方案详情
文
Metal–organic frameworks assembled from flexible alicyclic carboxylate and bipyridyl ligands for sensing of nitroaromatic explosives
方案详情

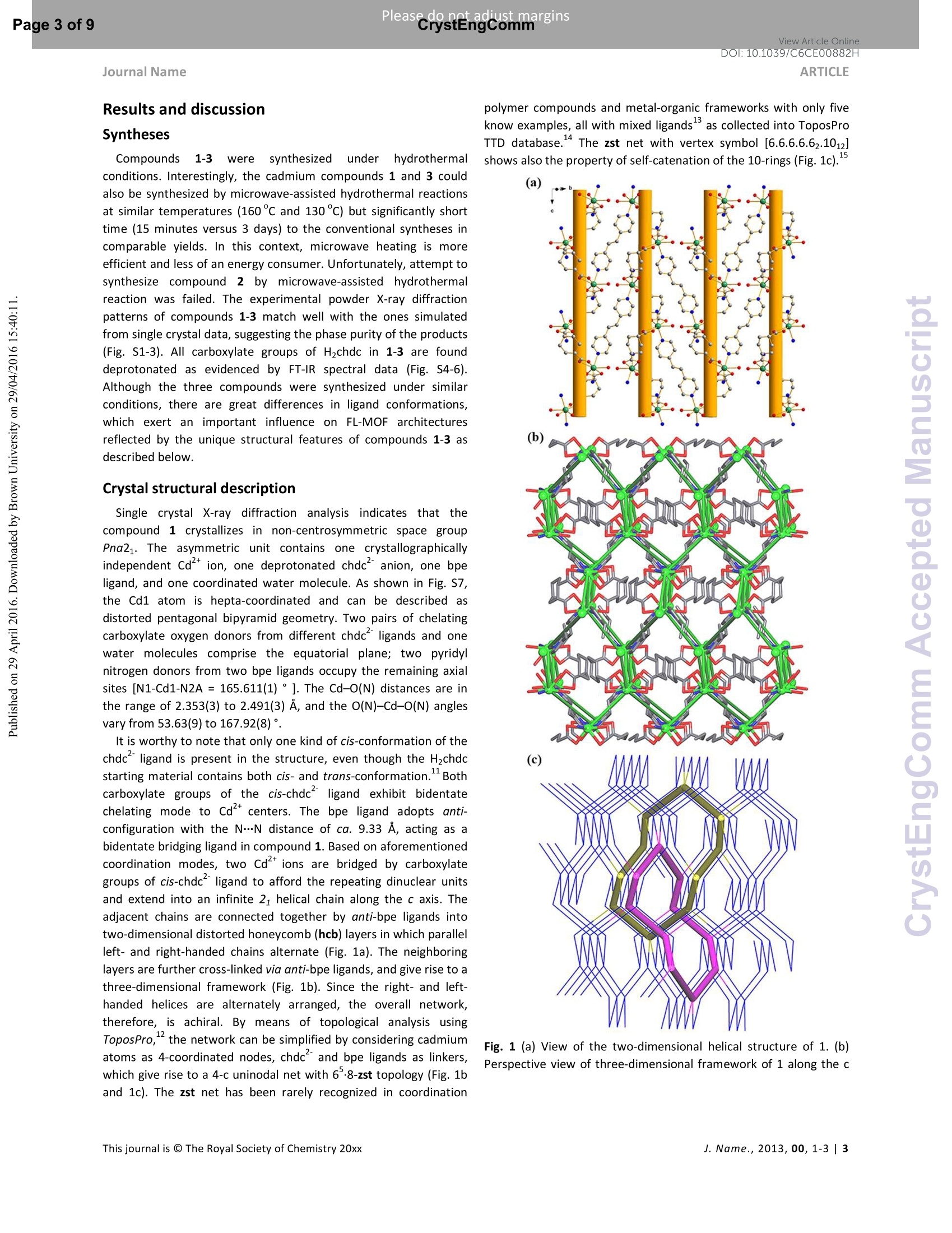
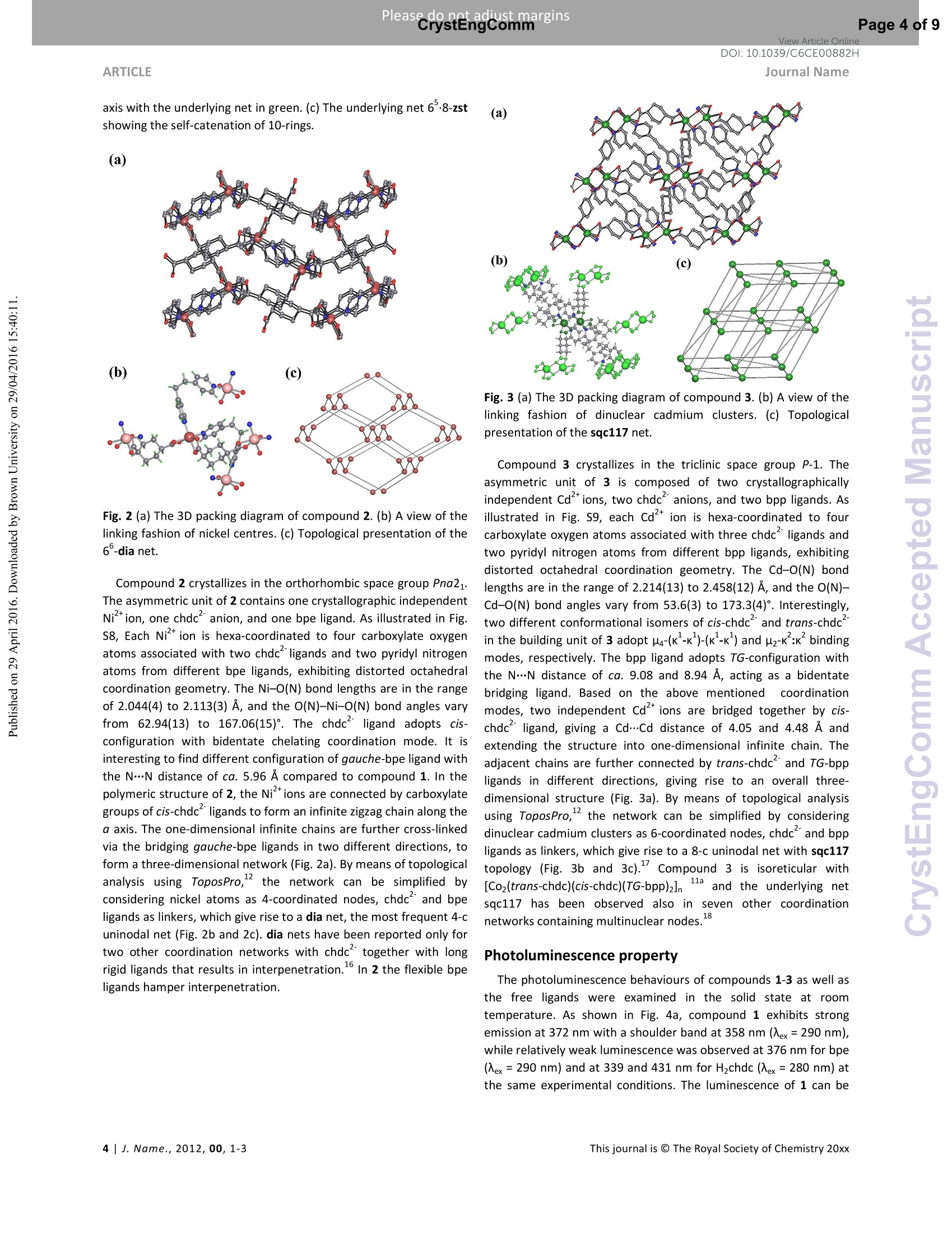
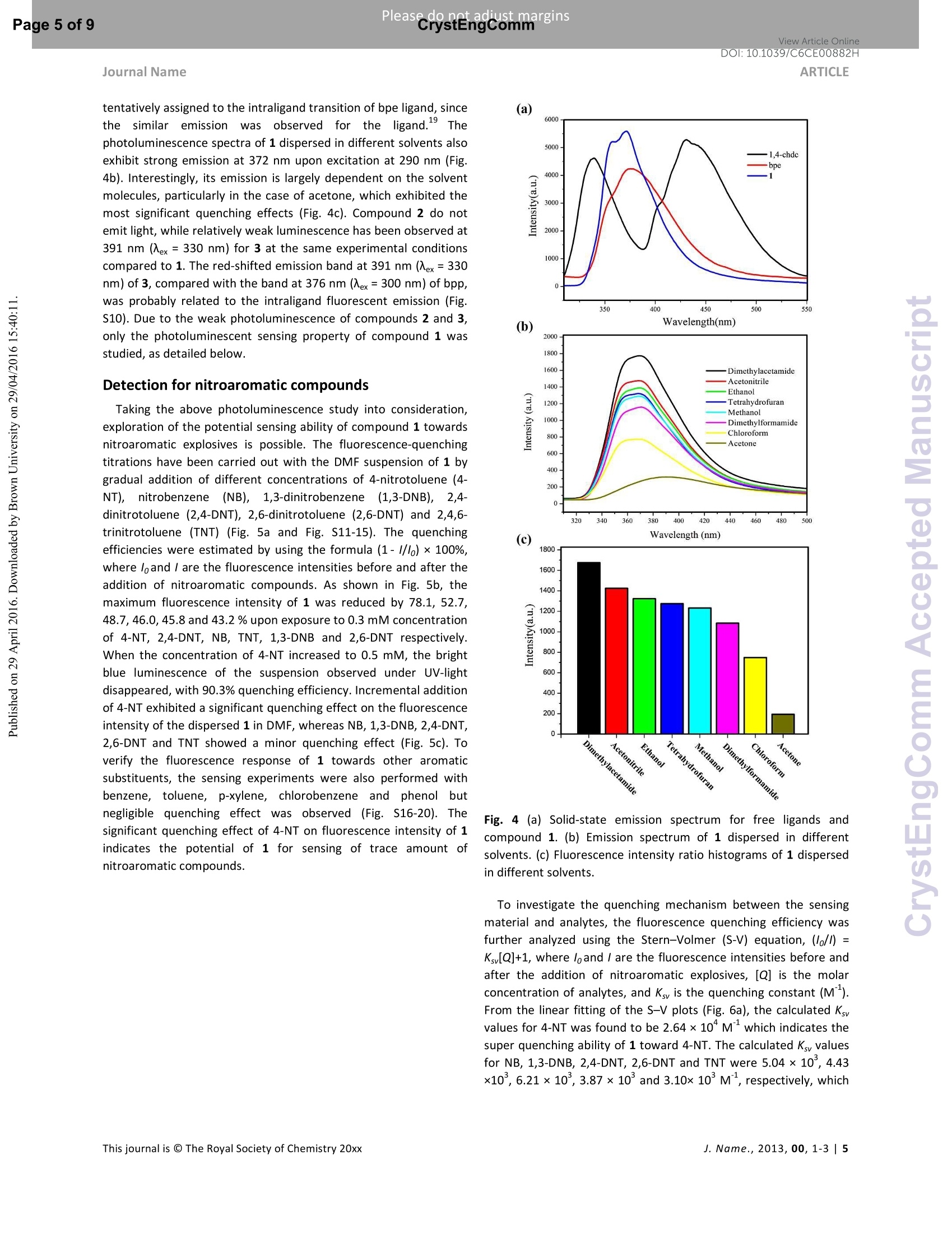
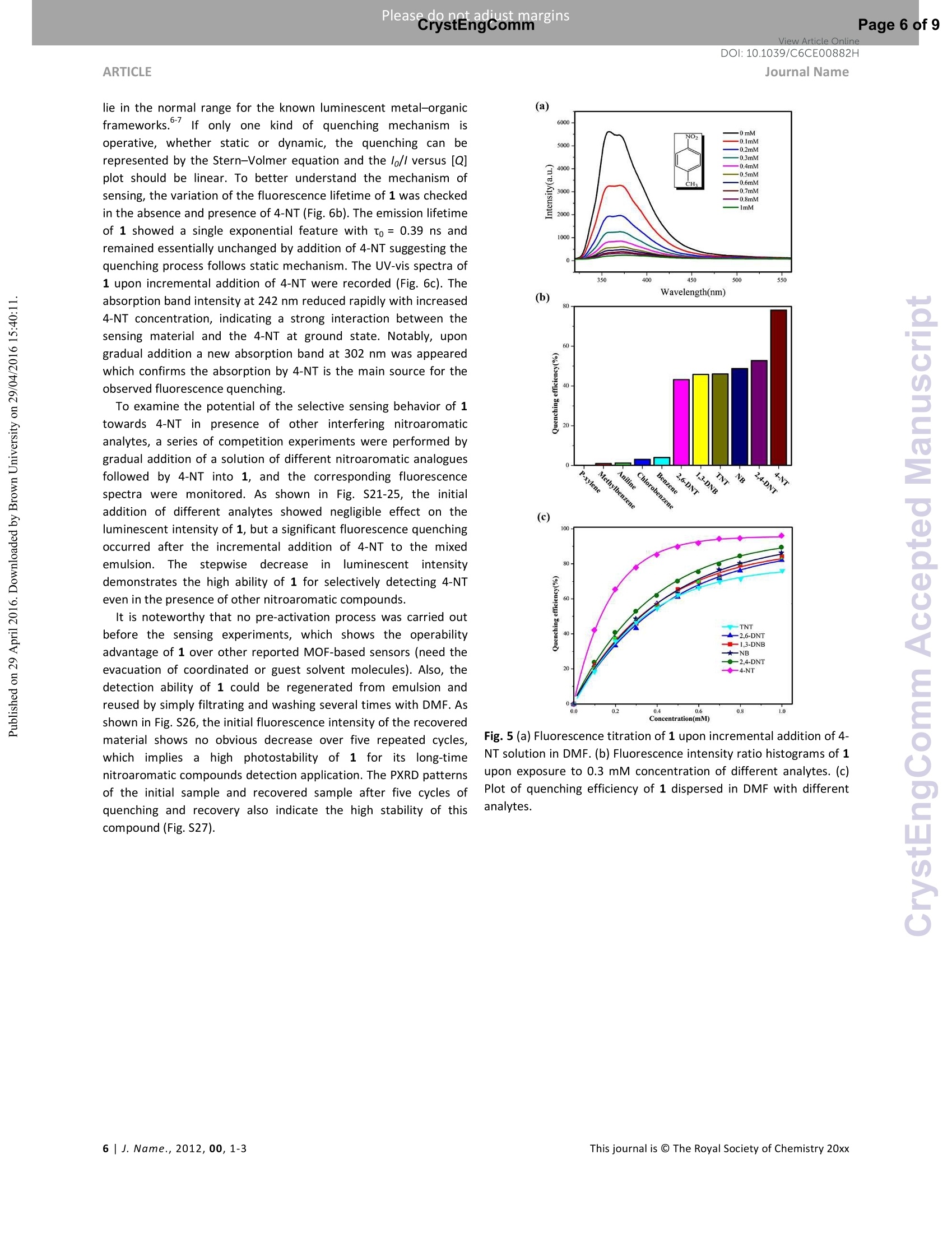
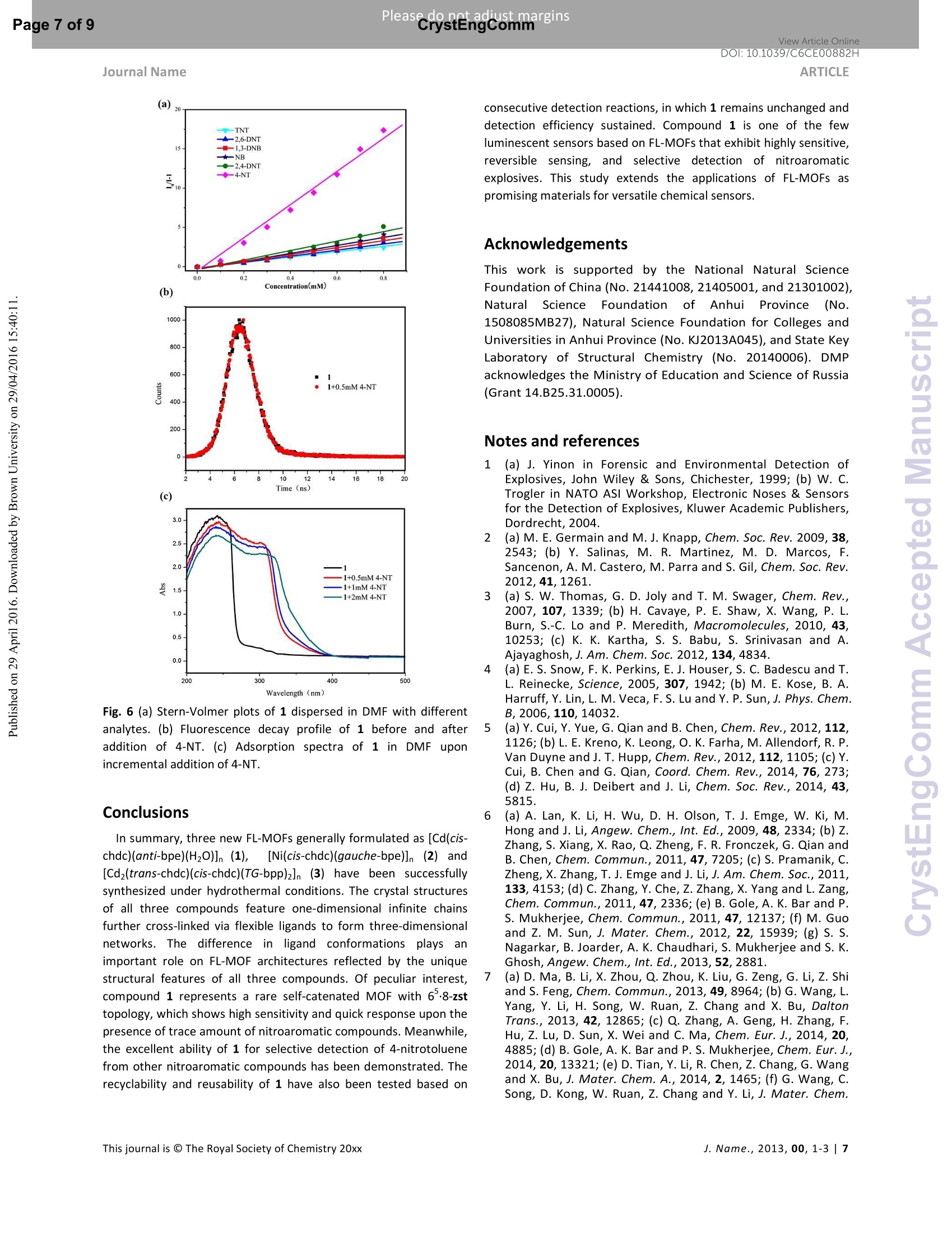
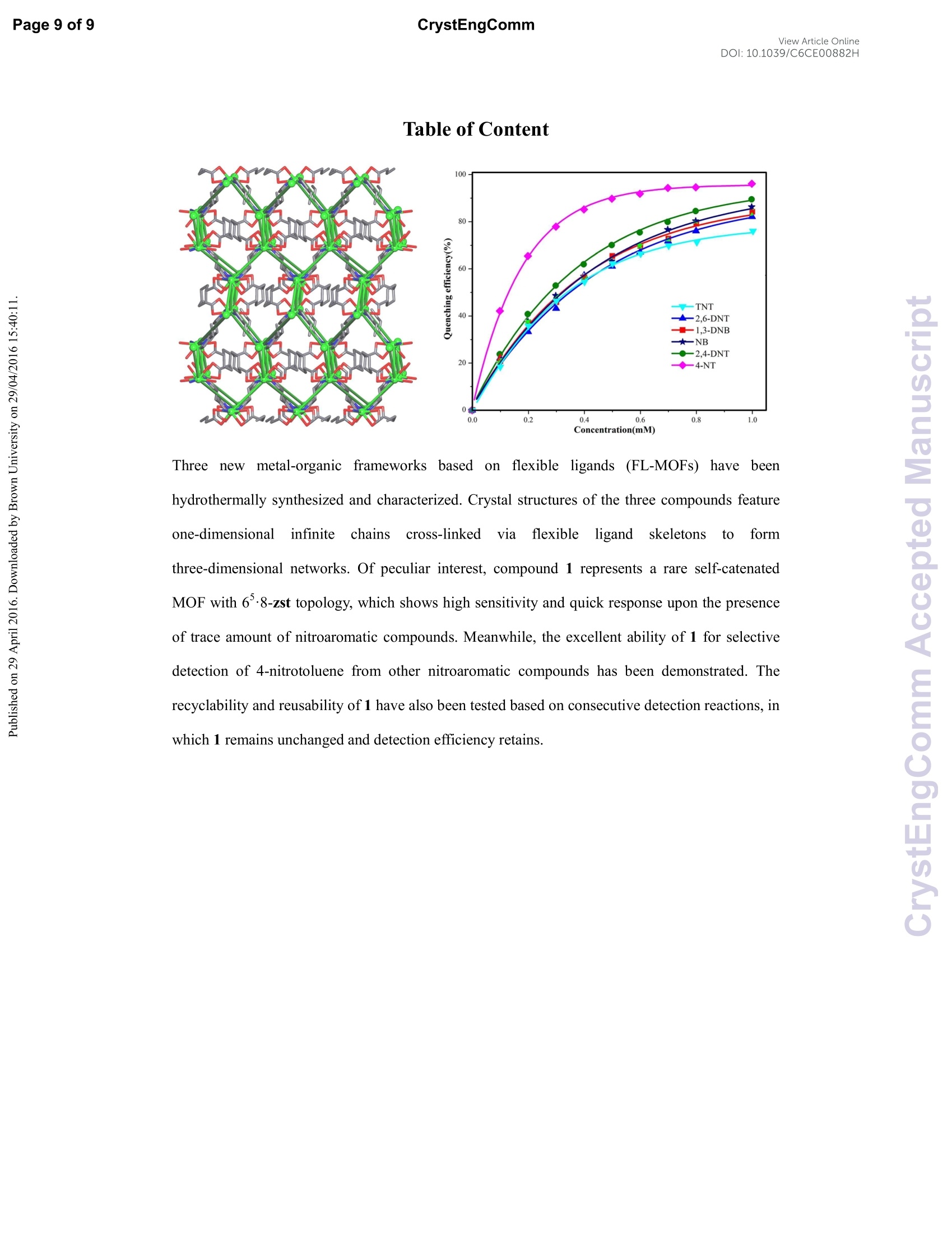
View Article OnlineView Journal Please do net aojust marginsPage 1 of 9View Article OnlineDOI:10.1039/C6CE00882H CrystEngComm Accepted Manuscript This article can be cited before page numbers have been issued, to do this please use: X. Zhu, Y. Li, W.Zhou, R. Liu, Y. Ding, J. Lu and D. M. Proserpio, CrystEngComm,2016,DOI: 10.1039/C6CE00882H. Volume1Numbor1 Jan2013 Pages 1-100 This is an Accepted Manuscript, which has been through theRoyal Society of Chemistry peer review process and has beenaccepted for publication. Accepted Manuscripts are published online shortly afteracceptance, before technical editing, formatting and proof reading.Using this free service, authors can make their results availableto the community, in citable form, before we publish the editedarticle. We will replace this Accepted Manuscript with the editedand formatted Advance Article as soon as it is available. You can find more information about Accepted Manuscripts in theInformation for Authors. Please note that technical editing may introduce minor changesto the text and/or graphics, which may alter content. The journal'sstandard Terms 8 Conditions and the Ethical guidelines stillapply. In no event shall the Royal Society of Chemistry be heldresponsible for any errors or omissions in this Accepted Manuscriptor any consequences arising from the use of any information itcontains. Received 0Oth January 20xx,Accepted 0Oth January 20xx Metal-organic frameworks assembled from flexible alicycliccarboxylate and bipyridyl ligands for sensing ofnitroaromatic explosives DOI: 10.1039/x0xx00000xWww.rsc.org/ Xian-Dong Zhu,*a,bYong Li, Wei-Xiang Zhou, Rong-Mei Liu, Yu-Jie Ding, Jian Lu*bd and Davide M.Proserpio Three new metal-organic frameworks based on flexible ligands (FL-MOFs) generally formulated as [Cd(cis-chdc)(anti-bpe)(H2O)]n (1), [Ni(cis-chdc)(gauche-bpe)]n(2) and[Cd2(trans-chdc)(cis-chdc)(TG-bpp)2]n(3) [H2chdc = 1,4-cyclohexanedicarboxylic acid, bpe = 1,2-bis(4-pyridyl)ethane and bpp = 1,3-bis(4-pyridyl)propane], have beenhydrothermally synthesized and characterized. Crystal structures of the three compounds feature one-dimensional infinitechains cross-linked via flexible ligand skeletons to form three-dimensional networks. Of peculiar interest, compound 1represents a rare self-catenated MOF with 6-8-zst topology, which shows high sensitivity and quick response upon thepresence of trace amount of nitroaromatic compounds. Meanwhile, the excellent ability of 1 for selective detection of 4-nitrotoluene from other nitroaromatic compounds has been demonstrated. The recyclability and reusability of 1 have alsobeen tested based on consecutive detection reactions, in which 1 remains unchanged and detection efficiency retains. Introduction Nitroaromaticccompounds, such as dinitrobenzene (DNB),dinitrotoluene (DNT), 2,4,6-trinitrotoluenee(TNT)and 2,4,6-trinitrophenol (TNP) are the major components of high explosives.Selective and rapid detection of these chemical explosives is ofimmense interest owing to the ever-increasing concerns such ascivilian safety, military operation and environmental management.Recently, fluorescence-based detection methods based on theelectron transfer and/or energy transfer mechanism have attractedincreasing attention due to their high sensitivity, portability, shortresponse time, and dual compatibility in both solid state and a.School of Biological & Chemical Engineering,Anhui Polytechnic University, Wuhu 241000, P. R.China, E-mail:zhuxd@ahpu.edu.cn. ( b . State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou ) ( 350002, P . R. C hina, E-mail: lujian05@fjirsm.ac.cn. L Dipartimento di Chimica, Universita degli Studi di Milano,Milano 20133, Italy. ) ( d . Samara Center for Theoretical Materials Science (SCTMS), Samara National Research University, Samara 443086, Russia. tElectronic S upplementary Inf o rmation (ESI) avai l able: crys t al data , stru c tural information, characterizati o n da t a. CCDC 1417406, 141707 and 1449851. See DOI: 10.1039/x0xx00000x ) solution media. A large number of fluorescent materials includingconjugated polymers,3 nanoparticles, and metal-organicframeworks (MOFs) have been employed for explosive detection.MOFs are particularly attractive for this purpose because, inprinciple, they are able to detect specific analytes based onstructural design, thus offering good selectivity and exhibiting highdensity of analyte adsorption/binding sites, thereby enabling lowerdetection limits. Detectable change in luminescence by tuningseveral parameters such as porosity, surface area, and host-guestinteractions makes MOFs promising candidates for sensingapplications. The pioneering work of Li et al., Ghosh et al.and othergroups has demonstrated the potentials of luminescent MOFs inexplosive detection.Up to now, most of the reported MOF-basedsensorrSsare constructe:ddwith rigidl or semi-rigid aromaticcarboxylate ligands,which can be explained by the increase ofelectrostatic interactionsbetweenframeworksanddelectrondeficient nitroaromatics due to the delocalized n-electrons ofligands. Further, the photo-induced electron transfer from excitedMOFs to the guest molecules results in fluorescence quenching ofthe MOF materials. However, using flexible carboxylate ligands asbuilding blocks for the construction of FL-MOFsensors still remainsundeveloped. This is also a promising strategy to enhance thesensing ability, since the flexible frameworks could adjust theirconfigurations to adapt to analyte molecules." On the other hand,selectivity is critical for fruitful detection in practical applications.Most of the reported MOF-based sensors exhibit highly sensitiveand reversible sensing properties. However,the selective detectionof specific nitroaromatic explosive from a mixture of others (withdifferent numbers of-NOz groups) has rarely been reported.6g.7h-k Based on the above considerations, we have selected 1,4-cyclohexanedicarboxylic acid (H2chdc) as a primary ligand coupledto auxiliary ligands, 1,2-bis(4-pyridyl)ethane (bpe) and 1,3-bis(4-pyridyl)propane (bpp), to construct new FL-MOFs by the adjustmentof metal nodes with various connection modes. In this contribution,we report the preparation and crystal structures of three FL-MOFsformulated as [Cd(cis-chdc)(anti-bpe)(HzO)]n (1), [Ni(cis-chdc)(gauche-bpe)]n (2), and [Cdz(trans-chdc)(cis-chdc)(TG-bpp)2]n(3), which feature one-dimensional infinite chains further cross-linked via flexible ligand skeletons to form three-dimensionalnetworks. The difference inligand conformationss playsanimportant role on FL-MOF architectures reflected by the uniquestructural features of all three compounds. Of peculiar interest,compound 1 represents a rare self-catenated MOF with 6·8-zsttopology, which shows high sensitivity and quick response to traceamount of nitroaromatic compounds present. Experimental Materials and general methods All commercially available reagents and starting materials wereof reagent-grade quality and used without further purification.Microwave-assisted syntheses were carried out in a microwaveoven(XH-800S-10, 0-1200W,Beijing Xianghu Corp.). UV-Visabsorption spectra were measured using a Jingke L6S spectrometer.Elemental analyses (C, H, N) were carried out on an Elementar VarioEL Ill analyzer. Powder X-ray diffraction (PXRD) data were collectedon a Bruker D8 ADVANCE diffractometer with Cu-Ka radiation (=1.5418 A). Fourier transform infrared (FT-IR) spectra were recordedon PerkinElmer Spectrum One with KBr pellets in the range 4000-400 cm. Thermogravimetric analysis (TGA) was performed undernitrogen atmosphere with a heating rate of 10 ℃ minusing anNETZSCH STA 449C unit. Synthesis of compound [Cd(cis-chdc)(anti-bpe)(Hz0)]n(1) As a typical synthetic procedure, a mixture of Cd(NO3)24H20(0.030 g, 0.1 mmol), Hzchdc (0.017 g, 0.1 mmol), bpe (0.019 g, 0.1mmol), NaOH (0.004g, 0.1 mmol) and 10 ml distilled water wassealed in a 25ml Teflon-lined stainless steel autoclave, and heatedat 160C for 72 hours, then cooled naturally to room temperature.Colourless block crystals of 1 were collected by filtration, washedwith distilled water, and dried in air at ambient temperature. Yield:36% (based on Cd). Calcd for C20H24N205Cd (484.82): C 49.55, H4.99, N 5.78; found: C 48.93, H 4.90, N 5.66. IR (KBr, cm=): 3413(s,br), 2913 (m), 2856 (w), 1610 (s), 1550 (vs), 1417 (s), 1014 (m), 837(m), 547 (m). Synthesis of compound [Ni(cis-chdc)(gauche-bpe)]n (2) Compound 2 was synthesized with a same recipe as that of 1,using Ni(NO3)26H20 (0.029 g, 0.1 mmol) instead of Cd(NO3)24H20.Green block crystals of 2 were collected by filtration, washed withdistilled water, and dried in air at ambient temperature. Yield: 52%(based on Ni). Calcd for C20H22N204Ni (413.09): C 58.15, H 5.37, N6.78; found: C 57.85, H 5.28, N 6.72. IR (KBr, cm): 3436 (m, br), 3050 (m), 2937 (s),2867 (w), 1610 (vs), 1535 (vs), 1430 (s), 1222(m),1025(m),931(w), 817(m), 557(m). Synthesis of compound [Cd2(trans-chdc)(cis-chdc)(TG-bpp)z]n (3) Compound 3 was synthesized with a similar procedure as that of1, using less amount of NaOH (0.001g, 0.025 mmol) at lowertemperature of 130°C. Colourless block crystals of 3 were collectedby filtration, washed with distilled water, and dried in air at ambienttemperature. Yield:52% (based on Cd). Calcd for C21H24N204Cd(480.84):C 52.45, H 5.03, N 5.82; found: C 52.31, H 4.72, N 5.37. IR(KBr, cm): 3469 (s, br), 2919 (m), 2859 (w), 1610 (s), 1573 (vs),1411 (s), 1018 (m),817(m). X-ray crystallographic study Data collection for 1-3 was performed on a Rigaku Mercury 2 CCDdiffractometer equipped with graphite-monochromated Mo-Karadiation (=0.71073 A) at room temperature. Both data sets werecorrected for Lorentz and polarization factors as well as forabsorption by a multiscan method.The structures were solved bydirect methods and refined by the full-matrix least-squares on Fusing the SHELXTL-97 program. All non-hydrogen atoms weretreated anisotropically. The positions of hydrogen atoms attachedto carbon atoms were generated geometrically. Idealized positionsof hydrogen atoms of water molecules were located from Fourierdifferencee rmaps and irefined isotropically. The date set ofcompound 1 shows a Flack parameter of 0.56(3) that indicate theexistence of racemic twinning. This is supported by the refinementsof several data sets collected on different crystals. We have alsotried to solve this structure in the centrosymmetric space groupPnma, but Ri (about 0.169) is too high and many atoms have ADPnonpositive-definite. Therefore, we finally chose space group Pna21,which gave a satisfactory refinement with R1= 0.0237 for 4214unique reflections with />2a (/). Compound 3 has disorder aboutthe dipyridyl ligand that was modeled successfully via the PART andISOR commands to restrain the carbon atoms at appropriate bondlengths. Crystallographic data and structure refinements for 1-3 arelisted in Table S1. Fluorescence sensing measurement The photoluminescence excitation and emission spectra ofcompounds 1-3 were recorded on a Hitachi F-4500spectrophotometer. The emission lifetimes were measured on anEdinburgh FLS-920 fluorescence spectrometer. The fluorescenceproperties of the sample in solid state and various solventsuspensions were examined at room temperature. The 1-solventsuspensions were prepared by introducing 20 mg fine grindingsample of 1 immersed in different organic solvents (20 mL), treatedby ultrasonication for 30 minutes, and then aged for 24 hours toform stable emulsions. In typical fluorescence quenchingexperimental setup, 2 mL suspension of 1 in DMF was placed in a 1cm quartz cuvette and the fluorescence response upon excitation at290 nm was measured in situ after incremental addition of freshlyprepared 20 mM analyte solution in DMF in the range of 320-550nm. Syntheses Compounds 1-3 were synthesized under hydrothermalconditions. Interestingly, the cadmium compounds 1 and 3 couldalso be synthesized by microwave-assisted hydrothermal reactionsat similar temperatures (160℃ and 130℃) but significantly shorttime (15 minutes versus 3 days) to the conventional syntheses incomparable yields. In this context, microwave heating is moreefficient and less of an energy consumer. Unfortunately, attempt tosynthesize compound 2 by microwave-assisted hydrothermalreaction was failed. The experimental powder X-ray diffractionpatterns of compounds 1-3 match well with the ones simulatedfrom single crystal data, suggesting the phase purity of the products(Fig. S1-3). All carboxylate groups of H2chdc in 1-3 are founddeprotonated as evidenced by FT-IR spectral data (Fig. S4-6).Although the three compounds were synthesized under similarconditions, there are great differences in ligand conformations,which exert an important influence on FL-MOF architecturesreflected by the unique structural features of compounds 1-3 asdescribed below. Crystal structural description Single crystal X-ray diffraction analysis indicates that thecompound 1 crystallizes in non-centrosymmetric space groupPna21. The asymmetric unit contains one crystallographicallyindependent Cd" ion, one deprotonated chdc anion, one bpeligand, and one coordinated water molecule. As shown in Fig. S7,the Cd1 atom is hepta-coordinated and can be described asdistorted pentagonal bipyramid geometry. Two pairs of chelatingcarboxylate oxygen donors from different chdc ligands and onewater molecules comprise the equatorial plane; two pyridylnitrogen donors from two bpe ligands occupy the remaining axialsites [N1-Cd1-N2A=165.611(1)°]. The Cd-O(N) distances are inthe range of 2.353(3) to 2.491(3) A, and the O(N)-Cd-O(N) anglesvary from 53.63(9)to167.92(8)°. It is worthy to note that only one kind of cis-conformation of thechdc ligand is present in the structure, even though the Hzchdcstarting material contains both cis- and trans-conformation.Bothcarboxylate groups of the cis-chdcliligand exhibit bidentatechelating mode to Cdcenters. The bpe ligand adopts anti-configuration with the N…N distance of ca. 9.33 A, acting as abidentate bridging ligand in compound 1. Based on aforementionedcoordination modes, two Cdions are bridged by carboxylategroups of cis-chdc ligand to afford the repeating dinuclear unitsand extend into an infinite 21 helical chain along the c axis. Theadjacent chains are connected together by anti-bpe ligands intotwo-dimensional distorted honeycomb (hcb) layers in which parallelleft- and right-handed chains alternate (Fig. 1a). The neighboringlayers are further cross-linked via anti-bpe ligands, and give rise to athree-dimensional framework (Fig. 1b). Since the right- and left-handed helices are alternately arranged, the overall network,therefore, is achiral. By means of topological analysis usingToposPro, the network can be simplified by considering cadmiumatoms as 4-coordinated nodes, chdc and bpe ligands as linkers,which give rise to a 4-c uninodal net with 68-zst topology (Fig. 1band 1c). The zst net has been rarely recognized in coordination polymer compounds and metal-organic frameworks with only fiveknow examples, all with mixed ligandsas collected into ToposProTTD database." The zst net with vertex symbol [6.6.6.6.6z.1012]shows also the property of self-catenation of the 10-rings (Fig. 1c)." (a) Fig. 1 (a) View of the two-dimensional helical structure of 1. (b)Perspective view of three-dimensional framework of 1 along the c axis with the underlying net in green. (c) The underlying net 68-zstshowing the self-catenation of 10-rings. (a) Fig. 2 (a) The 3D packing diagram of compound 2. (b) A view of thelinking fashion of nickel centres. (c) Topological presentation of the6-dia net. Compound 2 crystallizes in the orthorhombic space group Pna21.The asymmetric unit of 2 contains one crystallographic independentNi*ion,one chdcanion, and one bpe ligand. As illustrated in Fig.S8, Each Ni’ion is hexa-coordinated to four carboxylate oxygenatoms associated with two chdc ligands and two pyridyl nitrogenatoms from different bpe ligands, exhibiting distorted octahedralcoordination geometry. The Ni-O(N) bond lengths are in the rangeof 2.044(4) to 2.113(3) A, and the O(N)-Ni-O(N) bond angles varyfrom 62.94(13) to 167.06(15)°. The chdc ligand adopts cis-configuration with bidentate chelating coordination mode. It isinteresting to find different configuration of gauche-bpe ligand withthe N…N distance of ca. 5.96 A compared to compound 1. In thepolymeric structure of 2,the Ni’tions are connected by carboxylategroups of cis-chdc ligands to form an infinite zigzag chain along thea axis. The one-dimensional infinite chains are further cross-linkedvia the bridging gauche-bpe ligands in two different directions, toform a three-dimensional network (Fig.2a). By means of topologicalanalysis using ToposPro, the network can be simplified byconsidering nickel atoms as 4-coordinated nodes, chdc and bpeligands as linkers, which give rise to a dia net, the most frequent 4-cuninodal net (Fig. 2b and 2c). dia nets have been reported only fortwo other coordination networks with chdc together with longrigid ligands that results in interpenetration. In 2 the flexible bpeligands hamper interpenetration. Journal Name Fig. 3 (a) The 3D packing diagram of compound 3. (b) A view of thelinking fashion of dinuclear cadmium clusters. (c) Topologicalpresentation of the sqc117 net. Compound 3 crystallizes in the triclinic space group P-1. Theasymmetric unit of 3 is composed of two crystallographicallyindependent Cd ions, two chdc anions, and two bpp ligands. Asillustrated in Fig. S9, each Cd" ion is hexa-coordinated to fourcarboxylate oxygen atoms associated with three chdc ligands andtwo pyridyl nitrogen atoms from different bpp ligands, exhibitingdistorted octahedral coordination geometry. The Cd-O(N) bondlengths are in the range of 2.214(13) to 2.458(12) A, and the O(N)-Cd-O(N) bond angles vary from 53.6(3) to 173.3(4)°. Interestingly,two different conformational isomers of cis-chdc’ and trans-chdcin the building unit of 3 adopt p4-(K--k")-(K-K") and pz-K :Kbindingmodes, respectively. The bpp ligand adopts TG-configuration withthe N…N distance of ca. 9.08 and 8.94 A, acting as a bidentatebridging ligand. Based on the above mentioned coordinationmodes, two independent Cdions are bridged together by cis-chdc ligand, giving a Cd……Cd distance of 4.05 and 4.48 A andextending the structure into one-dimensional infinite chain. Theadjacent chains are further connected by trans-chdc and TG-bppligands in different directions, giving rise to an overall three-dimensional structure (Fig. 3a). By means of topological analysisusing ToposPro, the network can be simplified by consideringdinuclear cadmium clusters as 6-coordinated nodes, chdc and bppligands as linkers, which give rise to a 8-c uninodal net with sqc117topology (Fig. 3b and 3c)." Compound 3 is isoreticular with[Co2(trans-chdc)(cis-chdc)(TG-bpp)2]n11a and the underlying netsqc117 has been observed also in seven other coordinationnetworks containing multinuclear nodes. Photoluminescence property The photoluminescence behaviours of compounds 1-3 as well asthe free ligands were examined in the solid state at roomtemperature. As shown in Fig. 4a, compound 1 exhibits strongemission at 372 nm with a shoulder band at 358 nm (ex=290 nm),while relatively weak luminescence was observed at 376 nm for bpe(Xex=290 nm) and at 339 and 431 nm for H2chdc (Nex=280 nm) atthe same experimental conditions. The luminescence of 1 can be tentatively assigned to the intraligand transition of bpe ligand, sincethe similar emissionwasobserved ff(orthe liligand.Thephotoluminescence spectra of 1 dispersed in different solvents alsoexhibit strong emission at 372 nm upon excitation at 290 nm (Fig.4b). Interestingly, its emission is largely dependent on the solventmolecules, particularly in the case of acetone, which exhibited themost significant quenching effects (Fig. 4c). Compound 2 do notemit light, while relatively weak luminescence has been observed at391 nm (Xex= 330 nm) for 3 at the same experimental conditionscompared to 1. The red-shifted emission band at 391 nm (Xex= 330nm) of 3, compared with the band at 376 nm (入ex=300 nm) of bpp,was probably related to the intraligand fluorescent emission (Fig.S10). Due to the weak photoluminescence of compounds 2 and 3,only the photoluminescent sensing property of compound 1 wasstudied, as detailed below. Detection for nitroaromatic compounds Taking the above photoluminescence study into consideration,exploration of the potential sensing ability of compound 1 towardsnitroaromatic explosives is possible. The fluorescence-quenchingtitrations have been carried out with the DMF suspension of 1 bygradual addition of different concentrations of 4-nitrotoluene (4-NT),,nitrobenzene(NB),1,3-dinitrobenzene (1,3-DNB),l,2,4-dinitrotoluene (2,4-DNT), 2,6-dinitrotoluene (2,6-DNT) and 2,4,6-trinitrotoluene (TNT) (Fig. 5a and Fig. S11-15). The quenchingefficiencies were estimated by using the formula (1-//1o)×100%,where loand / are the fluorescence intensities before and after theaddition of nitroaromatic compounds. As shown in Fig. 5b, themaximum fluorescence intensity of 1 was reduced by 78.1, 52.7,48.7, 46.0,45.8 and 43.2 % upon exposure to 0.3 mM concentrationof 4-NT, 2,4-DNT, NB, TNT, 1,3-DNB and 2,6-DNT respectively.When the concentration of 4-NT increased to 0.5 mM, the brightblue luminescence of the suspension observed under UV-lightdisappeared, with 90.3% quenching efficiency.Incremental additionof 4-NT exhibited a significant quenching effect on the fluorescenceintensity of the dispersed 1 in DMF, whereas NB, 1,3-DNB, 2,4-DNT,2,6-DNT and TNT showed a minor quenching effect (Fig. 5c). Toverify the fluorescence response of 1 towards other aromaticsubstituents, the sensing experiments were also performed withbenzene, toluene, p-xylene,,Cchlorobenzeneandphenolo1butnegligible quenching effect was observed(Fig. S16-20). Thesignificant quenching effect of 4-NT on fluorescence intensity of 1indicates the potential of 1 for sensing of trace amount ofnitroaromatic compounds. Fig. 4 (a) Solid-state emission spectrum for free ligands andcompound 1. (b) Emission spectrum of 1 dispersed in differentsolvents. (c) Fluorescence intensity ratio histograms of 1 dispersedin different solvents. To investigate the quenching mechanism between the sensingmaterial and analytes, the fluorescence quenching efficiency wasfurther analyzed using the Stern-Volmer (S-V) equation, (/o/l) =Ksv[Q]+1, where loand / are the fluorescence intensities before andafter the addition of nitroaromatic explosives, [Q] is the molarconcentration of analytes, and K, is the quenching constant (M").From the linear fitting of the S-V plots (Fig. 6a), the calculated Ksvalues for 4-NT was found to be 2.64× 10 M which indicates thesuper quenching ability of 1 toward 4-NT. The calculated Ksy valuesfor NB, 1,3-DNB, 2,4-DNT, 2,6-DNT and TNT were 5.04×10,4.43x10 ,6.21×10,3.87×10° and 3.10x 10°Ml, respectively, which lie in the normal range for the known luminescent metal-organicframeworks. If only one kind of quenching mechanism isoperative, whether static or dynamic, the quenching can berepresented by the Stern-Volmer equation and the 1o/I versus [Q]plot should be linear. To better understand the mechanism ofsensing, the variation of the fluorescence lifetime of 1 was checkedin the absence and presence of 4-NT (Fig.6b). The emission lifetimeof 1 showed a single exponential feature with to= 0.39 ns andremained essentially unchanged by addition of 4-NT suggesting thequenching process follows static mechanism. The UV-vis spectra of1 upon incremental addition of 4-NT were recorded (Fig. 6c). Theabsorption band intensity at 242 nm reduced rapidly with increased4-NT concentration, indicating a strong interaction between thesensing material and the 4-NT at ground state. Notably, upongradual addition a new absorption band at 302 nm was appearedwhich confirms the absorption by 4-NT is the main source for theobserved fluorescence quenching. To examine the potential of the selective sensing behavior of 1towards 4-NT in presence of other interfering nitroaromaticanalytes, a series of competition experiments were performed bygradual addition of a solution of different nitroaromatic analoguesfollowed by 4-NT into 1, and the corresponding fluorescencespectra were monitored. As shown in Fig. S21-25, the initialaddition of different analytes showed negligible effect on theluminescent intensity of 1, but a significant fluorescence quenchingoccurred after the incremental addition of 4-NT to the mixedemulsion.The stepwisedecreaseinnluminescentiirntensitydemonstrates the high ability of 1 for selectively detecting 4-NTeven in the presence of other nitroaromatic compounds. It is noteworthy that no pre-activation process was carried outbefore the sensing experiments, which shows the operabilityadvantage of 1 over other reported MOF-based sensors (need theevacuation of coordinated or guest solvent molecules). Also, thedetection ability of 1 could be regenerated from emulsion andreused by simply filtrating and washing several times with DMF. Asshown in Fig. S26, the initial fluorescence intensity of the recoveredmaterial shows no obvious decrease over five repeated cycles,which implies a high photostability of 1 for its long-timenitroaromatic compounds detection application. The PXRD patternsof the initial sample and recovered sample after five cycles ofquenching and recovery also indicate the high stability of thiscompound (Fig.S27). (a) 6000 0mM NO2 0.1mM 5000 0.2mM 0.3mM Concentration(mM) Fig.5 (a) Fluorescence titration of 1 upon incremental addition of 4-NT solution in DMF. (b) Fluorescence intensity ratio histograms of 1upon exposure to 0.3 mM concentration of different analytes. (c)Plot of quenching efficiency of 1 dispersed in DMF with differentanalytes. 500 Wavelength (nm) Fig. 6 (a) Stern-Volmer plots of 1 dispersed in DMF with differentanalytes. (b) Fluorescence decay profile of 1 before and afteraddition of 4-NT. (c) Adsorption spectra of 1 in DMF uponincremental addition of 4-NT. Conclusions In summary, three new FL-MOFs generally formulated as [Cd(cis-chdc)(anti-bpe)(HzO)]n (1), [Ni(cis-chdc)(gauche-bpe)]n (2) and[Cd2(trans-chdc)(cis-chdc)(TG-bpp)2]n (3) have been successfullysynthesized under hydrothermal conditions. The crystal structuresof all three compounds feature one-dimensional infinite chainsfurther cross-linked via flexible ligands to form three-dimensionalnetworks. The difference innligand conformations plays animportant role on FL-MOF architectures reflected by the uniquestructural features of all three compounds. Of peculiar interest,compound 1 represents a rare self-catenated MOF with 6·8-zsttopology,which shows high sensitivity and quick response upon thepresence of trace amount of nitroaromatic compounds. Meanwhile,the excellent ability of 1 for selective detection of 4-nitrotoluenefrom other nitroaromatic compounds has been demonstrated. Therecyclability and reusability of 1 have also been tested based on consecutive detection reactions, in which 1 remains unchanged anddetection efficiency sustained. Compound 1 is one of the fewluminescent sensors based on FL-MOFs that exhibit highly sensitive,reversible sensing,andSselective detection of nitroaromaticexplosives. This study extends the applications of FL-MOFs aspromising materials for versatile chemical sensors. Acknowledgements This work is supported by the National Natural ScienceFoundation of China (No. 21441008,21405001, and 21301002),NaturaI Science Foundationn ofAnhui Province (No.1508085MB27), Natural Science Foundation for Colleges andUniversities in Anhui Province (No.KJ2013A045), and State KeyLaboratory of Structural Chemistry (No. 20140006). DMPacknowledges the Ministry of Education and Science of Russia(Grant 14.B25.31.0005). Notes and references 1 (a) J. Yinon in Forensic and Environmental Detection ofExplosives, John Wiley & Sons, Chichester, 1999;(b) W. C.Trogler in NATO ASI Workshop, Electronic Noses & Sensorsfor the Detection of Explosives, Kluwer Academic Publishers,Dordrecht,2004. 2 (a) M. E. Germain and M. J. Knapp, Chem. Soc. Rev. 2009,38,2543; (b) Y. Salinas, M. R. Martinez, M. D. Marcos, F.Sancenon, A. M.Castero, M. Parra and S. Gil, Chem. Soc. Rev.2012,41,1261. 3 (a) S. W. Thomas, G. D. Joly and T. M. Swager, Chem. Rev.,2007, 107, 1339; (b) H. Cavaye, P. E. Shaw, X. Wang, P. L.Burn, S.-C. Lo and P. Meredith, Macromolecules, 2010, 43,10253; (c) K. K. Kartha, S. S. Babu, S. Srinivasan and A.Ajayaghosh, J. Am. Chem. Soc. 2012, 134,4834. 4 (a) E. S. Snow, F. K. Perkins, E. J. Houser, S. C. Badescu and T.L. Reinecke, Science, 2005, 307, 1942; (b) M. E. Kose, B. A.Harruff, Y. Lin, L. M. Veca, F. S. Lu and Y.P. Sun, J. Phys. Chem.B, 2006, 110,14032. 5 (a)Y. Cui, Y. Yue, G. Qian and B. Chen, Chem. Rev.,2012, 112,1126; (b) L. E. Kreno,K. Leong, O. K. Farha, M. Allendorf, R. P.Van Duyne and J. T. Hupp, Chem. Rev.,2012, 112, 1105; (c)Y.Cui, B. Chen and G. Qian, Coord. Chem. Rev.,2014,76,273;(d) Z. Hu, B. J. Deibert and J. Li, Chem. Soc. Rev., 2014, 43,5815. ( 6 (a) A. Lan, K. Li, H. Wu, D. H . Olson, T . J . Emge, W . K i , M. Hong and J. Li, Angew. C h em., Int. E d .,2009,48, 233 4 ; (b) Z. Zhang, S. Xiang, X. Rao, Q. Zheng, F. R. Fronczek, G. Qian andB. Chen, Chem. Commun.,2011,4 7 ,7205;(c) S. P ramanik, C.Zheng, X. Zhang, T. J . Emge and J. Li , J. Am. C h em. So c ., 201 1 ,133 , 4153;(d ) C. Z h ang, Y . Che, Z. Z hang, X.Yang and L. Zang, Chem. Commun., 20 1 1,47, 2336;(e) B. Gole, A. K. B a r and P. S. Mukherjee,Chem. Commun., 2011, 47, 1 2137; (f) M. Guoand Z . M. S un, J. Mater. Chem., 2 012, 22, 1 5939; (g) S . S.Nagarkar, B. Joarder, A. K. Chaudhari, S. Mukherjee and S . K.Ghosh,Angew. Chem., Int. Ed., 2013,52,2881. ) ( 7 (a) D. M a, B. L i , X . Zhou, Q. Z h ou, K. Li u , G. Zeng, G. Li, Z. Shi and S. Feng, Chem. Commun., 2013, 49,8964;(b) G. Wang,L. Yang, Y . L i, H. Song, W. R uan, Z . C hang and X. B u , D a lton T rans., 2013, 42, 1 2865; (c) Q . Zhang, A. Geng, H . Z h ang, F.Hu, Z . Lu, D. Sun, X. Wei and C. Ma, Chem. Eur.J. , 2014, 20,4885;(d) B . Gole, A. K. Bar and P. S. M u kherjee, Chem. Eur. J.,2014,20, 1 3 321;(e) D. Ti a n, Y. Li, R . Chen, Z. Chang, G. Wangand X. B u, J. Mater. C hem. A., 2 014, 2, 1 465; (f) G. Wang, C.Song, D . K ong, W. R u an, Z. C h ang an d Y. Li , J . M ater. C h em. ) View Article Online DOI: 10.1039/C6CE00882HJournal Name A., 2014, 2, 2213; (g) D. Singh and C. M. Nagaraja, DaltonTrans.,2014, 43, 17912; (h) K. S. Asha, K. Bhattacharyya andS. Mandal, J. Mater. Chem. C., 2014, 2, 10073;(i) S. Sanda, S.Parshamoni, S. Biswasa and S. Konar, Chem. Commun., 2015,51, 6576; (j) J. Ye, L. Zhao, R. F. Bogale, Y. Gao, X. Wang, X.Qian, S. Guo, J. Zhao and G. Ning, Chem. Eur. J., 2015, 21,2029; (k) C. Zhang, L. Sun, Y. Yan, J. Li, X. Song, Y. Liu and Z.Liang, Dalton Trans.,2015, 44,230. 8 (a) Z. Lin and M.-L. Tong, Coord. Chem. Rev.,2011, 255, 421;(b) Z. Lin, J. Lu, M. Hong and R. Cao, Chem. Soc. Rev., 2014,43, 5867;(c) X. Zhu, J. Lu, X. Li, S. Gao, G. Li, F. Xiao and R.Cao, Cryst. Growth Des., 2008, 8, 1897-1901;(d) X. Zhu, Z. Lin,T. Liu, B. Xu and R. Cao, Cryst. Growth Des., 2012, 12, 4708. CrystalClear, version 1.3.5; Rigaku Corp.: Woodlands, TX,1999. 10 G. M. Sheldrick, SHELXTL, Crystallographic Software Package,version 5.1; Bruker-AXS: Madison, Wl, 1998. 111((a) S.-C. Hsu, P.-S. Chiang, H.-K. Liu, S.-H. Lo and C.-H. Lin, J.Chin. Chem. Soc.,2012,59, 18;(b) X. Wang, W. Yao, Y.-F. Qi,M.-F. Luo, Y.-H. Wang, H.-W. Xie, Y. Yu, R.-Y. Ma and Y.-G. Li,CrystEngComm, 2011,13,2542. 12 V. A. Blatov, A. P. Shevchenko and D. M. Proserpio, Cryst.Growth Des., 2014, 14. 3576-3586; see alsohttp://www.topospro.com. ( 13 3 ( ( a) FISZIR: Q. Chang, X.-R. Meng, Y.-L. Song and H . -W. Hou,Inorg. Chim. Acta, 2005, 358, 2117;(b) ALIGOS:S.-T. Zheng, Y . Li, T. Wu, R. A. Nieto, P. Feng and X. B u, Chem. Eur. J . , 2 010,16, 13035; (c) YIGZIZ, YIGZOF, YIGZUL: G.-Z. Liu, S.-H. Li, X.-L. Li, L.-Y. Xin and L.-Y. Wang, CrystEngComm, 2 0 13, 15,4571. ) 14 EE. V. Alexandrov, V. A. Blatov, A. V. Kochetkov and D. M.Proserpio, CrystEngComm, 2011, 13,3947. ( 15 ( ( i a) L. Carlucci, G . Cia n i an d D . M. Proserpio, Coord. Chem. Rev.,2003, 246, 2 4 7; (b) V. A. Blatov, M. O ' Keeffe and D. M.Proserpio, CrystEngComm, 2 0 10, 12, 44. ) ( 16 (a) ZAWNUI: S.-S. Ch e n, Y. Zhao, J. Fan, T. Ok a mura, Z.-S. Bai,Z.-H. Chen, W.-Y. Sun, CrystEngComm, 2012, 14, 3564-3576;(b) KINHAS: Z . -Q. S h i, Y.-Z. L i, Z.-J . Guo, H.-G. Zheng, Cryst. Growth Des., 2013, 13,3078-3086. ) ( 17 S . J. Ramsden, V. R obins, S. T.Hyde, Acta C r yst. A, 2 009, 65, 81; see http://epinet.anu.edu.au/sqc117. ) ( 3 (a) ANIDIL: E. Y . Kim Y. J. Song, H. G. Koo, J. H. Lee, H. M.Park, C. Kim, T.-H. Kwon, S . Huh, S.-J. K im, Y. K im, Polyhedron,2010, 2 9, 3335;(b) DEGSOY: J. Zhang, Y. Kang, J. Zhang, Z.-J.Li, Y.-Y. Qin, Y.-G. Yao, Eur. J . Inor g . Chem.,2006, 1 2,2 2 53;(c)DEGSOY01: H. S. Huh, S . W. Lee,J.Mol. Struct., 2007,829, 44;(d) SIRDOO: J . -J. Wang, T.- T . W ang, L. Tang, X . -Y. Hou, L.-J.Gao, F. Fu, M.-L. Zhang, J. Coord. Chem.,2 0 13, 6 6 , 3 9 79; (e) TEDDOW: R. - Q. Fa n g, Y. - F. Z h ao, X.-M. Zhang, Inorg. Chim. Acta, 2006, 359, 2023;(f) TEDDOW01: Y . F eng, J. Peng, X.Che, Y.Gao, J. Coord . Chem.,2006,59,1349;(g) VIVDOV:J.-J.Wang, T.-T. Wang, L. Tang, X. - Y. Hou, M. - L. Zhang, L.- J . Ga o , F.Fu,Y.-X. Ren, Z. Anorg. Allg. Chem.,2014, 6 4 0, 4 8 3. ) ( 19( ( : a) M. Y u, L . Xie, S. Liu , C. Wang, H. Cheng, Y. Ren and Z. S u ,Inorg.Chim. A c ta, 2007, 3 6 0,31 0 8; (b) E.-C Yang, H.-K Zhao,B. Ding, X .-G. Wang, and X . -J. Zhao,Cryst. G rowth Des., 2007, 7,2009. ) Table of Content Three newmetal-organicframeworksbased on 1flexible ligands(FL-MOFs) have beenhydrothermally synthesized and characterized. Crystal structures of the three compounds featureone-dimensional infinite chains cross-linked via flexible ligandskeletons to formthree-dimensional networks. Of peculiar interest, compound 1 represents a rare self-catenatedMOF with 6’8-zst topology, which shows high sensitivity and quick response upon the presenceof trace amount of nitroaromatic compounds. Meanwhile, the excellent ability of 1 for selectivedetection of 4-nitrotoluene from other nitroaromatic compounds has been demonstrated.Therecyclability and reusability of 1 have also been tested based on consecutive detection reactions, inwhich 1 remains unchanged and detection efficiency retains. www.rsc.org/crystengcomm This journal is C The Royal Society of Chemistry xJ. Name., 金属-有机骨架组装自柔性脂环族甲酸酯和双环戊二烯基配体用于硝基芳族化合物的检测Metal–organic frameworks assembled from flexible alicyclic carboxylate and bipyridyl ligands for sensing of nitroaromatic explosives
确定




还剩8页未读,是否继续阅读?
北京祥鹄科技发展有限公司为您提供《金属有机骨架中金属含量检测方案(微波合成仪)》,该方案主要用于其他中含量分析检测,参考标准--,《金属有机骨架中金属含量检测方案(微波合成仪)》用到的仪器有电脑微波超声波紫外光组合催化合成仪
推荐专场
相关方案
更多
















