方案详情
文
■ 单内标校正,极大的简化了定量分析,无基体影响;
■ 对于任何基体的样品可单独进行校准和定量分析;
■ V, Al等多元素实时分析,可进行痕量和超痕量分析;
■ 不受样品的类型和不同应用需求影响;
■ 独特的液体或固体样品的微量分析,分析所需样品量小;
■ 优秀的检出限水平;
■ 出色的动态线性范围;
■ 无需任何化学前处理,无记忆效应;
■ 非破坏性分析,运行成本低廉。
方案详情

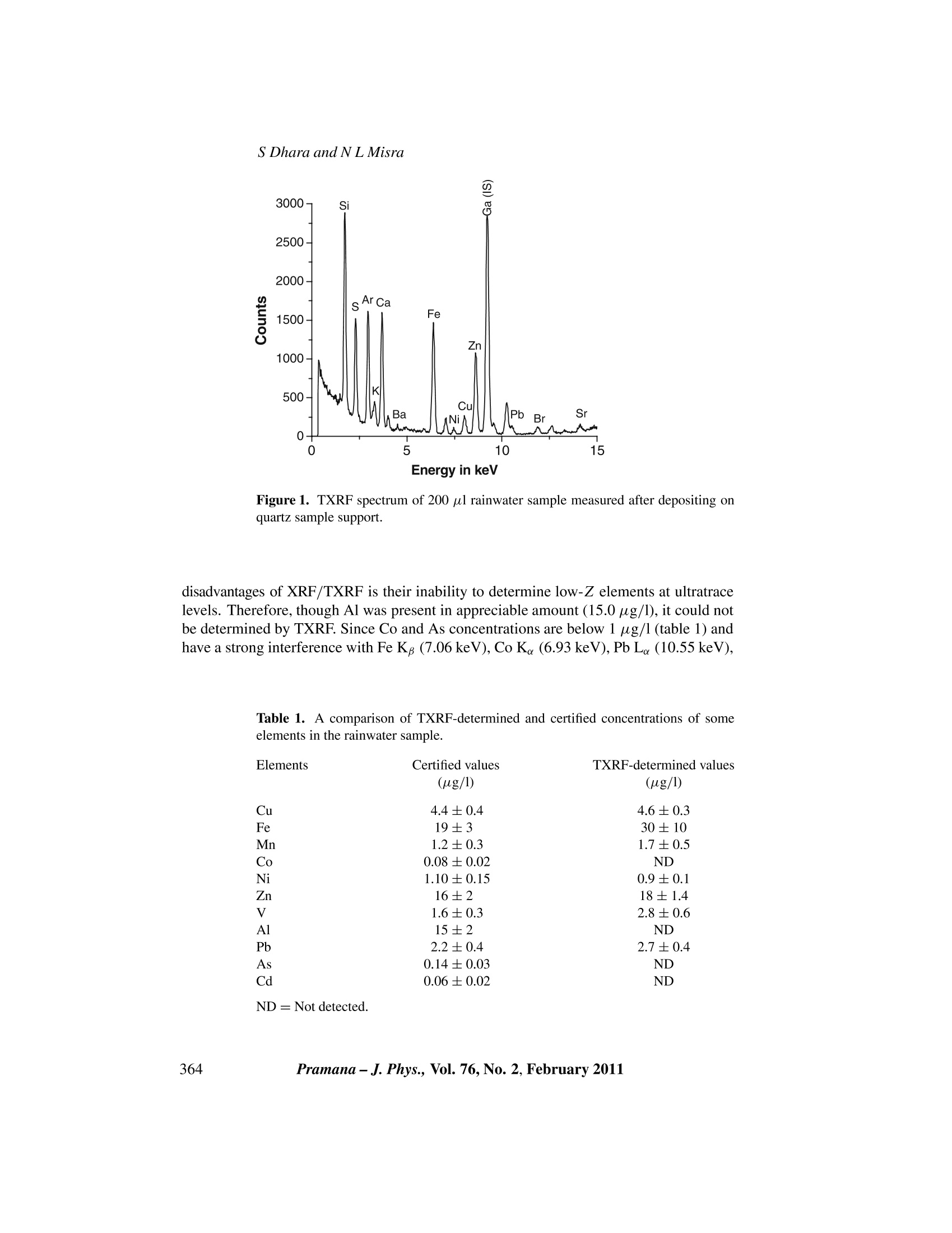
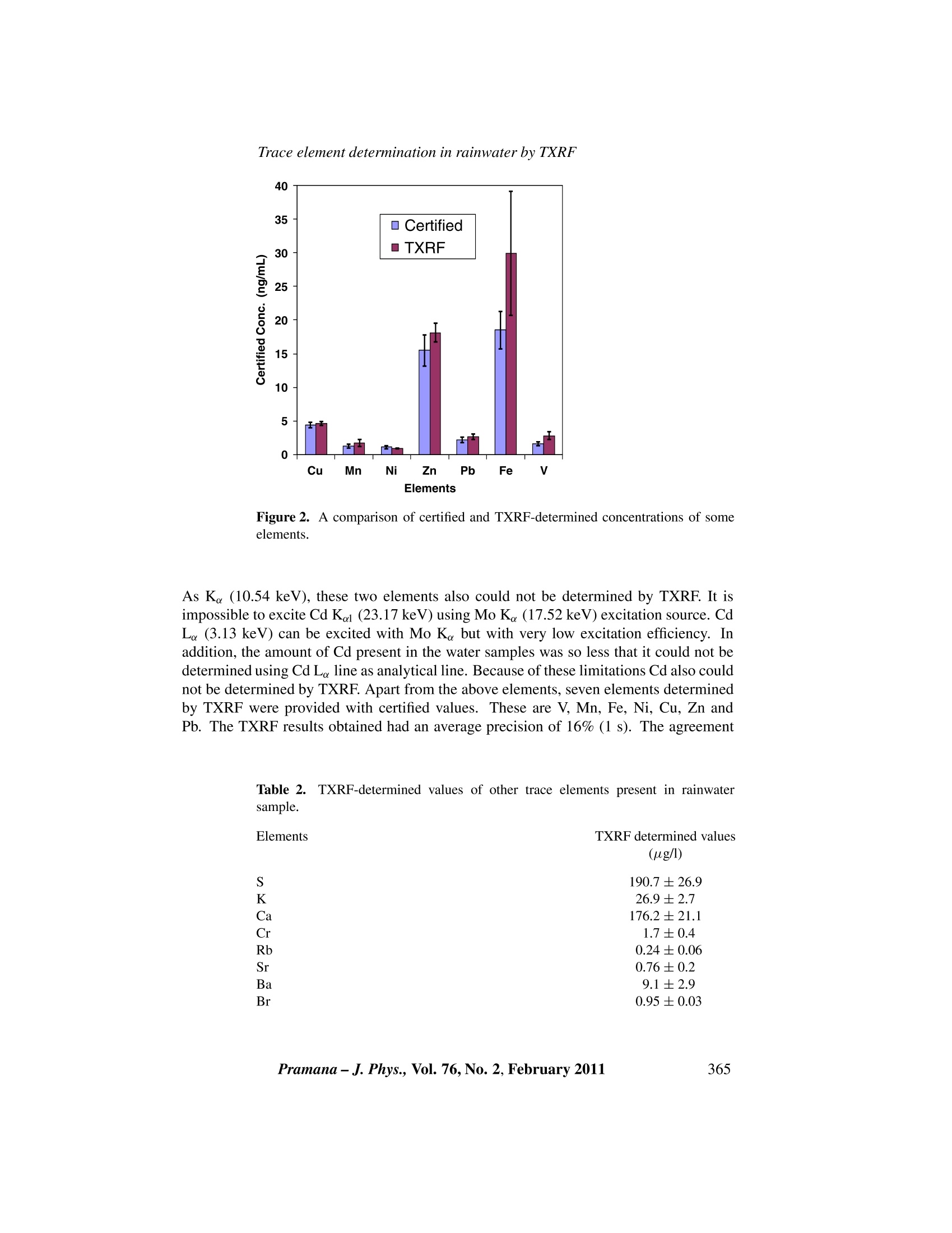
C Indian Academy of SciencesVol.76,No. 2February 2011pp. 361-366PRAMANA-journal ofphysics S Dhara and N L Misra Application of total reflection X-ray fluorescencespectrometry for trace elemental analysis of rainwater SANGITA DHARA and NL MSRA* Fuel Chemistry Division, Bhabha Atomic Research Centre, Mumbai 400 085, India *Corresponding author. E-mail: nlmisra@barc.gov.in; nlmisra@yahoo.com Abstract. Applicability of total reflection X-ray fluorescence (TXRF) spectrometry for traceelemental analysis of rainwater samples was studied. The study was used to develop these samplesas rainwater standards by the National University of Singapore (NUS). Our laboratory was oneof the participants to use TXRF for this study. The rainwater sample obtained from NUS wasanalysed by TXRF and the trace elements Mn, Fe, Ni, Cu, Zn, V and Pb were determined asrequired by the NUS. The average precision was found to be within 16% and the TXRF-determinedelemental concentrations of these elements were below 20 ug/1. The average deviation of TXRF-determined values from the certified values were 20% (excluding the deviation for Fe and V whichwere comparatively high). Apart from the above elements, S, K, Ca, Rb, Sr, Ba and Br were alsodetermined by TXRF and were found to be in the range of 0.2 to 191 ug/l.TXRF-determined valuesof our laboratory played an important role in the certification of concentration of seven elements inthis rainwater sample which was later developed as a rainwater standard. Keywords. Rainwater; total reflection X-ray fluorescence; interlaboratory study; trace elements. PACS Nos 92.40.Ea;78.70.En; 91.67.Pq 1..Introduction Determination of trace elements present in environmental samples is of considerable in-terest because of the increased awareness about environmental pollution and human healthas some of the trace elements are toxic whereas some are considered as nutrients for liv-ing population. Monitoring and accurate determination of these trace elements are veryimportant because some elements which are nutrients, can also act as a toxin if they arepresent above certain limits. Analysis of various environmental samples such as soil,rocks, vegetation, sea water, rainwater, ground water, etc., helps in studying the originand nature of deposition of trace and major elements [1-3]. Determination of trace aswell as major elements present in rainwater gives a direct indication about the nutrientand toxic elements present in the atmosphere. The presence of heavy elements indicatesthe level of pollution over that region. For monitoring rainwater quality, it is essentialto ensure that the results produced from a particular laboratory by a particular technique are sufficiently accurate and precise. This requires the availability of suitable certifiedreference materials (CRMs) [4]. Interlaboratory studies (ILS) play a major role in thedevelopment of these certified standards. The certification of trace element concentrationin a particular CRM depends on the number of laboratories participating and the numberof techniques used for the determination of a particular element. As rainwater contains a number of elements at ultratrace levels it is seldom possibleto detect and determine all the elements by a single technique. The sample collectionand storage also play a vital role as there are great chances of contamination during theseprocesses. Various techniques used for the elemental characterization of trace elementsin rainwater are, inductively coupled plasma-mass spectrometry (ICPMS), inductivelycoupled plasma-atomic emission spectrometry (ICP-AES), atomic absorption spectrom-etry (AAS), X-ray fluorescence (XRF), total reflection X-ray fluorescence (TXRF), neu-tron activation analysis (NAA), electroanalytical techniques, etc. [5-7]. Total reflectionX-ray fluorescence (TXRF) is an upcoming technique for trace and ultratrace analysis.In TXRF, the X-ray beam falls on the sample at an angle less than the critical angle andgets totally reflected after touching the sample and thus has a minimum interaction withthe sample support over which a few microlitres/micrograms of the sample is deposited.This unique geometry makes TXRF an advanced variant of energy-dispersive X-ray flu-orescence (EDXRF) and leads to excitation of the sample by incoming as well as totallyreflected beams. Since the penetration of the beam inside the sample support is almostnegligible, background in TXRF is very low. Both these features i.e., sample excitationby incoming and totally reflected beams and lower background result in comparativelybetter detection limits [8] in TXRF than in XRF by several orders of magnitude. Also,due to almost negligible matrix effects a single element can be used as internal standardfor all elements. Moreover, since the sample is deposited on a limited area of the TXRFsample support, sample contamination can be controlled. All these features make TXRF apotential analytical technique for the analysis of environmental, biological and industrialsamples [9]. Though monitoring of rainwater is very important, the number of rainwater standardsavailable is limited. Moreover, because of the presence of trace elements in rainwater atultratrace levels (ppt-ppb), quantification of these elements without much sample prepara-tion is a challenging task. National University of Singapore conducted an interlaboratorystudy (ILS) to develop such rainwater CRM and Fuel Chemistry Division,BARC, partic-ipated in this ILS by determining trace elements in rainwater samples by TXRF [10]. Inthis paper we present the TXRF methodology and elemental analysis results obtained bythe analysis of this rainwater sample. 2. Experimental procedure 2.1 Instrumentation An Ital Structures TXRF spectrometer TX-2000 with Mo-W dual target tube was usedfor TXRF measurements. Mo Keradiation obtained from the tube operated at 40 kVand 30 mA and monochromatized by a W-C multilayer was used for the excitation of theelements in sample. The characteristic X-rays emitted from the samples were detected by a Si(Li) detector having a resolution of 139 eV at 5.9 keV. The TXRF spectra wererecorded by TXRFACQ 32 and processed using the program EDXRFACQ32 providedwith the instrument. 2.2Sample preparation The collection and processing of rainwater samples are described elsewhere [10]. Ho-mogeneity and stability tests were also performed on these rainwater samples and thendistributed to various laboratories participating in this interlaboratory study. Our labo-ratory also received this processed sample to be analysed by TXRF. For TXRF determi-nations, the relative sensitivities of different elements were determined using a MERCKICP multielement standard solution IV. All the standards were diluted in 1.5% suprapureHNO3. Five millilitres of rainwater samples were taken separately in two thoroughlycleaned glass bottles (SCHOTT DURAN). These glass bottles were earlier immersed in1.5% suprapure HNO3 solution overnight and then rinsed with the suprapure dilute nitricacid solution thrice. After this, 1 ml of the acid solution was put in the bottles and againleft overnight. This solution was analysed for any trace impurity leaching out from thebottles. No impurity was found in any of the bottles. 50 ul of Ga internal standard solu-tion having 5 ug/ml concentration was mixed with 5 ml of rainwater samples. Aliquotsof 20 ul were deposited ten times independently on six quartz sample supports so that200 ul of the above rainwater sample was deposited and dried under an IR lamp. Thequartz sample supports were also cleaned with 1.5% suprapure nitric acid and checkedfor any contamination by measuring their TXRF spectrum before the sample deposition.The TXRF spectrum for each sample was recorded for 1000 s. 3. Results and discussion The excitation energy of Mo K (17.48keV) from the Mo-W dual target tube was used forsample excitation as it can excite the K lines of medium and L lines of high Z elementsvery efficiently. In rainwater, major matrix is water which gets evaporated on the TXRFsample support after drying, leaving behind elemental constituents in concentrated form,so that it is fit for direct analysis by TXRF. The sample deposition on quartz support wasdone in steps of 20 ul to avoid the uneven spreading of the sample on the sample support.This ensured less spreading and formation of a very thin film in about 2 mm radius andallowed the Si (Li) detector to receive almost all the excited X-rays. Depositing samplein this way also acted as a sample preconcentration step. A typical TXRF spectrum ofsuch rainwater sample is shown in figure 1. As TXRF is a multielemental analyticaltechnique applicable to metals and nonmetals equally, both metals and nonmetals presentin the sample could be analysed simultaneously. The Si Ke peak in the spectrum is dueto the sample support and Ar Ka peak is from the air. Gallium was chosen as internalstandard as it was not present in rainwater. Trace elements S, K, Ca, V, Cr, Mn, Fe, Ni,Cu,Br, Rb, Sr, Ba and Pb could be determined by TXRF and were found to be in therange of 0.2 to 191 ug/l. The concentration of trace elements determined by TXRF isgiven in table 1 along with final certified concentrations [10]. The various techniquesused in this ILS were ICP-MS, GFAAS, ICP-AES, DPASV and TXRF. One of the major Figure 1. TXRF spectrum of 200 ul rainwater sample measured after depositing onquartz sample support. disadvantages of XRF/TXRF is their inability to determine low-Z elements at ultratracelevels. Therefore, though Al was present in appreciable amount (15.0ug/l), it could notbe determined by TXRF. Since Co and As concentrations are below 1 ug/l (table 1) andhave a strong interference with Fe Kg (7.06 keV), Co K。 (6.93 keV), Pb L (10.55keV), Table 1. A comparison of TXRF-determined and certified concentrations of someelements in the rainwater sample. Elements Certified values Mn (ug/l) (ug/l) 4.4±0.4 4.6±0.3 19±3 30±10 1.2±0.3 1.7±0.5 0.08±0.02 ND 1.10±0.15 0.9±0.1 16±2 18±1.4 1.6±0.3 2.8±0.6 15±2 ND 2.2±0.4 2.7±0.4 0.14±0.03 ND 0.06±0.02 ND ND= Not detected. Trace element determination in rainwater by TXRF Figure 2. A comparison of certified and TXRF-determined concentrations of someelements. As K (10.54 keV), these two elements also could not be determined by TXRF. It isimpossible to excite Cd Ka (23.17 keV) using Mo Ke (17.52keV) excitation source. CdLe (3.13 keV) can be excited with Mo K but with very low excitation efficiency. Inaddition, the amount of Cd present in the water samples was so less that it could not bedetermined using Cd Le line as analytical line. Because of these limitations Cd also couldnot be determined by TXRF. Apart from the above elements, seven elements determinedby TXRF were provided with certified values. These are V, Mn, Fe, Ni, Cu, Zn andPb. The TXRF results obtained had an average precision of 16% (1 s). The agreement Table 2..TXRF-determined values of other trace elements present in rainwatersample. Elements TXRF determined values (ug/l) S K 190.7 ±26.9 26.9十2.7 176.2±21.1 1.7±0.4 0.24±0.06 0.76±0.2 9.1±2.9 0.95±0.03 between the TXRF-determined and the certified values is shown as a bar graph in figure 2.Except Fe and V all the elements are within the analytical uncertainty of the certifiedvalues. The average deviation of TXRF-determined values from the certified values are20%. Hence, out of the 11 elements which were assigned the certified values, TXRFplayed an important role in the certification of the concentrations of seven elements. Theanalytical results of TXRF-determined values of other trace elements which were notassigned any certified values due to the lack of sufficient analysis data are given in table 2. 4. Conclusions The role of TXRF in the development of rainwater standards was established. TXRFis one of the multielements analytical techniques which requires a very simple samplepreparation method and uses a single internal standard for quantification of all the ele-ments present in the sample. Out of the eleven elements which were assigned certifiedvalues, TXRF results from our laboratory for seven elements have a good agreement withthe certified values. Acknowledgements The authors would like to thank Dr V Venugopal, Director, Radiochemistry and IsotopeGroup, BARC,DrSK Aggarwal, Head, Fuel Chemistry Division and Dr S Kannan, Head,X-ray and Structural Studies Section for their interest in this work. References ( [1] M Baka, Instrum. Sci. T e chnol. 3 2 , 5 5 5 (2004) ) ( [2] M Nimmo and R Chester, Sci. Total Environ. 135,153(1993) ) ( [3] KB Pelig-Ba, Water, Air, S o il Pollut. 1 03, 71 (1998) ) ( [4] K A ndersen, JMerry, H van der Jagt and P h Quevauviller, Sci. Total Environ. 220,223 ( 1 998) ) ( [5] F B enda, V Filistein, F Hezina and J Musil, Int.J . Environ. Anal. C h em. 50 , 9(1 9 9 3 ) ) ( [6] J B Luten, J . R adioanal. Nucl. C hem. 37, 897 (1977) ) ( [7] H Emons, A B a ade and MJ Schoning, Electroanalysis 15 , 11 7 1 (2 0 00) ) ( [8] R K lockenkaemper, To t al reflection X-ray fluorescence a n alysis, c hemical a n alysis ( J ohn Wiley & Sons, New York, 1996) Vol. 140 ) ( [9] R -P Stossel and A Prange, Anal. C hem. 5 7 , 2880 ( 19 85) ) ( [10] S K arthikeyan and R Balasubramanian, Anal. Chim. Acta 576 , 9 ( 2 0 0 6) ) ramana-J. Phys., Vol. No., February 本文章研究了全反射X射线荧光光谱法(TXRF)用于雨水样品痕量元素分析的适用性。这项研究被用来开发新加坡国立大学的雨水标样,通过测试得出痕量元素Mn,Fe,Ni,Cu,Zn,V和Pb的平均精密度在16%以内,浓度低于20μg/L,测定值与标样的平均偏差为20%(不包括Fe和V的相对较高的偏差)。除上述元素外,还测定了S,K,Ca,Rb,Sr,Ba和Br,发现其范围为0.2-191μg/L。这些数据后来在雨水标准的七种元素浓度认证中发挥了重要参考作用。
确定




还剩4页未读,是否继续阅读?
利曼中国为您提供《雨水中V, Al检测方案(能散型XRF)》,该方案主要用于环境水(除海水)中(类)金属及其化合物检测,参考标准--,《雨水中V, Al检测方案(能散型XRF)》用到的仪器有TX 2000 全反射X荧光光谱仪
推荐专场
X荧光光谱、XRF(能量色散型X荧光光谱仪)
相关方案
更多
该厂商其他方案
更多
















