本文次以自然纳米结构(贝壳和骨质中的矿物质颗粒)的化学鉴定,证明通过红外近场显微镜技术能够解决以上问题。 纳米傅立叶红外光谱(nano-FTIR)是通过将傅立叶变换红外光谱技术(FTIR) 与散射式扫描近场光学显微技术(s-SNOM)结合获得的。对紫贻贝贝壳横截面抛光处理后,通过Nano-FTIR可以重复的观察到生物钙质微晶体的声子共振,以及生物文石质上明显不同的光谱特征。更重要的是,本研究次在紫贻贝贝壳中发现了尺寸为20nm左右、稀疏分布的纳米颗粒,其显著不同的光谱特征表明这些纳米颗粒为磷化物晶体。对人类牙齿界面的研究观察到了多组分磷酸盐的红外吸收峰。这些光谱在牙本质小管附近有明显的特征变换,证明了磷灰石纳米晶体的化学与结构的变化。红外光谱峰的强弱对应矿物质浓度变化,这点通过电镜得到印证。Nano-FTIR对结构的畸变反应敏感,因此非常适用于对生物矿物质形成和老化的研究。总体来说nano-FTIR适用于从微纳加工到临床骨科研究等多种学科中涉及复合材料的分析和鉴定工作。
方案详情

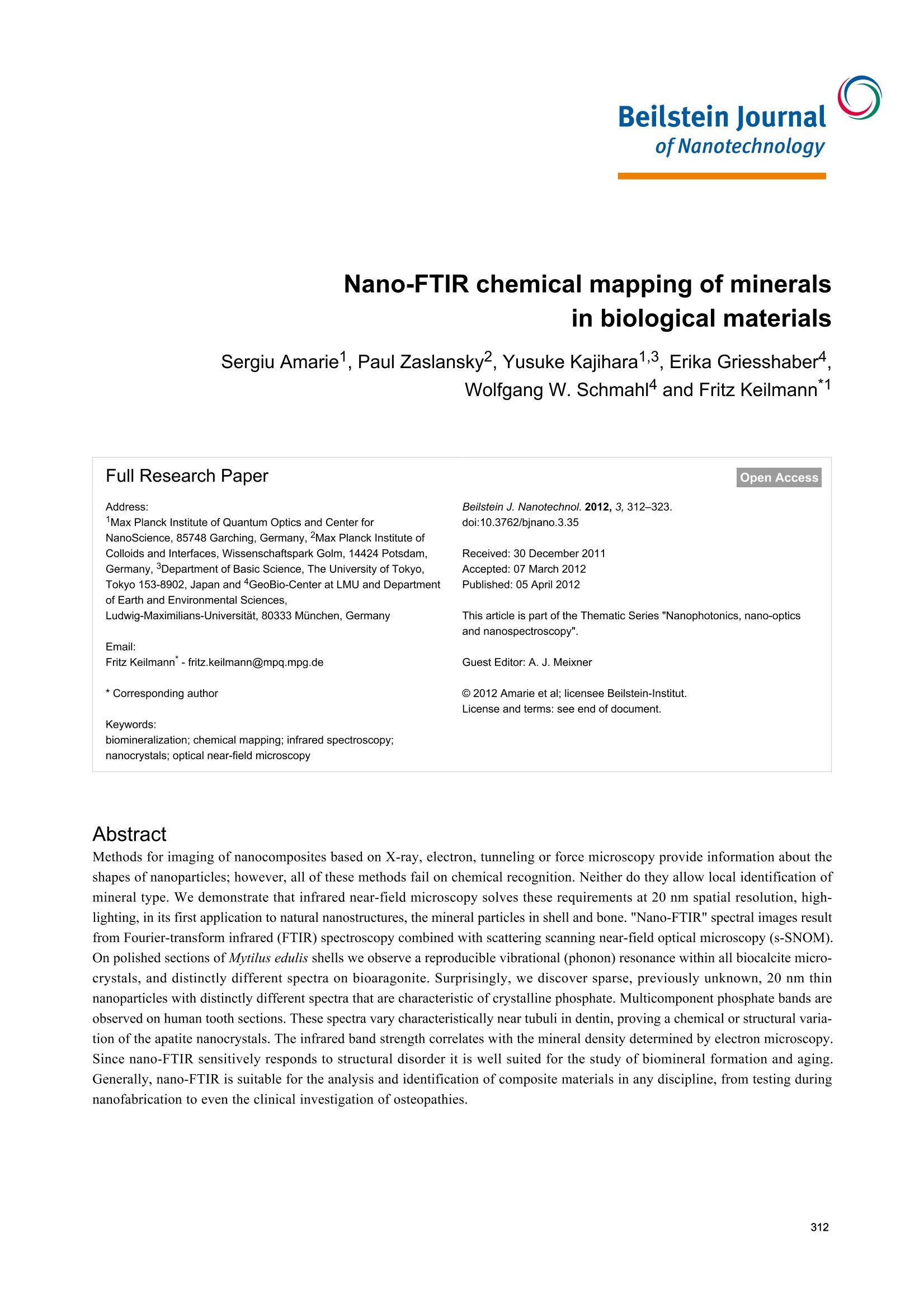
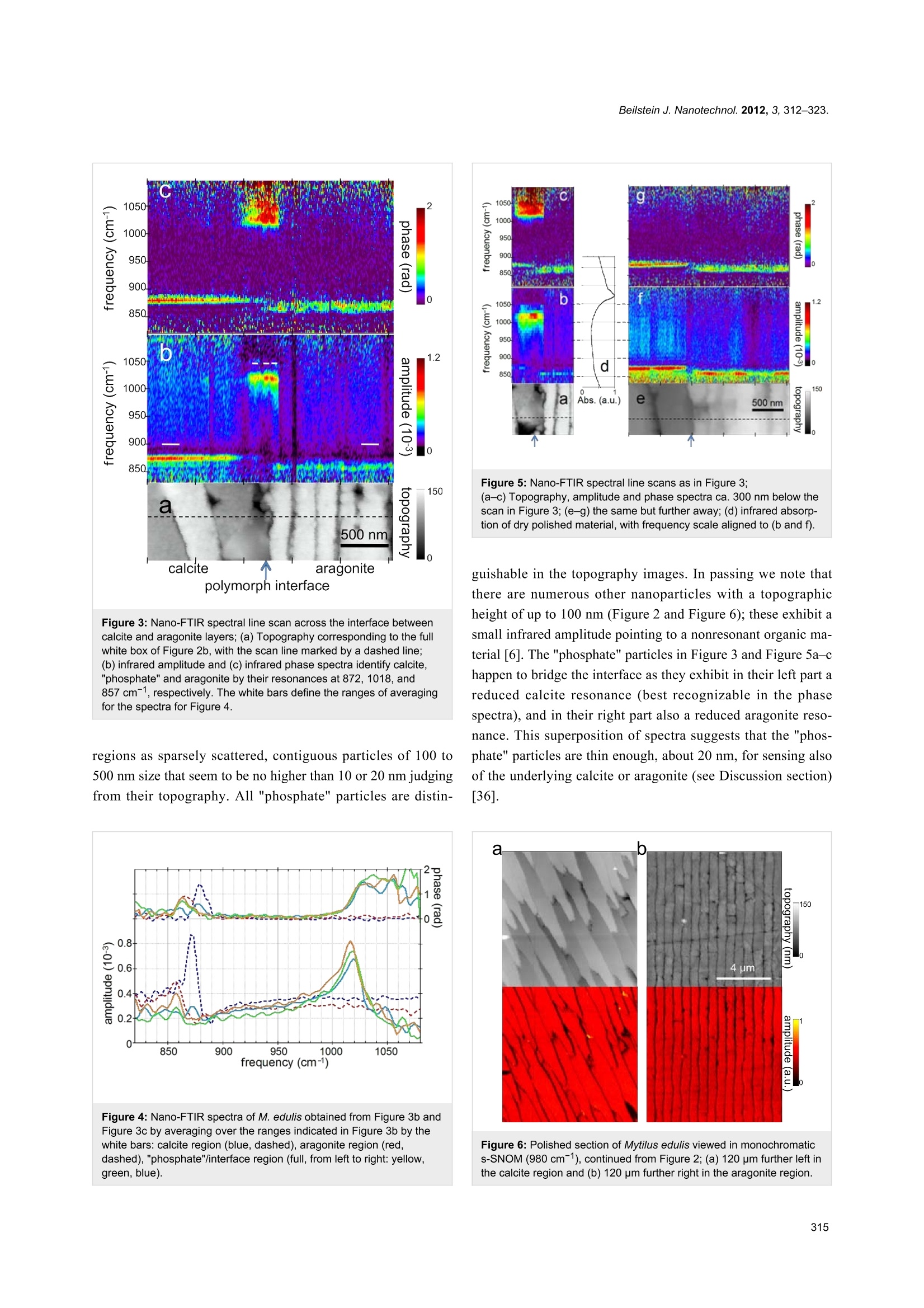
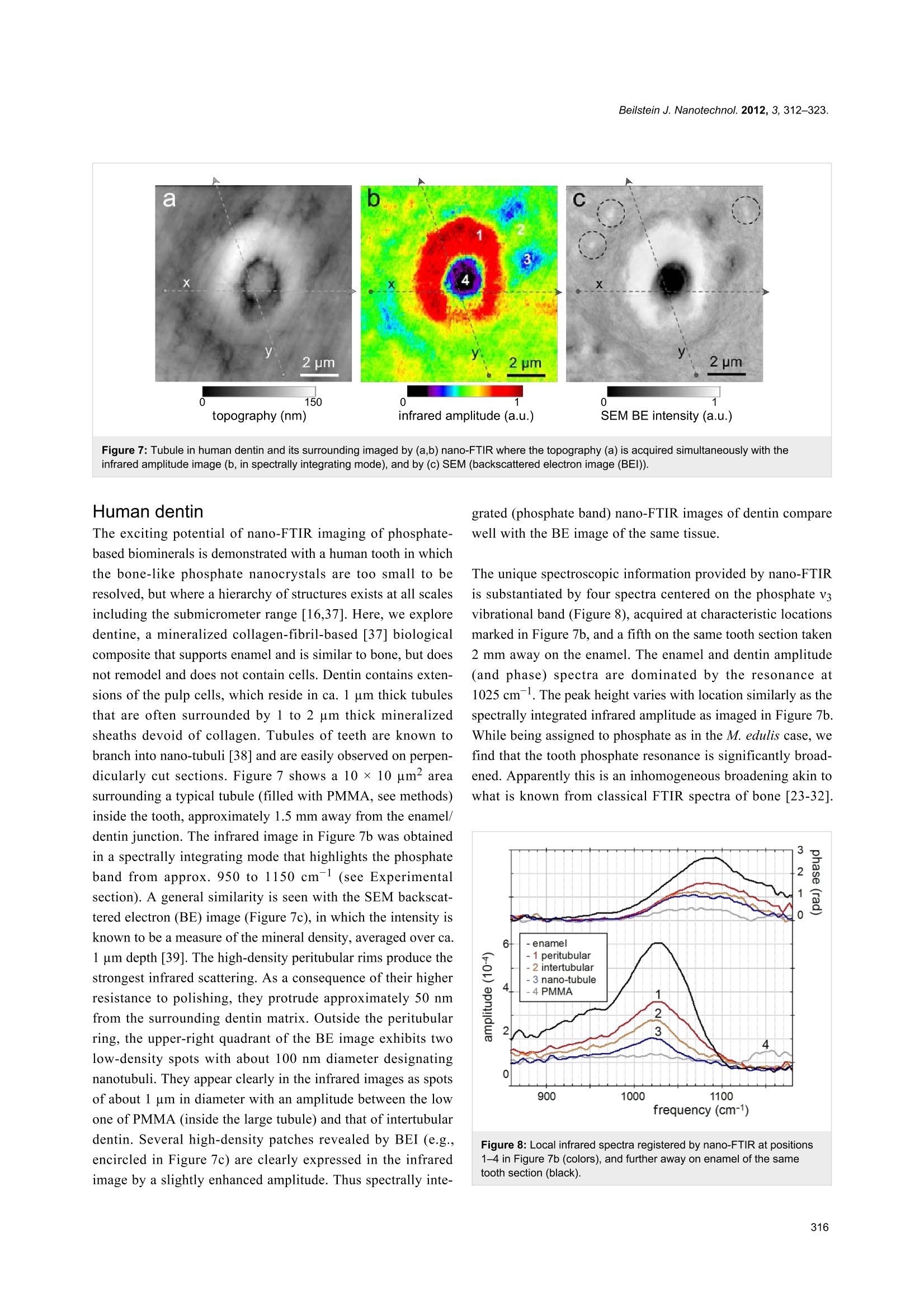
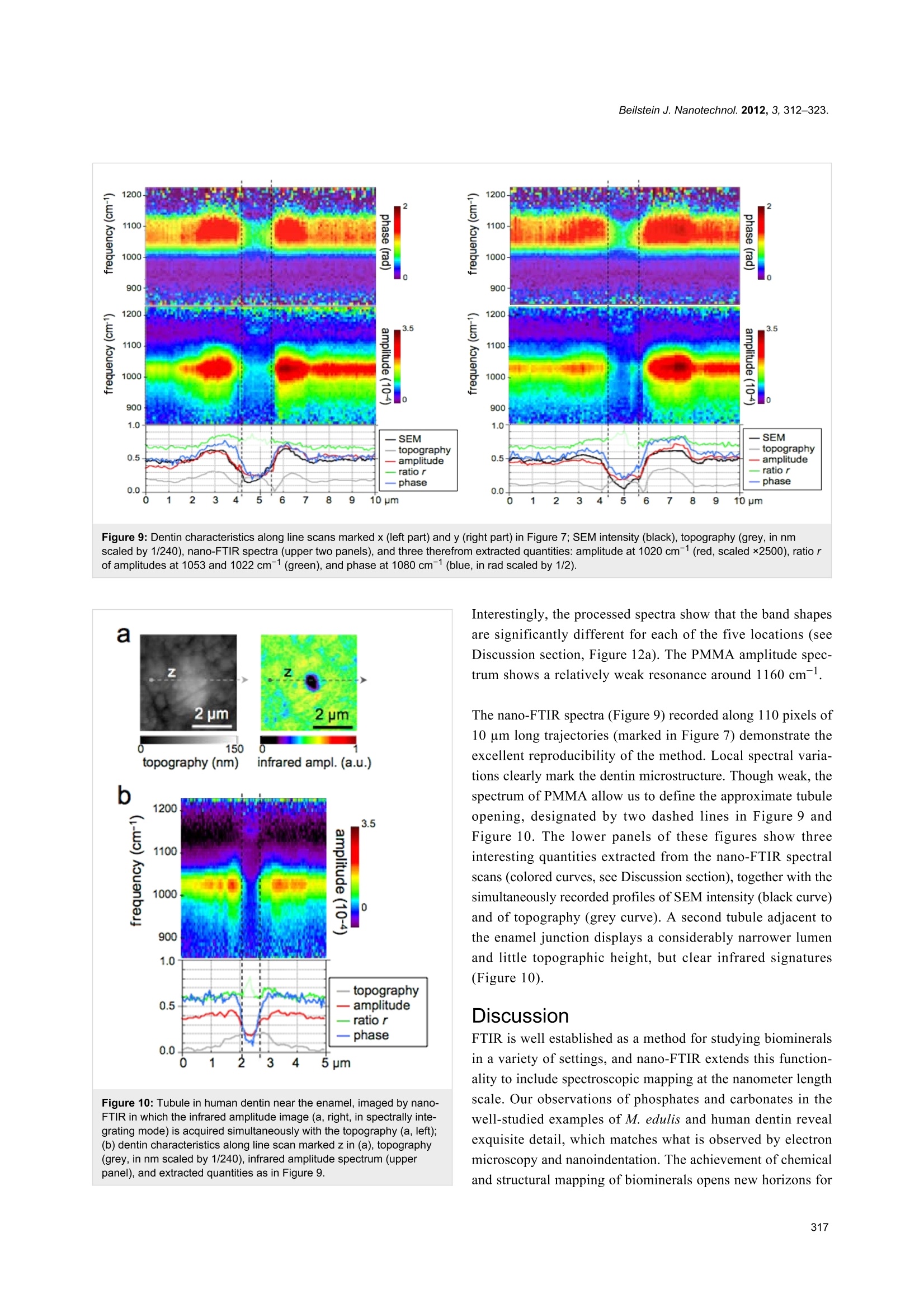
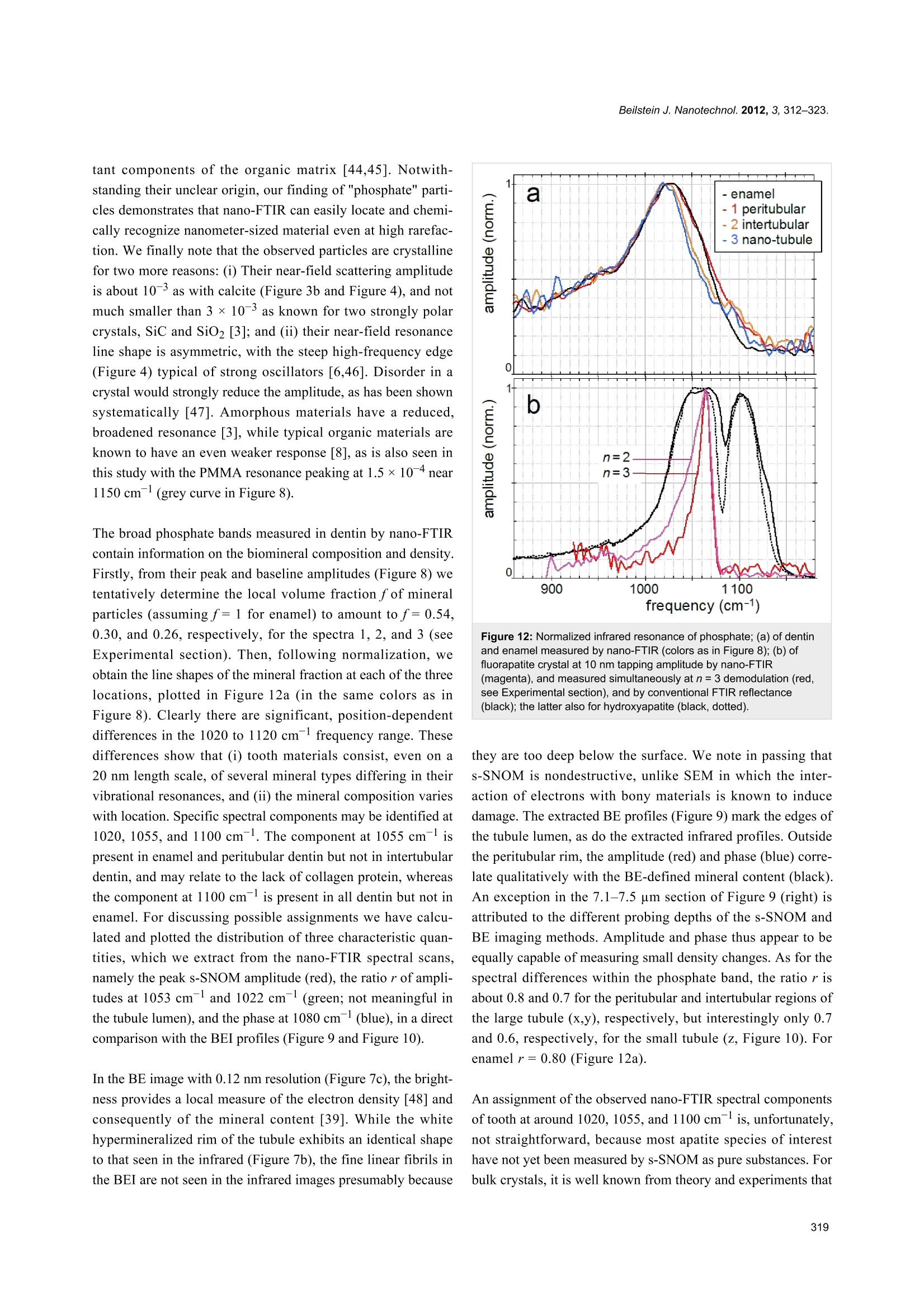
Beilstein JournalofNanotechnology Beilstein J. Nanotechnol. 2012,3, 312-323. Nano-FTIR chemical mapping of mineralsin biological materials Sergiu Amarie1, Paul Zaslansky2, Yusuke Kajihara1,3, Erika Griesshaber4,Wolfgang W. Schmahl4 and Fritz Keilmann Address: Max Planck Institute of Quantum Optics and Center for NanoScience, 85748 Garching, Germany,2Max Planck Institute ofColloids and Interfaces, Wissenschaftspark Golm, 14424 Potsdam,Germany,3Department of Basic Science, The University of Tokyo,Tokyo 153-8902, Japan and 4GeoBio-Center at LMU and Departmentof Earth and Environmental Sciences. Ludwig-Maximilians-Universitat, 80333 Munchen, Germany Email: Fritz Keilmann"-fritz.keilmann@mpq.mpg.de *Corresponding author Keywords: biomineralization; chemical mapping; infrared spectroscopy;nanocrystals; optical near-field microscopy Beilstein J. Nanotechnol. 2012, 3, 312-323.doi:10.3762/bjnano.3.35 Received: 30 December 2011 Accepted: 07 March 2012 Published: 05 April 2012 This article is part of the Thematic Series "Nanophotonics, nano-opticsand nanospectroscopy". Guest Editor: A. J. Meixner C 2012 Amarie et al; licensee Beilstein-Institut.License and terms: see end of document. Abstract Methods for imaging of nanocomposites based on X-ray, electron, tunneling or force microscopy provide information about theshapes of nanoparticles; however,all of these methods fail on chemical recognition. Neither do they allow local identification ofmineral type. We demonstrate that infrared near-field microscopy solves these requirements at 20 nm spatial resolution, high-lighting, in its first application to natural nanostructures, the mineral particles in shell and bone. "Nano-FTIR" spectral images resultfrom Fourier-transform infrared (FTIR) spectroscopy combined with scattering scanning near-field optical microscopy (s-SNOM).On polished sections of Mytilus edulis shells we observe a reproducible vibrational (phonon) resonance within all biocalcite micro-crystals, and distinctly different spectra on bioaragonite. Surprisingly, we discover sparse, previously unknown, 20 nm thinnanoparticles with distinctly different spectra that are characteristic of crystalline phosphate. Multicomponent phosphate bands areobserved on human tooth sections. These spectra vary characteristically near tubuli in dentin, proving a chemical or structural varia-tion of the apatite nanocrystals. The infrared band strength correlates with the mineral density determined by electron microscopy.Since nano-FTIR sensitively responds to structural disorder it is well suited for the study of biomineral formation and aging.Generally, nano-FTIR is suitable for the analysis and identification of composite materials in any discipline, from testing duringnanofabrication to even the clinical investigation of osteopathies. Introduction Fourier-transform infrared spectroscopy (FTIR) [1] is a stan-dard tool in chemical analysis. It can identify virtually anysubstance through the "fingerprint" of the molecular vibrationalabsorption spectrum in the 3-30 um wavelength region. Nano-FTIR spectroscopic near-field microscopy is a fascinatingrecent advance [2-4]. It enables scattering near-field opticalmicroscopes (s-SNOM) [5,6] to operate at ultrahigh spatialresolution over a broad mid-infrared spectrum emitted fromeither a coherent supercontinuum source [2,3] or an incoherentthermal source [4]. The s-SNOM uses a metalized AFM tip as alight-concentrating antenna such that the sample is probed witha nanofocused light field (Figure 1). The nanofocus is a lightspot of the same size as the tip radius, which thus defines boththe optical and the topographic resolutions of s-SNOM. Detec-tion of the backscattered light reveals local optical information.The probed volume extends typically 20 nm laterally, as well asinto the sample (sometimes even less than 10 nm) [7]. The highresolution is independent of the wavelength. This enables theutilization of long wavelengths corresponding to the infraredfingerprint vibrations. s-SNOM has been successfully operatedwith visible, infrared and terahertz illumination, and has beenapplied to organic [8,9] and inorganic [10] materials, in suchdiverse fields as nanoelectronics [11], the physics of phase tran-sitions [12], or material identification [13]. The underlyingnear-field interaction has been theoretically modeled and exper-imentally verified. The observable contrasts and spectra can bederived from the complex dielectric function of the sample ma-terial [6,14], and include both the absolute efficiency and thephase of the scattering [3]. Nano-FTIR has, up to now, beendemonstrated with flat test samples only, consisting of metals,semiconductors and polar crystals [3,4]. Hard biological tissues are highly textured composites ofsubmicrometric inorganic particles embedded in organicmatrices [15-17]. Major tissues of interest include the phos-phatic (bone) family of materials, and the carbonatic family asfound, e.g., in mollusc shells. Within the phylum Brachiopoda,both strategies of hybrid shell architecture have evolved:Calcium carbonate crystals in an organic matrix [18-20], andlaminates of calcium phosphate nanoparticle reinforced chitinfibers [21,22].FTIR spectroscopic microscopy is a well-estab-lished method and has been extensively used to study bonebiominerals at several micrometers spatial resolution [23-30].Its strength in the study of bone biopsies, mineralized tendons,dentin or ivory is mainly due to a broad absorption bandbetween 950 and 1150 cm- assigned to the V3 vibration of thePO43-ion of apatite. It is thus possible to acquire maps ofmineral concentration, and to relate mineral to protein(collagen) or carbonate distributions, usually revealing consid-erable spatial variation. Moreover, the apatite band exhibits a Figure 1: Nano-FTIR basic interaction. Focused infrared light incidentfrom the upper left excites a nanofocus at the metal tip, symbolized asa star, which interacts with the scanning sample. The backscatteredinfrared light carries local information. Here, the infrared response(color code as in Figure 2b) is overlaid on a pseudo-3D rendering ofthe topography, which is simultaneously recorded, of a 1.4×1.6 pmzoom area designated in Figure 2b by the dashed box on the left.Topographic height differences ca. 50 nm. weak spectral substructure, evident from Fourier self-deconvo-lution [1]. It reveals relative weights of apatite species that areassigned, with the help of chemical and X-ray analyses, toMg, F or CO32substitution, or differing particle size, orcrystal imperfections [24,31,32]. In this study we demonstratethe power of nano-FTIR to map naturally formed mineralizednanostructures. We show that we obtain fingerprint informationon two example systems of biominerals. Experimental compari-son is made with electron microscopy (SEM) to verify thatnano-FTIR perfectly matches what is already known about thestructures, and that the method indeed provides rich chemicaland structural contrasts at the 20 nm scale. Results Marine shell as an example of a carbonate-forming organism We demonstrate nano-FTIR near-field microscopy on a Mytilusedulis (M. edulis) shell specimen, which exhibits easily resolv-able fibrous biocalcite microcrystals and tablet-shaped bioarag-onite nanocrystals. On a polished section, the expected charac-teristic interface [33] between an outer calcite layer and an inneraragonite layer is readily located with the help of an overviewmicroscope (0.7 um resolution) built into the commercials-SNOM used (neaspec.com). On the inner side there is aninterlayer, ca. 2 um wide, with modified bioaragonite crystals[34]. Three adjoining s-SNOM images of 10 ×10 um areaeach were consecutively acquired and stitched together. Thetopography (Figure 2a) exhibits the arrangement of (i) fiber- Figure 2: Polished section of Mytilus edulis viewed in monochromatic s-SNOM (980 cm-1). (a) Topography of the interface between two calciumcarbonate polymorphs, biocalcite crystals (left) and bioaragonite crystals (right);(b) the backscattered infrared amplitude (n=3) contrasts the organicmatrix at a relatively low level; a few, unexpected particles highlighted by their enhanced amplitude are chemically different; we refer to them as"phosphate" crystals because their spectra (Figure 3 and Figure 4) are characteristic of phosphate. shaped biocalcite crystals with slightly oblique, flat surfaces at afew distinctly different heights, (ii) deep depressions mainly inthe interlayer, and (iii) bioaragonite crystals with flat surfaces atequal height, some (in the interlayer) as narrow as 100 nm. For chemical mapping we collected 300 nano-FTIR spectraalong a 2.5 um line marked in Figure 3a, across the interfaceregion designated by a full white rectangle in Figure 2b(Figure 3, additional scans are shown in Figure 5). The spectrain Figure 3b and Figure 3c (and also the extracted averagedspectral profiles in Figure 4) are dominated by a single, sharpresonance, which differs in frequency position for orthorhombicaragonite (855 cm-) and trigonal calcite (873cm), and thusboth calcium carbonate polymorphs can be readily distin-guished. The biocalcite spectra show no spectral shift within agiven crystal, either upon comparison of neighboring crystals ofthe same type, or with changes in topographic height as seenwith the three leftmost (biocalcite) crystals in Figure 3. Intrigu-ingly, we notice on close inspection of all biocarbonatesurfaces, e.g., in Figure 1, a shallow amplitude modulation on a50-200 nm lateral scale, which we tentatively explain to bedue to a mesocrystalline substructure that has been recentlyobserved by SEM [35]. In Figure 3 and Figure 5 the infrared resonance is not as repeat-able on the bioaragonite as on the biocalcite crystals, both withregard to spectral position and height.Further away from theinterface layer bioaragonite has a more stable spectrum (notshown). This indicates that the interlayer carbonate (i) is truelybioaragonite but (ii) has a reduced, changeable mineral content.The interlayer bioaragonite crystals are clearly smaller and lesswell ordered (Figure 2). Surprisingly, we find in Figure 3 a 350 nm long section witha similarly strong and sharp resonance at a much higherfrequency of about 1018 cm, which we tentatively assign tobe phosphate (see Discussion section). In order to specificallymap its occurrence we acquired monochromatic s-SNOMimages (Figure 2 and Figure 6) at 980 cm, which is a CO2laser frequency at which the scattering signal is still weaklyenhanced by the "phosphate" resonance (see amplitude spectrain Figure 3b, Figure 4 and Figure 5b)."Phosphate" occurs at afew spots only, in the calcite region up to and including theinterlayer, but not further out in the aragonite region; in thecalcite region its occurrence diminishes with distance from theinterface (Figure 6). Additional zoomed images such asFigure 1 (see also Figure 11) unveil individual "phosphate" Figure 3:Nano-FTIR spectral line scan across the interface betweencalcite and aragonite layers; (a) Topography corresponding to the fullwhite box of Figure 2b, with the scan line marked by a dashed line;(b) infrared amplitude and (c) infrared phase spectra identify calcite,"phosphate" and aragonite by their resonances at 872, 1018,and857 cm-1, respectively. The white bars define the ranges of averagingfor the spectra for Figure 4. regions as sparsely scattered, contiguous particles of 100 to500 nm size that seem to be no higher than 10 or 20 nm judgingfrom their topography. All "phosphate" particles are distin- Figure 4: Nano-FTIR spectra of M. edulis obtained from Figure 3b andFigure 3c by averaging over the ranges indicated in Figure 3b by thewhite bars: calcite region (blue, dashed), aragonite region (red,dashed),"phosphate"/interface region (full, from left to right: yellow,green,blue). Figure 5:Nano-FTIR spectral line scans as in Figure 3;(a-c) Topography, amplitude and phase spectra ca. 300 nm below thescan in Figure 3; (e-g) the same but further away; (d) infrared absorp-tion of dry polished material, with frequency scale aligned to (b and f). guishable in the topography images. In passing we note thatthere are numerous other nanoparticles with a topographicheight of up to 100 nm (Figure 2 and Figure 6); these exhibit asmall infrared amplitude pointing to a nonresonant organic ma-terial [6]. The "phosphate" particles in Figure 3 and Figure 5a-chappen to bridge the interface as they exhibit in their left part areduced calcite resonance (best recognizable in the phasespectra), and in their right part also a reduced aragonite reso-nance. This superposition of spectra suggests that the "phos-phate" particles are thin enough, about 20 nm, for sensing alsoof the underlying calcite or aragonite (see Discussion section)[36]. Figure 6: Polished section of Mytilus edulis viewed in monochromatics-SNOM (980 cm-1), continued from Figure 2;(a) 120 pm further left inthe calcite region and (b) 120 pm further right in the aragonite region. Figure 7: Tubule in human dentin and its surrounding imaged by (a,b) nano-FTIR where the topography (a) is acquired simultaneously with theinfrared amplitude image (b, in spectrally integrating mode), and by (c) SEM (backscattered electron image (BEl)). Human dentin The exciting potential of nano-FTIR imaging of phosphate-based biominerals is demonstrated with a human tooth in whichthe bone-like phosphate nanocrystals are too small to beresolved, but where a hierarchy of structures exists at all scalesincluding the submicrometer range [16,37]. Here, we exploredentine, a mineralized collagen-fibril-based [37] biologicalcomposite that supports enamel and is similar to bone, but doesnot remodel and does not contain cells. Dentin contains exten-sions of the pulp cells, which reside in ca. 1 um thick tubulesthat are often surrounded by 1 to 2 um thick mineralizedsheaths devoid of collagen. Tubules of teeth are known tobranch into nano-tubuli [38] and are easily observed on perpen-dicularly cut sections. Figure 7 shows a 10× 10 um areasurrounding a typical tubule (filled with PMMA, see methods)inside the tooth, approximately 1.5 mm away from the enamel/dentin junction. The infrared image in Figure 7b was obtainedin a spectrally integrating mode that highlights the phosphateband from approx. 950 to 1150 cm- (see Experimentalsection). A general similarity is seen with the SEM backscat-tered electron (BE) image (Figure 7c), in which the intensity isknown to be a measure of the mineral density, averaged over ca.1 um depth [39]. The high-density peritubular rims produce thestrongest infrared scattering. As a consequence of their higherresistance to polishing, they protrude approximately 50 nmfrom the surrounding dentin matrix. Outside the peritubularring, the upper-right quadrant of the BE image exhibits twolow-density spots with about 100 nm diameter designatingnanotubuli. They appear clearly in the infrared images as spotsof about 1 um in diameter with an amplitude between the lowone of PMMA (inside the large tubule) and that of intertubulardentin. Several high-density patches revealed by BEI (e.g.,encircled in Figure 7c) are clearly expressed in the infraredimage by a slightly enhanced amplitude. Thus spectrally inte- grated (phosphate band) nano-FTIR images of dentin comparewell with the BE image of the same tissue. The unique spectroscopic information provided by nano-FTIRis substantiated by four spectra centered on the phosphate v3vibrational band (Figure 8), acquired at characteristic locationsmarked in Figure 7b, and a fifth on the same tooth section taken2 mm away on the enamel. The enamel and dentin amplitude(and phase) spectra are dominated by the resonance at1025 cm-. The peak height varies with location similarly as thespectrally integrated infrared amplitude as imaged in Figure 7b.While being assigned to phosphate as in the M. edulis case, wefind that the tooth phosphate resonance is significantly broad-ened. Apparently this is an inhomogeneous broadening akin towhat is known from classical FTIR spectra of bone [23-32]. Figure 8: Local infrared spectra registered by nano-FTIR at positions1-4 in Figure 7b (colors), and further away on enamel of the sametooth section (black). Figure 9: Dentin characteristics along line scans marked x (left part) and y (right part) in Figure 7; SEM intensity (black), topography (grey, in nmscaled by 1/240), nano-FTIR spectra (upper two panels), and three therefrom extracted quantities: amplitude at 1020 cm-1 (red, scaled ×2500), ratio rof amplitudes at 1053 and 1022 cm1(green), and phase at 1080 cm1 (blue, in rad scaled by 1/2). Figure 10: Tubule in human dentin near the enamel, imaged by nano-FTIR in which the infrared amplitude image (a, right, in spectrally inte-grating mode) is acquired simultaneously with the topography (a, left);(b) dentin characteristics along line scan marked z in (a), topography(grey, in nm scaled by 1/240), infrared amplitude spectrum (upperpanel), and extracted quantities as in Figure 9. Interestingly, the processed spectra show that the band shapesare significantly different for each of the five locations (seeDiscussion section, Figure 12a). The PMMA amplitude spec-trum shows a relatively weak resonance around 1160 cm. The nano-FTIR spectra (Figure 9) recorded along 110 pixels of10 um long trajectories (marked in Figure 7) demonstrate theexcellent reproducibility of the method. Local spectral varia-tions clearly mark the dentin microstructure. Though weak, thespectrum of PMMA allow us to define the approximate tubuleopening, designated by two dashed lines in Figure 9 andFigure 10. The lower panels of these figures show threeinteresting quantities extracted from the nano-FTIR spectralscans (colored curves, see Discussion section), together with thesimultaneously recorded profiles of SEM intensity (black curve)and of topography (grey curve). A second tubule adjacent tothe enamel junction displays a considerably narrower lumenand little topographic height, but clear infrared signatures(Figure 10). Discussion FTIR is well established as a method for studying biomineralsin a variety of settings, and nano-FTIR extends this function-ality to include spectroscopic mapping at the nanometer lengthscale. Our observations of phosphates and carbonates in thewell-studied examples of M. edulis and human dentin revealexquisite detail, which matches what is observed by electronmicroscopy and nanoindentation. The achievement of chemicaland structural mapping of biominerals opens new horizons for our understanding of mineral arrangements and variability inbiological systems. Intricate carbonate-based natural skeletons,that may include transient and stabilized amorphous phases, cannow be mapped within and across interfaces by a noncontactand nondestructive imaging technique. With respect to apatitestudies, our method is directly applicable to the investigation ofhealthy and diseased forms of vertebrate bones and teeth.Mineral precipitation, aggregation and aging can now beanalyzed and quantified in submicrometer detail, to betterunderstand the biological processes of bone formation,abnormal development, and healing in response to drug treat-ment. Several technical advantages of surface scanning make thenano-FTIR approach extremely robust and useful for the studyof biological materials. The samples need not be thin, onlyreasonably flat, thus avoiding thin-section preparations, whichare prone to damage. Unavoidable topographic obstaclesresulting from the cutting and polishing procedures are of littleconsequence: Height variations of 100 nm do not change theoff-resonant infrared amplitude (Figure 2) nor the resonantresponse in amplitude and phase, as demonstrated for exampleby the repeatability ofthe carbonate resonance spectra withinthe sample region containing biocalcite (Figure 3). At steeptopographic edges though, the s-SNOM amplitude is known tobe reduced over a width equal to the spatial resolution, resultingin "edge darkening" [6].This effect probably contributes to thedark regions seen between calcite crystals in Figure 2b andremains to be further investigated. The impressive spatial reso-lution of nano-FTIR can be judged from the edges of the biocal-cite crystals (Figure 3a) that demonstrate a mechanical (AFM)resolution certainly below 30 nm. Abrupt edges of the nano-FTIR line section showing the phosphate resonance(Figure 3b,c and Figure 5b,c) prove that the infrared resolutionis better than 20 nm. The "phosphate" particles in M. edulis are clearly recognizedfrom their spectral signature (Figures3-5), but would have beenbarely detected based on their topographic appearance alone(interestingly, their surfaces (see also Figure 1 and Figure 11)appear smoother than the neighboring biocarbonate crystals).Note that the nano-FTIR spectra of the "phosphate"particles additionally show one of the carbonate resonances(Figures 3-5), obviously originating from the crystals under-neath [36,40]. To understand this effect, we recall that the basicnear-field interaction probes the sample to a depth on the orderof the tip radius (or somewhat deeper when one chooses thetapping amplitude or the average tip-to-sample distance to belarger than the tip radius)[6]. Thus buried objects may affectthe backscattering provided that the covering layer is not thickerthan a few times the tip radius [36]. Based on this effect, even a tomographic mapping capability of s-SNOM has beensuggested [6,41]. Our present observation is the first report todistinguish different phonon resonances in both the coveringlayer and the buried material. We estimate from the observedamplitudes that the thickness of the "phosphate" particles is onthe order of 10-30 nm, in agreement with their topographicappearance. Figure 11:"Phosphate" in M. edulis. High-resolution images (similar toFigure 1) of the dotted-box areas, 1.0×1.2 pm2 of Figure 2. Backscat-teredinfrared amplitude in color code as in Figure 2b, overlaid on apseudo-3D rendering of the topography. The origin of the "phosphate" particles remains unclear in thisproof-of-principle study. Their erratic distribution may suggestsome unknown preparation artifact. The material could be amodification of materials in the organic matrix; however, this isnot highlighted in the infrared images. Nevertheless, it is clearthat the particles could not simply be dried polishing material(Struers OP-A) since this shows a weak FTIR absorption at1073 cm (Figure 5d) but no discernible nano-FTIR resonancein the frequency range of interest. A strong argument for theassignment of the particles as crystalline phosphate is theobserved high spectral phase effect of about 80°, exceeding thatof bioaragonite (50°) and biocalcite (70°). Typically the spec-tral phase effect is on the order of 30° for strong polymer vibra-tions [8,9] but on the order of 400° for strong crystal phonons[3,6]. For molluscs the employment of phosphate in shell archi-tecture has not been reported, but the radula (tooth structure) ofthe chitons is known to contain calcium phosphate [42,43]. Inbones, phosphorylated proteins have been suggested as impor- tant components of the organic matrix [44,45]. Notwith-standing their unclear origin, our finding of "phosphate" parti-cles demonstrates that nano-FTIR can easily locate and chemi-cally recognize nanometer-sized material even at high rarefac-tion. We finally note that the observed particles are crystallinefor two more reasons: (i) Their near-field scattering amplitudeis about 10-3 as with calcite (Figure 3b and Figure 4), and notmuch smaller than 3× 10-3 as known for two strongly polarcrystals, SiC and SiO2 [3]; and (ii) their near-field resonanceline shape is asymmetric, with the steep high-frequency edge(Figure 4) typical of strong oscillators [6,46]. Disorder in acrystal would strongly reduce the amplitude, as has been shownsystematically [47]. Amorphous materials have a reduced,broadened resonance [3], while typical organic materials areknown to have an even weaker response [8], as is also seen inthis study with the PMMA resonance peaking at 1.5×10-4 near1150 cm(grey curve in Figure 8). The broad phosphate bands measured in dentin by nano-FTIRcontain information on the biomineral composition and density.Firstly, from their peak and baseline amplitudes (Figure 8) wetentatively determine the local volume fraction fof mineralparticles (assuming f=1 for enamel) to amount to f= 0.54,0.30, and 0.26, respectively, for the spectra 1, 2, and 3 (seeExperimental section). Then, following normalization, weobtain the line shapes of the mineral fraction at each of the threelocations, plotted in Figure 12a (in the same colors as inFigure 8). Clearly there are significant, position-dependentdifferences in the 1020 to 1120 cm- frequency range. Thesedifferences show that (i) tooth materials consist, even on a20 nm length scale, of several mineral types differing in theirvibrational resonances, and (ii) the mineral composition varieswith location. Specific spectral components may be identified at1020, 1055, and 1100 cm-. The component at 1055 cmispresent in enamel and peritubular dentin but not in intertubulardentin, and may relate to the lack of collagen protein, whereasthe component at 1100 cm-is present in all dentin but not inenamel. For discussing possible assignments we have calcu-lated and plotted the distribution of three characteristic quan-tities, which we extract from the nano-FTIR spectral scans,namely the peak s-SNOM amplitude (red), the ratio r of ampli-tudes at 1053 cm- and 1022 cm- (green; not meaningful inthe tubule lumen), and the phase at 1080 cm(blue), in a directcomparison with the BEI profiles (Figure 9 and Figure 10). In the BE image with 0.12 nm resolution (Figure 7c), the bright-ness provides a local measure of the electron density [48] andconsequently of the mineral content [39]. While the whitehypermineralized rim of the tubule exhibits an identical shapeto that seen in the infrared (Figure 7b), the fine linear fibrils inthe BEI are not seen in the infrared images presumably because Figure 12: Normalized infrared resonance of phosphate;(a) of dentinand enamel measured by nano-FTIR (colors as in Figure 8); (b) offluorapatite crystal at 10 nm tapping amplitude by nano-FTIR(magenta), and measured simultaneously at n=3 demodulation (red,see Experimental section), and by conventional FTIR reflectance(black); the latter also for hydroxyapatite (black, dotted). they are too deep below the surface. We note in passing thats-SNOM is nondestructive,unlike SEM in which the inter-action of electrons with bony materials is known to inducedamage. The extracted BE profiles (Figure 9) mark the edges ofthe tubule lumen, as do the extracted infrared profiles. Outsidethe peritubular rim, the amplitude (red) and phase (blue) corre-late qualitatively with the BE-defined mineral content (black).An exception in the 7.1-7.5 um section of Figure 9 (right) isattributed to the different probing depths of the s-SNOM andBE imaging methods. Amplitude and phase thus appear to beequally capable of measuring small density changes. As for thespectral differences within the phosphate band, the ratio r isabout 0.8 and 0.7 for the peritubular and intertubular regions ofthe large tubule (x,y), respectively, but interestingly only 0.7and 0.6, respectively, for the small tubule (z, Figure 10). Forenamelr=0.80 (Figure 12a). An assignment of the observed nano-FTIR spectral componentsof tooth at around 1020, 1055, and 1100 cmis, unfortunately,not straightforward, because most apatite species of interesthave not yet been measured by s-SNOM as pure substances. Forbulk crystals, it is well known from theory and experiments that the near-field resonance in the case of a strong oscillator isup-shifted from the transverse phonon frequency that marks theinfrared absorption [6]. The up-shift nearly to the longitudinalphonon frequency amounts to 62 cm-for SiO2 [3], and even to120 cm- for the exceptionally strong phonon of SiC [46]. Forfluorapatite, infrared-active modes are known to be at1030 cm-(strong), 1042.5 cm- (weak), and 1091 cm-(medium) [49], while nano-FTIR registers a strong resonance at1063 cm- (as also in hydroxyapatite) and a weak one at1090 cm, as shown in Figure 12b (for comparison we alsoshow reflectivity spectra that nearly match for both apatites).The strong near-field resonance obviously comes from thestrong infrared-active mode at 1030 cm-l, and thus is up-shiftedby 33 cm-. Naively one would expect that the near-fieldcomponents observed at 1020, 1055, and 1100 cm- in toothmaterials connect to correspondingly lower-frequency, stronginfrared absorption components. But this seems not to be thecase, because the experimental FTIR absorption of dentinexhibits peaks at 1039,1069,1108 [30], or 1040, 1060,1092 cm-[28]. A down-shift of the 1040 line to 1014 cm-was reported for caries-affected dentin [26]. Theoretically it hasnot been explored for the case of small particles as to whether,and in which direction, the near-field resonance should shiftfrom a given far-field absorption peak. Our experiments showthat the near-field resonance in enamel and dentin exhibits apeak near 1020 cm, which is 43 cmbelow the near-fieldresonance of apatite (Figure 12). Generally, the interpretation of infrared absorption observed inbone should be extended to include the influence of the parti-cles' shape through depolarization effects [50,51]. Recently,density functional theory has been applied specifically to theapatite v3 vibrational infrared absorption, predicting strongspectral distortion and splitting (up to ±50 cm-) due to thesemacroscopic electrostatic effects (not to be confused withmicroscopic distortion of lattice cells), depending on whetherthe particles are spherical, needle-like or plate-like[52]. Powdermeasurements with classical FTIR displayed absorption peaksat 1038, 1067, 1097 cm-l for fluorapatite, and at 1034, 1053,1105 cm-for hydroxyapatite, where indeed the last two peakswere found to be strongly split by the nonspherical shape of theparticles [52]. Similar values were reported in other studies[24,53,54]. As the mineral in dentin and bone consists ofisolated, locally ordered apatite platelets, strong depolarizationeffects probably distort the infrared spectra in the v3 phosphateresonance region. Clearly a systematic study is warranted inwhich near-field and far-field infrared apatite bands areacquired for various shapes of chemically and structurally well-defined nanocrystals. Such a study should also cover the weakervi phosphate band, which is less affected by electrostaticeffects, as are all Raman lines [52]. Figure 12b directly illustrates the spectral discriminationprovided by nano-FTIR [46], which has great potential formineral research.The measured near-field response is seento drop within 7 cm- (between 90% and 10% of the peakamplitude); the response is even sharper because our presentinstrumental resolution is about 6 cm-[3]. Additionally,Figure 12b shows that the resonance becomes narrowedsimply by choosing a higher order n of signal demodulation (seeExperimental section)[6]. This would result in a virtual"tip sharpening" and improve the spatial resolution of theS-SNOM [6,55,56]. As for the spectral resolution, a discrimin-ation of components differing by just a few cm- is certainlyachievable. Experimentals-SNOM near-field microscope We employed a commercial scattering near-field microscopebased on AFM (NeaSNOM, neaspec.com) equipped with astandard metalized tip (NCPt arrow, nanoandmore.com). It isoperated in AFM tapping mode to modulate the near-field inter-action between the tip and sample, and records the backscat-tered infrared signal simultaneously with the topography.Typical tapping amplitudes are 50-60 nm. Lock-in detection atthe n=2 harmonic (default) of the tapping frequency (approx.300 kHz) provides background-free near-field imaging. Moni-toring of the infrared signal versus tip-sample separation (ap-proach curves) was used to ensure the optimal working settingsof the tapping amplitude,the demodulation order n, and thefocusing. In the monochromatic infrared near-field imagingmode of the s-SNOM a line-tunable CO, laser attenuated to10 mW is used for illumination. The acquisition time was 5 msper pixel, requiring several minutes for a 128 × 128 sizedimage. Nano-FTIR mode of s-SNOM The nano-FTIR spectroscopic mode of s-SNOM uses illumina-tion by a coherent broadband mid-infrared beam (here 25 uW)from a difference-frequency source [3] driven by a femtosecond(<100 fs) Er fiber laser (FFS.SYS-2B and FFS-CONT,toptica.com). Detection and spectral analysis of the backscat-tered light is by an asymmetric Michelson interferometer thatgenerates, by online Fourier transformation, infrared amplitudeand phase spectra simultaneously; a switchable reference pathensures an absolute quantification of backscattering [3]. Notethat while common FTIR spectrometers are not equipped todetermine the complete,complex material response, the nano-FTIR phase spectra valuably complement the amplitude spectra[6]. For example, the phase change on resonance can be takenas a measure of the resonance strength.Nano-FTIR spectra canbe monitored in real time at 3 Hz rate allowing the optimalfocus adjustment on the tip. The usual acquisition time was 10 s per pixel for obtaining highly resolved spectra as in Figure 3,Figure 5 and Figure 8. The spectroscopic line scans in Figure 9and Figure 10b were obtained with a reduced spectral resolu-tion of about 8 cm-, and the shown result is an average overfive consecutive scans. Figure 12b illustrates that the use of the n=3 instead of then =2 demodulation order reduces the apatite resonancehalfwidth by 40%.However, this is paid for by a five-foldreduction ofthe amplitude, as noted with other crystals previ-ously [3]. Higher power than the presently available 25 pWwould certainly allow for routine use of n=3 and higher. Up to10 mW is desirable (at which point tip heating starts to reducethe AFM stability) and would thus increase the present signallevels by 400x, or alternatively, reduce the acquisition time by160,000× for a constant S/N ratio. Note that this positiveperspective is in sharp contrast to tip-enhanced Raman scat-tering (TERS) for which up to 10 mW is readily available, butintrinsically weak cross sections leave little room for futuresignal improvement [57]. A spectrally integrated mode of nano-FTIR is also introduced inthis study. It employs a fixed interferometer setting at a (free-induction-decay)[2] fringe maximum (ca. 150-300 fs delay).The detector amplitude signal then represents the background-suppressed near-field signal response averaged over a widespectral band around the peak of the backscattered spectrum.Again the routine scanning is at a rate of 5 ms per pixel,requiring several minutes for a 128 ×128 pixel image. Sample preparation The shell valve of M. edulis was sectioned longitudinally into200 um thick wafers. These were polished on both sides andetched for 45 s with a suspension of alumina nanoparticles(Struers OP-A), then cleaned and dried. Tooth samples wereembedded in PMMA following dehydration by a graded ethanoland PMMA exchange solution; samples were cut perpendicu-larly to the tubules, serially ground and polished by usingdiamond slurry down to 1 pm [58]. Line-shape determination of mineral compo-nent in a composite A theory of near-field interaction for the dentin and bone casesof mixed particles that are smaller than the tip radius is not yetavailable. A straightforward solution would be to calculate aneffective dielectric function, by using composite-medium theory[59], as a weighted average of the dielectric functions of theindividual components (and taking proper account of depolar-ization), and then to apply the point-dipole or, better, the finite-dipole model of near-field interaction [3]. While composite-medium theory traditionally assumes spherical particles, an extension to ellipsoids is available [60]. Since individual dielec-tric functions are not known, however, we attempt here, for thefirst time in near-field microscopy, a simplified two-componentanalysis to extract the spectral contribution due to minerals.First, we determine the volume fraction fof mineral nanoparti-cles by extracting f from the spectra in Figure 8 in the followingway. We assume the total scattering amplitude s to be aweighted sum of a mineral and an organic part, sM and s, res-pectively, s=fsM+(1-f)s. We assume f=1 for enamel,which consists nearly entirely of hydroxyapatite nanocrystals.For simplicity we assume a flat spectrum s. By setting s=0.00006 we then determine f= 0.54, 0.30, and 0.26 for thespectra 1, 2, and 3, respectively. With these values we computethe mineral component normalized amplitude spectra,sM=(1-(1-f) s /s)/f shown in Figure 12a. Other settings ofs would give less agreement of the spectra outside the phos-phate band. Conclusion We have quite generally demonstrated the achievement ofchemical identification—a central need for nanoscience—by aninfrared nanoscope, at 20 nm resolution. We show both thehighlighting of a selected compound in a scanned image, aswell the measurement of local FTIR spectra. Our method isnondestructive and needs no vacuum or special sample prepar-ation. Nano-FTIR is widely valuable for studying promisingnanostructures, be it in nanotechnology, the pharmaceuticalindustry, or solid-state physics. For this study we have chosenbiominerals over other obvious candidates because biomineral-ization is unexplored in its nanometer-scale detail but is yet ofgreat medical importance. Author contributions F. K. conceived this study, P. Z.,W. W. S. and E. G. identifiedand characterized the biomineral samples. F. K. and S. A.designed the SNOM experiments and analyzed the results.S. A., P. Z. and Y. K. performed the experiments. F. K. wrotethe draft, and all authors contributed to the manuscript. Acknowledgements The authors are indebted to P. Fratzl for his long-term interestand support of this study. They acknowledge discussions withP. Hansma, U. Schade and A. Roseler. Supported by DeutscheForschungsgemeinschaft through the Cluster of ExcellenceMunich Centre for Advanced Photonics. References 1. Griffiths, P. R.; de Haseth, J. A. Fourier Transform InfraredSpectroscopy; Wiley: New York, 2007. doi:10.1002/047010631X ( 2 . Amarie, S.; G anz, T.; Keilmann, F . Opt. Express 2009, 1 7,21794 doi:10.1364/OE.17.021794 ) ( 3. A marie, S.; Keilmann, F . Phys. Rev.B 2011,83 , 45404.doi:10.1103/PhysRevB.83.045404 ) ( 4. H uth, F.; Schnell, M.; Wi t tborn, J.; Ocelic, N.; Hillenbrand, R.Nat. Mater. 2011, 10, 352. doi:10.1038/nmat3006 ) ( 5. K noll, B.;Keilmann, F. Nature 1999, 399, 134. doi:10.1038/20154 ) ( 6. K eilmann, F.; Hillenbrand, R. In Nano-Optics and Near-Field OpticalMicroscopy; Richards, D.; Zayats, A., E d s.; A r tech House: Boston, London, 2009 ) ( 7 . C vitkovic, A.; Ocelic, N.; Hillenbrand, R. Nano Lett. 2 007, 7, 3 177. doi:10.1021/nl071775+ ) ( 8. T aubner, T. ; Hillenbrand, R. ; Ke i lmann, F. App l . Phys. Lett . 2004, 85, 5064. doi:10 . 1063/1.1827334 ) ( 9 . . Brehm, M .; Ta u bner, T.; Hillenbrand, R.; K eilmann, F . Nano Lett. 2006, 6, 1 307.doi:10.1021/nl0610836 ) ( 10. Kim, Z. H. ; Liu, B.; Leone, S. R. J. Phys. C hem. B 20 0 5, 109 , 8503.doi:10.1021/jp047425i ) ( 11. Huber, A. J . ; Keilmann, F.; Wi t tborn, J.; Ai z purua, J.; Hil l enbrand, R. Nano Lett. 2008, 8,3766. doi:10.1021/nl802086x ) ( 12. Qazilbash, M. M. ; Brehm, M.; C hae, B.-G.;Ho, P.-C.; Andreev, G. O.; Kim, B.-J.; Yun, S. J.; Balatsky, A. V .; Maple, M. B . ; Keilmann, F.; Kim, H.-T.; Basov, D. N. Science 2007, 318,1750.doi:10.1126/science.1150124 ) ( 13.Hillenbrand, R .; Keilmann, F. Appl. Phys. Lett.2002, 80, 2 5 . doi:10.1063/1.1428767 ) ( 14. Hillenbrand, R.; Keilmann, F. Phys. Rev. L ett. 2000, 85, 3 029. doi:10.1103/PhysRevLett.85.3029 ) ( 15.Lowenstam, H. A. Science 1981, 211, 1126. doi:10.1126/science.7008198 ) ( 16.Weiner, S.; Wagner, H. D. Annu. Rev. Mater. Sci. 1998, 28,271. doi:10.1146/annurev.matsci.28.1.271 ) ( 17.Meldrum, F. C.; Colfen,H. Chem. Rev. 2008, 108,4 3 32. doi:10.1021/cr8002856 ) ( 18. Schmahl, W. W.; Griesshaber,E. ; Neuser, R. ; Lenze, A.;Job, R.;Brand, U. Eur. J . Mineral.2004, 16,693. doi:10.1127/0935-1221/2004/0016-0693 ) ( .19. Griesshaber, E.; Schmahl, W. W .;Neuser, R.; Pettke, T.; Blum, M.;Mutterlose, J.;Brand, U. Am. Mineral. 2007, 92,7 2 2. doi:10.2138/am.2007.2220 ) ( 20. Goetz, A. J.;Steinmetz, D . R . ; Griesshaber, E.;Z a effere, S.; Raabe, D.; Kelm, K.; I rsen, S.;Sehrbock, A.; Schmahl, W. W. .Acta Biomater. 2011, 7 ,2237. d o i:10.1016/j.actbio.2011.0 1 .035 ) ( 21.Merkel, C.;Griesshaber, E.; Ke l m, K.; Neuser, R.; Jordan, G.; ) ( Logan, A .; Mader, W.; Schmahl, W. W. J. Geophys. Res. 2007, 112, G02008.doi:10.1029/2006JG000253 ) ( 2 2. Schmahl, W. W.; Griesshaber,E.; Merkel, C.; Ke l m, K.; Deuschle, J.;Neuser, R. D.; Goetz, A. J.; Sehrbrock, A.; Mader, W. Mineral. Mag. 2008,72, 541. doi:10.1180/minmag.2008.072.2.541 ) ( 23.Rey, C.; Shimizu, M.; Col l ins, B.; Gli m cher, M. J. Calcif. Tissue Int.1991,49, 383. doi:10.1007/BF02555847 ) ( 24.Pleshko,N.; B oskey, A .; Mendelsohn, R. Biophys. J.1991, 60, 786doi:10.1016/S0006-3495(91)82113-0 ) ( 25. Carden, A.; M orris, M. D. J . Biomed.Op t . 20 0 0, 5, 25 9 . doi:10.1117/1.429994 ) ( 26. Spencer, P . ; Wang, Y.; Ka t z, J. L.; M i sra, A. J. B i omed.Opt. 2005, 10, 031104. d oi:10.1117/1.1914844 ) ( 27. Boskey, A.; Mendelsohn, R . J. Biomed. O pt. 2005, 1 0, 0 31102 doi:10.1117/1.1922927 ) ( .28.Abraham, J . A.; Sanchez, H. J . ; M arceli, C. A.; Grenon, M.; ) ( G uidi, M. C.; Piccinin i , M.Anal. Bioana l . Chem.2011,399,1699. doi:10.1007/s00216-010-4430-0 ) ( 29.Paschalis, E. P .; Mendelsohn, R.; Boskey, A. L.Clin. Orthop. Relat . Res. 20 1 1, 469,2170. doi:10.1007/s11999-010-1751-4 ) ( 3 0.Tesch, W . ; E i delman, N.;Ro s chger, P.; G o ldenberg, F.; Klaushofer, K.; F ratzl, P . Calcif. Tissue I nt. 2001,69, 1 4 7. doi:10.1007/s00223-001-2012-z ) ( 3 1.Paschalis, E. P.; DiCarlo, E .; Betts, F. ; Sherman, P.; M endelsohn, R. ; B oskey, A . L. Calcif. Tissue Int. 1996,59, 480. doi:10.1007/BF00369214 ) ( 3 2.Gourion-Arsiquaud, S.; F a ibish, D.; M yers, E .; Spevak, L.; . Compston, J.;Hodsman, A.;S h ane, E.; Recker, R . R . ; Boskey,E. R.;Boskey, A. L. J. Bone Miner. Res. 2009, 24, 1565. doi:10.1359/jbmr.090414 ) ( 33.Dalbeck, P.; England, J.; Cusack, M.; Lee, M.R.; Fallick, A. E . Eur. J. Mineral. 2006, 18,601.doi:10.1127/0935-1221/2006/0018-0601 ) ( 3 4.Feng, Q. L.; Li , H. B.; P u , G.; Zhang, D. M .; Cui, F. Z.; Li, H. D.; Kim,T. N. J. Mater. Sci. 2000, 35,3337. doi:10.1023/A:1004843900161 ) ( 3 5.Griesshaber,E.; Ke l m, K.; Jordan, G. ; Xu, D. ; Schmahl, W. W. I n . preparation. ) ( 3 6. Taubner, T.; Keilmann,F. ; Hillenbrand, R. Opt. Ex p ress 2005, 13, 8893. doi:10.1364/OPEX.13.008893 ) ( 37.Zaslansky, P. In C ollagen: S tructure and Mechanics; Fratzl, P ., Ed.;Springer: Berlin, Heidelberg, 2008. 3 8.Mjor, I. A.; Nordahl, I. Arch. Ora l Bio l . 199 6 ,41,401 . doi:10.1016/0003-9969(96)00008-8 ) ( 3 9.Roschger, P.; Fratzl, P.; Eschberger, J.; Klaushofer, K. Bone 1998, 23,319. doi:10.1016/S8756-3282(98)00112-4 ) 40.Raschke, M. B.; Lienau, C. Appl. Phys. Lett. 2003, 83,5089 doi:10.1063/1.1632023 41. Sun, J.; Schotland, J. C.; Hillenbrand, R.; Carney, P. S. Appl. Phys. Lett. 2009, 95, 121108. doi:10.1063/1.3224177 42.Lowenstam, H. A.;Weiner,S. Science 1985, 227,51. doi:10.1126/science.227.4682.51 43. Lee, A. P.; Brooker, L. R.; Macey, D. J.;van Bronswijk, W.; Webb, J. Calcif. Tissue Int. 2000, 67,408.doi:10.1007/s002230001156 . 44. Thurner, P. J.; Lam, S.;Weaver, J. C.; Morse, D. E.; Hansma, P. K J. Adhes. 2009, 85, 526. doi:10.1080/00218460902996424 45. Becker, A.;Ziegler, A.;Epple, M. Dalton Trans. 2005, 1814 doi:10.1039/b412062k 46.Hillenbrand, R.; Taubner, T.; Keilmann, F. Nature 2002, 418, 159. doi:10.1038/nature00899 47.Ocelic, N.; Hillenbrand, R. Nat. Mater. 2004, 3, 606. doi:10.1038/nmat1194 48.Wells, O. C. Scanning Electron Microsc. 1977, 1,747. 49.Kravitz, L. C.; Kingsley, J. D.; Elkin, E. L. J. Chem. Phys. 1968, 49, 4600. doi:10.1063/1.1669918 50. Fuchs, R. Phys.Rev.B 1975, 11, 1732. doi:10.1103/PhysRevB.11.1732 51.Bohren, C. F.; Huffmann, D. R. Absorption and Scattering of Light by Small Particles; Wiley: New York, 1983. 52. Balan, E.; Delattre, S.; Roche,D.;Segalen, L.; Morin, G.; Guillaumet, M.; Blanchard, M.; Lazzeri, M.; Brouder, C.; Salje, E. K. H Phys. Chem. Miner. 2011, 38, 111. doi:10.1007/s00269-010-0388-x .53.Penel, G.; Leroy, G.; Rey, C.; Sombret,B.; Huvenne, J. P.; Bres, E J.Mater. Sci.:Mater. Med. 1997, 8,271. doi:10.1023/A:1018504126866 54. Leroy, G.; Leroy, N.; Penel, G.; Rey,C.; Lafforgue, P.; Bres, E. .Appl. Spectrosc. 2000, 54, 1521.doi:10.1366/0003702001948448 55.Knoll, B.; Keilmann, F. Opt. Commun. 2000, 182, 321.doi:10.1016/S0030-4018(00)00826-9 56. Giessibl, F. J. Science 1995,267, 68. doi:10.1126/science.267.5194.68 57.Richter, M.; Hedegaard, M.;Deckert-Gaudig, T.;Lampen, P.;Deckert, V. Small 2011, 7, 209. doi:10.1002/smll.201001503 ( 58.Zaslansky, P.; Z a bler, S . ; Fratzl, P. Dent. Mater. 2 010, 26, e1.doi:10.1016/j.dental.2009.09.007 ) 59.Bruggeman, D. A. G. Ann. Phys. (Berlin, Ger.) 1935, 416,636.doi:10.1002/andp.19354160705 ( 60. Hinrichs, K.; Roseler,A.; Roodenko, K.; Rappich,J. Appl. Spectrosc.2008,62, 121.doi:10.1366/000370208783412744 ) License and Terms This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), whichpermits unrestricted use, distribution, and reproduction inany medium, provided the original work is properly cited. The license is subject to the Beilstein Journal ofNanotechnology terms and conditions: (http://www.beilstein-journals.org/bjnano) The definitive version of this article is the electronic onewhich can be found at: doi:10.3762/bjnano.3.35 本文第一次以自然纳米结构(贝壳和骨质中的矿物质颗粒)的化学鉴定,证明通过红外近场显微镜技术能够解决以上问题。 纳米傅立叶红外光谱(nano-FTIR)是通过将傅立叶变换红外光谱技术(FTIR) 与散射式扫描近场光学显微技术(s-SNOM)结合获得的。对紫贻贝贝壳横截面抛光处理后,通过Nano-FTIR可以重复的观察到生物钙质微晶体的声子共振,以及生物文石质上明显不同的光谱特征。更重要的是,本研究第一次在紫贻贝贝壳中发现了尺寸为20nm左右、稀疏分布的纳米颗粒,其显著不同的光谱特征表明这些纳米颗粒为磷化物晶体。对人类牙齿界面的研究观察到了多组分磷酸盐的红外吸收峰。这些光谱在牙本质小管附近有明显的特征变换,证明了磷灰石纳米晶体的化学与结构的变化。红外光谱峰的强弱对应矿物质浓度变化,这点通过电镜得到印证。Nano-FTIR对结构的畸变反应敏感,因此非常适用于对生物矿物质形成和老化的研究。总体来说nano-FTIR适用于从微纳加工到临床骨科研究等多种学科中涉及复合材料的分析和鉴定工作。参考文献:Amarie S , Keilmann F , Zaslansky P , et al. Nano-FTIR chemical mapping of minerals in biological materials[J]. Microscopy & Microanalysis, 2012, 18(S2):32-33.
确定







还剩10页未读,是否继续阅读?
QUANTUM量子科学仪器贸易(北京)有限公司为您提供《紫贻贝贝壳及人类牙齿中矿物质检测方案(红外光谱仪)》,该方案主要用于牙齿中矿物质检测,参考标准--,《紫贻贝贝壳及人类牙齿中矿物质检测方案(红外光谱仪)》用到的仪器有德国Neaspec纳米傅里叶红外光谱仪nano-FTIR
推荐专场
相关方案
更多
该厂商其他方案
更多















