方案详情
文
自动电位滴定仪测定银合金首饰中含银量
使用日本京都电子公司(KEM)-自动电位滴定仪(AT-510),测定银合金首饰中含银量的应用资料。
相关标准规范:
GB/T 17832-2008 银合金首饰银含量的测定溴化钾容量法(电位滴定法)。
GB/T 18996-2003 银合金首饰中含银量的测定氯化钠或氯化钾容量法(电位滴定法)。
GB/T 15072.2-2008 贵金属合金化学分析方法银合金中银量的测定氯化钠电位滴定法。
ISO 11427:1993 Determination of silver in silver jewellery alloys - Volumetric (potentiometric) method using potassium bromide.(银合金首饰中含银量的测定 溴化钾容量法(电位滴定))。
ISO 13756:1997 Determination of silver in silver jewellery alloys - Volumetric (potentiometric) method using sodium chloride or potassium chloride.(银合金中含银量的测定 氯化钠或氯化钾容量法(电位滴定))。
JIS H6311:2002 Methods of determination of silver in silver jewellery alloys.(银饰用合金中银含量的测定方法)。
方案详情

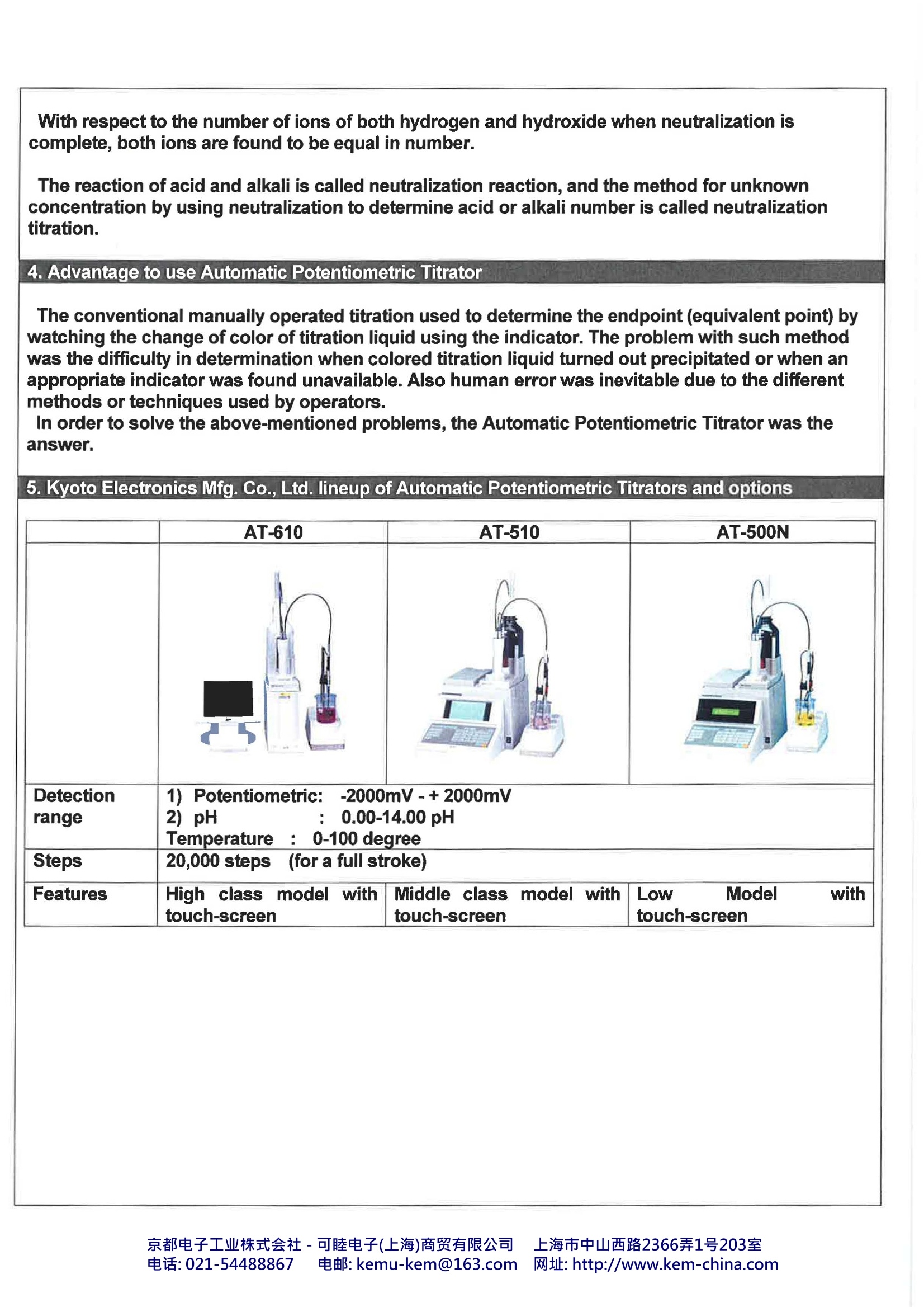
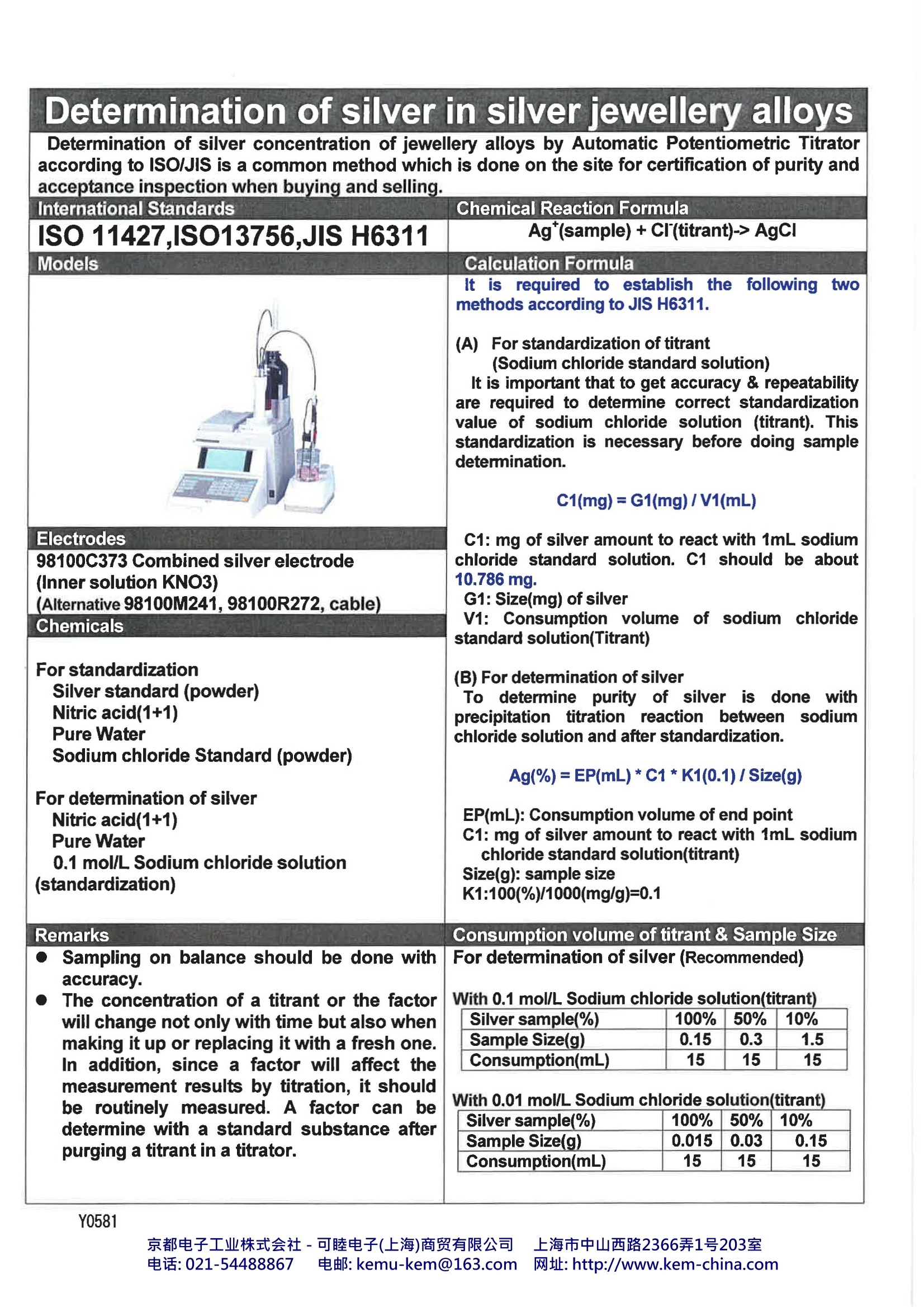
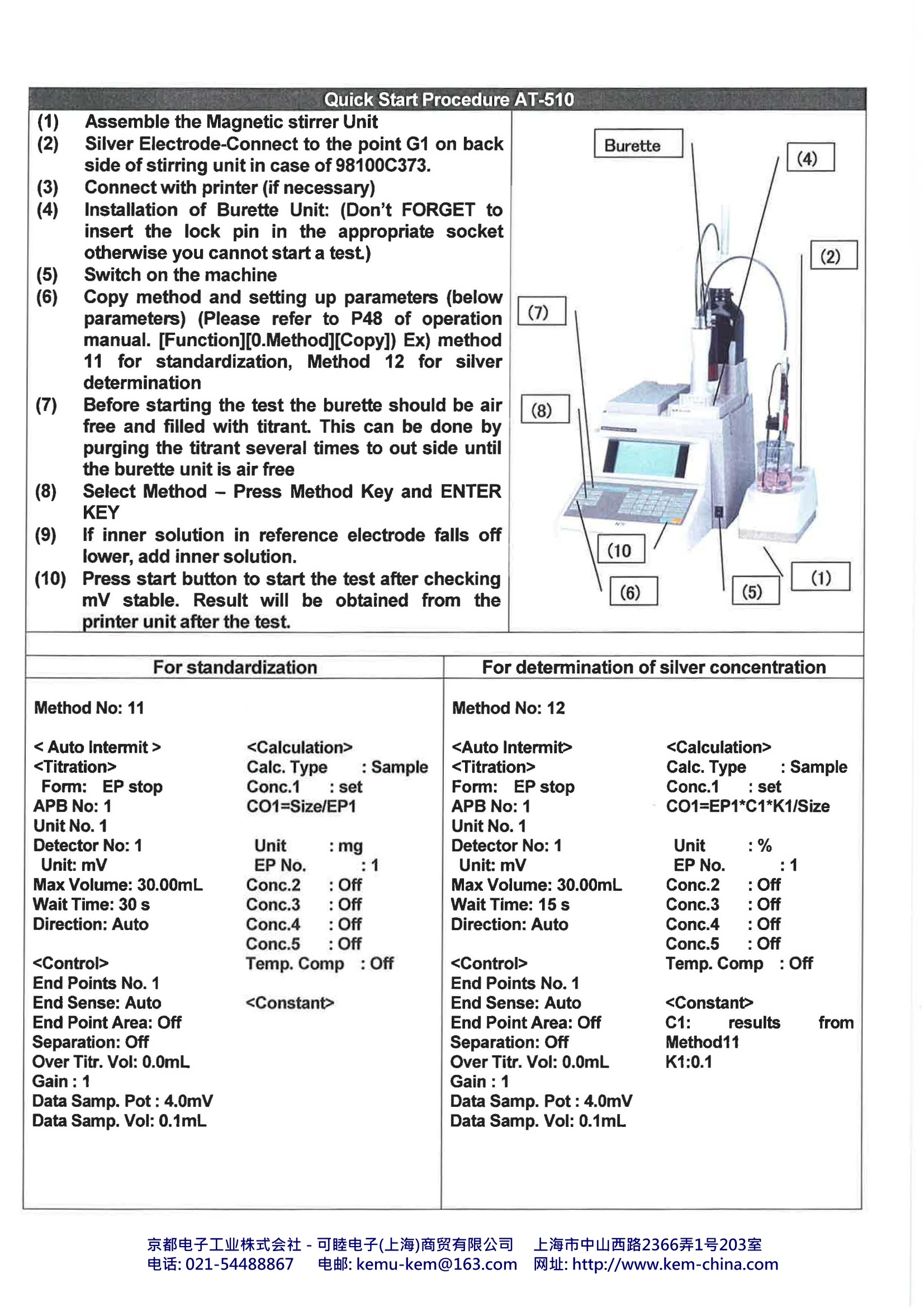
Automatic Potentiometric TitratorKyoto Electronics Manufacturing., Co.Ltd, Japan 1. What is Automatic Potentiometric Titrator?? The Automatic Potentiometric Titrator is an automated titration system as a method of chemicalanalysis, generally to obtain concentration of a specific material in a sample material. Goodexamples are shown in measurements of acidity and salinity of food products, neutralization value ofpetroleum products or chlorinity of tap water. 2. Industries The Automatic Potentiometric Titrator is used in a wide range of industry segments, particularlychemical, pharmaceutical and foods and beverage industry. It is mostly used for quality control attesting laboratory or QC unit for the purpose of determination of purity or concentration of liquid orsolid samples. Not only concentration of products but also of cleaning solvents is controlled,thus the AutomaticPotentiometric Titrator is widely used in production lines. The concentration of sample materials canbe measured by not just direct titration method but also density or refractive index measuredaccording to working curve. 3.Principle of Titration Titration goes on by controlling the power burette automatically from the potential differencebetween the electrodes until the endpoint (equivalent point) is reached. From the titratedvolume, concentration of the sample solution, for example, can be obtained.Typical substances are called acids which are hydrochloric acid, nitric acid and sulfuric acid. These acids change litmus paper from blue to red, and react with magnesium, thus generating hydrogen.The acid is ionized as below, and generates hydrogen ion (H*). For example, hydrochloric acidchanges: HCI→H*+cr The alkaline substance not only changes the color of litmus paper but reacts with acid, where theacid substance loses its own nature of acidity. Typical alkaline substances are sodium hydroxide,potassium hydroxide and calcium hydroxide. The alkaline substance is ionized as below, andgenerates hydroxide ion (OH). For example, sodium hydroxide changes: When NaOH solution is gradually added to HCl, the acidity fades away, ending up with a statuswhere both characters of acid and alkali are lost. This point is called neutralization. H*+OH→H2O Y0581 京都电子工业株式会社-可睦电子(上海)商贸有限公司 上海市中山西路2366弄1号203室 电话:021-54488867 电邮:kemu-kem@163.com网址: http://www.kem-china.com With respect to the number of ions of both hydrogen and hydroxide when neutralization iscomplete, both ions are found to be equal in number. The reaction of acid and alkali is called neutralization reaction,and the method for unknownconcentration by using neutralization to determine acid or alkali number is called neutralizationtitration. 4. Advantage to use Automatic Potentiometric Titrator The conventional manually operated titration used to determine the endpoint (equivalent point) bywatching the change of color of titration liquid using the indicator. The problem with such methodwas the difficulty in determination when colored titration liquid turned out precipitated or when anappropriate indicator was found unavailable. Also human error was inevitable due to the differentmethods or techniques used by operators. In order to solve the above-mentioned problems, the Automatic Potentiometric Titrator was theanswer. 5.Kyoto Electronics Mfg. Co., Ltd. lineup of Automatic Potentiometric Titrators and options AT-610 AT-510 AT-500N Detection range 12)1 Potentiometric: -2000mV-+2000mV2) pH : 0.00-14.00 pHTemperature : 0-100 degree Steps 20,000 steps (for a full stroke) Features High class model withtouch-screen Middle class model withtouch-screen LOW Model withtouch-screen Silver sample(%) 100% 50% 10% Sample Size(g) 0.015 0.03 0.15 Consumption(mL) 15 15 15 ISO 11427,ISO13756,JIS H6311 Ag*(sample)+CI (titrant)-> AgCl Models Calculation Formula Itisrequiredto establishthefollowingtwomethods according to JIS H6311. (A)For standardization of titrant (Sodium chloride standard solution)It is important that to get accuracy & repeatabilityare required to determine correct standardizationvalue of sodium chloride solution (titrant). Thisstandardization is necessary before doing sampledetermination. C1(mg)=G1(mg)/V1(mL) C1: mg of silver amount to react with 1mL sodiumchloride standard solution. C1 should be about10.786 mg. G1:Size(mg) of silver V1: Consumption volume of sodium chloridestandard solution(Titrant) (B) For determination of silver To determine purity of silver is done withprecipitation titration reaction between sodium chloride solution and after standardization. Ag(%)=EP(mL)*C1*K1(0.1)/ Size(g) EP(mL): Consumption volume of end pointC1: mg of silver amount to react with 1mL sodiumchloride standard solution(titrant)Size(g): sample size K1:100(%)/1000(mg/g)=0.1 Electrodes 98100C373 Combined silver electrode (Inner solution KNO3) (Alternative 98100M241,98100R272,cable) Chemicals For standardization Silver standard (powder)Nitric acid(1+1) Pure Water Sodium chloride Standard (powder) For determination of silverNitric acid(1+1) Pure Water 0.1 mol/L Sodium chloride solution (standardization) Remarks Sampling on balance should be done withaccuracy. The concentration of a titrant or the factorwill change not only with time but also whenmaking it up or replacing it with a fresh one.In addition, since a factor will affect themeasurement results by titration, it shouldbe routinely measured. A factor can bedetermine with a standard substance after purging a titrant in a titrator. (1) Assemble the Magnetic stirrer Unit (2) Silver Electrode-Connect to the point G1 on back side of stirring unit in case of 98100C373. (3) Connect with printer (if necessary)(4) Installation of Burette Unit: (Don’t FORGET toinsert the lock pin in the appropriate socketotherwise you cannot start a test.) (5) Switch on the machine(6) Copy method and setting up parameters (belowparameters) (Please refer to P48 of operationmanual. [Function][0.Method][Copy]) Ex) method11 for standardization, Method 12 for silverdetermination (7) Before starting the test the burette should be airfree and filled with titrant. This can be done bypurging the titrant several times to out side until the burette unit is air free (8) Select Method - Press Method Key and ENTERKEY (9) If inner solution in reference electrode falls offlower, add inner solution. (10)Press start button to start the test after checkingmV stable. Result will be obtained from the printer unit after the test. For standardization For determination of silver concentration Method No: 11 Calc. Type :Sample Form: EP stop Conc.1 : set APB No: 1 CO1=Size/EP1 Unit No.1 Detector No: 1 Unit :mg Unit: mV EP No. :1 Max Volume: 30.00mL Conc.2 : Off Wait Time: 30 s Conc.3 : Off Direction: Auto Conc.4 : Off Conc.5 : Off Temp. Comp: Off End Points No. 1 End Sense: Auto End Point Area: Off Separation: Off Over Titr. Vol: 0.0mL Gain : 1 Data Samp. Pot: 4.0mV Data Samp. Vol: 0.1mL Method No: 12 Form: EP stop Calc. Type : SampleConc.1 : set APB No: 1 CO1=EP1*C1*K1/Size Unit No. 1 Detector No: 1 Unit :% Unit: mV EP No. :1 Max Volume: 30.00mL Conc.2 : Off Wait Time: 15 s Conc.3 : Off Direction: Auto Conc.4 : Off Conc.5 : Off Temp. Comp : Off End Points No. 1 End Sense: Auto End Point Area: Off C1: results from Separation: Off Method11 Over Titr. Vol: 0.0mL K1:0.1 Gain : 1 Data Samp. Pot: 4.0mV Data Samp. Vol:0.1mL 京都电子工业株式会社-可睦电子(上海)商贸有限公司 上海市中山西路电话: 电邮: kemu-kem@com网址: http://www.kem-china.com
确定

还剩2页未读,是否继续阅读?
可睦电子(上海)商贸有限公司-日本京都电子(KEM)为您提供《银合金首饰中银检测方案(自动电位滴定)》,该方案主要用于珠宝/玉石中银检测,参考标准--,《银合金首饰中银检测方案(自动电位滴定)》用到的仪器有AT-710B简易实用型自动电位滴定仪、AT-710S豪华型自动电位滴定仪、AT-710M四通道旗舰型自动电位滴定仪
推荐专场
相关方案
更多