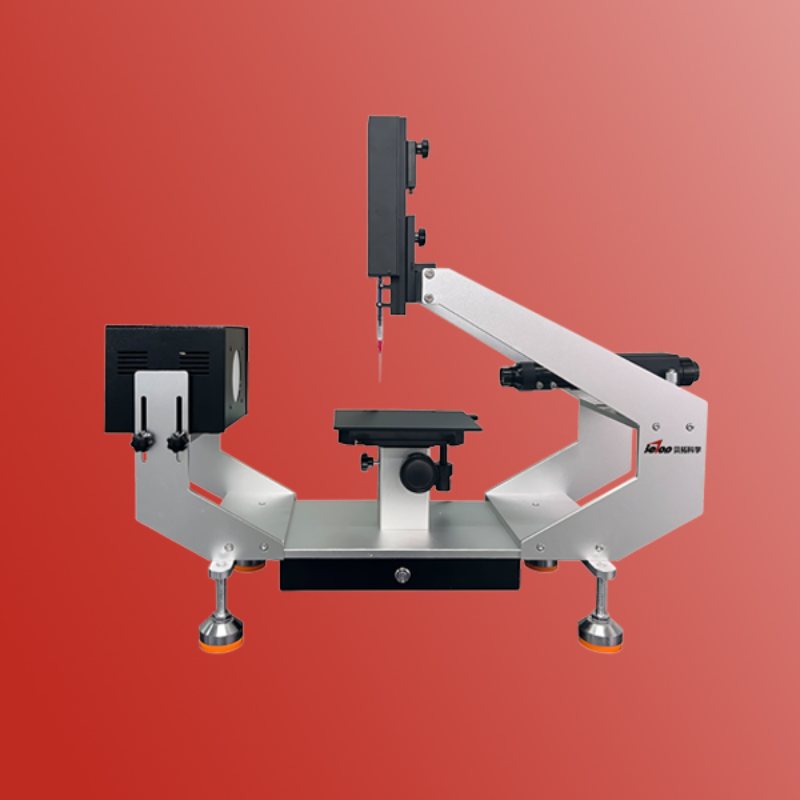
某些情况特别是在完成金属涂层或绘画及冲压润滑油残留时,使用室温测得的表面能数值会产生一些问题。因此我们提供了高温表面自由能测量方法。
方案详情

KRUSS Application Report Effect of Temperature on the Surface Energy of Solids Application report: AR250e Industry section: Adhesives, Lubricants, Metals Author: IChris Rulison Augustine Scientific, USA Date: December 2005 Method: SFE Force Tensiometer- K100 Drop Shape Analyzer-DSA100 Keywords: Adhesives, Surface Energy, Temperature, Sessile Drop, Contact Angle, Adhesion, Surface Tension,Metal Effect of Temperature on the Surface Energy of Solids- Sometimes It DoesMatter (Probe Liquid Recommendations) Abstract Surface energy values are rarely measured for solids at elevated temperatures. Even when the goal is understandingspreading and/or adhesion between a molten material (such as a hot melt adhesive) and a solid surface, most commonlysurface energy values for the solid surface are assumed to be those measurable at room temperature. Here we suggestthat such an approach can be problematic particularly when you have finish coatings on metals or the remnants ofdrawing and stamping lubricants, which is quite common for metals. So, we share a probe liquid set (withcharacterization) which can be used for high temperature solid surface energy in two-components up to 200°C. Method The Kruss Drop Shape Analyzer-DSA100 is used alongwith an elevated temperature chamber to measure thesurface energies of chrome plated steel and aluminumfoil at 40C and 160° by the Fowkes method using con-tact angles obtained by the sessile drop method. Forpurposes of making such measurements, at atmosphericpressure, high boiling point contact angle probe liquidsare necessary and must be previously characterized. Weused the Fowkes method to characterize 1-bromo-naphthalene and ethylene carbonate (1,3 dioxlan-2-one)for surface tension with polar and dispersive componentsat a variety of temperatures. Overall surface tensionswere measured using a Kruss Force Tensiometer- K100and separated into components by testing for contactangle against poly(tetrafluoroethylene) (PTFE). The liquidswere then put to use at 40℃ and 160° to characterize thechrome plated and aluminum surfaces. Both liquids haveboiling points above 240℃. Experiment Based on plate method surface tension measurementswith a Force Tensiometer-K100 and sessile drop contactmeasurements for the liquids on PTFE the followingsurface energy characterizations were made for the twoprobe liquids of interest by Fowkes method. Temperature 1-bromonaphthalene Overall Surface Tension (mN/m) 1-bromonaphthaleneSurface Polarity(%) 40 43.93 1.07 60 43.00 1.44 80 42.14 1.78 100 41.34 2.09 120 40.59 2.39 140 39.88 2.67 160 39.22 2.94 180 38.60 3.18 200 38.02 3.42 Table 1: Surface Tension Data-1-bromonaphthalene Temperature ethylene carbonate OverallSurface Tension (mN/m) ethylene carbonate SurfacePolarity (%) 40 53.74 63.41 60 51.92 60.99 80 50.32 58.98 100 48.84 57.22 120 47.50 55.68 140 46.27 54.34 160 45.14 53.16 180 44.11 52.13 200 43.16 51.23 Table 2: Surface Tension Data- ethylene carbonate The following contact angles were measured and surfaceenergies determined for thesurfaces attheetwotemperatures of interest. Sample ChromePlated Steel Chrome Plated Steel Temperature 40℃ 160°℃ Contact Angle with 1-bromonaphthalene 54.7° 41.4° Contact Angle with Ethylene Carbonate 42.2° 22.6° Overall Surface Energy (mJ/m‘) 42.16 42.02 Polar Component (mJ/m3) 19.01 18.91 Dispersive Component(mJ/m) 23.15 23.11 Surface Polarity(%) 45.11 45.01 Table 3: Chrome Plated Steel- Surface Energy Data Sample Aluminum Foil Aluminum Foil Temperature 40℃ 160℃ Contact Angle with 1-bromonaphthalene 44.1° 35.2° Contact Angle with Ethylene Carbonate 39.0° 38.2° Overall Surface Energy (mJ/m) 45.31 38.21 Polar Component (mJ/m ) 17.30 11.25 Dispersive Component (mJ/m²) 28.01 26.96 Surface Polarity (%) 38.19 29.44 Table 4: Aluminum Foil- Surface Energy Data Results The chrome plated steel was of interest for surfaceenergy analysis at high temperature because it forms thedie head for a polymer extrusion process, and it wasdesired to have molten polymer adhesion to be as low aspossible. The aluminum foil sample was of interest forsurface energy because it is to be bonded with moltenpolymer (hot melt) to another polymer film in ourcustomer’s process to make holographic film. The findings were that the chrome plated steel had verysimilar surface energy values at 40C and 160℃. (42.16 mJ/m with 45.11% surface polarity at 40C)(42.02 mJ/mwith 45.01% surface polarity at 160℃) The foil sampleontthe other hand,decreasedsubstantially in terms of overall surface energyandsurface polarity at 160℃ versus at 40C. (45.31mJ/m with 38.19% surface polarity at 40℃)(38.21 mJ/m"with 29.64% surface polarity at 160℃) It is sometimes valid to use solid surface energy data atroom temperature as a legitimate estimation of thesurface energy of the same solid at elevated temperature(as with the chrome plated steel above). However, othertimes it is not. In the case of metals this may be due tosurface oxidation changes, rearrangement of a surfacecoating, or other aspects not necessary completelyunderstood. We have also seen the effect in sputtercoated surfaces such as gold, nickel, etc. In the case ofpolymers passing glass transition temperatures andsecondary transition temperatures, well before the meltpoint, can also change surface energy substantially.These changes may well be important to the completeunderstanding of your adhesion and wetting processes.Please feel free to use the probe liquids and data abovein your elevated temperature surface energy evaluations,or to contact us if you wish to have such measurementsmade on your systems. Summary Surface energies of solids are most commonly onlymeasured at room temperature and assumed to besimilar at elevatedd temperatures for most adhesionenergyr workk.HHowever,it is. ofteni ltheecasethatsubstantial differences exist with temperature in thesurface energies of real surfaces including metals thatmay contain surface coatings effected by temperatureand polymers. In this note we offer the surface tensioncomponents for two surface energy probe liquids1-bromonaphthalene and ethylene Ccarbonate(1,3 dioxlan-2-one) which can be used, along with sessiledrop contact angle measurements to determine thesurface energy of solids up to 200C. The example dataare for chrome plated steel (of interest for a dieapplication) and an aluminum foil (of interest for theformation of holographic film). We find the chromeplated steel to have similar surface energy at 40℃ and160°C. The aluminum foil, however, is found to decreasein surface energy by about 7 mj/m’ and in surfacepolarity by about 7% over the same temperature range. Literature [1]"So you Want to Measure Surface Energy?"KRUSStechnical note TN306e (available for download at www.kruss.de or www.augustinescientific.com) KRUSS GmbH|Borsteler Chaussee Hamburg|Germany|www.kruss.de|
确定

还剩1页未读,是否继续阅读?
克吕士科学仪器(上海)有限公司为您提供《固体中温度对表面能影响检测方案(接触角测量仪)》,该方案主要用于其他中温度对表面能影响检测,参考标准--,《固体中温度对表面能影响检测方案(接触角测量仪)》用到的仪器有KRUSS DSA100接触角测量仪
推荐专场
相关方案
更多