方案详情
文
Through the combination of the ICS-5000 ion chromatography system with the iCAP Qc ICP-MS, a sensitive, robust method for the speciation analysis of trace levels of Cr (III) and Cr (VI) in natural waters has been developed. The method developed enables fast and reliable speciation analysis of both Cr (III) and Cr (VI) species in water samples without prior incubation steps and with high purity nitric acid as mobile phase. The short, but highly ef?cient Dionex AG-7 column, provides complete separation of both species in under 150 s, enabling high sample throughput for the routine analysis of water samples.
方案详情

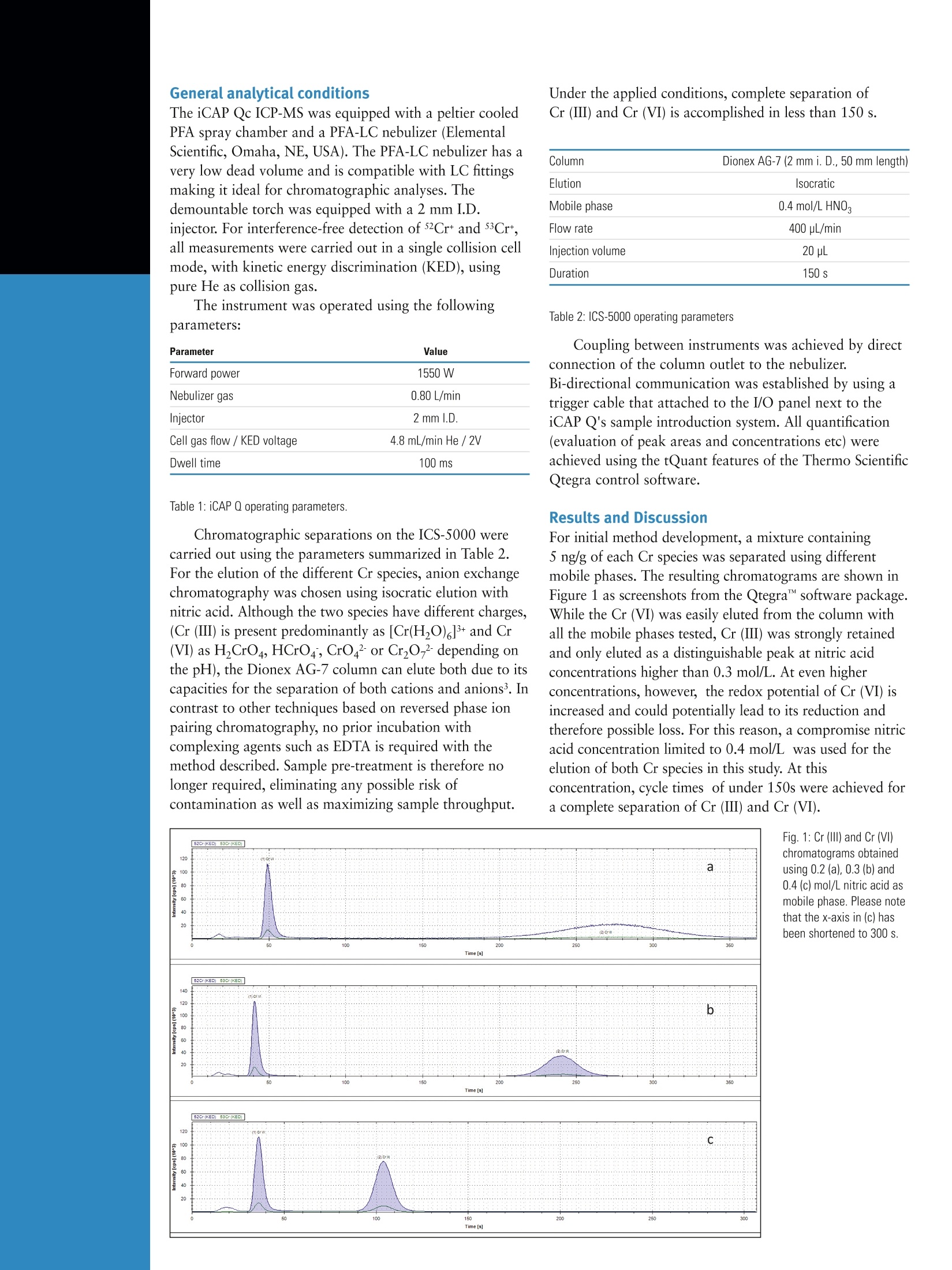
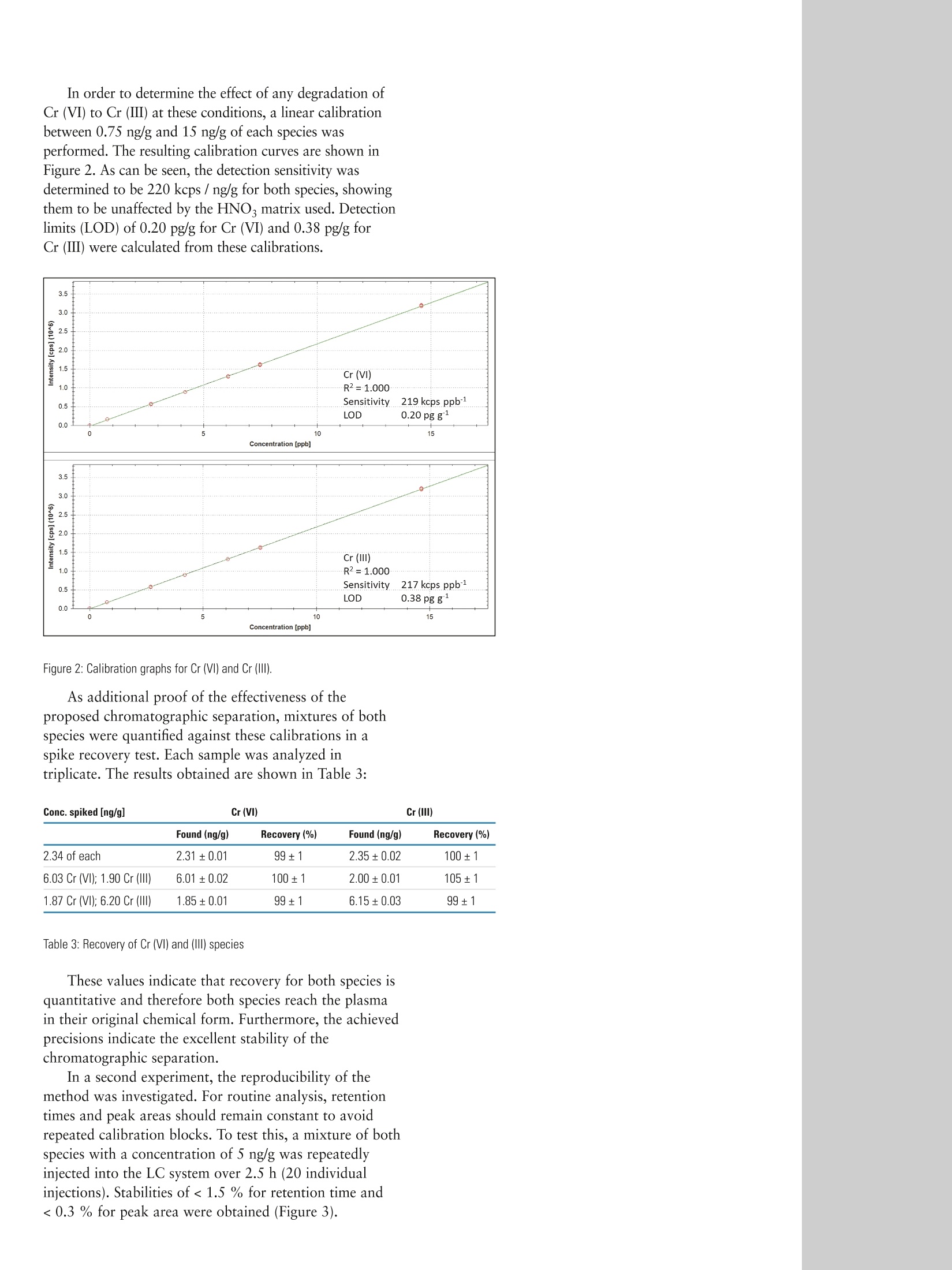
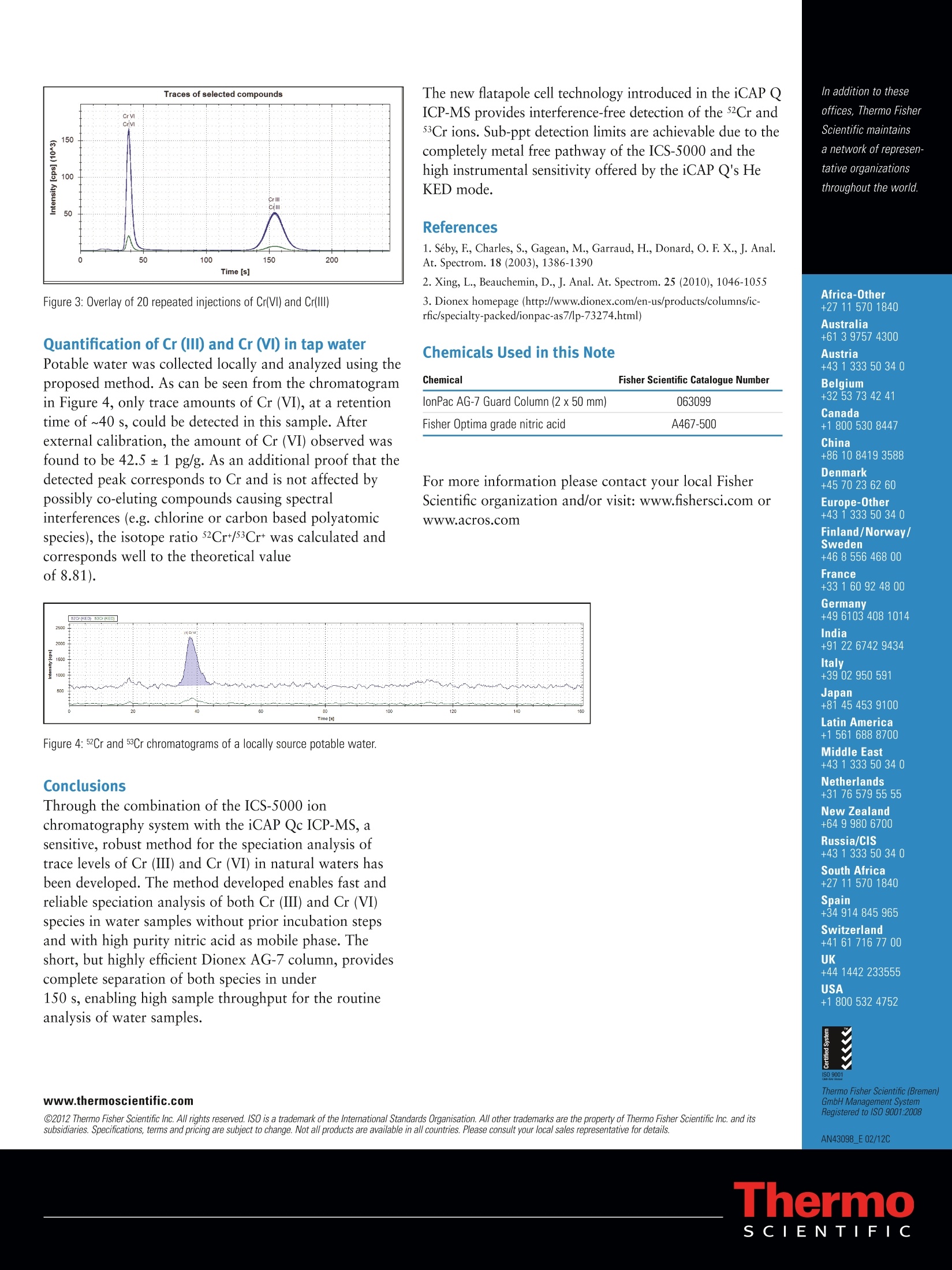
Daniel Kutscher, Shona McSheeby, Julian Wills, Thermo Fisher Scientific, Germany, Detlef Jensen, Thermo Fisher Scientific, Switzerlandwww.thermoscientific.comC2012 Thermo Fisher Scientific Inc. All rights reserved. ISO is a trademark of the International Standards Organisation. All other trademarks are the property of Thermo Fisher Scientific Inc. and itssubsidiaries.Specifications, terms and pricing are subject to change. Not all products are available in all countries. Please consult your local sales representative for details. Speciation analysis of Cr (III) and Cr (VI) indrinking waters using anion exchangechromatography coupled to theThermo Scientific iCAP Q ICP-MS Introduction Due to it widespread use in industrial applications such aschromium plating, dye manufacturing and preservation ofwood and leather materials, chromium concentrations inenvironmental samples are monitored on a routine basis.Both the United States EPA and the European Union havespecified maximum admissible chromium concentrationsin their respective drinking water directives. As with manyother trace elements, chromium (Cr) is typically found inmore than one chemical form, each of which withdifferent chemical properties and behavior, such asbioavailability and toxicity. For chromium, Cr (III) isessential to human beings and involved in differentprocesses in the body while Cr (VI) is highly toxic. TotalCr content therefore in, for example, a drinking watersample does not provide sufficient information to evaluatepotential hazards to populations exposed to it. In order toprovide this critical information a supporting speciationanalysis is required to determine the amounts of thedifferent Cr species in the sample. The speciation analysisof Cr however is a challenging task, since the stability ofdifferent Cr species is easily affected by conditions duringsample collection and treatment . For example, low pHvalues may lead to the degradation of Cr (VI) to Cr (III)due to the increased redox potential, while high pH valuesmay lead to the precipitation of Cr (III) as Cr(OH)32. Anadditional difficulty in the accurate speciation analysis ofCr by ICP-MS are the numerous spectral interferences (e.g.35Cl16O1H+ or 40Arl2C+) on the most abundant chromiumisotope,52Cr. Sample and calibration solution preparationDaily working standards were prepared by diluting theappropriate quantity of commercially available stocksolutions (1000 pg/mL) of each chromium standard in a0.1 mol/L ammonium nitrate solution adjusted to a pH of 4.Drinking water was collected in a PFA bottle previouslyrinsed with high purity nitric acid. The water was analyzeddirectly without dilution or pH adjustment in order to keepthe species unchanged before analysis. Instrument configuration Chromatographic separations were carried out using theThermo Scientific Dionex ICS-5000 ion chromatographysystem. Due to its completely metal-free solvent pathway,this system is non-contaminating and is therefore perfectlysuited for elemental speciation studies at the trace levelsrequired by this application. For the separation of the twoCr species, a Thermo Scientific Dionex AG-7 anionexchange column (2x 50mm) was used throughout thisstudy. Although this column is designed to be used as aguard column, its highly effective separation mediumcontains capacities for the separation of both cationic andanionic species and it is therefore able to completelyseparate both Cr species in less than three minutes. AThermo Scientific iCAP Qc ICP-MS was used as a highperforming elemental detector of the Cr species elutedfrom the ICS-5000. Due to the use of flatapole technologyin the Thermo Scientific QCell collision cell, the iCAP Qseries of ICP-MS instruments offer the selectivity tosuppress spectral interferences while maintaining the highsensitivity for trace metal detection in coupledapplications such as IC-ICP-MS. General analytical conditions The iCAP Qc ICP-MS was equipped with a peltier cooledPFA spray chamber and a PFA-LC nebulizer (ElementalScientific, Omaha, NE, USA). The PFA-LC nebulizer has avery low dead volume and is compatible with LC fittingsmaking it ideal for chromatographic analyses. Thedemountable torch was equipped with a 2 mm I.D.injector. For interference-free detection of 52Crt and 53Cr*,all measurements were carried out in a single collision cellmode, with kinetic energy discrimination (KED), usingpure He as collision gas. The instrument was operated using the followingparameters: Parameter Value Forward power 1550 W Nebulizer gas 0.80 L/min Injector 2 mm l.D. Cell gas flow / KED voltage 4.8 mL/min He /2V Dwell time 100 ms Table 1: iCAP Q operating parameters. Chromatographic separations on the ICS-5000 werecarried out using the parameters summarized in Table 2.For the elution of the different Cr species, anion exchangechromatography was chosen using isocratic elution withnitric acid. Although the two species have different charges,(Cr (III) is present predominantly as [Cr(HO) ]t and Cr(VI) as H,CrO4, HCrO4, CrO4 or Cr2O7 depending onthe pH), the Dionex AG-7 column can elute both due to itscapacities for the separation of both cations and anions. Incontrast to other techniques based on reversed phase ionpairing chromatography, no prior incubation withcomplexing agents such as EDTA is required with themethod described. Sample pre-treatment is therefore nolonger required, eliminating any possible risk ofcontamination as well as maximizing sample throughput. Under the applied conditions, complete separation ofCr (III) and Cr (VI) is accomplished in less than 150 s. Column Dionex AG-7 (2 mm i. D., 50 mm length) Elution Isocratic Mobile phase 0.4 mol/L HNO, Flow rate 400 pL/min Injection volume 20 pL Duration 150 s Table 2: ICS-5000 operating parameters Coupling between instruments was achieved by directconnection of the column outlet to the nebulizer.Bi-directional communication was established by using atrigger cable that attached to the I/O panel next to theiCAP Q's sample introduction system. All quantification(evaluation of peak areas and concentrations etc) wereachieved using the tQuant features of the Thermo ScientificQtegra control software. For initial method development, a mixture containing5 ng/g of each Cr species was separated using differentmobile phases. The resulting chromatograms are shown inFigure 1 as screenshots from the Qtegra software package.While the Cr (VI) was easily eluted from the column withall the mobile phases tested, Cr(III) was strongly retainedand only eluted as a distinguishable peak at nitric acidconcentrations higher than 0.3 mol/L. At even higherconcentrations, however, the redox potential of Cr (VI) isincreased and could potentially lead to its reduction andtherefore possible loss. For this reason, a compromise nitricacid concentration limited to 0.4 mol/L was used for theelution of both Cr species in this study. At thisconcentration, cycle times of under 150s were achieved fora complete separation of Cr (ⅢII) and Cr (VI). In order to determine the effect of any degradation ofCr (VI) to Cr (III) at these conditions, a linear calibrationbetween 0.75 ng/g and 15 ng/g of each species wasperformed. The resulting calibration curves are shown inFigure 2. As can be seen, the detection sensitivity wasdetermined to be 220 kcps/ng/g for both species, showingthem to be unaffected by the HNO matrix used. Detectionlimits (LOD) of 0.20 pg/g for Cr (VI) and 0.38 pg/g forCr (ⅢI) were calculated from these calibrations. Figure 2: Calibration graphs for Cr (VI) and Cr (III). As additional proof of the effectiveness of theproposed chromatographic separation, mixtures of bothspecies were quantified against these calibrations in aspike recovery test. Each sample was analyzed intriplicate. The results obtained are shown in Table 3: Found (ng/g) Recovery(%) Found (ng/g) Recovery(%) 2.34 of each 2.31±0.01 99±1 2.35±0.02 100±1 6.03 Cr (VI); 1.90 Cr (III) 6.01±0.02 100±1 2.00±0.01 105±1 1.87 Cr (VI); 6.20 Cr (II) 1.85±0.01 99±1 6.15±0.03 99±1 Table 3: Recovery of Cr (VI) and (III) species These values indicate that recovery for both species isquantitative and therefore both species reach the plasmain their original chemical form. Furthermore, the achievedprecisions indicate the excellent stability of thechromatographic separation. In a second experiment, the reproducibility of themethod was investigated. For routine analysis, retentiontimes and peak areas should remain constant to avoidrepeated calibration blocks. To test this, a mixture of bothspecies with a concentration of 5 ng/g was repeatedlyinjected into the LC system over 2.5 h (20 individualinjections). Stabilities of <1.5 % for retention time and<0.3 % for peak area were obtained (Figure 3). Figure 3: Overlay of 20 repeated injections of Cr(Vl) and Cr(III) Quantification of Cr (III) and Cr (VI) in tap waterPotable water was collected locally and analyzed using theproposed method. As can be seen from the chromatogramin Figure 4, only trace amounts of Cr (VI), at a retentiontime of ~40 s, could be detected in this sample. Afterexternal calibration, the amount of Cr (VI) observed wasfound to be 42.5±1 pg/g. As an additional proof that thedetected peak corresponds to Cr and is not affected bypossibly co-eluting compounds causing spectralinterferences (e.g. chlorine or carbon based polyatomicspecies), the isotope ratio 52Cr+/53Cr was calculated andcorresponds well to the theoretical valueof 8.81). The new flatapole cell technology introduced in the iCAP Q[CP-MS provides interference-free detection of the 52Cr and53Cr ions. Sub-ppt detection limits are achievable due to thecompletely metal free pathway of the ICS-5000 and thehigh instrumental sensitivity offered by the iCAP Q's HeKED mode. References 1. Seby, F, Charles, S., Gagean, M., Garraud, H.,Donard, O. F. X., J. Anal.At. Spectrom. 18 (2003),1386-1390 2. Xing, L., Beauchemin, D.,J. Anal. At. Spectrom. 25 (2010), 1046-1055 3. Dionex homepage (http://www.dionex.com/en-us/products/columns/ic-rfic/specialty-packed/ionpac-as7/lp-73274.html) Chemicals Used in this Note lonPac AG-7 Guard Column (2 x50 mm) 063099 Fisher Optima grade nitric acid A467-500 For more information please contact your local FisherScientific organization and/or visit: www.fishersci.com orWww.acros.com Daily working standards were prepared by diluting the appropriate quantity of commercially available stocksolutions (1000 µg/mL) of each chromium standard in a 0.1 mol/L ammonium nitrate solution adjusted to a pH of 4.Drinking water was collected in a PFA bottle previouslyrinsed with high purity nitric acid. The water was analyzed directly without dilution or pH adjustment in order to keep the species unchanged before analysis.Through the combination of the ICS-5000 ion chromatography system with the iCAP Qc ICP-MS, a sensitive, robust method for the speciation analysis of trace levels of Cr (III) and Cr (VI) in natural waters has been developed. The method developed enables fast and reliable speciation analysis of both Cr (III) and Cr (VI) species in water samples without prior incubation steps and with high purity nitric acid as mobile phase. The short, but highly efficient Dionex AG-7 column, provides complete separation of both species in under 150 s, enabling high sample throughput for the routine analysis of water samples.
确定
还剩2页未读,是否继续阅读?
赛默飞色谱与质谱为您提供《drinking waters中Speciation analysis of Cr (III) and Cr (VI)检测方案(离子色谱仪)》,该方案主要用于饮用水中(类)金属及其化合物检测,参考标准--,《drinking waters中Speciation analysis of Cr (III) and Cr (VI)检测方案(离子色谱仪)》用到的仪器有Dionex ICS-5000+ ED 电化学检测器、赛默飞iCAP TQ电感耦合等离子体质谱仪
推荐专场
ICP-MS电感耦合等离子体质谱
更多
相关方案
更多
该厂商其他方案
更多