
石膏墙板也叫石膏板、灰板或干墙(drywall)。最近关于“中国进口的石膏墙板质量纠纷”在美愈演愈烈。美国消费品安全委员会说,已经接到大约 1500个投诉报告,涉及数十亿美元的赔偿投诉。投诉者称,他们所居住新房子上的中国制石膏墙板散发出类似臭鸡蛋味儿的含硫气体,腐蚀电缆铜线和金属管 道,并且时常使人出现头痛、喉咙痛等症状。为此,美国太平洋大学的太平洋质谱实验室受委托应急检测了中美两国生产的石膏板,分析采用DART和精确质量 TOF质谱,TOF分辨率为7000。样品未经萃取或任何化学前处理,直接放置于DART源和质谱接口处。结果显示,在室温下,美国和中国生产的石膏板没 有显著区别,都没有含硫化合物离子存在。当DART快速升温至250℃时,中国产石膏板明显释放出臭味。质谱鉴定,中国石膏板含有的硫化物是美国产品的 46倍。其中含有的组分包括 S2-(63.9440 Da)、 SO2-(63.9615 Da)、 SO3-、S2O-、S3-、 S4-等。
方案详情

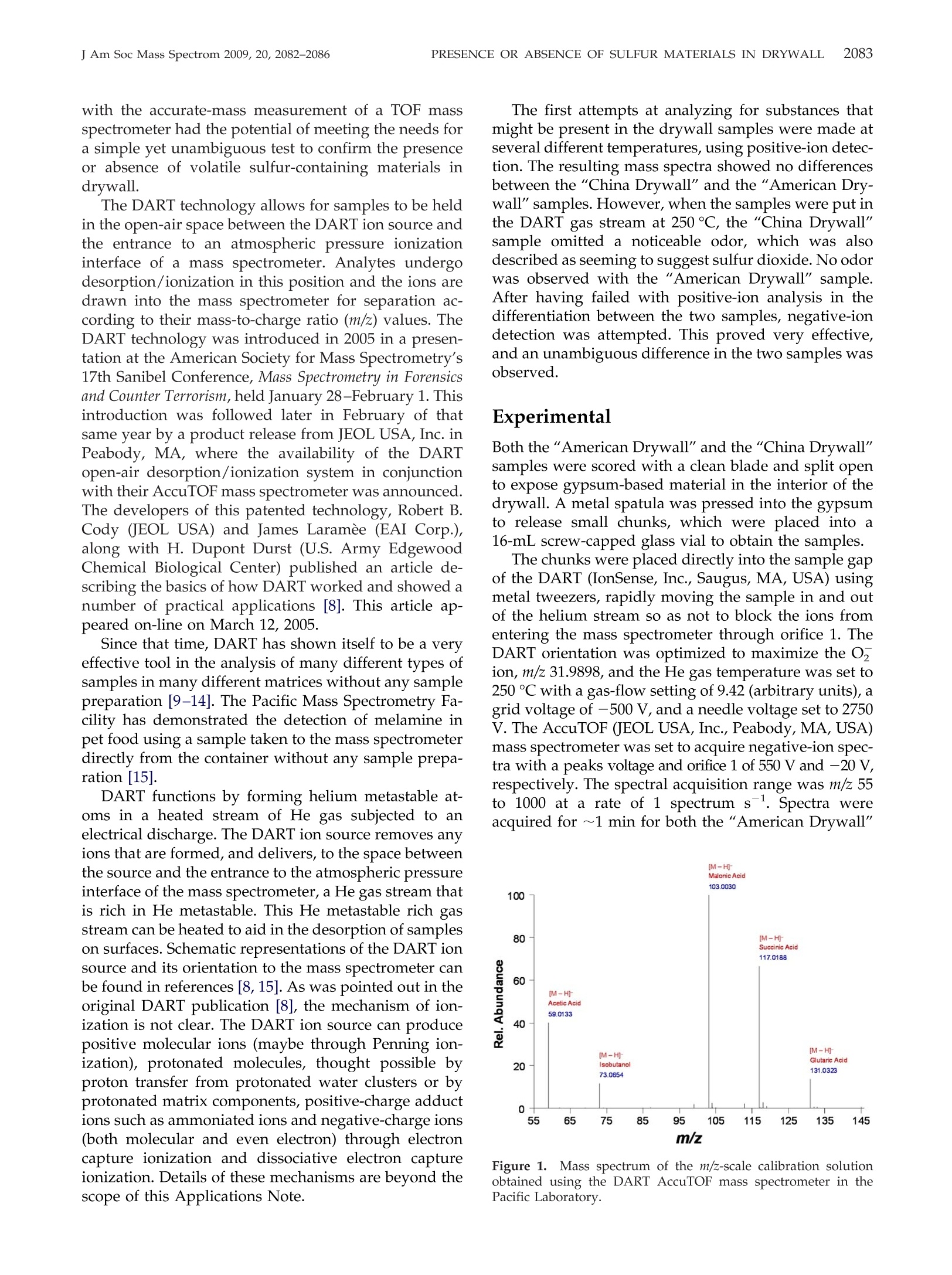
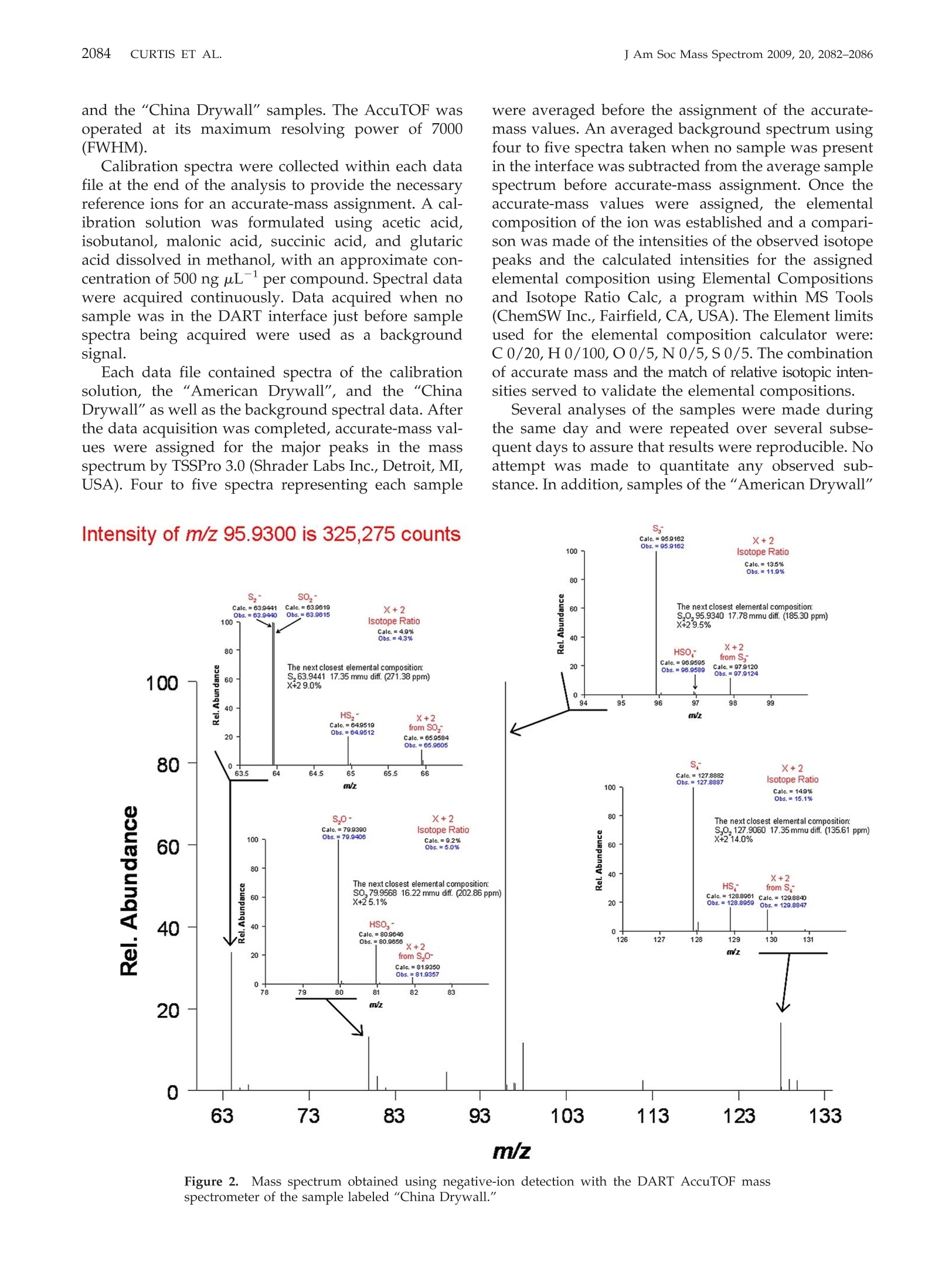
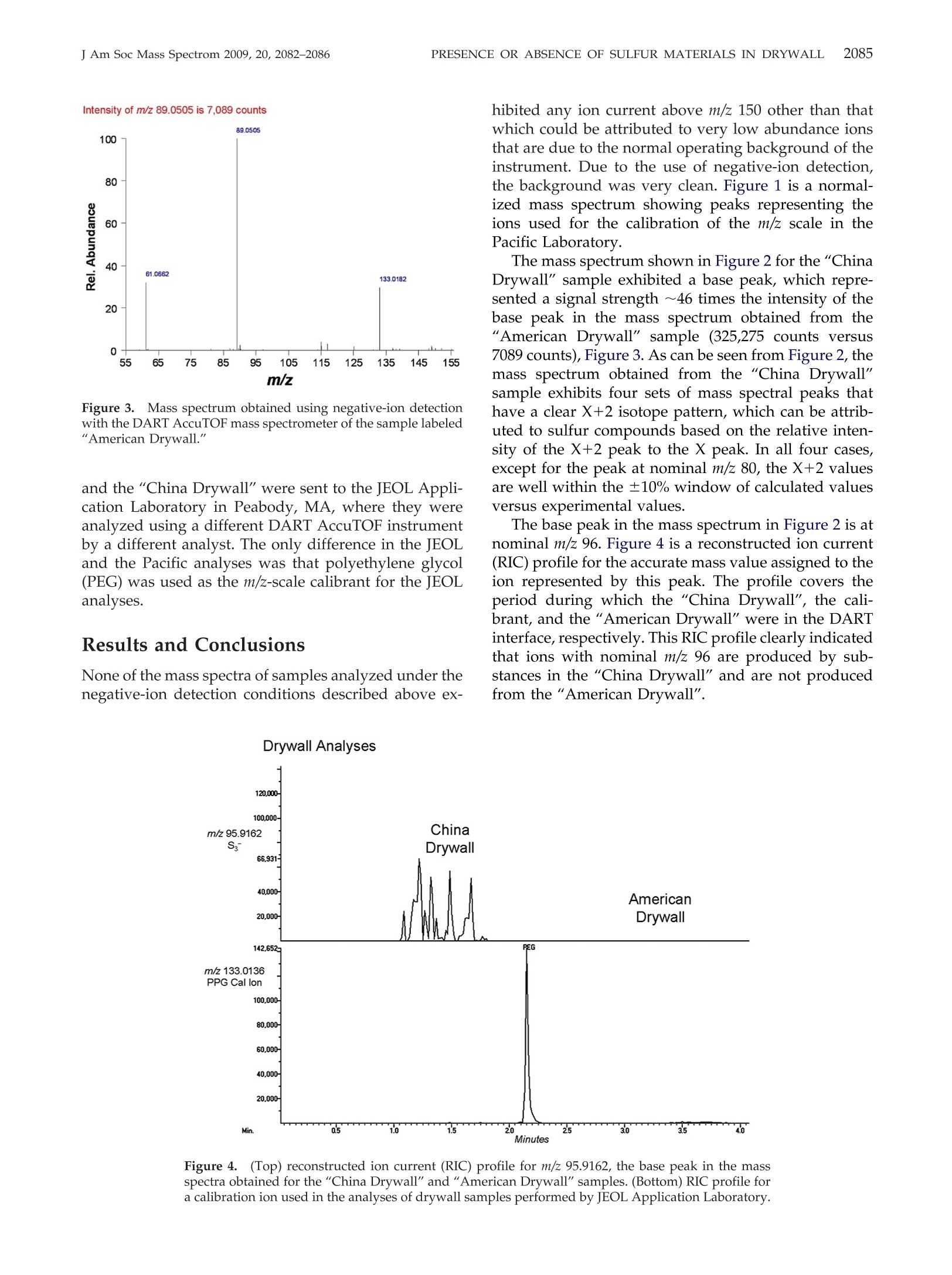
APPLICATION NOTE 2083PRESENCE OR ABSENCE OF SULFUR MATERIALS IN DRYWALLJ Am Soc Mass Spectrom 2009, 20, 2082-2086 Published online July 19, 2009Received June 15, 2009Revised July 10, 2009Accepted July 13, 2009doi:10.1016/j.jasms.2009.07.012 Determination of the Presence or Absence ofSulfur Materials in Drywall Using DirectAnalysis in Real Time in Conjunction with anAccurate-Mass Time-of-Flight Mass Spectrometer Matthew E. Curtis, Patrick R. Jones, O. David Sparkman, andRobert B. Cody Pacific Mass Spectrometry Facility, Department of Chemistry, College of the Pacific, University of thePacific, Stockton, California, USA"JEOL USA, Inc., Peabody, Massachusetts, USA Based on the concern about the presence of sulfur materials being in drywall (wallboard),a quick and reliable test to confirm the presence or absence of these materials using directanalysis in real time (DART) mass spectrometry in conjunction with an accurate-masstime-of-flight (TOF) mass spectrometer has been developed and is described here. (J AmSoc Mass Spectrom 2009, 20, 2082-2086) O 2009 American Society for Mass Spectrometry n recent weeks, there have been a number of reportsin the legitimate press about problems homeowners_have been having with what is believed to beemission from drywall that was reported to be manu-factured in China [1-7]. Various tests have been per-formed, such as the one conducted by Unified Engi-neering, Inc., Aurora, IL, for the Florida Department ofHealth, Division of Environmental Health, and re-ported on March 17, 2009. All of these tests have beenless than conclusive. The Unified Engineering reportdid state that hydrogen sulfide, carbonyl sulfide, andcarbon disulfide were detected at levels between 100and 1 ppm with a GC-AED (gas chromatograph-atomicemission detector) headspace analysis technique ap-plied to samples of"bulk drywall material with theoutside papers removed" after a 24-h exposure to 95%humidity followed by setting a sample in a septum vialcontaining dry nitrogen for another 24 h. It was alsoreported by Unified Engineering that there was "adistinct sulfur odor from all three Chinese drywallsamples." The Pacific Mass Spectrometry Facility was con-tacted by an East Coast developer looking for a quick,simple, and inexpensive test that could conclusivelydetermine if there was a potential problem based on thepresence of sulfur or sulfur-containing compounds. Theconcern was based on analytical tests that had beenconducted on reported odors observed by homeownersand investigators, and the observation of copper mate-rials that had turned black in the homes. These tests ( Address r e print requests to Dr. O. D. Sparkman, Department of Chemistry, P acific Mass Spectrometry Facility, U n iversity of the Pa c ific, 3 6 01 Pacific Ave., Stockton, CA 95211, USA. E-mail: osparkman@pacific.edu ) supported that the probable source of problems wassome sulfur-containing material in the drywall material. The Pacific Mass Spectrometry Facility was providedwith drywall samples labeled“American Drywall" and"China Drywall". Both samples were identified bylabels written on one of the two paper coverings with agreen felt-tip pen. The “American Drywall" samplemeasured 18 cm × 11.5 cm × 1.7 cm (slightly greaterthan 5/8 in. thick). The “China Drywall" sample mea-sured 13.3 cm x 11 cm x 1.4 cm (slightly greater than1/2 in. thick. The contractor sent the 5/8-in. AmericanDrywall sample because no 5/8-in. drywall had beenimported from China. After removing some of the ma-terial between the paper coverings comprising the out-side covers of the drywall, it was observed that thematerial from the "China Drywall" sample was a grayish-brown color and had a much coarser grain than the"American Drywall" material. The “American Dry-wall" material was almost a pure white in color. Sam-ples of the material removed from between the surfacecoverings were placed in 16-mL glass vials with Teflon-lined caps and allowed to stand tightly sealed overnightat room temperature. There was no special effort tochange the air in the vials to an inert gas. The next day,when each vial was opened, the "China Drywall"sample emitted a distinct sulfur odor that could best bedescribed as that of sulfur dioxide. This odor was notpresent from the vial containing the "American Dry-wall" material. Direct analysis in real time (DART), in conjunctionwith accurate-mass mass spectrometry, has been usedfor the determination of many analytes in complexmatrices without any extraction or other sample prep-aration. Combining the speed of sampling by DART ( @ 2009 American Society f o r Mass Spectrometry. P u blished b y Elsevier Inc.1044-0305/09/$32.00 ) with the accurate-mass measurement of a TOF massspectrometer had the potential of meeting the needs fora simple yet unambiguous test to confirm the presenceor absence of volatile sulfur-containing materials indrywall. The DART technology allows for samples to be heldin the open-air space between the DART ion source andthe entrance to an atmospheric pressure ionizationinterface of a mass spectrometer. Analytes undergodesorption/ionization in this position and the ions aredrawn into the mass spectrometer for separation ac-cording to their mass-to-charge ratio (m/z) values. TheDART technology was introduced in 2005 in a presen-tation at the American Society for Mass Spectrometry's17th Sanibel Conference, Mass Spectrometry in Forensicsand Counter Terrorism, held January 28-February 1. Thisintroduction was followed later in February of thatsame year by a product release from JEOL USA, Inc. inPeabody, MA, where the availability of the DARTopen-air desorption/ionization system in conjunctionwith their AccuTOF mass spectrometer was announced.The developers of this patented technology, Robert B.Cody (JEOL USA) and James Laramee (EAI Corp.),along with H. Dupont Durst (U.S. Army EdgewoodChemical Biological Center) published an article de-scribing the basics of how DART worked and showed anumber of practical applications [8]. This article ap-peared on-line on March 12, 2005. Since that time, DART has shown itself to be a veryeffective tool in the analysis of many different types ofsamples in many different matrices without any samplepreparation [9-14]. The Pacific Mass Spectrometry Fa-cility has demonstrated the detection of melamine inpet food using a sample taken to the mass spectrometerdirectly from the container without any sample prepa-ration [15]. DART functions by forming helium metastable at-oms in a heated stream of He gas subjected to anelectrical discharge. The DART ion source removes anyions that are formed, and delivers, to the space betweenthe source and the entrance to the atmospheric pressureinterface of the mass spectrometer, a He gas stream thatis rich in He metastable. This He metastable rich gasstream can be heated to aid in the desorption of sampleson surfaces. Schematic representations of the DART ionsource and its orientation to the mass spectrometer canbe found in references [8, 15]. As was pointed out in theoriginal DART publication [8], the mechanism of ion-ization is not clear. The DART ion source can producepositive molecular ions (maybe through Penning ion-ization), protonated molecules, thought possible byproton transfer from protonated water clusters or byprotonated matrix components, positive-charge adductions such as ammoniated ions and negative-charge ions(both molecular and even electron) through electroncapture ionization and dissociative electron captureionization. Details of these mechanisms are beyond thescope of this Applications Note. The first attempts at analyzing for substances thatmight be present in the drywall samples were made atseveral different temperatures, using positive-ion detec-tion. The resulting mass spectra showed no differencesbetween the "China Drywall" and the "American Dry-wall" samples. However, when the samples were put inthe DART gas stream at 250 ℃, the "China Drywall"sample omitted a noticeable odor, which was alsodescribed as seeming to suggest sulfur dioxide. No odorwas observed with the "American Drywall" sample.After having failed with positive-ion analysis in thedifferentiation between the two samples, negative-iondetection was attempted. This proved very effective,and an unambiguous difference in the two samples wasobserved. Experimental Both the "American Drywall" and the "China Drywall"samples were scored with a clean blade and split opento expose gypsum-based material in the interior of thedrywall. A metal spatula was pressed into the gypsumto release small chunks, which were placed into a16-mL screw-capped glass via to obtain the samples. The chunks were placed directly into the sample gapof the DART (IonSense, Inc., Saugus, MA, USA) usingmetal tweezers, rapidly moving the sample in and outof the helium stream so as not to block the ions fromentering the mass spectrometer through orifice 1. TheDART orientation was optimized to maximize the O,ion, m/z 31.9898, and the He gas temperature was set to250 ℃ with a gas-flow setting of 9.42 (arbitrary units), agrid voltage of -500 V, and a needle voltage set to 2750V. The AccuTOF (JEOL USA, Inc., Peabody,MA, USA)mass spectrometer was set to acquire negative-ion spec-tra with a peaks voltage and orifice 1 of 550 V and-20V,respectively. The spectral acquisition range was m/z 55to 1000 at a rate of 1 spectrum s.Spectra wereacquired for ~1 min for both the “American Drywall" Figure 1. Mass spectrum of the m/z-scale calibration solutionobtained using the DART AccuTOF mass spectrometer in thePacific Laboratory. and the "China Drywall" samples. The AccuTOF wasoperated at its maximum resolving power of 7000(FWHM). Calibration spectra were collected within each datafile at the end of the analysis to provide the necessaryreference ions for an accurate-mass assignment. A cal-ibration solution was formulated using acetic acid,isobutanol, malonic acid, succinic acid, and glutaricacid dissolved in methanol, with an approximate con-centration of 500 ng uL-per compound. Spectral datawere acquired continuously. Data acquired when nosample was in the DART interface just before samplespectra being acquired were used as a backgroundsignal. Each data file contained spectra of the calibrationsolution, the “American Drywall", and the “ChinaDrywall" as well as the background spectral data. Afterthe data acquisition was completed,accurate-mass val-ues were assigned for the major peaks in the massspectrum by TSSPro 3.0 (Shrader Labs Inc., Detroit, MI,USA). Four to five spectra representing each sample m/z Figure 2.Mass spectrum obtained using negative-ion detection with the DART AccuTOF massspectrometer of the sample labeled "China Drywall." Intensity of m/z 89.0505 is 7,089 counts Figure 3. Mass spectrum obtained using negative-ion detectionwith the DART AccuTOF mass spectrometer of the sample labeled"American Drywall." and the "China Drywall" were sent to the JEOL Appli-cation Laboratory in Peabody,MA, where they wereanalyzed using a different DART AccuTOF instrumentby a different analyst. The only difference in the JEOLand the Pacific analyses was that polyethylene glycol(PEG) was used as the m/z-scale calibrant for the JEOLanalyses. Results and Conclusions None of the mass spectra of samples analyzed under thenegative-ion detection conditions described above ex- hibited any ion current above m/z 150 other than thatwhich could be attributed to very low abundance ionsthat are due to the normal operating background of theinstrument. Due to the use of negative-ion detection,the background was very clean. Figure 1 is a normal-ized mass spectrum showing peaks representing theions used for the calibration of the m/z scale in thePacific Laboratory. The mass spectrum shown in Figure 2 for the "ChinaDrywall" sample exhibited a base peak, which repre-sented a signal strength ~46 times the intensity of thebase peak in the mass spectrum obtained from the“American Drywall" sample (325,275 counts versus7089 counts), Figure 3. As can be seen from Figure 2, themass spectrum obtained from the “China Drywall"sample exhibits four sets of mass spectral peaks thathave a clear X+2 isotope pattern, which can be attrib-uted to sulfur compounds based on the relative inten-sity of the X+2 peak to the X peak. In all four cases,except for the peak at nominal m/z 80, the X+2 valuesare well within the ±10% window of calculated valuesversus experimental values. The base peak in the mass spectrum in Figure 2 is atnominal m/z 96. Figure 4 is a reconstructed ion current(RIC) profile for the accurate mass value assigned to theion represented by this peak. The profile covers theperiod during which the "China Drywall", the cali-brant, and the"American Drywall"were in the DARTinterface, respectively. This RIC profile clearly indicatedthat ions with nominal m/z 96 are produced by sub-stances in the "China Drywall" and are not producedfrom the “American Drywall". Figure 4.(Top) reconstructed ion current (RIC) profile for m/z 95.9162, the base peak in the massspectra obtained for the "China Drywall" and"American Drywall" samples.(Bottom) RIC profile fora calibration ion used in the analyses of drywall samples performed by JEOL Application Laboratory. The peak at nominal m/z 64 is a resolved doubletrepresenting Sz (63.9440 Da) and SOz (63.9615 Da) ions;a respective difference between measured and calcu-lated masses of 0.1 and 0 mmu). The peak at nominalm/z 65 not only represents the S, ion where one of theSatoms is 33s, and the SO, ion where the S is 34s, or oneof the O atoms is O; but based on the measured mass,it mainly represents the HS, ion. This is supported bythe doublet at nominal m/z 66. The peak at nominal m/z 80 is an unresolved doubletrepresenting SO, and S,O .Even though the differencein the calculated mass of the S,Oion and the measuredvalue for the peaks supports this elemental composi-tion, the X+2 intensity supports the SOs composition(calculated relative intensity of 9.2% compared with anobserved value of 5.0%). Both the agreement between the calculated andmeasured mass for the Ss (zero difference) and theagreement between the calculated and observed valuesfor the X+2 intensity (11.9% observed versus 13.5%calculated) for the peak at nominal m/z 96 support thiscomposition. It is also seen from the resolved doubletassociated with the peak with nominal m/z 97 thatHSO is formed. The calculated and measured massesassociated with series of peaks with nominal m/z 128,129, and 130 support elemental compositions of S andHS4. Based on the mass spectra, clearly some substance inthe "China Drywall" produces ions that contain atomsof sulfur. Based on the RIC profile for the ion withnominal m/z 96 and the mass spectra, clearly these ionsare not produced by the "American Drywall" whensubjected to the same analytical conditions using theDART AccuTOF mass spectrometer. This mass spectro-metric method can be used to confirm whether a drywall sample contains substances that will emit ionscontaining sulfur. References 1. Corkery, M. Homeowner Problems with Chinese-Made Drywall Spread; TheWall Street Journal: New York, April 17,2009,Real Estate, HomeownerProblems with Chinese-Made Drywall Spread-WSJ.com. 2. Skoloff, B. Chinese Drywall Poses Potential Risks; AP IMPACT: Saturday,April 11, 2009 11:30 AM EDT. ( 3. S 1 k oloff, B. F lorida t o te s t air i n homes with Chine s e drywall; USA T o day: h ttp://www.usatodayeconomy/h o u s ing/2009-04-17-chi n ese-drywall _ N.htm. ) ( .4 . . Schmit, J . Drywall from China blamed for problems in homes ; USA Today: Updated 3/17/2009 3:20 PM. ) ( 5. N orfolk v otes to ban Chinese drywall; USA Today: Posted 5/19/2009 6 :45 PM ET. ) ( 6. Thorner, J . F l orida b u ilders b r ace for suits over od o r-emitting Chinese drywall; St . Peterburg Times: Saturday M arch 2 1 , 2009, http://www . t am p aba y .com/n e ws/business / real e st at e / artic l e985827.ece. ) ( 7. B radley, L . U.S. Hearings Open Today on Chinese Drywall: Another I m portMade with Reckless Abandon; USA Today: May 21, 2009 5:00 AM. ) 8. Cody, R. B.; Laramee,J. A.; DuPont Durst, H. Versatile New Ion Sourcefor the Analysis of Materials in Open Air under Ambient Conditions.Anal. Chem. 2005,77(8),2297-2302. ( 9 . Haefliger, O. P.; Jeckelmann, N. Direct Mass Spectrometric Analysis of Flavors and Fragrances in Real Applications Using DART. R apid Com- m un. Mass Spectrom. 2 0 07, 21, 1361-1366. ) ( 10. Kpegba, K.; S padaro, T.; Cody, R. B .; Nesnas, N. ; Olson, J . A. Analysis of Self-Assembled Monolayers on Gold Surfaces U sing D irect Analysisin Real Time Mass Spectrometry. Anal. C h em. 2007, 79,5479-5483. ) ( 11. Madhusudanan, K. P. ; Banerjee, S . ; Khanuja, S . P. S .; Chattopadhyay, S. K. Analysis of Hairy Root Culture of Rauvolfia serpentina Using DirectAnalysis in Real Time Mass Spectrometric T echnique. B iomed. Chro- matogr. 2008, 22, 596-600. ) 12. Morlock, G.; Ueda, Y. New Coupling of Planar Chromatography withDirect Analysis in Real Time Mass Spectrometry. J. Chromatogr. A 2007,1143,243-251. ( 1 3. Petucci, C.; Diffendal, J. ; Kaufman, D.; Mekonnen, B.; T e refenko, G.; Musselman, B. Direct Analysis in R eal Time for Reaction Monitoring in I D r ug Discovery. A nal. Chem. 2007, 79, 5 064-5070. ) ( 14. Grange, A. H.; Sovocool, G. W. Automated D e termination of Precursor Ion, Product Ion, and Neutral Loss Compositions and D econvolution ofComposite M a ss Spectra U s ing Ion C o rrelation Based on E x act M a sses and Relative Isotopic Abundances. Rapid Commun. Mass Spectrom. 2 0 08, 2 2,2375-2390. ) 15. Vail, T. M.; Sparkman, O. D.; Jones, P. R. Rapid and UnambiguousIdentification of Melamine in Contaminated Pet Food Based on MassSpectrometry with Four Degrees of Confirmation. J. Anal. Toxicol. 2007,31,304-312.
确定


还剩3页未读,是否继续阅读?
华质泰科生物技术(北京)有限公司为您提供《石膏板中硫化物检测方案(液质联用仪)》,该方案主要用于砖/瓦/石材中硫化物检测,参考标准--,《石膏板中硫化物检测方案(液质联用仪)》用到的仪器有
相关方案
更多







