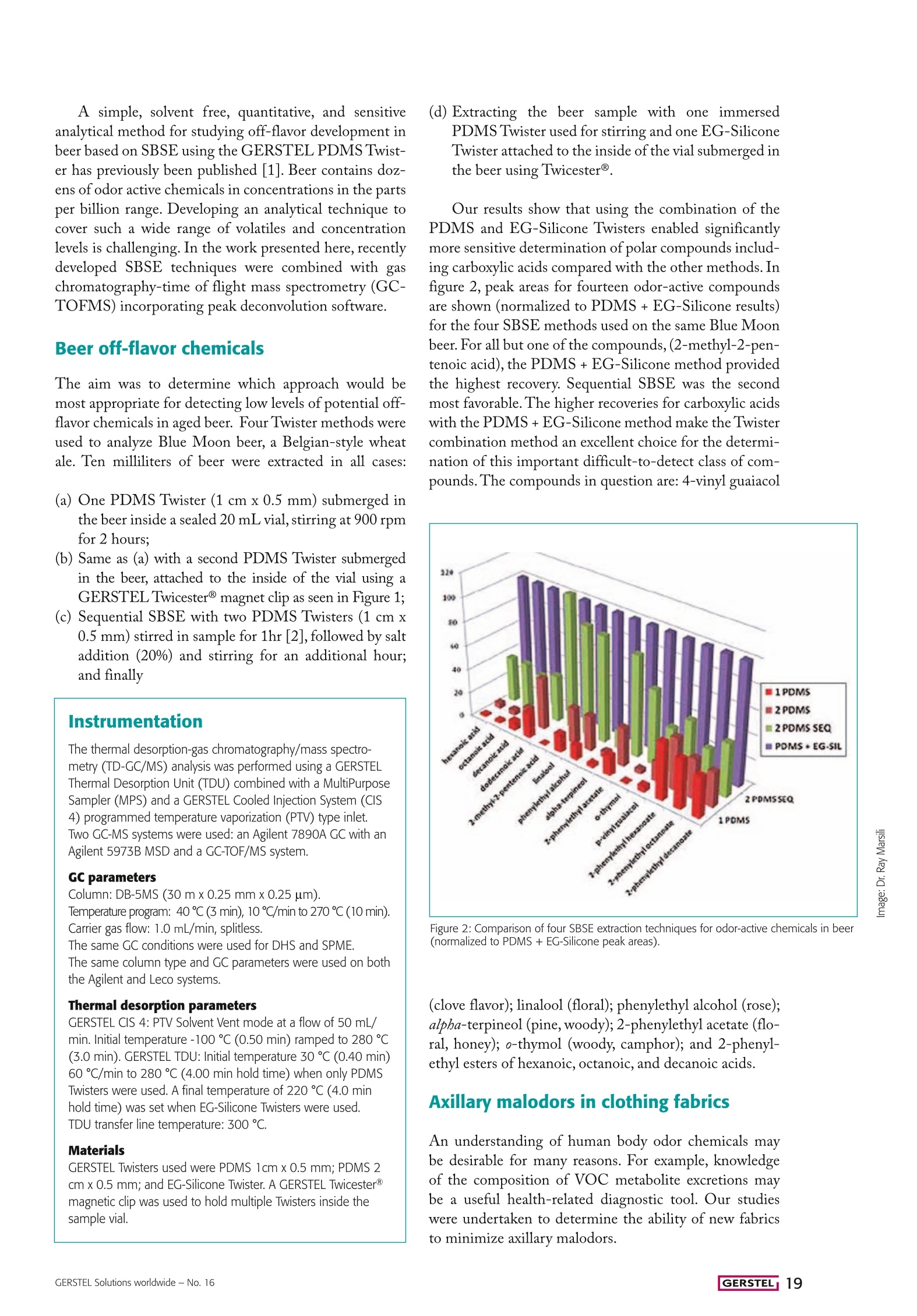
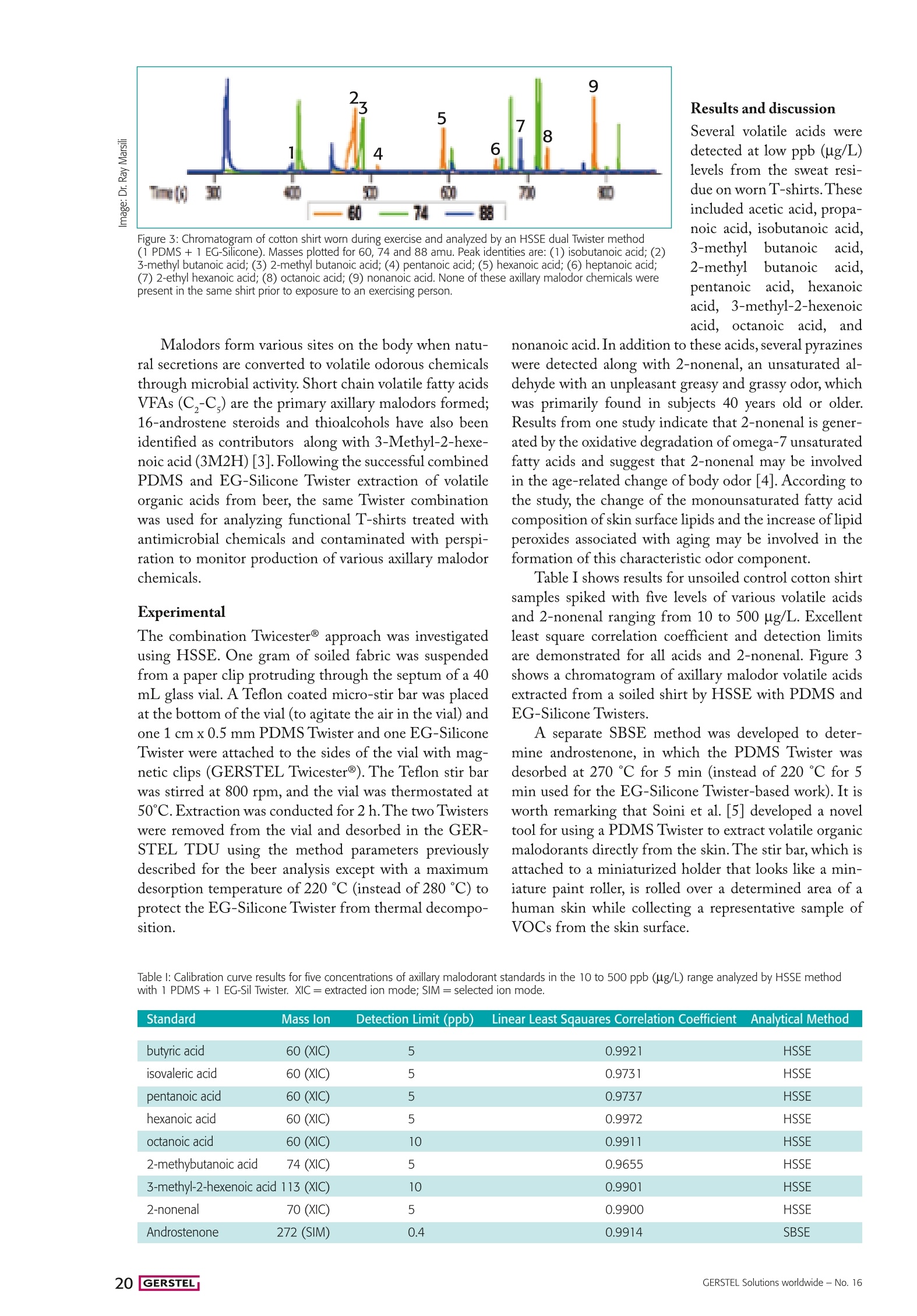
Flavor, Fragrance, and Odor Analysis A new Twist: Free acids and phenols Sensitive SBSE determination of free acids and phenols- along with the usual suspects By Ray Marsili and Charles Laskonis, Rockford University, Rockford, Illinois, USA. tir Bar Sorptive Extraction (SBSE) and HeadspaceSorptive Extraction (HSSE) have regularly been applied ticle. Versatile application of Twister technology combinedwith Thermal desorption - GC/MS and peak deconvolu- tion are adding up to a sig.nificant analytical tool-kitto assist the flavor chem-ist in off-flavor and mal-odor elucidation, detectingboth non-polar and polaranalytes spanning a widerange of volatilities andconcentrations. successfully to the analysisof odor-active chemicals in awide variety of sample matri-ces at concentrations as low asultra-trace levels.The additionof the Ethylene Glycol-Sili-cone (EG-Silicone) Twister@has significantly enhanced theusefulness of these techniquesby enabling the efficient ex-traction ofpolar hydrogenbond donors like phenols andcarboxylic acids - importantodor and flavor compounds.Various SBSE and HISSEextraction techniques us-inggdifferent combinationsof PDMS and EG-SiliconeTwisters were used to analyzesamples discussed in this ar- Figure 1:Schematic diagram of the multi stir bar extraction process usingTwicester@ (A) Magnetic positioning of up to three Twisters using Twices-ter Showing 1 Twister in HS & 1 stirring (B)MSBSE using two Twisterssubmerged in liquid (C)Transfer of Twisters to TDU Tube (D) Simultane-ous thermal desorption of the Twisters, cryofocusing in the CIS and GC/MS analysis. Examples presented inthis article include the de-termination of off odors inBeer, Pretzels, and Casein,a widely used food ingre-dient. Last but not least,axillary off odors weredetermined in well-wornT-shirts to determine theodor-reducing impact ofantibacterial fabrics. A simple, solvent free, quantitative, and sensitiveanalytical method for studying off-flavor development inbeer based on SBSE using the GERSTEL PDMS Twist-er has previously been published [1]. Beer contains doz-ens of odor active chemicals in concentrations in the partsper billion range. Developing an analytical technique tocover such a wide range of volatiles and concentrationlevels is challenging. In the work presented here, recentlydeveloped SBSE techniques were combined with gaschromatography-time of flight mass spectrometry (GC-TOFMS) incorporating peak deconvolution software. Beer off-flavor chemicals The aim was to determine which approach would bemost appropriate for detecting low levels of potential off-flavor chemicals in aged beer. Four Twister methods wereused to analyze Blue Moon beer, a Belgian-style wheatale. Ten milliliters of beer were extracted in all cases: (a) One PDMS Twister (1 cm x 0.5 mm) submerged inthe beer inside a sealed 20 mL vial, stirring at 900 rpmfor 2 hours; (b) Same as (a) with a second PDMS Twister submergedin the beer, attached to the inside of the vial using aGERSTEL Twicester@ magnet clip as seen in Figure 1; (c) Sequential SBSE with two PDMS Twisters (1 cm x0.5 mm) stirred in sample for 1hr [2], followed by saltaddition (20%) and stirring for an additional hour;and finally Instrumentation The thermal desorption-gas chromatography/mass spectro-metry (TD-GC/MS) analysis was performed using a GERSTELThermal Desorption Unit (TDU) combined with a MultiPurposeSampler (MPS) and a GERSTEL Cooled Injection System (CIS4) programmed temperature vaporization (PTV) type inlet.Two GC-MS systems were used: an Agilent 7890A GC with anAgilent 5973B MSD and a GC-TOF/MS system. GC parameters Column: DB-5MS (30 m x0.25 mmx0.25 um). Temperature program: 40℃(3min), 10℃/min to 270 ℃ (10 min).Carrier gas flow: 1.0 mL/min, splitless. The same GC conditions were used for DHS and SPME. The same column type and GC parameters were used on boththe Agilent and Leco systems. Thermal desorption parameters GERSTEL CIS 4: PTV Solvent Vent mode at a flow of 50 mL/min. Initial temperature -100℃(0.50 min) ramped to 280 ℃(3.0 min). GERSTEL TDU: Initial temperature 30 ℃ (0.40 min)60℃/min to 280 ℃ (4.00 min hold time) when only PDMSTwisters were used. A final temperature of 220 ℃ (4.0 minhold time) was set when EG-Silicone Twisters were used.TDU transfer line temperature: 300 ℃. Materials GERSTEL Twisters used were PDMS 1cm x 0.5 mm; PDMS 2cm x 0.5 mm; and EG-Silicone Twister. A GERSTEL TwicesterBmagnetic clip was used to hold multiple Twisters inside thesample vial. (d) Extracting the beer sample with one immersedPDMS Twister used for stirring and one EG-SiliconeTwister attached to the inside of the vial submerged inthe beer using Twicester@. Our results show that using the combination of thePDMS and EG-Silicone Twisters enabled significantlymore sensitive determination of polar compounds includ-ing carboxylic acids compared with the other methods. Infigure 2, peak areas for fourteen odor-active compoundsare shown (normalized to PDMS+EG-Silicone results)for the four SBSE methods used on the same Blue Moonbeer. For all but one of the compounds,(2-methyl-2-pen-tenoic acid), the PDMS+EG-Silicone method providedthe highest recovery. Sequential SBSE was the secondmost favorable. The higher recoveries for carboxylic acidswith the PDMS+EG-Silicone method make the Twistercombination method an excellent choice for the determi-nation of this important difficult-to-detect class of com-pounds. The compounds in question are: 4-vinyl guaiacol Figure 2: Comparison of four SBSE extraction techniques for odor-active chemicals in beer(normalized to PDMS + EG-Silicone peak areas). (clove flavor); linalool (floral); phenylethyl alcohol(rose);alpba-terpineol (pine, woody); 2-phenylethyl acetate (flo-ral, honey); o-thymol (woody, camphor); and 2-phenyl-ethyl esters of hexanoic, octanoic, and decanoic acids. Axillary malodors in clothing fabrics An understanding of human body odor chemicals maybe desirable for many reasons. For example, knowledgeof the composition of VOC metabolite excretions maybe a useful health-related diagnostic tool. Our studieswere undertaken to determine the ability of new fabricsto minimize axillary malodors. Figure 3: Chromatogram of cotton shirt worn during exercise and analyzed by an HSSE dual Twister method(1 PDMS + 1 EG-Silicone). Masses plotted for 60, 74 and 88 amu. Peak identities are: (1) isobutanoic acid; (2)3-methyl butanoic acid; (3) 2-methyl butanoic acid; (4) pentanoic acid; (5) hexanoic acid; (6) heptanoic acid;(7) 2-ethyl hexanoic acid; (8) octanoic acid; (9) nonanoic acid. None of these axillary malodor chemicals werepresent in the same shirt prior to exposure to an exercising person. Results and discussionSeveral volatile acids weredetected at low ppb (ug/L)levels from the sweat resi-due on worn T-shirts.Theseincluded acetic acid, propa-noic acid, isobutanoic acid,3-methylbutanoic aacid2-methylbutanoic acid,pentanoic acid, hexanoicacid, 3-methyl-2-hexenoicacid, octanoic acid, and Malodors form various sites on the body when natu-ral secretions are converted to volatile odorous chemicalsthrough microbial activity. Short chain volatile fatty acidsVFAs (C,-C,) are the primary axillary malodors formed;16-androstene steroids and thioalcohols have also beenidentified as contributors along with 3-Methyl-2-hexe-noic acid (3M2H) [3]. Following the successful combinedPDMS and EG-Silicone Twister extraction of volatileorganic acids from beer, the same Twister combinationwas used for analyzing functional T-shirts treated withantimicrobial chemicals and contaminated with perspi-ration to monitor production of various axillary malodorchemicals. Experimental The combination Twicester@ approach was investigatedusing HSSE. One gram of soiled fabric was suspendedfrom a paper clip protruding through the septum of a 40mL glass vial. A Teflon coated micro-stir bar was placedat the bottom of the vial (to agitate the air in the vial) andone 1 cmx 0.5 mm PDMS Twister and one EG-SiliconeTwister were attached to the sides of the vial with mag-netic clips (GERSTEL Twicester@). The Teflon stir barwas stirred at 800 rpm, and the vial was thermostated at50℃.Extraction was conducted for 2h. The two Twisterswere removed from the vial and desorbed in the GER-STEL TDU using the method parameters previouslydescribed for the beer analysis except with a maximumdesorption temperature of220 ℃ (instead of 280℃) toprotect the EG-Silicone Twister from thermal decompo-sition. nonanoic acid. In addition to these acids, several pyrazineswere detected along with 2-nonenal, an unsaturated al-dehyde with an unpleasant greasy and grassy odor, whichwas primarily found in subjects 40 years old or older.Results from one study indicate that 2-nonenal is gener-ated by the oxidative degradation of omega-7 unsaturatedfatty acids and suggest that 2-nonenal may be involvedin the age-related change of body odor [4]. According tothe study, the change of the monounsaturated fatty acidcomposition of skin surface lipids and the increase oflipidperoxides associated with aging may be involved in theformation of this characteristic odor component. Table I shows results for unsoiled control cotton shirtsamples spiked with five levels of various volatile acidsand 2-nonenal ranging from 10 to 500 ug/L. Excellentleast square correlation coefficient and detection limitsare demonstrated for all acids and 2-nonenal. Figure 3shows a chromatogram of axillary malodor volatile acidsextracted from a soiled shirt by HSSE with PDMS andEG-Silicone Twisters. A separate SBSE method was developed to deter-mine androstenone, in which the PDMS Twister wasdesorbed at 270℃ for 5 min (instead of 220 C for 5min used for the EG-Silicone Twister-based work). It isworth remarking that Soini et al. [5] developed a noveltool for using a PDMS Twister to extract volatile organicmalodorants directly from the skin. The stir bar, which isattached to a miniaturized holder that looks like a min-iature paint roller, is rolled over a determined area of ahuman skin while collecting a representative sample ofVOCs from the skin surface. Table l: Calibration curve results for five concentrations of axillary malodorant standards in the 10 to 500 ppb (ug/L) range analyzed by HSSE methodwith 1 PDMS + 1 EG-Sil Twister. XIC= extracted ion mode; SIM= selected ion mode. butyric acid 60 (XIC) 5 0.9921 HSSE isovaleric acid 60 (XIC) 5 0.9731 HSSE pentanoic acid 60 (XIC) 5 0.9737 HSSE hexanoic acid 60 (XIC) 5 0.9972 HSSE octanoic acid 60 (XIC) 10 0.9911 HSSE 2-methybutanoic acid 74 (XIC) 5 0.9655 HSSE 3-methyl-2-hexenoic acid 113 (XIC) 10 0.9901 HSSE 2-nonenal 70 (XIC) 5 0.9900 HSSE Androstenone 272 (SIM) 0.4 0.9914 SBSE Due to its flavor stability and functional properties, ca-sein powder is commonly used in cheese analogues,bakery products, meat products, confectionery products,desserts, nondairy coffee creamers, and beverages. Severalcomplaint casein powders from various international foodand beverage companies were received in our laboratory.These had extreme musty taints and were implicated ascauses of expensive product recalls involving cereal bars,nutritional beverages, a chocolate-flavored beverage, andnondairy coffee creamers in the U.S. Musty off-flavors are commonly associated withmicrobial metabolites such as trihaloanisoles, geosmin(trans-1,10-dimethyl-trans-9-decalol), 2-methylisobor-neol, and 2-isopropyl-3-methoxy pyrazine. These com-pounds have such low odor detection thresholds that theymust be detected in the ng/L range making determina-tion challenging (2,4,6-trichloroanisole odor threshold:0.15-2.0 ng/L; geosmin: 1-10 ng/L). These compoundshave relatively large log Ko/w values, however, allowingPDMS Twisters to extract them very efficiently from anaqueous sample or aqueous suspension of a solid sample.Indeed, SBSE GC-TOFMS was found to be a sensitiveand simple technique for the determination of these com-pounds in casein powders. Experimental 1 g of casein +25 mL water was stirred 30 min at room tem-perature with a Teflon coated stir bar, which was then replacedwith a2 cm x0.5 mm PDMS Twister and stirred for threehours. The Twisters were thermally desorbed in a GERSTELTDU using the parameters previously indicated. Results and discussion The primary musty off-flavor chemicals found in the ca-sein were trichloroanisole isomers formed by microbialmethylation of 2,4,6-trichlorophenol, a common ingre-dient in fungicides, pesticides, and wood preservatives.In extracted ion chromatograms at 212, 195, 196 and161 amu, haloanisole peaks were readily apparent. Themajor haloanisoles identified were 2,4-dichloroanisole,2,4,6-trichloroanisole (at 7 ug/L), and 2,3,6-trichloroan-isole contaminated with coeluters. In resolving the product recall lawsuits, it was impor-tant to provide supporting evidence that the substrate2,4,6-trichlorophenol was also present in the complaintcaseins. The source of the trichlorophenol was found tobe the wooden shipping pallets that the bags of caseinwere stacked on during overseas shipment. Interestingly, testing of additional complaint caseinsamples revealed the presence of 2,4,6-tribromoanisole(2,4,6-TBA), the source of which was identified as theslip sheets used when stacking the bags of casein duringtransport. These sheets were manufactured from recycledplastic, mainly high-density polyethylene, contaminatedwith tribromophenol (TBP), likely stemming from theuse of organobromine flame retardants, such as polybro- minated diphenyl ethers (PBDEs). Standard calibrationcurves for 2,4,6-TCA demonstrated correlation coef-ficients (R2 values) greater than 0.99. For TBA testing,samples were spiked with deuterated TBA at 133 ug/L.Stable Isotope Dilution Analysis (SIDA) is arguably oneof the most accurate quantitation techniques for the de-termination of organic compounds by GC-MS. The application examples presented in this article dem-onstrate that SBSE and HSSE have been successfully ex-tended to a wide variety of sample matrices. The new po-lar phase EG-Silicone Twister has significantly extendedthe application potential of SBSE and HSSE to includeanalytes that were difficult to extract at high recoverieswith PDMS. Twister technology combined with thermaldesorption-GCMS can be applied to a broad spectrum ofsample types using SBSE and HSSE, making the Twisterasignificant analytical tool to assist the flavor chemistin off-flavor and malodor elucidation of a wide profile ofnon-polar and polar analytes covering a broad range ofanalyte volatilities and concentrations. References ( [1]R R. Marsili, C . Laskonis, C. Kenaan "Evaluation of PDMS-based e xtraction techniques and G C-TOFMS for the analysis o f off-flavor chemicals in beer", Journal o f t he American Society of Brewing Chemists 2007,65(3), 129-137.DOI: 10.1094/ ASBCJ-2007-0617-01 ) ( 2] N. Ochiai, K. Sasamoto, H. Kanda, E. Pfannkoch "Sequential stir bar sorptive extraction for unifor m enrichment of trace amounts of organic pollutants i n water samples, Journal of the Ameri-can Society of Brewing Chemists 2008, 1200(1), 72-79. DOI: 1 0.1016/j.chroma.2008.05.069 ) ( 3| A . N atsch, H. Gfeller, P. G y gax, J. Schmid "lso l ation of a bacterialenzyme releasing axillary malodor and it s use as a screeningtarget for novel deodorant formulations", International J ournal of Cosmetic Science. 2005, 27, 1 15-122. DOI: 10.1111/j.1467-2494.2004.00255.x ) ( 4] S S. Haze,Y. Gozu, S Nakamura., Y. Kohno, K. Sawano, H.Ohta, K. Yamazaki“ 2 -Nonenal newly found in human body odor tends to i ncrease w ith aging" Journal of Investigati v e Dermatology.2001, 1 16,520-524.DOI:10.1046/j.0022-202x.2001.01287 ) ( 5] H . S oini, K. Bruce, I. Klouckova,R. Brereton, D. P e nn, M. No v otny " In situ surface sampling of biological objects and preconcentra- t ion of t heir volatiles for chromatographi c analysis", Anal. C h em. 2006, 78,7161-7168.DOI:10.1021/ac0606204 CCC ) Written from a practical perspec-tive, the second edition of Fla-vor, Fragrance, and Odor Analy-sis highlights the powerful SBSEtechnique and emphasizes therange of applications available. Ray Marsili (Edi.), Flavor, Fragrance, andOdor Analysis, Second Edition, 280 Pages, ISBN-10: 1439846731 and ISBN-13:978-1439846735. ERSTELIGERSTEL Solutions worldwide - No. 搅拌棒吸附萃取技术,SBSE,是一种无溶剂的用于萃取和浓缩痕量有机物的绿色萃取技术,原理类似于固相微萃取SPME。但是SBSE拥有更多的萃取吸附层,是SPME的50到250倍,进而在相同的萃取条件下,回收率可以达到SPME的100-1000倍,大大提高了检测的灵敏度。具有操作简单,高效,快速,重现性好,绿色无溶剂等优点。Twister®是其商品名,又称磁力搅拌棒(见下图),是一个包裹着萃取层/吸附层的磁力搅拌棒(见下图)。同一个Twister®可以重复利用超过200次以上。搅拌棒吸附萃取(SBSE)成功地应用于各种样品基质中用以检测和分析低至超痕量气味活性化学物质。极性吸附材料聚乙二醇-二甲基硅氧烷(EG-Silicone)Twister®显著增强了这项技术的实用性,使带氢键供体的极性化合物(如酚类和羧酸—重要的气味和风味化合物)的有效萃取成为可能。本文采用PDMS和EG-Silicone Twister® (磁力搅拌棒)的不同组合,采用不同的萃取技术对样品进行了分析。SBSE技术与热解析-气相色谱/质谱和峰反卷积相结合,构成了一个重要的多功能分析工具包,可帮助香料化学家进行异味和恶臭的分析。同时使用PDMS和EG-Silicone磁力搅拌棒萃取样品(如下图),可以同时检测非极性和极性分析物,覆盖广泛的挥发性和不同的浓度。本应用将近年来发展的SBSE技术与气相色谱飞行时间质谱(GCTOFMS)相结合,结合峰反卷积软件,来分析啤酒和酪蛋白中的异味。啤酒中的异味久置的啤酒会产生异味,影响产品质量。究竟是什么原因导致了啤酒质量的改变,又是什么化合物在从中作梗呢?作者分别研究了温度和光照两个影响因素,同时使用PDMS和EG-Silicone磁力搅拌棒萃取10ml样品,萃取时间为短短1小时。结果表明,长时间处在较高温度下的啤酒,会产生比控制样品更高浓度的糠醛,烟酸乙酯,苯乙酸乙酯和beta-大麻酮,并生成糠基乙醚,呋喃基羟甲基酮,(E,E)-2,4-十二碳二烯醛。而对光照条件下的啤酒分析发现,二甲基二硫醚,二甲基三硫醚,还有苯乙醛的含量相比控制样品浓度更高。酪蛋白粉中的霉味2007年夏天,2个案例发现酪蛋白粉中有三氯苯甲醚TCA污染,一个案例在酪蛋白粉发现三溴苯甲醚TBA污染,有霉臭味的酪蛋白被用来制造营养饮料,被多次起诉。土臭味素,三氯苯甲醚,三溴苯甲醚这类臭味物质的味觉阈值非常低(如下图),即痕量的纳克每升的浓度就可以被闻到,影响产品的质量。取1mg酪蛋白粉,加入25ml水,常温下用PDMS Twister萃取3小时,即可检测到此类物质。SBSE有着极高的灵敏度,而且加同位素后的标准曲线,线性也非常好,R2>0,994。结论本文给出的应用实例表明,SBSE已成功地扩展到多种样本基质。新的极性相EG-Silicone Twister显著地扩展了SBSE的应用潜力,对极性化合物回收率大大提高。SBSE技术与热脱附气质联用相结合,可应用于多种样品类型。使用Twister的SBSE技术是一个可以协助香料化学家分析异味和恶臭的重要的分析工具,涵盖了分析物广泛的挥发性和浓度范围。
确定


还剩2页未读,是否继续阅读?
GERSTEL(哲斯泰)为您提供《酒类,食品中风味物质,异味物质检测方案(热解吸仪)》,该方案主要用于啤酒中营养成分检测,参考标准--,《酒类,食品中风味物质,异味物质检测方案(热解吸仪)》用到的仪器有GERSTEL热脱附单元TDU2 (热解吸,热解析)
推荐专场
相关方案
更多
该厂商其他方案
更多