方案详情
文
主要讨论了根系分泌物对土壤中的碳的保护作用,研究过程中应用unisense氧气微电极对根际周围加入不同的渗入液(草酸、葡萄糖等)后对应的氧浓度剖面分布进行了测试,从而确定了根系分泌物对根际微区微生物呼吸速率的影响。获得的氧浓度剖面表明了草酸的加入显著降低了周围土壤微生物对O2的应用的有效性,其影响的最高深度可达5mm,而葡萄糖和乙酸的添加量则局限在1.5 mm处。草酸处理中的土壤内的微生物呼吸作用超过使用葡萄糖的效果。这也进一步了解根际碳矿化的加速(即启动效应)是否是由渗出物从保护性的矿物-有机结合中释放碳的能力所促进。并发现了一种由根、根相关的真菌和细菌产生的有机酸(草酸),具有较强的金属络合能力,对微生物的生物能利用有限。根际上述的相关实验结构,研究人员发现一种常见的根分泌物草酸会促进使有机化合物从与矿物的保护性联系中释放出来而造成的碳损失。而通过加强微生物与以前的矿物保护化合物,这种间接机制会加速碳损失的过程,该研究结果也为“启动”现象背后的生物-非生物耦合机制提供了一些见解,并挑战了关于矿物质相关的碳是受微生物保护的假设机制
方案详情

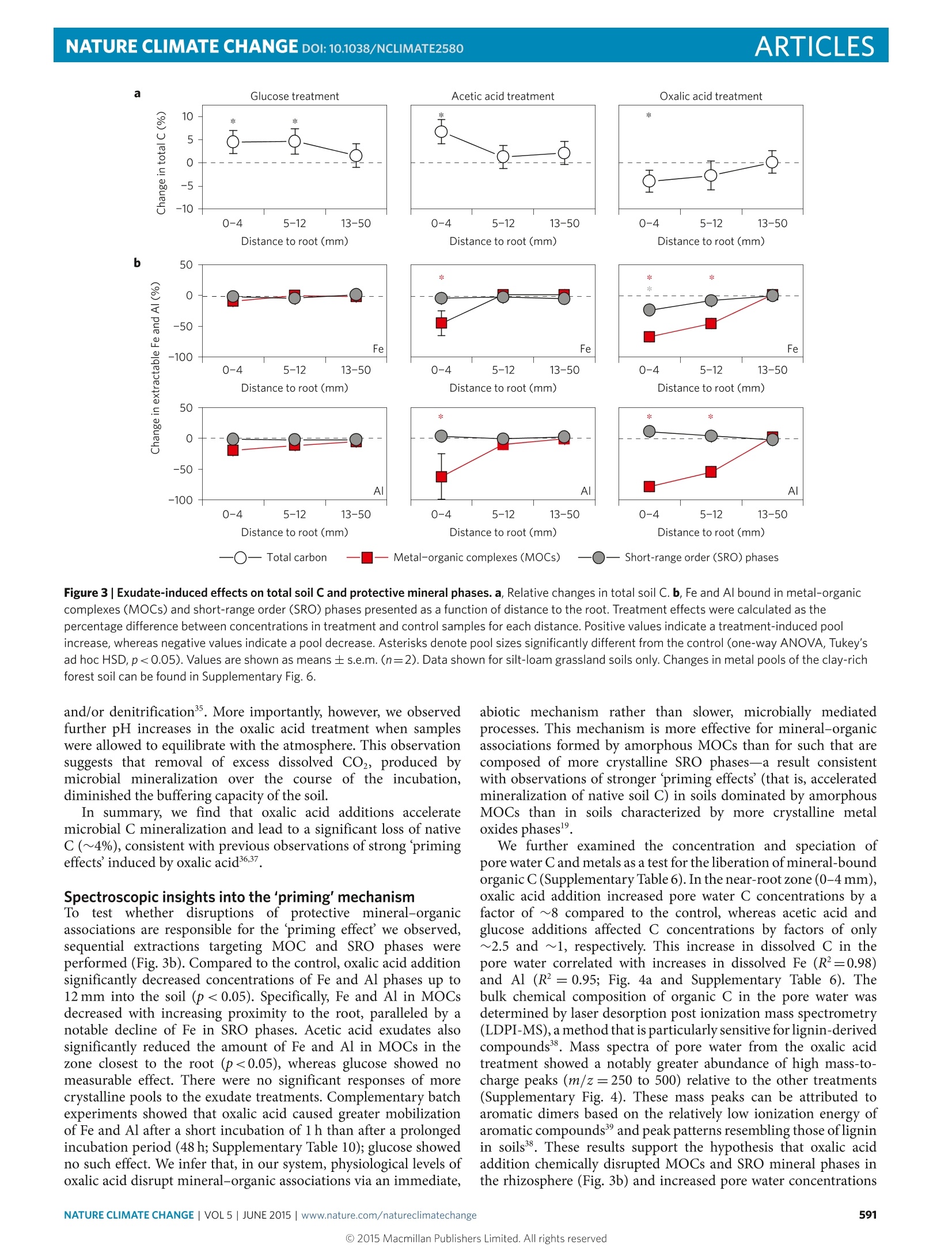
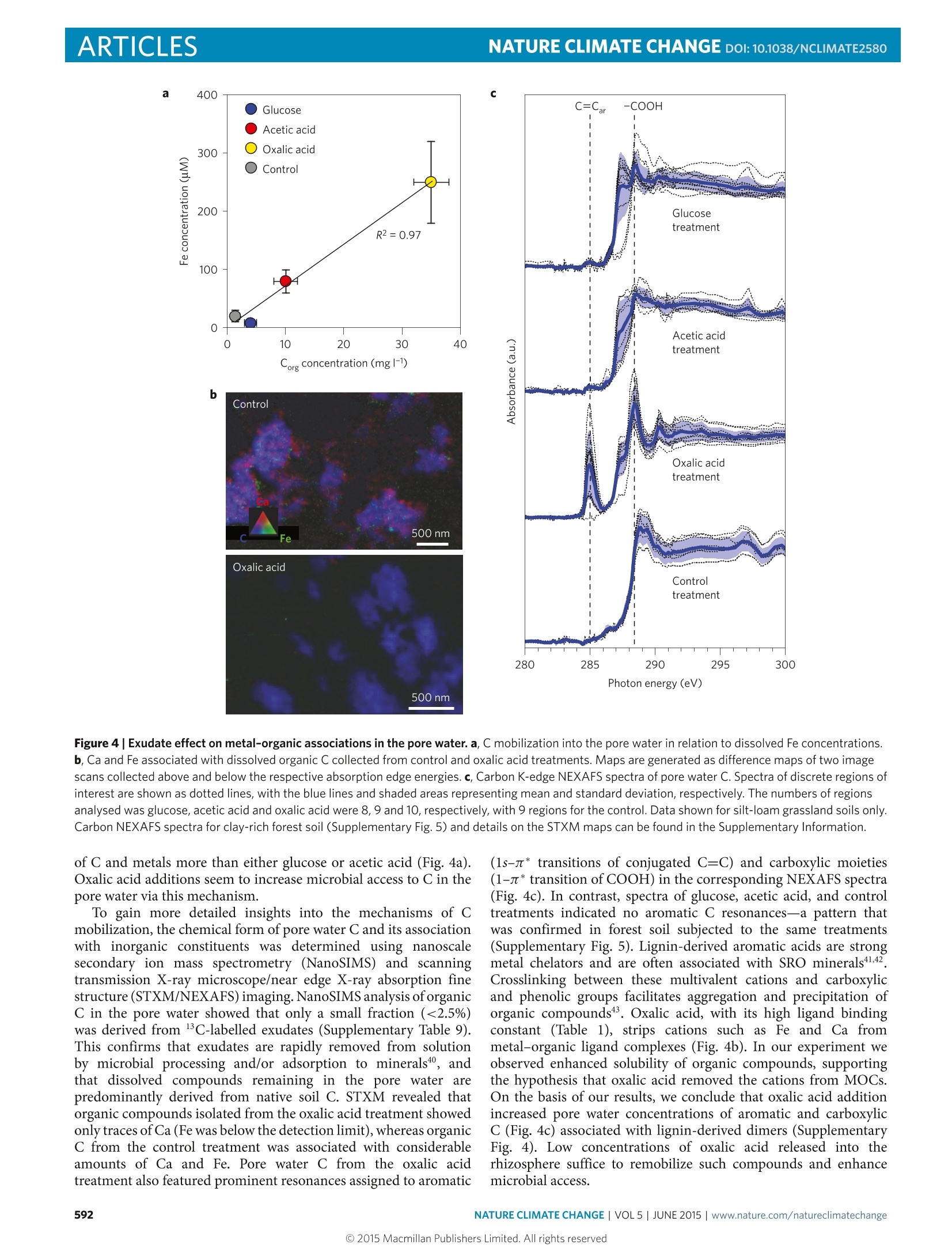
natureclimate changeARTICLESPUBLISHED ONLINE: 30 MARCH 2015|DOI:10.1038/NCLIMATE2580 ARTICLESNATURE CLIMATE CHANGE DOI:10.1038/NCLIMATE2580 Mineral protection of soil carbon counteracted byroot exudates Marco Keiluweitl,2*, Jeremy J. Bougoure2,3, Peter S. Nico+, Jennifer Pett-Ridge², Peter K. Weber’and Markus Kleber1,5 Multiple lines of existing evidence suggest that climate change enhances root exudation of organic compounds into soilsS.Recent experimental studies show that increased exudate inputs may cause a net loss of soil carbon. This stimulation ofmicrobial carbon mineralization ('priming') is commonly rationalized by the assumption that exudates provide a readilybio: SU ply of energy for the decomposition of native soil carbon (co-metabolism). Here we show that an alternatemechanism can cause carbon loss of equal or greater magnitude. We find that a common root exudate, oxalic acid,promotescarbon loss by liberating organic compounds from protective associations with minerals. By enhancing microbial access topreviously mineral-protected compounds, this indirect mechanism accelerated carbon loss more than simply increasing thesupply of energetically more favourable substrates.Our results provide insights into the coupled biotic-abiotic mechanismsunderlying the 'priming'phenomenon and challenge the assumptionthat mineral-associated carbon is protected from microbialcycling over millennial timescales. lants direct between 40-60% of photosynthetically fixedPcarbon (C) to roots and associated microorganisms viasloughed-off root cells, tissues, mucilage and a variety ofexuded organic compounds2. Elevated CO concentrations in theatmosphere are projected to increase the quantity3.4 and alter thecomposition5of root exudates released into the soil. It seems lessclear to what extent changing inputs will cause a net loss of native (orold) organic C (ref. 7). A better understanding of the mechanismunderlying soil Closs is pivotal in predicting how the large soil Cstocks may respond to global change. Exudate-induced soil C loss is commonly attributed to a primingeffect-that is, a short-term increase in microbial mineralizationof native soil C as a result of fresh carbon inputs to the soil.Although the process of priming’ has received great attentionin ecosystem sciences in recent years, our knowledge of theunderlying mechanism is limited. It is often supposed thatbioavailable exudate compounds induce greater microbial activityand enzyme production because they serve as co-metabolites8,10Co-metabolism is defined as the mineralization of a non-growthsubstrate(for example, certain forms of native soil organic C) duringgrowth of a microorganism on a bioavailable carbon and energysource (for example, exudate compounds). This mechanism isoften invoked to increase the physiological potential of decomposersfor the mineralization of native soil C (refs 8,10; Fig.la). However, asnoted by Kuzyakov and co-workers"?, direct experimental evidencein support of this mechanism is scarce because most studies haveaimed at identifying priming effects’ rather than the underlyingmechanism. Previous attempts to describe the mechanism(s) underlyingpriming effects’ have focused almost exclusively on biological phe-nomena. That approach, however, results in a conceptual conun-drum: how can observed priming effects’be explained solely by co-metabolism if microbial access to substrate C is notoriouslylimited in most soils? In mineral soil, the majority of organic com-pounds is intimately associated with reactive mineral phases13,14.Metal-organic complex (MOC) and short-range order (SRO)phases bind organic compounds through their large surface area andvarious bonding sites15,16. Such mineral-organic associations limitmicrobial and enzymatic access and are quantitatively the mostimportant mechanism protecting C from microbial use for centuriesor millennials. Rasmussen et al.l showed that the magnitude of thepriming effect'is at least partly controlled by the presence ofreactivemineral phases. Recent conceptual frameworks20.21 and numericalmodels therefore argue that C mineralization rates are generallyenhanced by mechanisms that facilitate the release of mineral-protected C into more accessible pools. Here we tested whether the acceleration of carbon mineralizationin the rhizosphere (that is, the priming effect) is promotedby the exudates ability to liberate C from protective mineral-organic associations, thereby increasing microbial access. In a well-controlled system, we investigated the effects of the two mostabundant exudate classes-organic acids and simple sugars23.24. Wehypothesized that direct dissolution of protective mineral phaseswould be promoted by oxalic acid (Fig.1b). Oxalic acid-an organicacid produced by roots, root-associated fungi and bacteria-is routinely found among the most abundant compounds inrhizosphere pore water23,24.Oxalic acid has strong metal-complexingabilities but is of limited bioenergetic use to microbes. In contrast,we expected bioenergetically more favourable sugars such as glucoseto act as a co-metabolite and stimulate microbial mineralizationof native soil C (Fig. 1a). A third common exudate, acetic acid,was expected to have an intermediate response, as it is less easilymetabolized than glucose and has a lower complexing capacity thanoxalic acid (Table 1). ( lDepartment of Crop and Soil Science, Oregon State U niversity, ALS Building 3017, Corvallis, Oregon 97331, USA. 2Chemical Sciences Division , LawrenceLivermore National Laboratory, 7000 East avenue, L- 2 31, Livermore, California 9 4 550, US A . 3School of Earth and Env i ronment, Univ e rsity of WesternAustralia, 35 Stirling Highway, Cr a wley, Western Aus t ralia 6009,Australia. 4Earth Scienc e s Divisio n , Lawrence Berkeley National Laboratory , 1 CyclotronRd, Berkeley, California 94720, USA. 5Institut fur Bodenlandschaftsforschung, Leibnitz-Zentrum fur Agrarlandschaftsforschung (ZALF) e.V. Eber s walder StraBse 84,15374 Muncheberg, Germany.*e-mail: ke i lu w e i t@uma ss.edu ) Figure 1| Proposed mechanisms for the exudate-induced acceleration of the microbial mineralization of native carbon ('priming effects') in therhizosphere. a, The traditional view is that reduced exudate compounds (for example,simple sugars) stimulate microbial growth and activity viaco-metabolism, and so increase the overall physiological potential of the decomposer community for carbon mineralization. Other factors such as theincreased microbial demand for nitrogen or successional shifts in the community structure may also contribute to increased mineralization rates8,12. b, Thealternative mechanism proposed here takes into account that large quantities of soil C are inaccessible to microbes owing to associations with mineralphases. Root exudates that can act as ligands (for example,organic acids) effectively liberate C through complexation and dissolution reactions withprotective mineral phases, thereby promoting its accessibility to microbes and accelerating its loss from the system through microbial mineralization.Microbial O2 consumption may further increase the accessibility of protected C by lowering the redox potential, Eh, and promoting reductive dissolution ofSRO minerals. Table 1|Exudate properties and their predicted and measured effect on microbial carbon-use efficiency (CUE) and biochemicaloxygen demand(BOD). Glucose Acetic acid Oxalic acid Exudate properties Acidity (pKa) n.a. 4.76 1.25 and 4.14 Complexation of Fe3+(logK)* n.a. 3.5 8.2 Predicted effect CUET (ratio) 0.4-0.7 0.4-0.6 0.05-0.25 stoichiometric BOD (molO2 mol-1 C) 1 1 0.25 Expected BOD* (mo1O2mol-1C) 0.3-0.6 0.4-0.6 0.23-0.30 Expected BODs 1.0-2.0 1.3-2.0 0.7-1.0 Measured effect Measured respiration rates silt-loam 1.0±0.3 0.5±0.0 1.5±0.0 Measured respiration rates clays 1.0±0.4 1.0±0.5 2.4±0.2 *Formation constants (logK) defined by the equilibrium constant K=[M][4]/([M]+[L]), where M is Fe+ and L is either glucose, acetic acid or oxalic acid52. tReported microbial CUEs in soilincubations27-29 expressed as the amount of C assimilated in new biomass relative to the amount of C used in cellular respiration.*Range of expected BOD in soils calculated as (1-CUE)xstoichiometric BOD.Expected BOD values and measured O2 respiration rates given relative to those for glucose. Recreating a rhizosphere environment We tested these hypotheses by delivering a continuous supply ofindividual exudate solutions through an artificial root (length =10 cm, diameter =2.5 mm) into unperturbed soil,recreating arhizosphere environment (Supplementary Fig. 1). Exudates weresupplied to a grassland soil (Supplementary Table 1) at root surface-normalized rates mimicking natural exudation rates of root tips(15 umol C cm-d-1; refs 25,26). For comparison, selected analyseswere replicated with a forest soil. To distinguish exudate C fromnative soil C, individual exudate solutions were isotopically labelledwith 13c (81C=8,800%). Exudate solutions or an inorganicnutrient solution (control) were provided over an incubation periodof 35 days to replicate a burst in root growth at the onset of the growing season when the highest exudation rates are expected.Compared to the control, all three exudate compounds inducedvisible physical gradients surrounding the artificial root after7-10 days, which remained stable until the end of the experiment.In the oxalic acid treatment, these pronounced effects developedaround the entire root and extended up to 5-10 mm into the soil(Fig.2a), but in soils receiving glucose and acetic acid additions werelimited to small patches around the root. If root exudates were to accelerate the microbial mineralizationof soil C (that is, cause priming effects) primarily because theyserve as a co-metabolite (that is, provide easily assimilable C andenergy; Fig. la), one would expect energetically favourable glucoseto cause a greater priming effect’ than less favourable substrates Exudatetreatments Oxalic acid Control b 250 Glucose treatment Acetic acid treatment 0Oxalic acid treatment—— Control treatment —Model fits Figure2|Exudate effects on artificial rhizosphere soil. a, Photographs ofthe rhizosphere effect caused by oxalic acid addition and the control forcomparison. White arrows indicate positions of the artificial root providingexudate solutions. b, O2 concentrations as a function of distance to the rootfor the different exudate treatments. Points represent mean ± s.e.m.(n=2). Asterisks denote locations with mean O2 concentrationssignificantly lower than the control (one-way ANOVA, Tukey's ad hoc HSDtest, p<0.05).Solid lines represent model fits used to calculate microbialrespiration based on Fick’s first law of diffusion53. The inset showsvolume-specific respiration rates in the rhizosphere for each exudatetreatment. See Supplementary Information for details on fitting parametersand rate calculations. such as oxalic acid (Table 1). However, we found that oxalic acidadditions led to greater microbial respiration, community shifts tofast growing, rapidly C-mineralizing microbial taxa, and loss of totalsoil C relative to the other treatments. This accelerated microbialC mineralization caused by oxalic acid exudates coincided withthe disruption of mineral-organic associations and increases in Caccessibility in the pore water. The following describes evidence thatthis priming effect’ is at least in part caused by the exudates’ abilityto enhance microbial access to previously protected soil C (Fig. 1b). Oxalic acid accelerated soil carbon mineralization To determine the effect of root exudates on microbial respirationrates in rhizosphere microsites, we used microsensors to recordO2 profiles in the soil surrounding the root (Fig. 2b). Theseprofiles showed that addition of oxalic acid significantly depleted O availability up to 5 mm into the surrounding soil (p<0.05;Fig. 2b), whereas the effects of glucose and acetic acid additionswere restricted to the first 1.5 mm. Across the entire rhizospherezone (0-15 mm), microbial respiration rates followed a consistentpattern: oxalic acid > glucose > acetic acid (Fig. 2b,inset). To testwhether this was a response specific to the grassland soil (silt-loam),we repeated the experiment with a forest soil (clay-loam) differing intexture and mineralogy (Supplementary Table 1) and observed thesame result (Supplementary Fig.1). Overall, microbial respirationin the oxalic acid treatment exceeded that of glucose by factors of~1.6 (silt-loam) and ~2.8(clay; Table 1). We compared the stoichiometry of microbial carbon-useefficiency (CUE) and biochemical oxygen demand (BOD) of oxalicacid versus glucose mineralization pathways and found that oxalicacid addition accelerated the mineralization of native soil C morethan glucose addition. Reported CUEs (the ratio of C assimilatedinto new biomass relative to the amount of C used in cellularrespiration) for oxalic acid (5-25%) and glucose (40-70%) insoils27-29 indicate that the fraction of oxalic acid C released as CO,(75-95%) is about twice that of glucose C (30-60%). Mineralization(that is, complete oxidation) of oxalic acid to CO, requires 0.25moles of O2 per unit C compared to 1 mole of O2 per unit C forglucose (Table 1). The BOD expected for the mineralization ofeachsubstrate is equal to: Expected BOD=(1-CUE)xstoichiometric BOD The CUE and stoichiometric BOD values used are shown inTable 1. These calculations demonstrate that the BOD expectedfor microbial mineralization of oxalic acid should be only half thevalue associated with glucose mineralization (Table1). Because ourmeasured respiration rates show the opposite trend, we concludethat mineralization of other, more reduced (non-exudate) soil Cmust have accounted for at least 50% of the respiration observed inthe oxalate treatment. In line with accelerated mineralization of native soil C,soilsreceiving oxalic acid additions experienced a net C loss in the zoneclosest to the root (p<0.05; Fig.3a). In contrast, addition of theenergetically more favourable substrates glucose and acetic acidresulted in a pronounced increase in total soil C content (Fig. 3a).This net C accumulation, possibly due to an increase in microbialbiomass C caused by microbial assimilation of these exudates, wasnot counterbalanced by concurrent increases in the mineralizationof native C. Pronounced compositional changes in microbial communitystructure also reflect greater carbon mineralization activity insoil receiving oxalic acid (Supplementary Fig. 3a). Near theroot (0-4mm), oxalic acid additions significantly increased therelative abundance of Bacteroides and Proteobacteria and reducedthat of Acidobacteria Firmicutes and Verrucomicrobia (p<0.05;Supplementary Fig. 3b). Microbial communities in soils amendedwith acetic acid soils shifted in a similar but less pronouncedmanner relative to soils treated with oxalic acid; glucose had verylittle effect. Taxa from the Bacteroidetes and Proteobacteria phylathat were strongly promoted by the oxalic acid treatment areoften characterized as copiotrophs, and positively correlated withincreased C bioavailability and mineralization rates30,31. Excess dissolved CO, measured in the pore water providesfurther evidence for accelerated C mineralization in the oxalic acidtreatment. Measured immediately after harvest, the pH of soil closeto the root was 1.5 and 0.7 units higher in oxalic and acetic acidtreatments, respectively, whereas glucose lowered the pH by 0.5units (Supplementary Table 6). This increased proton consumptioncan be attributed to one or a combination of factors, all of which arelinked to increased microbial activity: microbial decarboxylationmineral dissolution reactions, dissimilatory metal reduction34 O一 Short-rangeorder (SRO) phases Figure 3|Exudate-induced effects on total soil C and protective mineral phases. a, Relative changes in total soil C. b, Fe and Al bound in metal-organiccomplexes (MOCs) and short-range order (SRO) phases presented as a function of distance to the root. Treatment effects were calculated as thepercentage difference between concentrations in treatment and control samples for each distance. Positive values indicate a treatment-induced poolincrease, whereas negative values indicate a pool decrease. Asterisks denote pool sizes significantly different from the control (one-way ANOVA, Tukey'sadhoc HSD, p<0.05). Values are shown as means ± s.e.m. (n=2). Data shown for silt-loam grassland soils only. Changes in metal pools of the clay-richforest soil can be found in Supplementary Fig. 6. and/or denitrification35. More importantly, however, we observedfurther pH increases in the oxalic acid treatment when sampleswere allowed to equilibrate with the atmosphere. This observationsuggests that removal of excess dissolved CO, produced bymicrobial mineralization over the course of the incubation,diminished the buffering capacity of the soil. In summary, we find that oxalic acid additions acceleratemicrobial C mineralization and lead to a significant loss of nativeC (~4%), consistent with previous observations of strong primingeffects' induced by oxalic acid3637. Spectroscopic insights into the'priming'mechanism Totest whether disruptionss of protective mineral-organicassociations are responsible for the priming effect’we observed,sequential extractions targeting MOC and SRO phases wereperformed (Fig.3b). Compared to the control, oxalic acid additionsignificantly decreased concentrations of Fe and Al phases up to12 mm into the soil (p<0.05). Specifically, Fe and Al in MOCsdecreased with increasing proximity to the root, paralleled by anotable decline of Fe in SRO phases. Acetic acid exudates alsosignificantly reduced the amount of Fe and Al in MOCs in thezone closest to the root (p<0.05), whereas glucose showed nomeasurable effect. There were no significant responses of morecrystalline pools to the exudate treatments. Complementary batchexperiments showed that oxalic acid caused greater mobilizationof Fe and Al after a short incubation of 1 h than after a prolongedincubation period (48 h; Supplementary Table 10); glucose showedno such effect. We infer that, in our system, physiological levels ofoxalic acid disrupt mineral-organic associations via an immediate, abiotic mechanism rather than slower, microbially mediatedprocesses. This mechanism is more effective for mineral-organicassociations formed by amorphous MOCs than for such that arecomposed of more crystalline SRO phases-a result consistentwith observations of stronger priming effects’ (that is, acceleratedmineralization of native soil C) in soils dominated by amorphousMOCs than in soils characterized by more crystalline metaloxides phases9. We further examined the concentration and speciation ofpore water Cand metals as a test for the liberation of mineral-boundorganic C (Supplementary Table 6). In the near-root zone (0-4 mm),oxalic acid addition increased pore water C concentrations by afactor of ~8 compared to the control, whereas acetic acid andglucose additions affected C concentrations by factors of only~2.5 and ~1, respectively. This increase in dissolved C in thepore water correlated with increases in dissolved Fe (R²=0.98)and Al (R²=0.95; Fig. 4a and Supplementary Table 6). Thebulk chemical composition of organic C in the pore water wasdetermined by laser desorption post ionization mass spectrometry(LDPI-MS), a method that is particularly sensitive for lignin-derivedcompounds38. Mass spectra of pore water from the oxalic acidtreatment showed a notably greater abundance of high mass-to-charge peaks (m/z=250 to 500) relative to the other treatments(Supplementary Fig. 4). These mass peaks can be attributed toaromatic dimers based on the relatively low ionization energy ofaromatic compounds and peak patterns resembling those ofligninin soils38. These results support the hypothesis that oxalic acidaddition chemically disrupted MOCs and SRO mineral phases inthe rhizosphere (Fig.3b) and increased pore water concentrations Figure 4|Exudate effect on metal-organic associations in the pore water. a, C mobilization into the pore water in relation to dissolved Fe concentrations.b, Ca and Fe associated with dissolved organic C collected from control and oxalic acid treatments. Maps are generated as difference maps of two imagescans collected above and below the respective absorption edge energies. c, Carbon K-edge NEXAFS spectra of pore water C. Spectra of discrete regions ofinterest are shown as dotted lines, with the blue lines and shaded areas representing mean and standard deviation, respectively. The numbers of regionsanalysed was glucose, acetic acid and oxalic acid were 8, 9 and 10, respectively, with 9 regions for the control. Data shown for silt-loam grassland soils only.Carbon NEXAFS spectra for clay-rich forest soil (Supplementary Fig.5) and details on the STXM maps can be found in the Supplementary Information. of C and metals more than either glucose or acetic acid (Fig. 4a).Oxalic acid additions seem to increase microbial access to C in thepore water via this mechanism. To gain more detailed insights into the mechanisms of Cmobilization, the chemical form of pore water C and its associationwith inorganic constituents was determined using nanoscalesecondary ion mass spectrometry (NanoSIMS) and scanningtransmission X-ray microscope/near edge X-ray absorption finestructure (STXM/NEXAFS) imaging. NanoSIMS analysis oforganicC in the pore water showed that only a small fraction (<2.5%)was derived from C-labelled exudates (Supplementary Table 9).This confirms that exudates are rapidly removed from solutionby microbial processing and/or adsorption to minerals4, andthat dissolved compounds remaining in the pore water arepredominantly derived from native soil C. STXM revealed thatorganic compounds isolated from the oxalic acid treatment showedonly traces of Ca (Fe was below the detection limit), whereas organicC from the control treatment was associated with considerableamounts of Ca and Fe. Pore water C from the oxalic acidtreatment also featured prominent resonances assigned to aromatic (1s-n* transitions of conjugated C=C) and carboxylic moieties(1-n* transition of COOH) in the corresponding NEXAFS spectra(Fig. 4c). In contrast, spectra of glucose, acetic acid, and controltreatments indicated no aromatic C resonances-a pattern thatwas confirmed in forest soil subjected to the same treatments(Supplementary Fig. 5). Lignin-derived aromatic acids are strongmetal chelators and are often associated with SRO minerals41,42Crosslinking between these multivalent cations and carboxylicand phenolic groups facilitates aggregation and precipitation oforganic compounds43. Oxalic acid, with its high ligand bindingconstant (Table 1), strips cations such as Fe and Ca frommetal-organic ligand complexes (Fig. 4b). In our experiment weobserved enhanced solubility of organic compounds, supportingthe hypothesis that oxalic acid removed the cations from MOCs.On the basis of our results, we conclude that oxalic acid additionincreased pore water concentrations of aromatic and carboxylicC (Fig. 4c) associated with lignin-derived dimers (SupplementaryFig. 4). Low concentrations of oxalic acid released into therhizosphere suffice to remobilize such compounds and enhancemicrobial access. Implications for terrestrial carbon cycling As detailed above, we expected that an energetically more favourableroot exudate such as glucose should cause a greater priming effectthan a less attractive one such as oxalic acid (Fig. 1a). However,the inverse was observed: by improving the accessibility of Cpreviously protected in mineral-organic associations, oxalic acidinduced a much stronger priming effect’than glucose and aceticacid. Rather than via co-metabolism, organic ligands with metal-complexing abilities accelerate microbial mineralization of C in therhizosphere via an indirect, multipart mechanism(Fig.1b).Organicligands released into the rhizosphere (i) mobilize mineral-bound Cthrough complexation and dissolution of SRO phases and/or (ii)solubilize organic compounds through the removal of crosslinkingmetal cations from MOCs. Release from SRO or MOC phasespromotes the accessibility of organic compounds to microbes.Increased microbial access subsequently (iii) stimulates microbialmineralization and shifts the community structure towards phylaadapted to soil environments with greater accessibility of C. On thebasis of these results, we conclude that priming effects’ are not apurely biotic phenomenon and should be viewed as the sum of directbiotic co-metabolic (Fig. la) and indirect C mobilization (Fig. 1b)mechanisms. We suggest that future investigations into the causesof priming effects’consider both mechanisms simultaneouslyand focus on quantifying their relative contributions in differentsoil ecosystems. In our study, oxalic acid disrupted mineral-organic associationsin both grassland and forest soils. This observation is consistentwith root-induced weathering of protective mineral phases duringperiods of rapid root growth in arable4and forest soils45, andhighlights the general nature of the proposed mechanism. Toestimate the importance of the proposed mechanism at theecosystem-scale, we calculated the potential C loss that may beattributed to this mobilization mechanism in a forest ecosystem.The oxalic acid treatment resulted in an overall C loss of ~4% overour experimental period. If we assume that the total amount ofexudate C released into the soil in our experiments is comparableto that released over the course of an annual burst of root growthin times of high primary productivity26, and organic acids compriseup to ~25% of the mixture of compounds exuded over that timeframe², the annual soil C loss is estimated to be ~1% Cyr-. Bycomparison, Richter et al46 found that soil C in deep mineralhorizons, where C accessibility is probably low owing to mineral-organic associations, is rapidly mineralized on reforestation andexpansion of the rooting zone. In that study, the average loss overfour decades of reforestation was approximately 1.08% Cyr--arate strikingly close to the value in our experiment. Although simple,our calculation highlights the potential impact of the proposedpriming'mechanism on mineral-protected C at an ecosystem level. Conceptual frameworks of soil organic matter stabilization havelong considered C in mineral-organic associations permanentlyinaccessible to microbes, and thus protected from loss processesfor millennia or longerl3,14,18. But this paradigm is shifting, andmany now recognize that any natural organic compound canbe decomposed when the required resources are available tothe decomposer community21. Here we demonstrate a climate-dependent priming mechanism where plant exudates counteractthe strong protective effect of mineral-organic associations andfacilitate the loss of C from the soil system. This physiologicalcapacity of plant roots to effectively remobilize mineral-associatedsoil C will have to be factored into next-generation soil Ccycling models22.47 if root exudation rates respond to climatechange as predicted. Elevated Co concentrations may not onlystimulate exudation, they may also alter the composition ofexudate compounds released into soil6,48 and so increase metalmobilization in the rooting zone9. If shifts towards greaterexudation of reactive weathering agents such as oxalic acid can be confirmed, determining how changing exudation patterns impactmineral-protected C should be a high priority for future research Methods Soils were chosen to represent two important biomes, forests and grasslands.Medium-textured silt-loam (Typic Haploxeroll) under cultivation with Triticumspp. (Hermiston Agricultural Research & Extension Center) and volcanic-ash clay(Humic Dystrudepts) under old-growth Douglas-fir (H.J. Andrews ExperimentalForest, Oregon, USA). At both sites, soil cores and mixed samples of surfacehorizons characterized by dense root networks were collected and stored at fieldmoisture at 4°C until further use (see Supplementary Table 1 for soilcharacteristics and handling). Incubations were conducted in microcosm systems designed and fabricatedto deliver a continuous supply of exudate solution through an artificial root intothe soil (see Supplementary Information for details). Soils were sieved (2 mm),pre-incubated at 75% field capacity for one week, and packed into the frame atfield bulk density. The artificial roots were connected to a syringe pump equippedwith a multi-syringe feeding system, which provided sterile exudate solutionscontaining glucose, acetic acid or oxalic acid as well as inorganic nutrients forosmoregulation (330 uM KCl, 70 uM KH PO and 70 uM MgSO4).Concentrations of each exudate solution were normalized on a C-basis and thepump rate was adjusted such that exudates were delivered at a rate of15 umolC cm-’d- per microcosm at1 mld-1.13C-labelled substrates. (Cambridge Isotope Laboratories) were used to enrich exudate solutions in I’C(fina 83C=8,800%o). Incubations were carried out in the dark for 35 days,with temperature (25°C) and relative humidity (75%) controlled by anenvironmental chamber. Two replicate microcosms were assembled foreach treatment. O2 concentration profiles were measured after the incubation period usingClark-type microsensors (OX-100, Unisense). Microsensors had a 100-um tipsize, a stirring sensitivity of <2%, and a 90% response time of <1s. Linearcalibrations were performed in 0.1 M sodium ascorbate in 0.1 M NaOH (0%Osaturation) and air bubbled water (100%O2 saturation) before and betweenmeasurements. The microsensor was mounted on a micromanipulator(MM33-2, Unisense) and connected to a picoamperemeter (PA-2000, Unisense)to collect six replicate measurements at distances of 0.5, 1, 5 and 15 mmfrom the root in each microcosm (see Supplementary Informationfor details). After the incubation, microcosms were opened in an anaerobic glove box andthree zones on both sides of the root (0-4, 5-12 and 13-50 mm from the root)were carefully sampled for metal and DNA/RNA extractions, pH and total Cmeasurements. Metal pools in each zone were determined using a sequentialextraction procedure consisting of 0.1 M Na-pyrophosphate at pH 10 fororganically complexed pools and 0.2 M ammonium oxalate at pH 3 forshort-range order phases. Duplicate extractions were performed under anaerobicconditions in the dark. Elemental analysis of the supernatants was performed ona Perkin Elmer SCIEX Elan DRC II inductively coupled plasma massspectrometer (ICP-MS). Total RNA/DNA was extracted from 0.25 g soil of eachzone using a MoBio PowerSoil RNA Isolation Kit in combinationwith the PowerSoil DNA Elution Accessory Kit (MoBio Laboratories)following manufacturer’s instructions. Duplicate extracts for each samplewere prepared for pyrosequencing (see Supplementary Informationfor details). Soil pore water was extracted from the root zone (0-4 mm) using aWhatman Centrex MF Disposable Centrifugal Microfilter, adapting an existinglow-pressure centrifugal displacement technique. In the glove box, a 2 g aliquotof moist soil was transferred to the sample reservoir. Soil pore water wasdisplaced and filtered through pre-rinsed 0.45 um cellulose acetate membranesinto the collection tube by centrifugation at 2,000g for 60 min. Organic Cconcentrations in the pore water samples were determined by ultraviolet/visiblespectrometry (450 nm) and the chemical composition was analysed using laserdesorption synchrotron ionization (LDSI). LDSI was performed on a modifiedtime-of-flight secondary ion mass spectrometer (TOF.SIMS V; IonTOF) coupledto a synchrotron vacuum ultraviolet light port at beamline 9.0.2 of the AdvancedLight Source (ALS) at the Lawrence Berkeley National Laboratory. For combinedimaging analysis of pore water C and associated metals, 1 ul pore water aliquotswere dried on Si,N windows (Silson) in the glove box. STXM/NEXAFSspectromicoscopic analyses were performed at beamline 5.3.2.2 of the ALS(ref. 51). 813Cvalues of pore water C were acquired using high-resolutionsecondary ion mass spectrometry imaging performed on a NanoSIMS 50(Cameca) at the Lawrence Livermore National Laboratory. Details on imaginganalyses can be obtained in the Supplementary Information. Reported resultsfrom statistical tests were obtained with OriginPro (OriginLab), includingone-way ANOVA followed by post hoc analysis using Tukey’s HSD test (Figs 2band 3) and correlation analysis (Fig. 4a), with a p-value of less than 0.05indicating statistical significance. Received 14 February 2014; accepted 30 January 2015;published online 30 March 2015 References ( 1. H ogberg, P. et al.Large-scale forest girdling shows that c urrent p hotosynthesis d rives soil respiration. Nature 4 1 1, 789-792 ( 2 001). ) ( 2 . Clemmensen, K. E. et al. Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 339, 1 615-1618(20 1 3). ) ( 3 . Phillips, R. P, Finzi, A . C. & Bernhardt, E . S. Enhanced r o ot exudation in d ucesmicrobial feedbacks to N cycling in a pine forest under long-term CO f umigation. E c ol. Lett. 1 4, 187-1 9 4 (2011). ) ( 4. Carney, K. M., Hun g ate, B. A., Drake, B. G. & Megonigal,J. P. Altered soilmicrobial community at elevated CO, leads to loss of soil carbon. Proc. NatlAcad. S ci. USA 1 04, 4990-4995 (2007). ) ( 5 . D eLucia, E. H . , Callaway,R. M., Thomas, E. M . & Schlesinger, W. H. M echanisms of phosphorus acquisition for ponderosa pine seedlings un d er high CO and temperature. A n n. B o t. 79,1 1 1-120(1997). ) 6.Fransson, P. Elevated CO, impacts ectomycorrhiza-mediated forest soil carbonflow: Fungal biomass production, respiration and exudation. Fungal Ecol. 5,85-98(2012). ( 7. H eimann, M. & Reichstein,M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 451,289-292(2008). ) ( 8 K uzyakov, Y., Friedel,J. K. & Stahr, K. Review of mechanisms and ) quantification of priming effects. Soil Biol. Biochem. 32, 1485-1498(2000). ( 9.B i anchi, T. S . The ro l e of te r restrially derived organic carbon i n th e c o ast a locean: A changing paradigm and the priming effect. Proc. Natl Acad. Sci. US A 108, 1 9473-19481( 2 011). ) ( 10. Fontaine, S.,Mariotti, A. & Abbadie, L . The p r iming ef f ect o f organic matter: Aquestion of microbial competition? Soil Biol. Biochem. 35, 837-843 (2003). ) ( 11. Horvath, R. S. Microbial co-metabolism and the degradation of organic c ompounds in nature. Bacteriol. Rev. 36, 146-155(1972). ) ( 1 2. Blagodatskaya, E. & Kuzyakov, Y. Mechanisms of real and apparent priming effects and their dependence on soi l microbial biomass and community s tructure: Critical review. Biol. Fertil. Soils 45, 115-131 (2008). ) 13. Torn, M. S., Trumbore, S. E., Chadwick, O. A., Vitousek, P. M. & Hendricks, D.M. Mineral control of soil organic carbon storage and turnover. Nature 389,170-173(1997). ( 14. Baisden, W. T , Amundson, R. , Cook, A. C. & Brenner,D. L. T urnover and s torage of C and N in five d e nsity fr a ctions from California ann u al gras s land s urface soils. Glob. Biogeochem. Cycles 16 , 1117 (2002). ) ( 15. Mikutta, R. et al. Biodegradation of forest floor organic matter bound tominerals via different binding mechanisms. Geochim. Cosmochim. Acta 71, 2569-2590(2007). ) ( 16. Chorover, J . & Amistadi, M.K . R e action of forest floor organic matter at goethite, birnessite and smectite surfaces. Geochim. C osmochim. Ac t a 65, 95-109 (2001). ) ( 17. Conant, R. T . e t al. Temperature and soil organic matter decomposition r ates-synthesis o f current knowledge and a way f orward. Glob. Change Biol. 17,3392-3404(2011). ) ( 18. M ikutta, R., K l eber, M., Torn, M. S. & Jahn, R. S t ab i lization of soil organicmatter: Association with m inerals or chemical recalcitrance? B iogeochemistry 77,25-56(2006). ) ( 19. Rasmussen, C., Southard, R. J.& Horwath, W. R. S oil mineralogy affects coniferforest soil carbon source utilization and m i crobial priming. Soil Sci. Soc. A m . J.71,1141-1150(2007). ) ( 20. K emmitt, S. J. et al. Mineralization of native soil organic matter is not regulated by the size, activity or composition of the soil microbial biomass-a new p erspective. Soil Biol. Bioche m . 40,61-73 ( 2008). ) ( 21. S chmidt, M. W. I. et al. Persistence of soil organic matter as an e c osystem p roperty. Nature 478,49-56 (2011). ) ( 22. T ang, J. Y., R i ley, W. J., Kove n , C . D. & Subin, Z. M.CLM4-BeTR, a generic b iogeochemical t ransport and reaction module for CLM4: Model development, e valuation, a nd application. Geosci. M odel Dev. 6 , 127-140 (2013). ) ( 23. J ones, D. L., Dennis, P. G. & vanHees, P. A. W. Or g anic acid behavior insoils-misconceptions and knowledge gaps. Pla n t Soil 248, 31-41(200 3 ). ) ( 24. Neumann, G. & Roemheld, V. Th e Rhizosphere: Biochemistry and OrganicSubstances at the S oil-Plant I nterface (CRC P r ess, 2007). ) ( 25. P aterson, E. & Sim, A. Rhizodeposition an d C-partitioning of Lol i um perenne i n axenic culture affected by nitrogen supply and def o liation. Pla n t Soi l 216, 1 5 5- 1 64(1999). ) ( 2 6. P hillips, R. P, Erlitz , Y . , Bier, R. & Bernhardt, E . S. New approach for capturing soluble root exudates in f orest soils . Funct. Ecol. 22,990-999(2008). ) 27. Brant, J. B., Sulzman, E. W. & Myrold, D. D. Microbial community utilization ofadded carbon substrates in response to long-term carbon input manipulation.Soil Biol. Biochem. 38, 2219-2232(2006). 28. Frey, S. D., Lee, J., Melillo, J. M. & Six, J. The temperature response of soilmicrobial efficiency and its feedback to climate. Nature Clim. Change 3,395-398(2013). ( 29. S chneckenberger, K., Demin, D., Stahr, K. & Ku z yakov, Y. Microbial utilizationand mineralization of [1 4 C]glucose added in s i x orders of concentration to soil. Soil Biol. Biochem. 40, 1981-1988(2008). ) ( 30. F ierer,N., B radford,M. A. & Ja c kson, R. B. Toward an e co l ogical cla s sificationof soil bacteria. Ecology 88,1354-1364(2007). ) ( 3 1. Eilers, K. G . , Debenport, S., Anderson, S. &F i erer, N. D ig g ing deeper to find u nique microbial communities: The strong effect of depth on the s tructureof bacterial and archaeal communities in soil. So i l Biol. Biochem. 50, 58-65(2012). ) ( 32. Yan, F, Schubert, S. & Mengel, K. S o il pH increase due to biological decarboxylation o f organic anions. Soil Biol. Biochem.28,617-624(1996). ) ( 33. McBride, M. B. Environmental Chemistry of Soils (Oxford Univ. Press, 1994). ) ( 34. Lovley, D. R. Dissimilatory Fe(III ) and Mn(IV) reduction. Microbiol. Rev.55, 259-287(1991). ) ( 35. G linski, J ., Stahr, K., Stepniewska, Z. & Brzezinska, M . Changes of redox and p H conditions i n a flooded soil amended w i th glucose a n d nitrate underlaboratory conditions. J . Plant Nutr . Soil S c i.15 5 ,13-17(1992). ) ( 3 6. H amer, U. & Marschner, B. P ri m ing effects in d ifferent so i l types induced by f ructose, alanine, oxalic acid and catechol additions. Soi l Biol. Bi o chem. 37, 4 45-454(2005). ) ( 37. F alchini, L., Naumova, N., Kuikman, P. J . , Bl o em, J. & N annipieri, P. CO evolution and denaturing gradient gel electrophoresis profiles of b acterial communities in soil following addition of low molecular weight substrates to simulate root exudation. S o il Biol. B iochem. 35, 775-7 8 2 (2003). ) ( 38.L i u, S. Y.et al. Synchrotron-based m a ss spectrometry to investigate th e molecular properties of mineral-organic associations. Anal. Chem. 85, 6100-6106(2013). ) ( 39. Hanley, L. & Zimmermann,R. Light and molecular Ions: The emergence of vacuum U V single-photon ionization in MS. An a l. Chem. 81, 4174-4182(2009). ) ( 40. Fischer, H . , Ingwersen,J. & Kuzyakoy, Y. Microbial uptake oflow-molecular-weight organic substances out-competes sorption in soil. Eur . J.Soil Sci. 61,504-513(2010). ) ( 41. M ikutta, R. et al. Biogeochemistry of mineral-organic as s ociations across a long-term mineralogical soil gradient ( 0 .3-4100 kyr), Hawaiian Islands. Geochim. Cosmochim. Acta 73, 2034-2060(2009). ) ( 42. Kramer, M. G., Sanderman,J., Chadwick,O. A ., Ch o rover, J. & Vitousek, P. M. L ong-term carbon storage through retention of dissolved aromatic acids by r eactive p articles in soil. Glob . Change Biol. 18,2594-2605 (2012). ) ( 43. Kunhi Mouvenchery, Y. , K u cerik, J., Diehl, D. & Schaumann, G. E. Cation-mediated cross-linking in natural organic matter: A review. Rev.Environ. S ci. Biotechnol. 11, 41-54(2011). ) ( 4 4. F ischer, W . R ., F l essa, H. & Schaller, G. pH values and redox potentials inmicrosites of the rhizosphere. Z. Fir Pflanzenernahr. Bodenkd.1 5 2, 1 91-195 (1989). ) ( 45. Collignon, C., Ranger, J . & Turpault, M. P. Seasonal dynamics of Al-and F e- b earing secondary minerals in a n a c id forest soil: Influence of Norwayspruce roots (Picea abies (L.) K arst.). Eur. J. Soil Sci. 63, 592-602(2012). ) 46. Richter, D.D.,Markewitz, D., Trumbore, S. E. & Wells, C. G. Rapidaccumulation and turnover of soil carbon in a re-establishing forest. Nature400,56-58(1999). ( 4 7. Wieder, W. R., Grandy, A . S., Kallenbach, C. M. & Bonan, G. B. Integratingmicrobial physiology and physio-chemical principles in soils with the M icrobial-Mineral Carbon Stabilization (MIMICS) model. Biogeosciences 11, 3899-3917(2014). ) ( 48. H odge, A. et al. C haracterisation and microbial uti l isation of exudate material from the rhizosphere of Lolium perenne grown under CO2 enrichment. Soil B iol. Biochem. 30, 1 033-1 0 43 (1998). ) ( 49. Cheng, L. et al. Atmospheric COz enrichment facilitates cation release from s oil. Ecol. Lett. 13, 284-29 1 (2010). ) ( 50. P érez, D. V., de C ampos, R. C. & N ovaes, H. B. Soil solution charge balance for defining t he speed and time of centrifugation of two Brazilian soils. Commun. S oil Sci . Plant Anal. 33, 202 1 -2036(2002). ) ( 5 1. K ilcoyne, A. L. D . et al. Interferometer-controlled scanning transmission X-ray microscopes at the A dvanced Light Source. J . Synchrotron R adiat. 10, 1 2 5- 1 36(2003). ) 52. Smith,R. M. & Martell, A. E. Critical stability constants, enthalpies andentropies for the formation of metal complexes of aminopolycarboxylic acidsand carboxylic acids. Sci. Total Environ. 64,125-147(1987). 53. Hojberg, O. & Sorensen,J. Microgradients of microbial oxygen consumption ina barley rhizosphere model system. Appl. Environ. Microbiol.59,431-437 (1993). Acknowledgements The authors thank A.L.D. Kilcoyne (ALS beamline 5.3.2.2), S.Y. Liu and M. Ahmed (ALSbeamline 9.0.2) for their support. M.Keiluweit was supported by a Lawrence ScholarFellowship awarded through Lawrence Livermore National Laboratory (LLNL). M.Kleber acknowledges support through Research Agreement No. 2014-1918 with theInstitute of Soil Landscape Research,Leibniz-Center for Agricultural LandscapeResearch (ZALF), Muincheberg, Germany. This work was performed under the auspicesof the US Department of Energy by LLNL under Contract DE-AC52-07NA27344. Funding was provided by LLNL LDRD Microbes and Minerals: Imaging C Stabilizationand a US DOE Genomics Science program award SA-DOE-29318 to J.P-R. The work ofP.S.N. is supported by LBNL award No. IC006762 as sub-award from LLNL andDOE-BER Sustainable Systems SFA. The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the US DOE underContract No. DE-AC02-05CH11231. Author contributions M.Keiluweit performed microcosm set-up,laboratory analyses, synchrotron analyses,data analysis and wrote the manuscript. J.J.B. was responsible for DNA/RNA extractionsand data processing.J.J.B. and P.K.W. conducted NanoSIMS analyses.M.Kleber., J.P-R.,P.K.W.and P.S.N. supervised the project. All authors discussed the results and contributed to the manuscript. Additional information Supplementary information is available in the online version of the paper. Reprints andpermissions information is available online at www.nature.com/reprints.Correspondence and requests for materials should be addressed to M.K. Competing financial interests The authors declare no competing financial interests. NATURE CLIMATE CHANGE|VOL|JUNE www.nature.com/natureclimatechange Macmillan Publishers Limited. All rights reserved 植物通过脱落的根系细胞、组织、粘液和各种渗出的有机化合物将40-60%的光合固定碳(C)直接输送到根部和相关微生物中。大气中二氧化碳浓度的升高预计会增加根分泌物的数量,并改变根分泌物释放到土壤中的成分。目前科研人员似乎还不太清楚改变输入会在多大程度上导致原生(或“旧的”)有机碳的净损失。因此需要存在一种更好地理解土壤中碳损失的潜在机制,这对于预测大型土壤碳储量如何应对全球变化做出反应至关重要。来自美国俄亥俄州的科研人员通过测试了根际碳矿化的加速(即启动效应)是否是由渗出物从保护性的矿物-有机结合中释放碳的能力所促进的。并提出了另一种方法可导致等量或更大的碳损失的机制(图1所示)。 图1、根际土壤碳(“启动物”)的微生物矿化加速机制 研究人员主要研究了根系分泌物对土壤中的碳的保护作用,研究过程中应用unisense氧气微电极对根际周围加入不同的渗入液(草酸、葡萄糖等)后对应的氧浓度剖面分布进行了测试,从而确定了根系分泌物对根际微区微生物呼吸速率的影响。获得的氧浓度剖面表明了草酸的加入显著降低了周围土壤微生物对O2的应用的有效性,其影响的最高深度可达5mm,而葡萄糖和乙酸的添加量则局限在1.5 mm处(图2所示)。图2、根系分泌物对人工根际土壤的影响 草酸处理中的土壤内的微生物呼吸作用超过使用葡萄糖的效果。这也进一步了解根际碳矿化的加速(即启动效应)是否是由渗出物从保护性的矿物-有机结合中释放碳的能力所促进。并发现了一种由根、根相关的真菌和细菌产生的有机酸(草酸),具有较强的金属络合能力,对微生物的生物能利用有限。根际上述的相关实验结构,研究人员发现一种常见的根分泌物草酸会促进使有机化合物从与矿物的保护性联系中释放出来而造成的碳损失。而通过加强微生物与以前的矿物保护化合物,这种间接机制会加速碳损失的过程,该研究结果也为“启动”现象背后的生物-非生物耦合机制提供了一些见解,并挑战了关于矿物质相关的碳是受微生物保护的假设机制。从以上研究内容来看,unisense微电极剖面系统在根际相关的机制研究方面具有很好的应用价值,为相关研究人员探索植物根际的相关领域研究提供了一种重要的技术支持。
确定



还剩6页未读,是否继续阅读?
上海谓载科技有限公司为您提供《土壤碳中根系分泌物保护作用的机制检测方案(溶解氧测定仪)》,该方案主要用于其他中植物生理检测,参考标准--,《土壤碳中根系分泌物保护作用的机制检测方案(溶解氧测定仪)》用到的仪器有丹麦Unisense溶氧仪
推荐专场