
ΑIIbβ3 is a key mediator in the thrombotic process and has been studied extensively. This current work reports the first Raman spectrum of the protein, and examines changes in conformation during activation. Using three common agonists (DTT, Mn(II) and EDTA), the results indicate that in each case activation is caused by reduction of disulfide links, but suggests that different disulfides are activated according to the reagent used. The histopathological correlation to these findings has yet to be determined.
方案详情

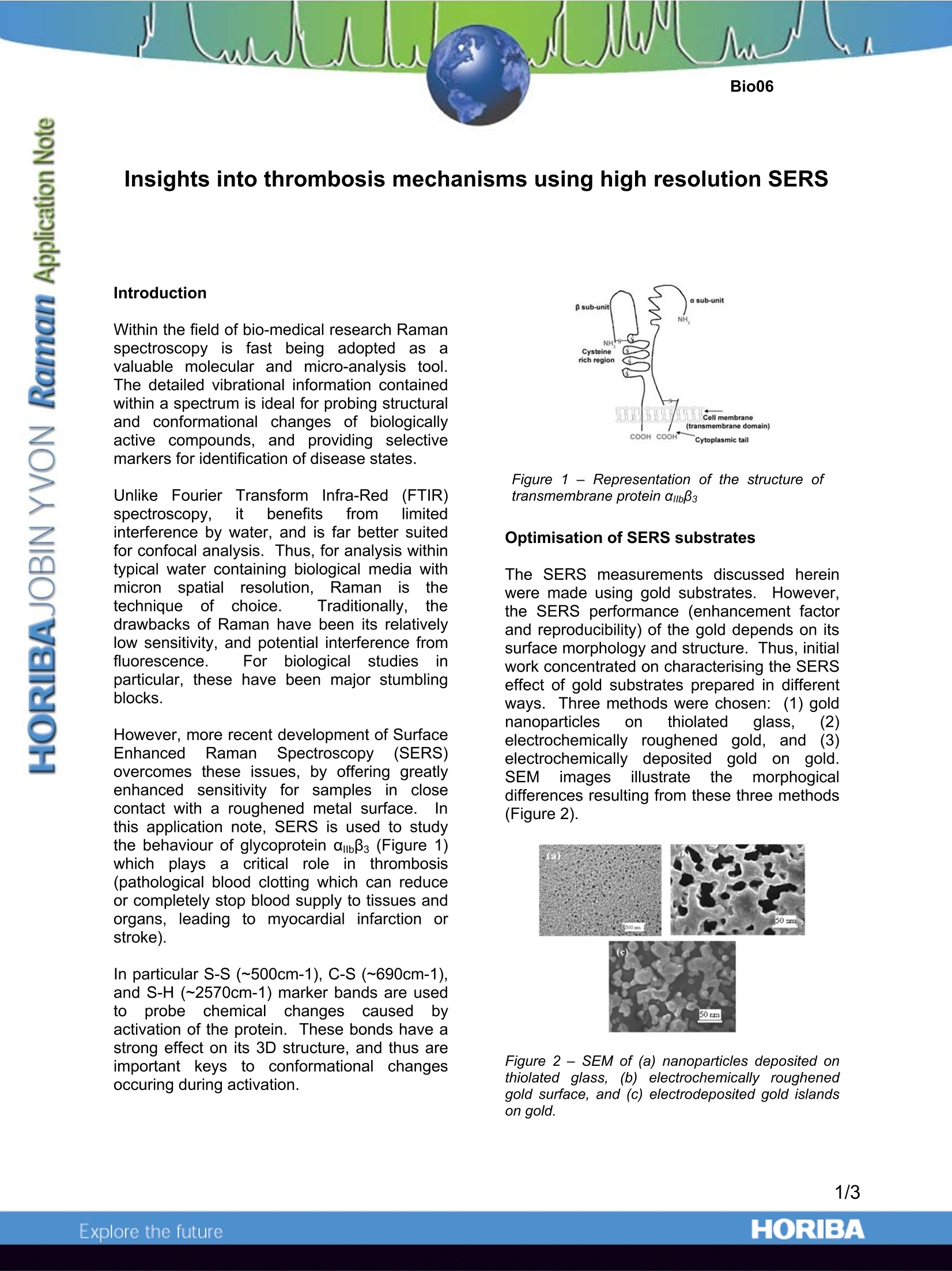
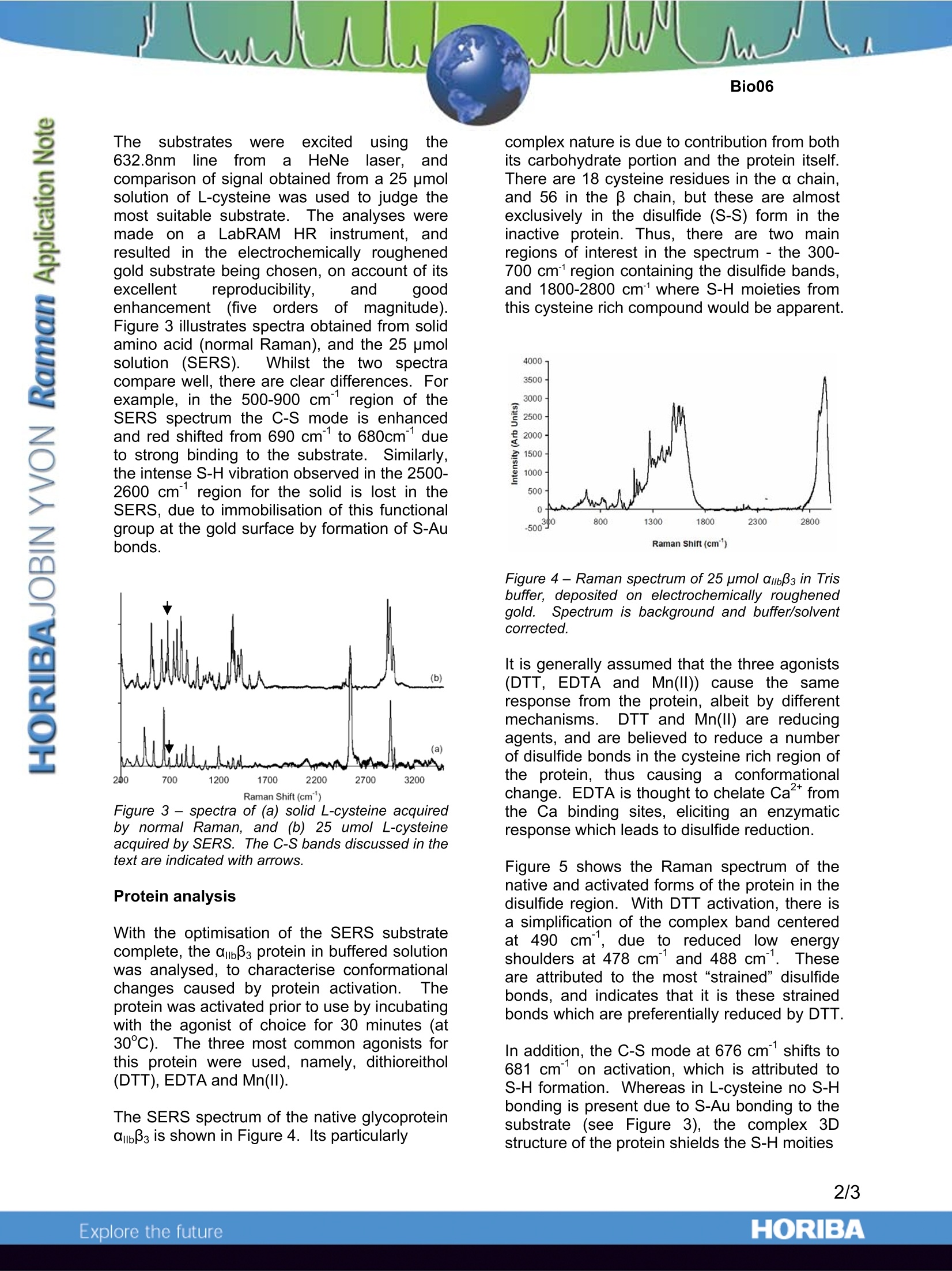
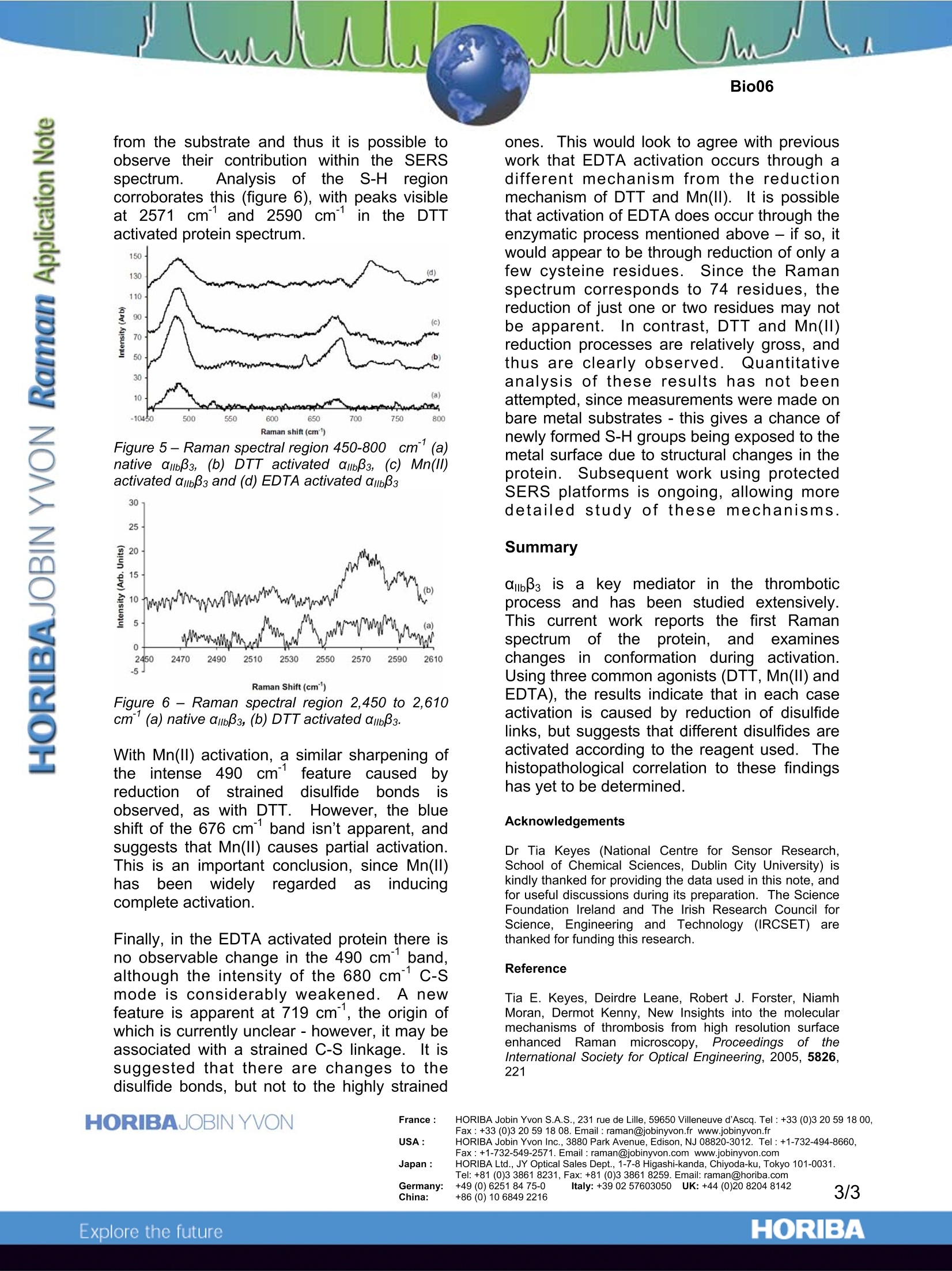
Insights into thrombosis mechanisms using high resolution SERS Introduction Within the field of bio-medical research Ramanspectroscopy is fast being adopted as; avaluable molecular and micro-analysis tool.The detailed vibrational information containedwithin a spectrum is ideal for probing structuraland conformational changes of biologicallyactive compounds, and providing selectivemarkers for identification of disease states. Unlike Fourier Transform Infra-Red(FTIR)spectroscopy,it benefitsffrom limitedinterference by water, and is far better suitedfor confocal analysis. Thus, for analysis withintypical water containing biological media withmicron spatial resolution, Raman iisnetechnique of choice. Traditionally,drawbacks of Raman have been its relativelylow sensitivity, and potential interference fromfluorescence. For biological studiesiparticular, these have been major stumblingblocks. However, more recent development of SurfaceEnhanced RamanSpectroscopy(SERS)overcomes these issues, by offering greatlyenhancedsensitivity for samplesin closecontact with a roughened metal surface.. Inthis application note, SERS is used to studythe behaviour of glycoprotein ambBs (Figure 1)which plays a critical role in11thrombosis(pathological blood clotting which can reduceor completely stop blood supply to tissues andorgans,, leading to myocardial infarction orstroke). In particular S-S (~500cm-1), C-S (~690cm-1),and S-H (~2570cm-1) marker bands are usedtoprobe chemical changescausedbyactivation of the protein. These bonds have astrong effect on its 3D structure, and thus areimportant keys to conformational changesoccuring during activation. Figure 1 -Representation of the structure oftransmembrane protein amb33 Optimisation of SERS substrates The SERS measurements discussed hereinwere made using gold substrates. However,the SERS performance (enhancement factorand reproducibility) of the gold depends on itssurface morphology and structure. Thus, initialwork concentrated on characterising the SERSeffect of gold substrates prepared in differentways. Three methods were chosen:(1) goldnanoparticles on thiolatedglass,electrocheemmiiccaallllyy roughenedgold, andelectrochemicallydepositedgold on gold.SEM imagesillustratethe morphogicaldifferences resulting from these three methods(Figure 2). Figure 2- SEM of (a) nanoparticles deposited onthiolated glass, (b) electrochemically roughenedgold surface, and (c) electrodeposited gold islandson gold. The substrates Were excited using the632.8nmline froma HeNelalaser,, andcomparison of signal obtained from a 25 pmolsolution of L-cysteine was used to judge themost suitable substrate.TThe analyses weremade on a LabRAM HR instrument, andresulted in the electrochemically roughenedgold substrate being chosen, on account of itsexcellent reproducibility, and goodenhancement(five orders of magnitude).Figure 3 illustrates spectra obtained from solidamino acid (normal Raman), and the 25 umolsolution(SERS). vWhilst the two spectracompare well, there are clear differences. Forexample, in the 500-900 cm’region of theSERS spectrum the C-S mode is enhancedand red shifted from 690 cm to 680cm° dueto strong binding to the substrate. Similarly,the intense S-H vibration observed in the 2500-2600 cm’ region for the solid is lost in theSERS, due to immobilisation of this functionalgroup at the gold surface by formation of S-Aubonds. Figure 3 - spectra of (a) solid L-cysteine acquiredby normal Raman, and (b) 25 umol L-cysteineacquired by SERS. The C-S bands discussed in thetext are indicated with arrows. Protein analysis With the optimisation of the SERS substratecomplete, the ambB3 protein in buffered solutionwas analysed, to characterise conformationalchanges caused by protein activation.l.Theprotein was activated prior to use by incubatingwith the agonist of choice for 30 minutes (at30℃). The three most common agonists forthis protein were used, namely, dithioreithol(DTT), EDTA and Mn(II). The SERS spectrum of the native glycoproteinabB3 is shown in Figure 4. Its particularly complex nature is due to contribution from bothits carbohydrate portion and the protein itself.There are 18 cysteine residues in the a chain,and 56 in the β chain, but these are almostexclusively in the disulfide (S-S) form in theinactive protein. Thus, there are two mainregions of interest in the spectrum - the 300-700 cm' region containing the disulfide bands,and 1800-2800 cm'where S-H moieties fromthis cysteine rich compound would be apparent. Figure 4-Raman spectrum of 25 pmol ambB3 in Trisbuffer, deposited on electrochemically roughenedgold..Spectrum is background and buffer/solventcorrected. It is generally assumed that the three agonists(DTT, EDTA and Mn(ll)) cause the sameresponse from the protein, albeit by differentmechanisms5..1DTT and Mn(ll) are reducingagents, and are believed to reduce a numberof disulfide bonds in the cysteine rich region ofthe protein, thus causing a conformationalchange. EDTA is thought to chelate Ca fromthe Ca binding sites, eliciting an enzymaticresponse which leads to disulfide reduction. Figure 5 shows the Raman spectrum of thenative and activated forms of the protein in thedisulfide region. With DTT activation, there isa simplification of the complex band centeredat 490 cm, due to reduced low energyshoulders at 478 cm and 488 cm1. Theseare attributed to the most “strained”disulfidebonds, and indicates that it is these strainedbonds which are preferentially reduced by DTT. In addition, the C-S mode at 676 cm shifts to681 cm on activation, which is attributed toS-H formation. Whereas in L-cysteine no S-Hbonding is present due to S-Au bonding to thesubstrate (see Figure 3), the complex 3Dstructure of the protein shields the S-H moities from the substrate and thus it is possible toobserve their contribution within the SERSspectrum. iAnalysis of ithe S-H regioncorroborates this (figure 6), with peaks visibleat 2571 cmand 2590 cm1in the DTTactivated protein spectrum. Figure 5- Raman spectral region 450-800) cm(a)native ambB3, (b) DTT activated ambB3, (c) Mn(ll)activated ambBs and (d) EDTA activated ambBs 30 Figure 6- Raman spectral region 2,450 to 2,610cm’(a) native ambB3, (b) DTT activated ambB3. With Mn(II) activation, a similar sharpening ofthe intense 490 cmfeature causedbreductionoffstraineddisulfideebondsobserved, as with DTT. However, the blueshift of the 676 cm band isn’t apparent, andsuggests that Mn(II) causes partial activation.This is an important conclusion, since Mn(II)hass beennwidely regardedas inducingcomplete activation. Finally, in the EDTA activated protein there isno observable change in the 490 cmband,although the intensity of the 680 cm°c-smode is considerably weakened. A newfeature is apparent at 719 cm, the origin ofwhich is currently unclear-however, it may beassociated with a strained C-S linkage. It issuggested that there are changes to thedisulfide bonds, but not to the highly strained ones.This would look to agree with previouswork that EDTA activation occurs through adifferent mechanism from the reductionmechanism of DTT and Mn(lI). It is possiblethat activation of EDTA does occur through theenzymatic process mentioned above - if so, itwould appear to be through reduction of only afew cysteine residues. Since the Ramanspectrum corresponds to 74 residues, thereduction of just one or two residues may notbe apparent.In contrast, DTT and Mn(II)reduction processes are relatively gross, andthus are clearly observed.. Quantitativeanalysis of these results has not beenattempted, since measurements were made onbare metal substrates - this gives a chance ofnewly formed S-H groups being exposed to themetal surface due to structural changes in theprotein. Subsequent work using protectedSERS platforms is ongoing, allowing moredetailed study of these rmechanisms. Summary abBs is a key mediator in the thromboticprocess andhas been studied extensively.This; current work reportsthe first Ramanspectrumofthe protein, andexamineschanges in conformation during activation.Using three common agonists (DTT, Mn(II) andEDTA), the results indicate that in each caseactivation is caused by reduction of disulfidelinks, but suggests that different disulfides areactivated according to the reagent used. Thehistopathological correlation to these findingshas yet to be determined. Acknowledgements Dr Tia Keyes (National Centre for Sensor Research,School of Chemical Sciences, Dublin City University) iskindly thanked for providing the data used in this note, andfor useful discussions during its preparation. The ScienceFoundation Ireland and The Irish Research Council forScience, Engineering and Technology (IRCSET) arethanked for funding this research. Reference Tia E. Keyes, Deirdre Leane, Robert J. Forster, NiamhMoran, Dermot Kenny, New Insights into the molecularmechanisms of thrombosis from high resolution surfaceenhanced Raman microscopy, Proceedings of ttInternational Society for Optical Engineering, 2005, 5826,221 ( France: HORIBA Jobin Yv o n S.A.S., 23 1 rue de Lille, 59650 Villeneuve d'Ascq. T e l:+3 3 (0)3 20 59 18 0 0,Fax: + 33 (0)3 20 5918 08.Email : raman@jobinyvon.fr w w w.jobinyvon.fr ) ( USA: HORIBA Jobin Yvon Inc., 3880 Park Avenue, Edison, NJ 08820-3012. Te l :+1-732-494-8660, ) Fax : +1-732-549-2571.Email :raman@jobinyvon.com www.jobinyvon.com ( Japan: HORIBA Ltd., JY Optical Sales Dept., 1-7-8 Higashi-kanda, Ch i yoda-ku, Tok y o 101-0031.Tel: +81 (0)3 3861 8231, Fax: +81 (0)3 3861 8259. Email: raman@horiba.com ) ( Germany: +49 (0)6251 8475-0 I taly: +39 02 5760305 0 U K : +44 (0)20 8204 8142 ) ORIBAExplore the future ΑIIbβ3 is a key mediator in the thrombotic process and has been studied extensively. This current work reports the first Raman spectrum of the protein, and examines changes in conformation during activation. Using three common agonists (DTT, Mn(II) and EDTA), the results indicate that in each case activation is caused by reduction of disulfide links, but suggests that different disulfides are activated according to the reagent used. The histopathological correlation to these findings has yet to be determined.
确定
还剩1页未读,是否继续阅读?
HORIBA(中国)为您提供《血栓中反应监控检测方案(激光拉曼光谱)》,该方案主要用于全血/血清/血浆中反应监控检测,参考标准--,《血栓中反应监控检测方案(激光拉曼光谱)》用到的仪器有
相关方案
更多
该厂商其他方案
更多








