方案详情
文
本文建立了用超临界萃取提取牛肝中的β-agonists兴奋剂的方法.此方法适用于不同的β-agonists激动剂类别.如:替代苯胺类(β肾上腺素受体激动剂clenbuterol)和酚类(β肾上腺素受体激动剂salbutamol).开发的实验采用超临界萃取和酶免疫测定技术,可以检测ppb级的激动剂的残留. 添加一定的甲醇改性剂和样品的除水对于极性比较强的salbutamol的萃取是必要的.实验结果表明了很好的回收率和低的误差.
方案详情

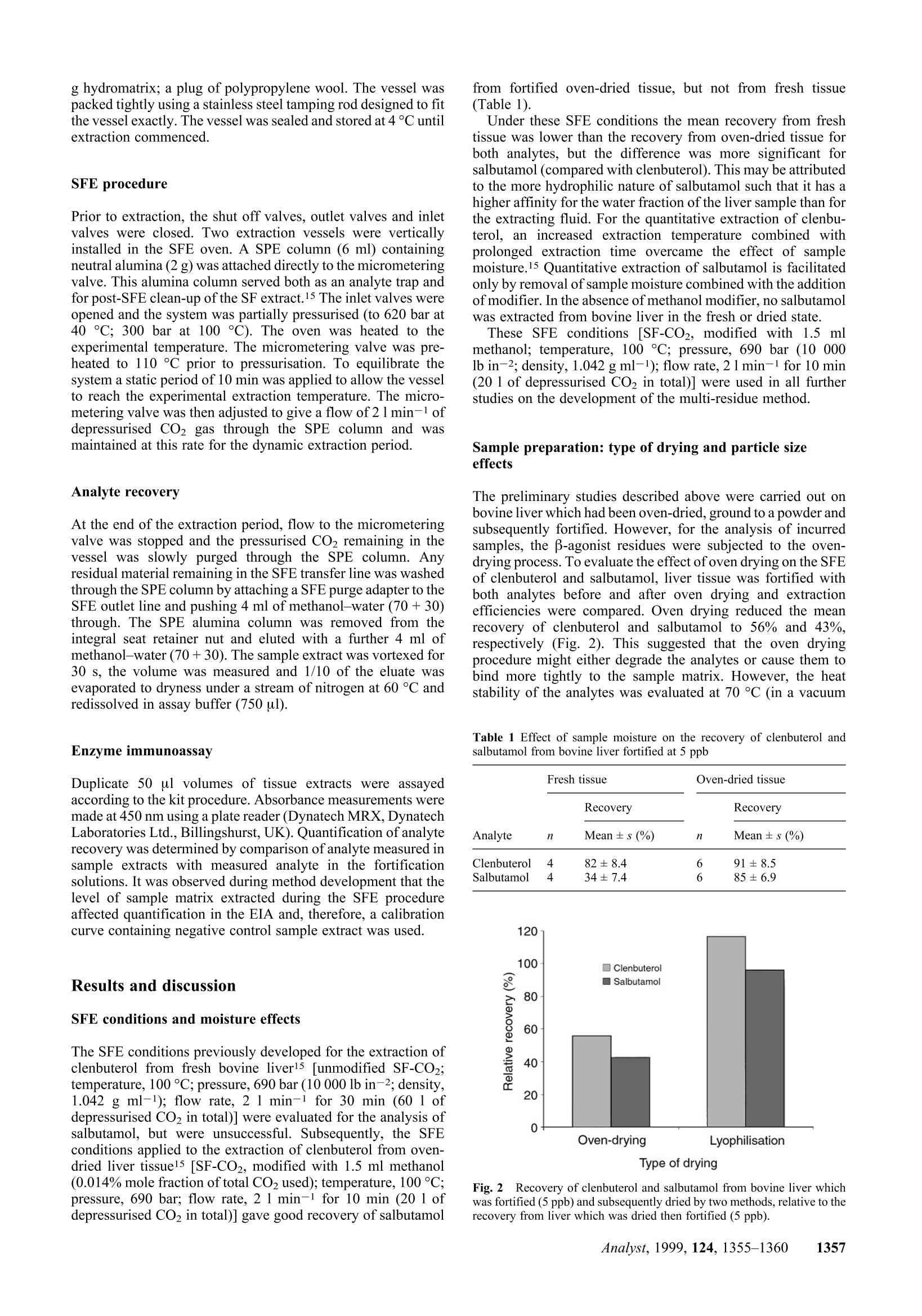
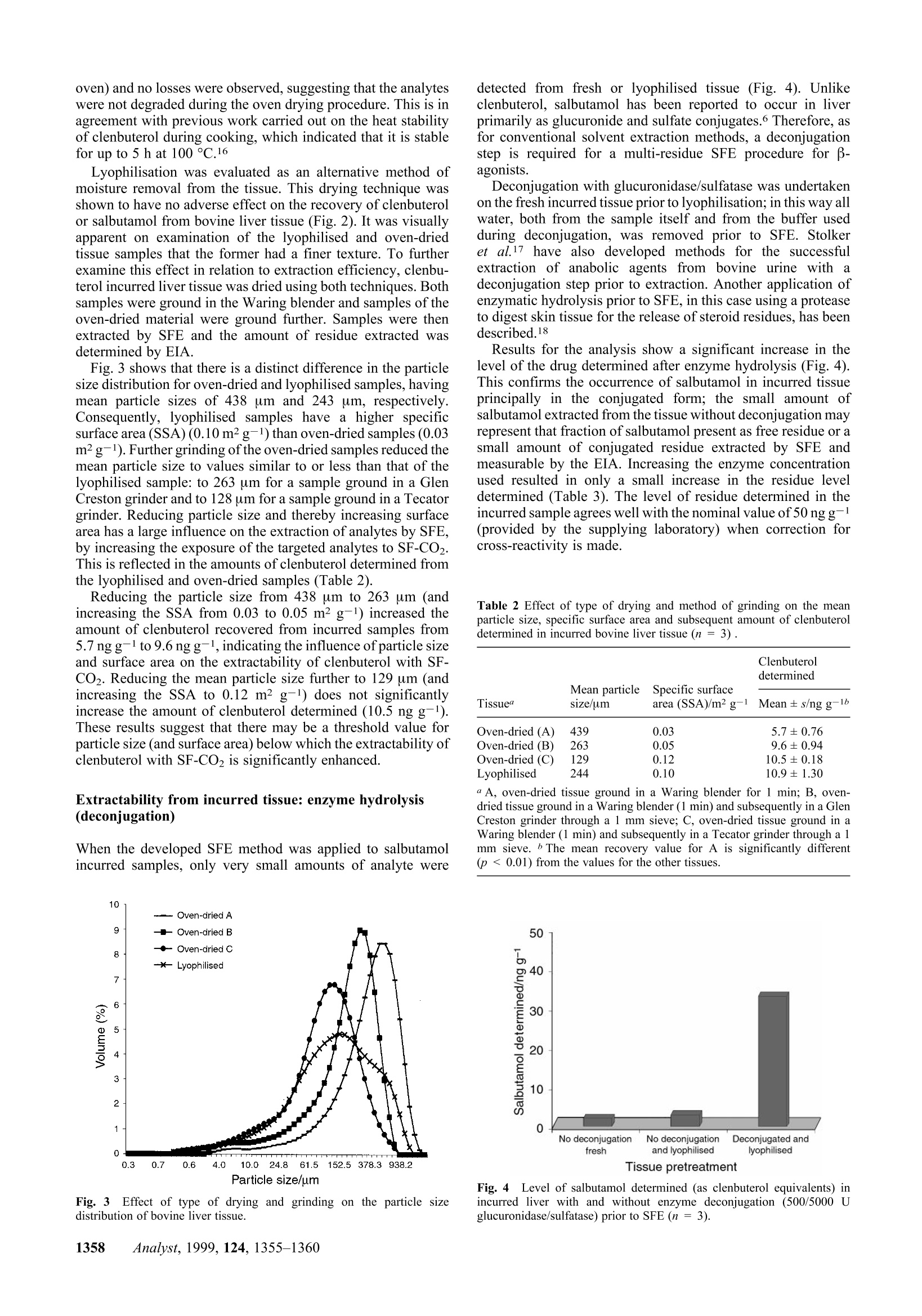
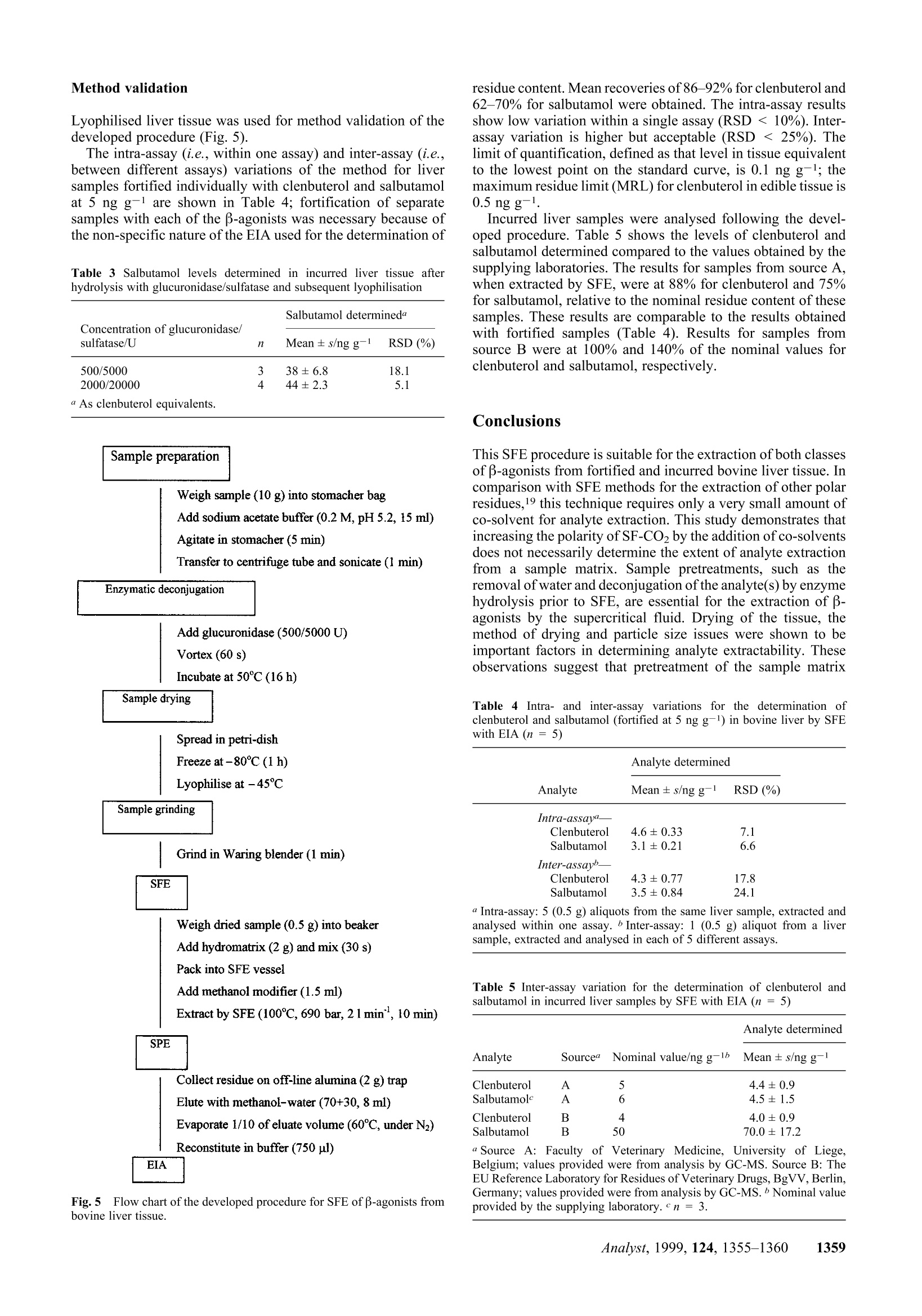
Method validation Supercritical fluid extraction (SFE) as a multi-residueextraction procedure for B-agonists in bovine liver tissue Mandy J.O'Keeffe, Michael O'Keeffe*a and Jeremy D. Glennon a Teagasc, The National Food Centre, Dunsinea, Castleknock,Dublin 15, Irelandb Department of Chemistry, University College Cork, Ireland Received 6th April 1999, Accepted 2nd July 1999 A supercritical fluid extraction procedure has been developed for the extraction of β-agonists in bovine liversamples. The method is suitable for compounds of different B-agonist classes: the substituted aniline-typecompounds (e.g. clenbuterol) and the phenolic-type compounds (e.g. salbutamol),including conjugated forms ofthe latter. The developed procedure involves a combination of supercritical fluid extraction with enzymeimmunoassay for the determination of clenbuterol and salbutamol residues at the low ppb level. Addition ofmethanol modifier and removal of sample moisture are necessary for the extraction of more polar analytes such assalbutamol. Method validation incorporating intra- and inter-assays was carried out on fortified liver tissue andshowed good recovery and low variation (RSD <15%). An enzyme hydrolysis procedure was incorporated intothe method for the deconjugation of conjugated residues. The developed procedure was shown to be successful forthe determination of both clenbuterol and salbutamol in incurred liver tissue. Introduction Supercritical fluid extraction (SFE) is finding wide acceptancein a number of analytical disciplines as a unique extractiontechnique due to inherent properties such as the avoidance oflarge quantities of organic solvents, speed of extraction and thecapability to manipulate solvent strength. Supercritical carbondioxide (SF-CO2) is the most widely used supercritical solvent,and in the area of residue analysis has been successfully appliedto the extraction of relatively non-polar analytes, such aspesticides,1,2 herbicides, and fungicides4 from a range ofmatrices. However, the most important limitation of SF-CO2 isthat its polarity is often too low to obtain efficient extraction,either because the analytes lack sufficient solubility or becausethe extractant has a poor ability to displace the analytes fromactive matrix sites. For the extraction of veterinary drugresidues and growth promoting agents, the polarity within aclass of compounds can vary over a wide range,thus limitingthe extraction of one or more of these analytes by SF-CO2. B-Agonists are synthetic derivatives of the naturally occur-ring catecholamines. Clenbuterol is the only B-agonist licensedfor the therapeutic treatment of respiratory conditions inanimals (with a maximum residue limit in edible tissue of 0.5ng g-1). At multiples of the recommended therapeutic dose, β-agonists are illegally used as growth promoting agents. Theyfunction as repartitioning agents by shifting the flow ofnutrients towards the production of muscle and away from theproduction of fat. The net result is the production of a leaneranimal carcass.5 There are two main classes of B-agonists: the substitutedanilines, which include clenbuterol and cimaterol, and thesubstituted phenols, which include salbutamol and terbutaline.These compounds possess a common β-hydroxyamino groupon the side chain, but are differentiated from each other byvaried substituents on the aryl moiety (Fig.1). The hydroxy andalkylhydroxy group(s) on the aromatic ring of the substitutedphenol type increase the polarity of the molecule and render itless extractable by SF-CO2. These group(s) are also a target forglucuronide or sulfate conjugate formation. Effective conventional methods for the analysis of thesecompounds in biological tissues have incorporated procedures such as immunoaffinity chromatography, matrix solid phasedispersion8 or solid phase extraction° (SPE) which are efficientenough to extract both groups of compounds. Detectionsystems, such as immunoassays |[radioimmunoassay7(RIA) andenzyme immunoassay10 (EIA)] or gas chromatography-massspectrometryl1 (GC-MS), are sensitive enough to detect thesecompounds at the low (ppb) levels required for the stringentmonitoring of their illegal use. The extraction of some polar analytes by SFE may beachieved by incorporating small amounts of polar organicsolvents, called modifiers or co-solvents, into the primaryfluid.12,13 The modifier exerts its effect in two ways: byinteracting with the analyte/matrix complex to promote rapiddesorption into the supercritical fluid and by enhancing thesolubility properties of SF-CO2. Lyophilisation has been usedextensively in analytical SFE of drug residues as a means toenable a larger sample size to be analysed by SFE, but moreimportantly to remove moisture from samples and to facilitateextraction.14 The purpose of this work was to develop an SFE proceduresuitable for the quantitative extraction of β-agonists from liversamples. Data on the recovery ofclenbuterol and salbutamol arepresented. This paper highlights the importance of modifiers forthe extraction of more polar analytes, such as salbutamol. Theeffect of sample moisture and of methods of moisture removalfrom tissue on analyte extractability is also examined. Theeffect of particle size on analyte extractability is illustrated. Fig.1 Structures of B-agonists B-Agonist R1 R2 R3 R4 Clenbuterol -CI -NHz -H -C(CH3)3 Salbutamol -CH2OH -OH -H -C(CH3)3 Cimaterol -CN -NH, -H -CH(CH3)2 Terbutaline -OH -H -OH -C(CH3)3 B-Agonists form conjugates which, being more polar than thefree residues, are therefore less extractable by SF-CO2. Asuitable deconjugation step is required for successful extractionby SF-CO2. The development of a hydrolysis step, followed byan efficient SFE procedure and its validation with fortified andincurred tissue are presented. The residues of B-agonists in thetissue extracts were determined by EIA. Experimental Supercritical fluid extraction system A Spe-ed 680 bar SFE extractor (Applied Separations Inc..Allentown, PA, USA) was used in these studies. Thisinstrument was configured for the parallel extraction of twoSFE vessels. The extraction vessels used (24 ml volume) wereconstructed of high pressure stainless steel and were capable ofwithstanding pressures up to 690 bar. The extraction vesselswere connected to the system using hand-tightenable slip-freeconnectors. The restrictors used were micrometering valvesencased in an aluminium block fitted with a heater and athermocouple. A commercial SPE cartridge (6 ml) was attacheddirectly to the micrometering valve using a seat retainer columnnut connected to the valve itself. A Floline SEF-51 flowmetertotalizer (Horiba, Sunnyvale, CA, USA) was used to measurethe flow of expanded CO2 gas. Materials Hydromatrix (Celite 566),polypropylene wool (filtration grade)and neutral alumina sorbent (Brockmann Grade 1, mesh 150)were obtained from Aldrich (Poole, Dorset, UK). Empty 6 mlSPE reservoir columns were supplied by Jones Chromatog-raphy (Hengoed, Mid-Glamorgan, UK). Foodd grade CO2(99.95% pure, liquid offtake with a diptube) was purchasedfrom Air Products (Basingstoke, UK). HPLC grade methanolwas obtained from Merck (Darmstadt, Germany). The EIA kitused for the determination of B-agonists was obtained fromLaboratoire d’Hormonologie (Marloie, Belgium) with anti-serum which had been raised against salbutamol and hadrelative cross-reactivity values for clenbuterol of 100% andsalbutamol of87.5%. The calibrant for the EIA was clenbuterol.Suc d'Helix pomatia solution, 100 000 U ml-1β-glucuronidaseand 1 000 000 U ml-1 sulfatase was supplied by Sepracor(Villeneuve la Garenne, France). Clenbuterol hydrochlorideand salbutamol, which were used for the fortification of liversamples, were obtained from Sigma (St. Louis, MO,USA). Samples Samples of bovine liver were homogenised in a Waring blenderand frozen until analysis. Liver samples of known history,which had tested negative for clenbuterol were used infortification studies. Incurred liver samples were provided bythe Faculty of Veterinary Medicine, University of Liege,Belgium, and also by the EU Reference Laboratory for Residuesof Veterinary Drugs (BgVV), Berlin, Germany. Fortification Fortification of samples for method development was carriedout by pipetting 25 ul volumes of methanolic solutionstcontaining clenbuterol or salbutamol at a concentration of 300ng ml-1 onto the surface of the tissue (1.5 g), resulting infortification levels of5 ng g-1. Bulk fortification was carriedout by pipetting 10 × 100 ul volumes of methanolic solutions containing clenbuterol or salbutamol at a concentration of 500ng ml-1 onto the surface of 100 g of liver tissue in a plasticbeaker. The fortified tissue (5 ng g-1) was mixed thorousghly for.:i+b5 min with a glass rod, by repeatedly folding the tissue awayfrom the sides of the beaker. The tissue was stored frozen untilanalysis. The method was validated by analysing aliquots of aliver sample which was bulk fortified. Enzyme hydrolysis Fifteen millilitres of sodium acetate buffer (0.2 M, pH 5.2) wasadded to 10 g of tissue preweighed into a stomacher bag. Thehomogenised liver was mixed for 5 min in the stomacher andthen transferred to a centrifuge tube and sonicated for 1 min. 0.5or 2.0 ml Helix pomatia solution (diluted 1 in 10 with bufferwas added and the homogenate vortexed for 60 s. The sampleswere incubated overnight at 50 C, and then vortexed for60 s. Drying of tissue For studies on oven-dried liver tissue, homogenised liver wasdried at 70°C in a vacuum oven (50mmHg) for 16 h. It was thenbrought to room temperature in a desiccator, after which it wasground to a powder (for 1 min) in a Waring blender using a 100ml stainless steel container with a lid. For studies on lyophilisedliver tissue, homogenised liver was thinly spread on Petri dishesand frozen at -80 °C for1 h; it was then lyophilised at -45Cfor 72 h, after which it was ground (as described for oven-driedliver). Grinding of tissue Where appropriate, after oven drying, the powdered tissueobtained from the Waring blender was ground further, through1 mm stainless steel sieves, using either a Glen Creston MicroHammer-Cutter Mill (Stanmore, Middlesex, UK) or a TecatorCyclone Sample Mill (Hoganas, Sweden). Particle sizing A Malvern Mastersizer 2000, with a Scirocco 2000 dry powderunit (Malvern Instruments Ltd., Malvern, Worcs., UK), wasused to obtain a particle size profile of dried and ground tissuesamples. Sample preparation for SFE The liver sample [1.5 g of fresh tissue or an equivalent weighof dried tissue (approximately 0.5 g)] was weighed into a 50 mlbeaker, fortified and left to stand for 15 min before furtherprocessing to allow the fortification solution to soak into thesample. Dried samples were mixed for 30 s with a metal spatula,post-fortification, to ensure that the clenbuterol or salbutamolrt1I1Csolution was dispersed uniformly throughout the tissue; 2.0 ghydromatrix was then blended with the sample, using a metalspatula, for 30 s. Packing of SFE vessels Vessels were filled in the following order: a plug of poly-propylene wool; the tissue/hydromatrix mixture; where used,methanol modifier (1.5 ml) was pipetted onto the surface of thetissue/hydromatrix mixture; a plug of polypropylene wool; 3.5 g hydromatrix; a plug of polypropylene wool. The vessel waspacked tightly using a stainless steel tamping rod designed to fitthe vessel exactly.The vessel was sealed and stored at 4°C untilextraction commenced. SFE procedure Prior to extraction, the shut off valves, outlet valves and inletvalves were closed. Two extraction vessels were verticallyinstalled in the SFE oven. A SPE column (6 ml) containingneutral alumina (2 g) was attached directly to the micrometeringvalve. This alumina column served both as an analyte trap andfor post-SFE clean-up of the SF extract.15 The inlet valves wereopened and the system was partially pressurised (to 620 bar at40 ℃; 300 bar at 100 C). The oven was heated to theexperimental temperature. The micrometering valve was pre-heated to 110°C prior to pressurisation. To equilibrate thesystem a static period of 10 min was applied to allow the vesselto reach the experimental extraction temperature. The micro-metering valve was then adjusted to give a flow of 2 1 min-1 ofdepressurised CO2 gas through the SPE column and wasmaintained at this rate for the dynamic extraction period. Analyte recovery At the end of the extraction period, flow to the micrometeringvalve was stopped and the pressurised CO2 remaining in thevessel was slowly purged through the SPE column. Anyresidual material remaining in the SFE transfer line was washedthrough the SPE column by attaching a SFE purge adapter to theSFE outlet line and pushing 4 ml of methanol-water (70+30)through. The SPE alumina column was removed from theintegral seat retainer nut and eluted with a further 4 ml ofmethanol-water (70+30). The sample extract was vortexed for30 s, the volume was measured and 1/10 of the eluate wasevaporated to dryness under a stream of nitrogen at 60°C andredissolved in assay buffer (750 ul). Enzyme immunoassay Duplicate 50 ul volumes of tissue extracts were assayedaccording to the kit procedure. Absorbance measurements weremade at 450 nm using a plate reader (Dynatech MRX, DynatechLaboratories Ltd., Billingshurst, UK). Quantification of analyterecovery was determined by comparison of analyte measured insample extracts with measured analyte in the fortificationsolutions. It was observed during method development that thelevel of sample matrix extracted during the SFE procedureaffected quantification in the EIA and, therefore, a calibrationcurve containing negative control sample extract was used. Results and discussion SFE conditions and moisture effects The SFE conditions previously developed for the extraction ofclenbuterol from fresh bovine liverl5 [unmodified SF-CO2;temperature, 100 °C; pressure, 690 bar (10 000 lb in-2; density,1.042 g ml-1); flow rate, 2 1 min-1 for 30 min (60 1 ofdepressurised CO2 in total)] were evaluated for the analysis ofsalbutamol, but were unsuccessful. Subsequently, the SFEconditions applied to the extraction of clenbuterol from oven-dried liver tissuel5 [SF-CO2, modified with 1.5 ml methanol(0.014% mole fraction of total CO2 used); temperature, 100°C;pressure, 690 bar; flow rate,2 1 min-1 for 10 min (20 1 ofdepressurised CO2 in total)] gave good recovery of salbutamol from fortified oven-dried tissue, but not from fresh tissue(Table 1). Under these SFE conditions the mean recovery from freshtissue was lower than the recovery from oven-dried tissue forboth analytes, but the difference was more significant forsalbutamol (compared with clenbuterol). This may be attributedto the more hydrophilic nature of salbutamol such that it has ahigher affinity for the water fraction of the liver sample than forthe extracting fluid. For the quantitative extraction of clenbu-terol, an increased extraction temperature combined withprolonged extraction time overcame the effect of samplemoisture.15 Quantitative extraction of salbutamol is facilitatedonly by removal of sample moisture combined with the additionof modifier. In the absence of methanol modifier. no salbutamolwas extracted from bovine liver in the fresh or dried state. These SFE conditions [SF-CO2, modified with 1.5 mlmethanol; temperature,100°C; pressure,690 bar (10 000lb in-2; density, 1.042 g ml-1); flow rate, 2 1min-1 for 10 min(20 1 of depressurised CO2 in total)] were used in all furtherstudies on the development of the multi-residue method. Sample preparation: type of drying and particle sizeeffects The preliminary studies described above were carried out onbovine liver which had been oven-dried, ground to a powder andsubsequently fortified. However, for the analysis of incurredsamples, the B-agonist residues were subjected to the oven-drying process. To evaluate the effect of oven drying on the SFEof clenbuterol and salbutamol, liver tissue was fortified withboth analytes before and after oven drying and extractionefficiencies were compared. Oven drying reduced the meanrecovery of clenbuterol and salbutamol to 56% and 43%,respectively (Fig. 2). This suggested that the oven dryingprocedure might either degrade the analytes or cause them tobind more tightly to the sample matrix. However, the heatstability of the analytes was evaluated at 70 C (in a vacuum Table 1 Effect of sample moisture on the recovery of clenbuterol andsalbutamol from bovine liver fortified at 5 ppb Fresh tissue Oven-dried tissue Recovery Recovery Analyte 11 Mean± s (%) 7 Mean ±s (%) Clenbuterol 82±8.4 6 91±8.5 Salbutamol 34±7.4 6 85±6.9 Fig.2Recovery of clenbuterol and salbutamol from bovine liver whichwas fortified (5 ppb) and subsequently dried by two methods, relative to therecovery from liver which was dried then fortified (5ppb). oven) and no losses were observed, suggesting that the analyteswere not degraded during the oven drying procedure. This is inagreement with previous work carried out on the heat stabilityof clenbuterol during cooking, which indicated that it is stablefor up to 5 h at100°C.16 Lyophilisation was evaluated as an alternative method ofmoisture removal from the tissue. This drying technique wasshown to have no adverse effect on the recovery of clenbuteroll or salbutamol from bovine liver tissue (Fig. 2). It was visuallyapparent on examination of the lyophilised and oven-driedtissue samples that the former had a finer texture. To furtherexamine this effect in relation to extraction efficiency, clenbu-terol incurred liver tissue was dried using both techniques. Bothsamples were ground in the Waring blender and samples oftheoven-dried material were ground further. Samples were thenextracted by SFE and the amount of residue extracted wasdetermined by EIA. Fig. 3 shows that there is a distinct difference in the particlesize distribution for oven-dried and lyophilised samples, havingmean particle sizes of 438 um and 243 um, respectively.Consequently, lyophilised samples have a higher specificsurface area (SSA) (0.10m² g-1) than oven-dried samples (0.03m²g-1). Further grinding of the oven-dried samples reduced themean particle size to values similar to or less than that of thelyophilised sample: to 263 um for a sample ground in a GlenCreston grinder and to 128 um for a sample ground in a Tecatorgrinder. Reducing particle size and thereby increasing surfacearea has a large influence on the extraction of analytes by SFE,by increasing the exposure of the targeted analytes to SF-CO2.This is reflected in the amounts of clenbuterol determined fromthe lyophilised and oven-dried samples (Table 2). Reducing the particle size from 438 um to 263 um (andincreasing the SSA from 0.03 to 0.05 m²g-l) increased theamount of clenbuterol recovered from incurred samples from5.7 ng g-l to 9.6 ng g-l, indicating the influence of particle sizeand surface area on the extractability of clenbuterol with SF-CO2. Reducing the mean particle size further to 129 um (andincreasing the SSA to 0.12 m² g-1) does not significantlyincrease the amount of clenbuterol determined (10.5 ng g-).These results suggest that there may be a threshold value forparticle size (and surface area) below which the extractability ofclenbuterol with SF-CO2 is significantly enhanced. Extractability from incurred tissue: enzyme hydrolysis(deconjugation) When the developed SFE method was applied to salbutamolincurred samples, only very small amounts of analyte were Fig. 3 Effect of type of drying and grinding on the particle sizedistribution of bovine liver tissue. detected from fresh or lyophilised tissue (Fig. 4). Unlikeclenbuterol, salbutamol has been reported to occur in liverprimarily as glucuronide and sulfate conjugates.6 Therefore, asfor conventional solvent extraction methods, a deconjugationstep is required for a multi-residue SFE procedure for B-agonists. Deconjugation with glucuronidase/sulfatase was undertakenon the fresh incurred tissue prior to lyophilisation; in this way allwater, both from the sample itself and from the buffer usedduring deconjugation, was removed prior to SFE. Stolkeret al.17 have also developed methods for the successfuextraction of anabolic agents from bovine urine with adeconjugation step prior to extraction. Another application ofenzymatic hydrolysis prior to SFE, in this case using a proteaseto digest skin tissue for the release of steroid residues, has beendescribed.18 Results for the analysis show a significant increase in thelevel of the drug determined after enzyme hydrolysis (Fig.4).This confirms the occurrence of salbutamol in incurred tissueprincipally in the conjugated form; the small amount ofsalbutamol extracted from the tissue without deconjugation mayrepresent that fraction of salbutamol present as free residue or asmall amount of conjugated residue extracted by SFE andmeasurable by the EIA. Increasing the enzyme concentrationused resulted in only a small increase in the residue leveldetermined (Table 3). The level of residue determined in theincurred sample agrees well with the nominal value of50 ng g--l1(provided by the supplying laboratory) when correction forcross-reactivity is made. Table 2 Effect of type of drying and method of grinding on the meanparticle size, specific surface area and subsequent amount of clenbuteroldetermined in incurred bovine liver tissue (n=3). Tissue size/um area (SSA)/m²g- Mean ± s/ng g-1 Oven-dried (A) 439 0.03 5.7±0.76 Oven-dried (B) 263 0.05 9.6±0.94 Oven-dried (C) 222 0.12 10.5±0.18 Lyophilised 0.10 10.9±1.30 aA, oven-dried tissue ground in a Waring blender for 1 min; B, oven-dried tissue ground in a Waring blender (1 min) and subsequently in a GlenCreston grinder through a 1 mm sieve; C,oven-dried tissue ground in aWaring blender (1 min) and subsequently in a Tecator grinder through a 1mm sieve. The mean recovery value for A is significantly different(p<0.01) from the values for the other tissues. Lyophilised liver tissue was used for method validation of thedeveloped procedure (Fig. 5). The intra-assay (i.e.,within one assay) and inter-assay (i.e.,between different assays) variations of the method for liversamples fortified individually with clenbuterol and salbutamolat 5 ng g-1 are shown in Table 4; fortification of separatesamples with each of the B-agonists was necessary because ofthe non-specific nature of the EIA used for the determination of Table 3 Salbutamol levels determined in incurred liver tissue afterhydrolysis with glucuronidase/sulfatase and subsequent lyophilisation Concentration of glucuronidase/ sulfatase/U Salbutamol determineda n Mean ± s/ng g-1 RSD (%) 500/5000 3 38±6.8 2000/20000 4 44±2.3 “ As clenbuterol equivalents. Fig.5Flow chart of the developed procedure for SFE of B-agonists frombovine liver tissue. residue content.Mean recoveries of86-92% for clenbuterol and62-70% for salbutamol were obtained. The intra-assay resultsshow low variation within a single assay (RSD <10%). Inter-assay variation is higher but acceptable (RSD < 25%). Thelimit of quantification, defined as that level in tissue equivalentto the lowest point on the standard curve, is 0.1 ng g-1; themaximum residue limit (MRL) for clenbuterol in edible tissue is0.5 ng g. Incurred liver samples were analysed following the devel-oped procedure. Table 5 shows the levels of clenbuterol andsalbutamol determined compared to the values obtained by thesupplying laboratories. The results for samples from source A,when extracted by SFE, were at 88% for clenbuterol and 75%for salbutamol, relative to the nominal residue content of thesesamples. These results are comparable to the results obtainedwith fortified samples (Table 4). Results for samples fromsource B were at 100% and 140% of the nominal values forclenbuterol and salbutamol, respectively. Conclusions This SFE procedure is suitable for the extraction of both classesof β-agonists from fortified and incurred bovine liver tissue.Incomparison with SFE methods for the extraction of other polarresidues,19 this technique requires only a very small amount ofco-solvent for analyte extraction. This study demonstrates thatincreasing the polarity of SF-CO2 by the addition of co-solventsdoes not necessarily determine the extent of analyte extractionfrom a sample matrix. Sample pretreatments, such as theremoval of water and deconjugation of the analyte(s) by enzymehydrolysis prior to SFE, are essential for the extraction of β-agonists by the supercritical fluid. Drying of the tissue, themethod of drying and particle size issues were shown to beimportant factors in determining analyte extractability. Theseobservations suggest that pretreatment of the sample matrix Table 4 Intra- and inter-assay variations for the determination ofclenbuterol and salbutamol (fortified at 5 ng g-) in bovine liver by SFEwith EIA (n = 5) Analyte determined Analyte Mean ± s/ng g-1 RSD (%) Intra-assay"一 Clenbuterol 4.6±0.33 7.1 Salbutamol 3.1±0.21 6.6 Inter-assayb— Clenbuterol 4.3±0.77 17.8 Salbutamol 3.5±0.84 24.1 Intra-assay: 5 (0.5g) aliquots from the same liver sample, extracted andanalysed within one assay. Inter-assay: 1 (0.5 g) aliquot from a liversample, extracted and analysed in each of 5 different assays. Table 5 Inter-assay variation for the determination of clenbuterol and salbutamol in incurred liver samples by SFE with EIA (n =5) Analyte determined Analyte Sourcea Nominal value/ng g-1b Mean ±s/ng g-1 Clenbuterol A 5 4.4±0.9 Salbutamolc 6 4.5±1.5 Clenbuterol 4.0±0.9 Salbutamol 70.0±17.2 “ Source A: Faculty of Veterinary Medicine, University of Liege,Belgium; values provided were from analysis by GC-MS. Source B: TheEU Reference Laboratory for Residues of Veterinary Drugs,BgVV, Berlin,iCYGermany; values provided were from analysis by GC-MS.Nominal valueprovided by the supplying laboratory.e n =3. prior to SFE is important in the application of SFE to residueanalysis. Acknowledgements The authors gratefully acknowledge the assistance of Prof. GuyMaghuin-Rogister (Faculty of Veterinary Medicine, Universityof Liege, Belgium), Dr. Linda Stolker [The National Institute ofPublic Health and Environmental Protection (RIVM), Bilth-oven, The Netherlands] and Dr. Bernd Jilicher (EU ReferenceLaboratory for Residues of Veterinary Drugs, BgVV, Berlin,Germany) for the supply of incurred liver samples. Dr. SeanQuilty (Particular Sciences, Castleknock, Dublin, Ireland) isthanked for his assistance with work carried out on the particlesize distribution of samples. This research has been part fundedby grant aid from the Department of Agriculture and Food,under the US-Ireland Co-operation Programme in AgriculturalScience and Technology. References ( 1 R. K. Juhler, Analyst , 1998,123 , 1551. 2 ) B. Van Bevel,M. Jaremo,L. Karlsson andG. Lindstrom, Anal. Chem.,1996, 68, 1279. ( I. A. S tuart,R. A. Ansell,J . Maclachan,P. A. Bather andW. P.Gardiner, Analyst, 1997, 122,303. ) 4 N. Aharonson,S. J. Lehotay andA. I. Medina,J. Agric. Food Chem.,1994,42,2817. 5 P. J. Buttery andJ. M. Dawson, in Beta-agonists and Their Effects onAnimal Growth and Carcass Quality, ed. J. P. Hanrahan,Elsevier,London,1987,p. 20. ( 6 R . F . WitkampandA. S.J. P. A. M. van Miert, in Proceedings ofFlairConcerted Action N o. 8 Workshop, Thessaloniki, G reece, O ctober1992, eds., H. Kuiper and L. A. P. Hoogenboom, R IKILT-DLO,Wageningen, T h e Netherlands, 1 9 92, p . 7 5 . ) 7 W. Haasnoot,M. E. Ploum,R. J. A. Paulessen,R. Schilt andF.A. Huf,J. Chromatogr., 1990,519,323. D. Boyd,M. O’Keeffe andM. R. Smyth, Analyst, 1994, 119, 1467. 89S. Collins,M. O'Keeffe andM. R. Smyth, Analyst, 1994, 119, 2671.0G. Degand,A. Bernes-Duyckaerts andG. Maghuin-Rogister,J. Agric.Food Chem.,1992, 40, 70. 11 M. Montrade,B. Le Bizec,F. Monteau,B. Siliart andF. Andre, Anal.Chim. Acta,1993,275,253. 12 J. J. Langenfeld,S. B. Hawthorne,D. J. Miller andJ. Pawlisyn, Anal.Chem., 1994, 66,909. 13 Y. Yang,A. Gharaibeh,S. B. Hawthorne andD. J.Miller, Anal. Chem.,1995,67,641. 14 N. Din,K. D. Bartle andA. A. Clifford,J. High Resolut. Chromatogr.,1996,19,465. 15 M. J.O’Keeffe,M.O’Keeffe,J. D. Glennon,A. R. Lightfield andR. J.Maxwell, Analyst, 1998,123,2711. 16 M. D. Rose,G. Shearer andW. H. H. Farrington, Food Addit. Contam.,1995, 12, 67. ( 17 A. A . M. S t olker,M. A . S i poli M a rques,P. W. Zoontjes,L. A. van Ginkel andR. J. Maxwell, Semin. F ood Anal., 1996, 1, 1 17. ) ( 18 A . A. M. Stolker,P. W. Zoontjes andL. A. va n Ginkel,Analyst, 1998, 1 23, 2671. ) ( 19 M . T . Combs,S. Boyd,M. Ashraf-Khorassani and L . T. T ay l or, J.Agric. Food Chem., 1997, 45 , 1779. ) ( Paper 9/02715G ) nalyst,
确定



还剩4页未读,是否继续阅读?
香港环球分析测试仪器有限公司为您提供《牛肝中β-agonists肾上腺素受体激动剂检测方案(超临界萃取)》,该方案主要用于畜禽肉及副产品中兽药残留检测,参考标准--,《牛肝中β-agonists肾上腺素受体激动剂检测方案(超临界萃取)》用到的仪器有









