方案详情
文
There are many fast optical imaging techniques available to the cell biologist which are widely used to analyse biological specimens. Emerging micro-spectroscopic techniques such as Raman microscopy open up an exciting new dimension for imaging, by combining spatial and spectroscopic information. Through the ability to investigate real cellular biochemistry it is possible to rapidly learn more about chemical processes, interactions, and behaviour, down to the single cell level.
方案详情

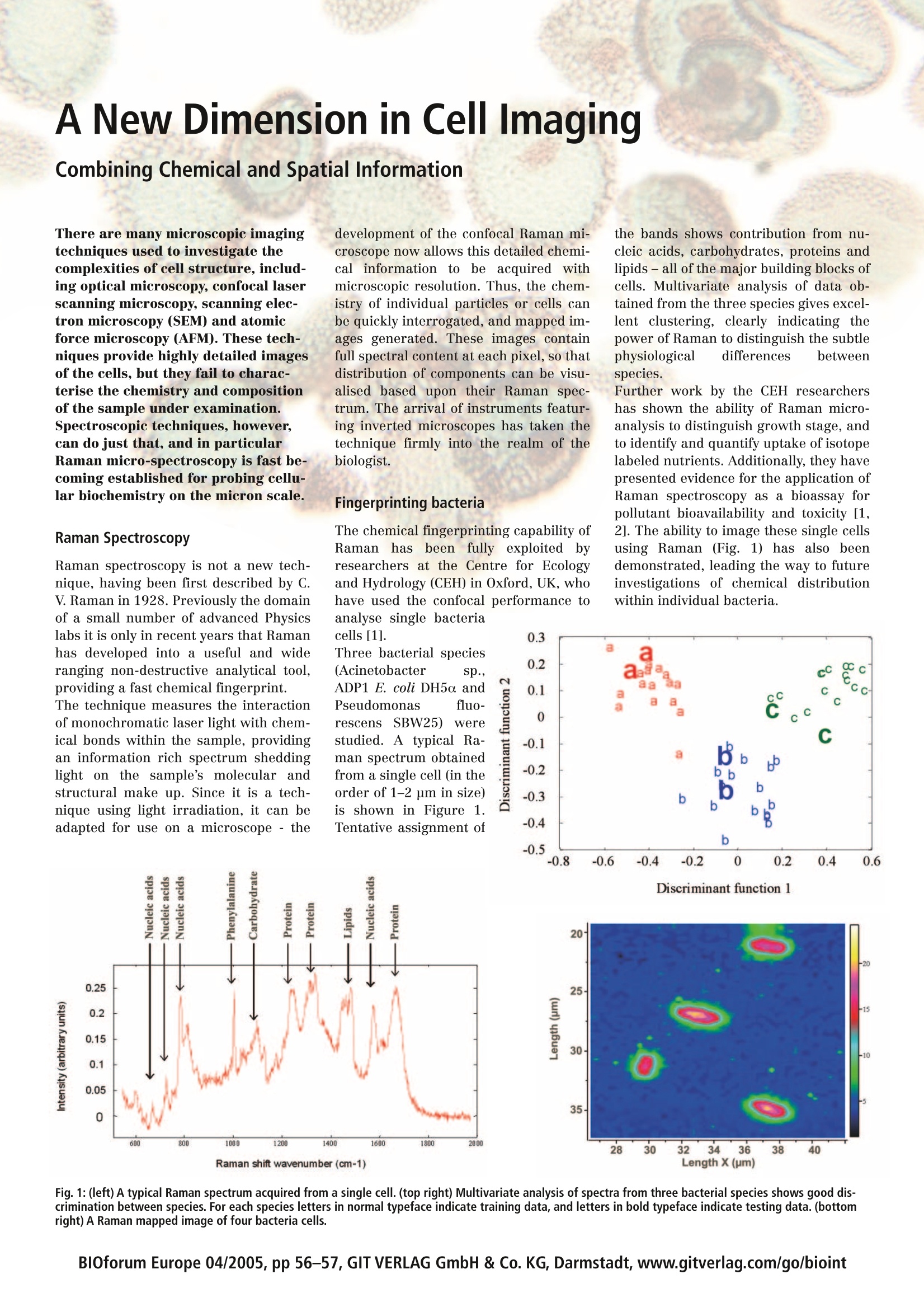
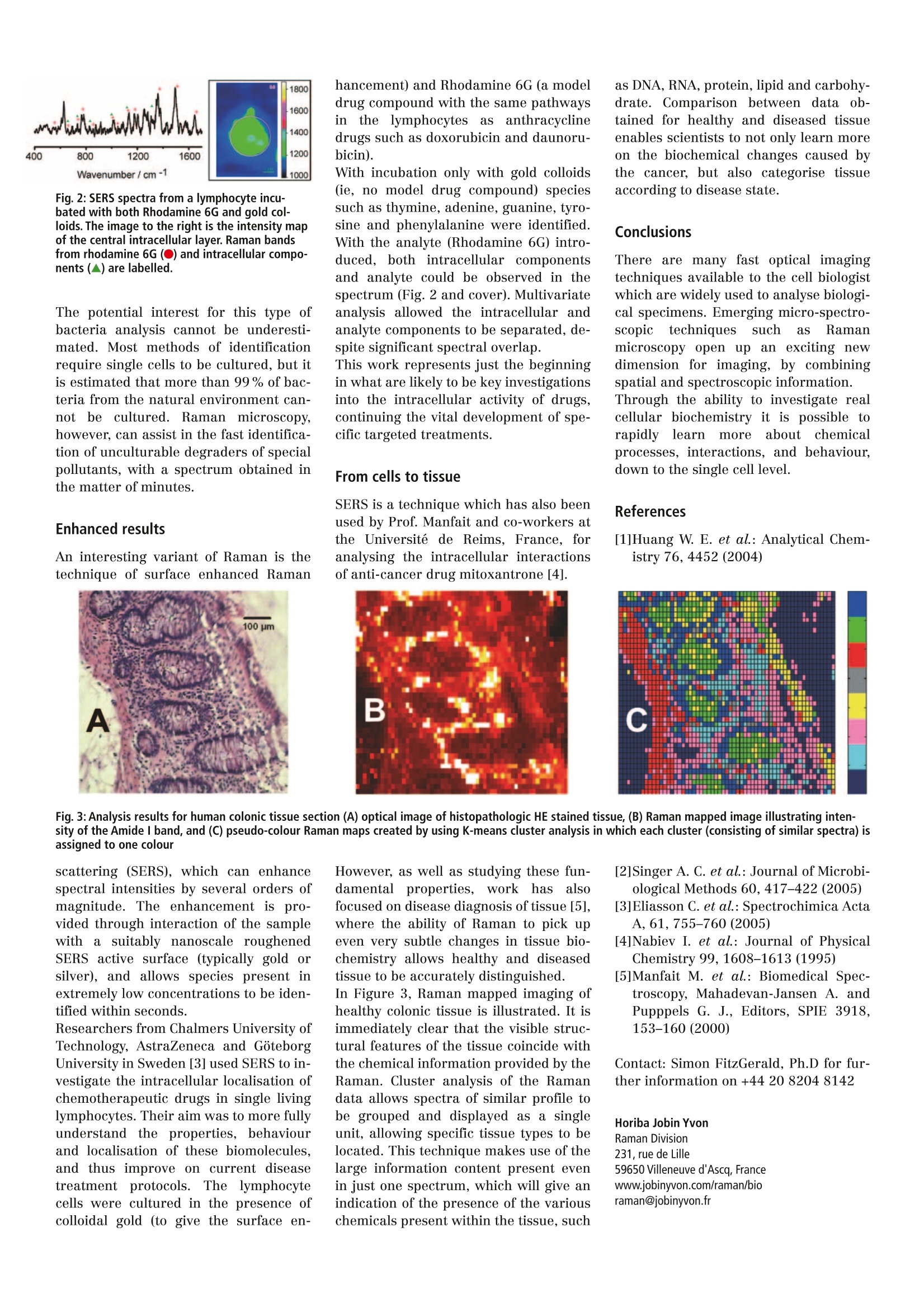
B30 Europe forum TRENDS & TECHNIQUES IN LIFE SCIENCE RESEARCH Volume 9June 2005pp56-57 4 400 800 1200 1600 vM. Wavenumber/cm-1 400 800 1200 1600 Wavenumber/cm-1 wwi 400 800 1200 1600 Wavenumber/cm-l Cell Biology Proteomics Country Focus UK Protein Engineering A New Dimension in Cell ImagingCombining Chemical and Spatial Information There are many microscopic imagingtechniques used to investigate thecomplexities of cell structure, includ-ing optical microscopy, confocal laserscanning microscopy, scanning elec-tron microscopy (SEM) and atomicforce microscopy (AFM). These tech-niques provide highly detailed imagesof the cells, but they fail to charac-terise the chemistry and compositionof the sample under examination.Spectroscopic techniques, however,can do just that, and in particularRaman micro-spectroscopy is fast be-coming established for probing cellu-lar biochemistry on the micron scale. Raman Spectroscopy Raman spectroscopy is not a new tech-nique, having been first described by C.V. Raman in 1928. Previously the domainof a small number of advanced Physicslabs it is only in recent years that Ramanhas developed into a useful and wideranging non-destructive analytical tool,providing a fast chemical fingerprint. The technique measures the interactionof monochromatic laser light with chem-ical bonds within the sample, providingan information rich spectrum sheddinglight on the sample’s molecular andstructural make up. Since it is a tech-nique using light irradiation, it can beadapted for use on a microscope -the development of the confocal Raman mii-croscope now allows this detailed chemi-calinformation to be acquired withmicroscopic resolution. Thus, the chem-istry of individual particles or cells canbe quickly interrogated, and mapped im-ages generated. These images containfull spectral content at each pixel, so thatdistribution of components can be visu-alised based upon their Raman spec-trum. The arrival of instruments featur-ing inverted microscopes has taken thetechnique firmly into the realm of thebiologist. Fingerprinting bacteria The chemical fingerprinting capability ofRamanhas been fully exploited byresearchers at the Centre for Ecologyand Hydrology (CEH) in Oxford, UK, whohave used the confocal performance toanalyse single bacteria the bands shows contribution from nu-cleic acids, carbohydrates, proteins andlipids-all of the major building blocks ofcells. Multivariate analysis of data ob-tained from the three species gives excel-lent clustering, clearly indicating thepower of Raman to distinguish the subtlephysiological differences betweenspecies. Further work by the CEH researchershas shown the ability of Raman micro-analysis to distinguish growth stage,andto identify and quantify uptake of isotopelabeled nutrients. Additionally, they havepresented evidence for the application ofRaman spectroscopy as a bioassay forpollutant bioavailability and toxicity [1,2]. The ability to image these single cellsusing Raman (Fig. 1) has also beendemonstrated, leading the way to futureinvestigations of chemical distributionwithin individual bacteria. Discriminant function 1 Fig.1:(left) A typical Raman spectrum acquired from a single cell. (top right) Multivariate analysis of spectra from three bacterial species shows good dis-crimination between species. For each species letters in normal typeface indicate training data, and letters in bold typeface indicate testing data. (bottomright) A Raman mapped image of four bacteria cells. 1600 1400 1200 Wavenumber/ cm-1 1000 Fig. 2: SERS spectra from a lymphocyte incu-bated with both Rhodamine 6G and gold col-loids. The image to the right is the intensity mapof the central intracellular layer. Raman bandsfrom rhodamine 6G (O)) and intracellular compo-nents (▲) are labelled. The potential interest for this type ofbacteria analysis cannot be underesti-mated. Most methods of identificationrequire single cells to be cultured, but itis estimated that more than 99% of bac-teria from the natural environment can-not be cultured.Raman microscopy,however, can assist in the fast identifica-tion of unculturable degraders of specialpollutants, with a spectrum obtained inthe matter of minutes. Enhanced results An interesting variant of Raman is thetechnique of surface enhanced Raman hancement) and Rhodamine 6G (a modeldrug compound with the same pathwaysini ttheelymphocytesiasanthracyclinedrugs such as doxorubicin and daunoru-bicin). With incubation only with gold colloids(ie, no model drug compound) speciessuch as thymine, adenine, guanine, tyro-sine and phenylalanine were identified.With the analyte (Rhodamine 6G) intro-duced, both intracellular componentsand analyte could be observed in thespectrum (Fig. 2 and cover). Multivariateanalysis allowed the intracellular andanalyte components to be separated, de-spite significant spectral overlap. This work represents just the beginningin what are likely to be key investigationsinto the intracellular activity of drugs,continuing the vital development of spe-cific targeted treatments. From cells to tissue SERS is a technique which has also beenused by Prof. Manfait and co-workers atthe Universite de Reims, France, foranalysing the intracellular interactionsof anti-cancer drug mitoxantrone [4]. as DNA, RNA, protein, lipid and carbohy-drate. Comparison between data ob-tained for healthy and diseased tissueenables scientists to not only learn moreon the biochemical changes caused bythe cancer, but also categorise tissueaccording to disease state. Conclusions There are many fast optical imagingtechniques available to the cell biologistwhich are widely used to analyse biologi-cal specimens. Emerging micro-spectro-scopictechniques such as Ramanmicroscopy open up an exciting newdimension for imaging, by combiningspatial and spectroscopic information.Through the ability to investigate realcellular biochemistry it is possible torapidly iearnmoreaboutitchemicalprocesses, interactions, and behaviour,down to the single cell level. References [1]Huang W. E. et al.: Analytical Chem-istry 76, 4452 (2004) Fig. 3: Analysis results for human colonic tissue section (A) optical image of histopathologic HE stained tissue, (B) Raman mapped image illustrating inten-sity of the Amide l band, and (C) pseudo-colour Raman maps created by using K-means cluster analysis in which each cluster (consisting of similar spectra) isassigned to one colour scattering (SERS), which can enhancespectral intensities by several orders ofmagnitude. The enhancement is pro-vided through interaction of the samplewith a suitably nanoscale roughenedSERS active surface (typically gold orsilver), and allows species present inextremely low concentrations to be iden-tified within seconds. Researchers from Chalmers University ofTechnology, AstraZeneca and GoteborgUniversity in Sweden [3] used SERS to in-vestigate the intracellular localisation ofchemotherapeutic drugs in single livinglymphocytes. Their aim was to more fullyunderstand the properties, behaviourand localisation of these biomolecules,and thus improve on current diseasetreatmentprotocols.Thelymphocytecells were cultured in the presence ofcolloidal gold (to give the surface en- However, as well as studying these fun-damentalproperties, work has alsofocused on disease diagnosis of tissue [5],where the ability of Raman to pick upeven very subtle changes in tissue bio-chemistry allows healthy and diseasedtissue to be accurately distinguished.In Figure 3,Raman mapped imaging ofhealthy colonic tissue is illustrated. It isimmediately clear that the visible struc-tural features of the tissue coincide withthe chemical information provided by theRaman. Cluster analysis of the Ramandata allows spectra of similar profile tobe grouped and displayed as a singleunit, allowing specific tissue types to belocated. This technique makes use of thelarge information content present evenin just one spectrum, which will give anindication of the presence of the various chemicals present within the tissue, such [2]Singer A. C. et al.: Journal of Microbi-ological Methods 60, 417-422 (2005) [3]Eliasson C. et al.: Spectrochimica ActaA, 61, 755-760 (2005) [4]Nabiev I. et al.: Journal of PhysicalChemistry 99, 1608-1613 (1995) [5]Manfait M. et al.: Biomedical Spec-troscopy, Mahadevan-Jansen A. andPupppels G. J., Editors, SPIE 3918,153-160(2000) Contact: Simon FitzGerald, Ph.D for fur-ther information on +44 20 82048142 Horiba Jobin YvonRaman Division231, rue de Lille59650Villeneuve d'Ascq, Francewww.jobinyvon.com/raman/bioraman@jobinyvon.fr HORIBAJOBIN YVON BlOforum Europe pp GIT VERLAG GmbH & Co. KG, Darmstadt, www.gitverlag.com/go/bioint There are many fast optical imaging techniques available to the cell biologist which are widely used to analyse biological specimens. Emerging micro-spectroscopic techniques such as Raman microscopy open up an exciting new dimension for imaging, by combining spatial and spectroscopic information. Through the ability to investigate real cellular biochemistry it is possible to rapidly learn more about chemical processes, interactions, and behaviour, down to the single cell level.
确定
还剩1页未读,是否继续阅读?
HORIBA(中国)为您提供《细菌,细胞中化学组成及分布检测方案(激光拉曼光谱)》,该方案主要用于其他中化学组成及分布检测,参考标准--,《细菌,细胞中化学组成及分布检测方案(激光拉曼光谱)》用到的仪器有
相关方案
更多
该厂商其他方案
更多









