方案详情
文
一般炸药和推进剂的稳定性和兼容性的调查需要远远超出了讨论范围。
在过去的100多年里,硝基一直被用作推进剂,设定了大量的测试来调查其稳定性。大多数的这些测试在本质上都很非常简单和实证的。然而,在过去10年左右的时间里,现代分析方法已被引入炸药领域,且常与旧方法一起使用。现代研究方法提供了有关该组分研究的更真实信息,但其结果是稳定的、兼容的且保持复杂混合物的稳定性,这在推进剂研究中是不容易的。
方案详情

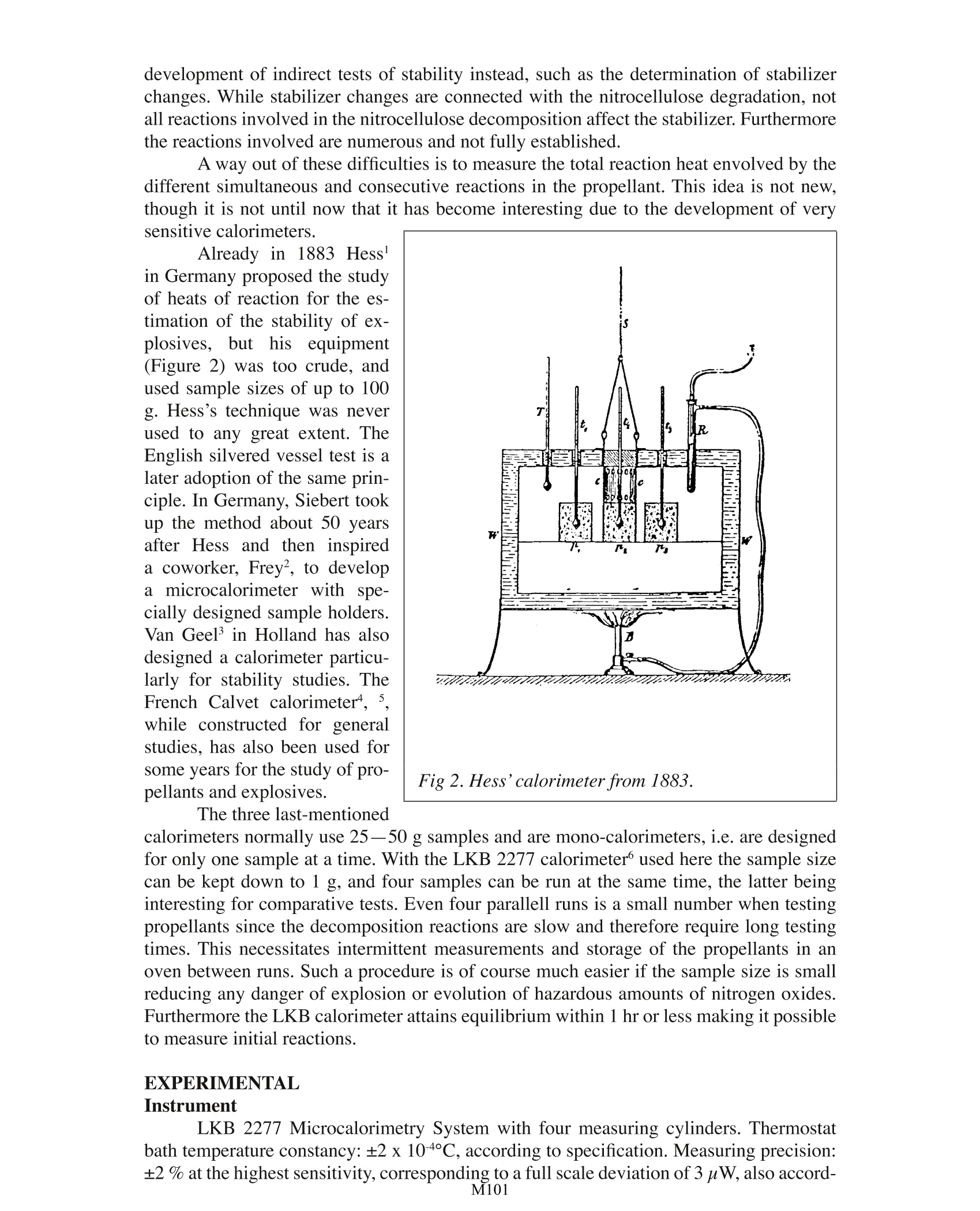
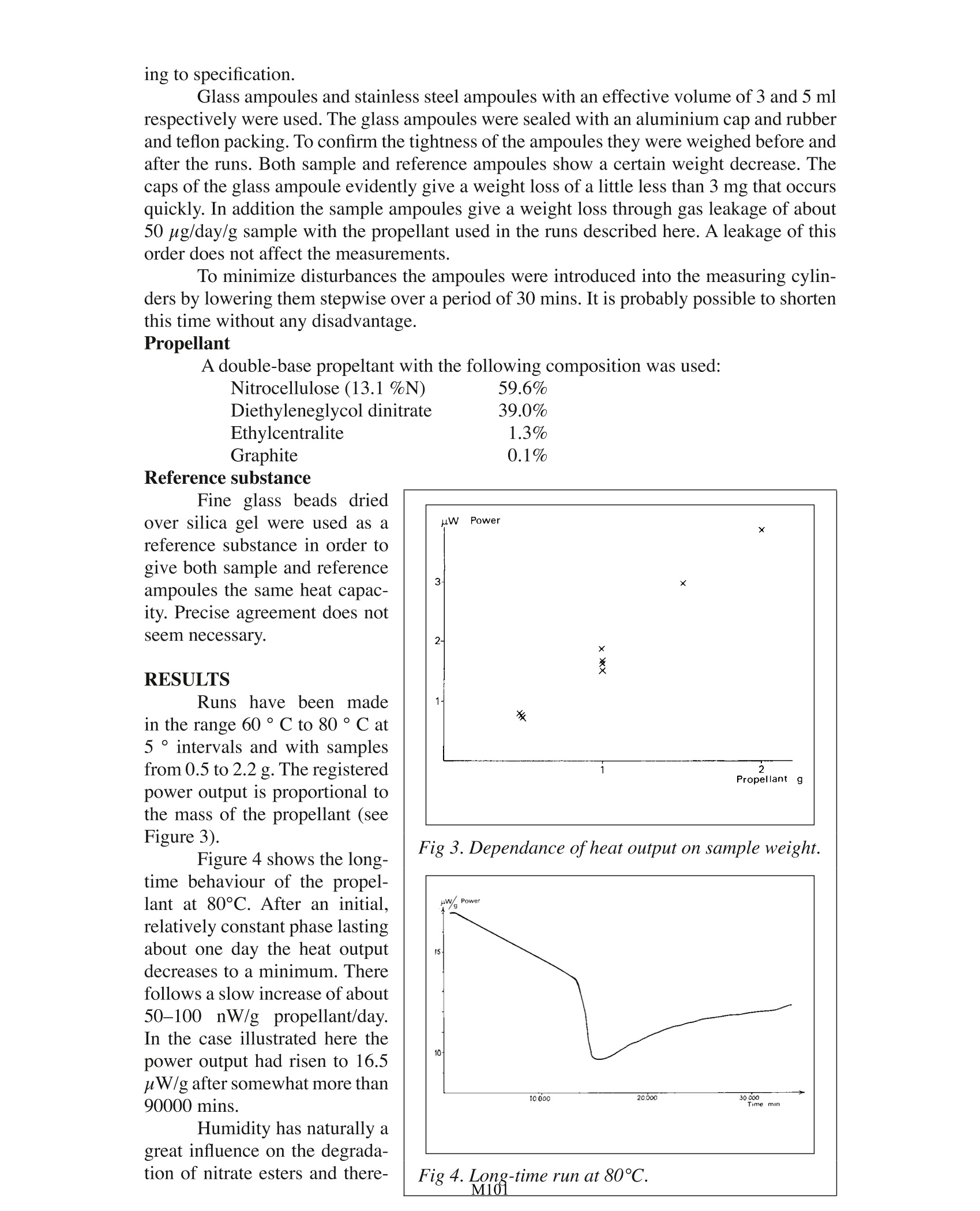
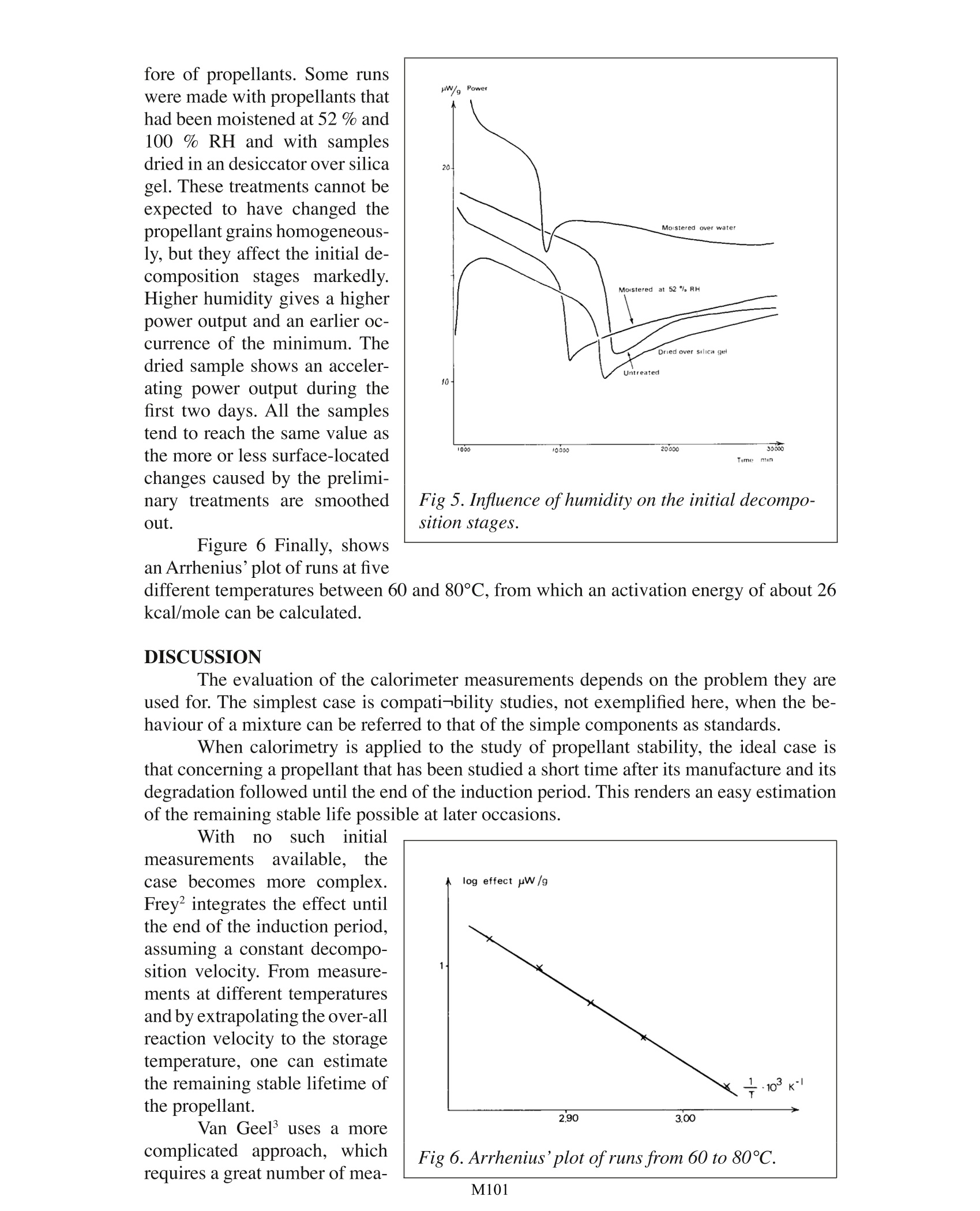
Testing Propellants by Calorimetry . Jan Hansson National Defence Res. Institute. Stockholm TA Instruments, 109 Lukens Drive,New Castle, DE 19720, USA Fig 1. The French battleship“Liberte” destroyed by a propellant explosion in 1911. The need to survey the stability and compatibility of explosives in general and pro-pellants in particular is beyond discussion During the more than 100 years that nitrocellulose has been in use as a propellant,an enormous number of tests have been devised to survey its stabiliiy. The majority of thesetests are rather simple and empirical in nature. During the last decade or so, however, mod-ern analytical methods have been introduced in the explosives field, and are often used inparallel with older methods. Modern methods give real information about the componentsstudied, but the translation of the result into terms of stability, compatibility and remainingstable life of the complex mixture of ingredients in a propellant is no easy task. Propellants are among the least stable and most difficult explosives to analyse. Themain component of propellants is the polymer is nitrocellulose, or, more precisely a de-liberate mixture of nitrocelluloses with varying molecular weights and varying of degreesesterification. To determine the molecular weight distribution and also the degree of es-terification of the different fractions is of course possible with modern methods. But tocarry out such determinations with the high precision needed to detect the small changesassociated with a dangerous deterioration of the propellant is a highly demanding task. Toturn the determinations into simple routine tests is not possible today. This has led to the development of indirect tests of stability instead, such as the determination of stabilizerchanges. While stabilizer changes are connected with the nitrocellulose degradation, notall reactions involved in the nitrocellulose decomposition affect the stabilizer. Furthermorethe reactions involved are numerous and not fully established. A way out of these difficulties is to measure the total reaction heat envolved by thedifferent simultaneous and consecutive reactions in the propellant. This idea is not new,though it is not until now that it has become interesting due to the development of verysensitive calorimeters. Already in 1883 Hessin Germany proposed the studyof heats of reaction for the es-timation of the stability of ex-plosives, but hisequipment(Figure 2) was too crude, andused sample sizes of up to 100g. Hess’s technique was neverused to any great extent. TheEnglish silvered vessel test is alater adoption of the same prin-ciple. In Germany, Siebert tookup the method about 50 yearsafter Hess and then inspireda coworker, Frey, to developa microcalorimeter with spe-cially designed sample holders.Van Geel in Holland has alsodesigned a calorimeter particu-larly for stability studies. TheFrench Calvet calorimeter, ,while constructed for generalstudies, has also been used forsome years for the study of pro-pellants and explosives. Fig 2. Hess’calorimeter from 1883. The three last-mentioned calorimeters normally use 25-50 g samples and are mono-calorimeters, i.e. are designedfor only one sample at a time. With the LKB 2277 calorimeter used here the sample sizecan be kept down to 1 g, and four samples can be run at the same time, the latter beinginteresting for comparative tests. Even four parallell runs is a small number when testingpropellants since the decomposition reactions are slow and therefore require long testingtimes. This necessitates intermittent measurements and storage of the propellants in anoven between runs. Such a procedure is of course much easier if the sample size is smallreducing any danger of explosion or evolution of hazardous amounts of nitrogen oxides.Furthermore the LKB calorimeter attains equilibrium within 1 hr or less making it possibleto measure initialreactions. EXPERIMENTAL Instrument LKB 2277 Microcalorimetry System with four measuring cylinders. Thermostatbath temperature constancy: ±2 x 10-4C, according to specification. Measuring precision:±2 % at the highest sensitivity, corresponding to a full scale deviation of 3 uW, also accord- Fine glass beads driedover silica gel were used as areference substance in order togive both sample and referenceampoules the same heat capac-ity. Precise agreement does notseem necessary. RESULTS Runshave been madein the range 60°C to 80°C at5°intervals and with samplesfrom 0.5 to 2.2 g. The registeredpower output is proportional tothe mass of the propellant (seeFigure3). Figure 4 shows the long-time behaviour of the propel-lant at 80°C. After an initial.relatively constant phase lastingabout one day the heat outputdecreases to a minimum. Therefollows a slow increase of about50-100 nW/g propellant/day.In the case illustrated here thepower output had risen to 16.5uW/g after somewhat more than90000 mins. Humidity has naturally agreat influence on the degrada-tion of nitrate esters and there- Fig 3. Dependance of heat output on sample weight. M101 fore of propellants. Some runswere made with propellants thathad been moistened at 52% and100 % RH and with samplesdried in an desiccator over silicagel. These treatments cannot beexpected to have changed thepropellant grains homogeneous-ly, but they affect the initial de-composition stages markedly.Higher humidity gives a higherpower output and an earlier oc-currence of the minimum. Thedried sample shows an acceler-ating power output during thefirst two days. All the samplestend to reach the same value asthe more or less surface-locatedchanges caused by the prelimi-nary treatments are smoothedout. Fig 5. Influence ofhumidity on the initial decompo-sition stages. Figure 6 Finally, showsan Arrhenius’plot of runs at five different temperatures between 60 and 80C, from which an activation energy of about 26kcal/mole can be calculated. DISCUSSION The evaluation of the calorimeter measurements depends on the problem they areused for. The simplest case is compati-bility studies, not exemplified here, when the be-haviour of a mixture can be referred to that of the simple components as standards. When calorimetry is applied to the study of propellant stability, the ideal case isthat concerning a propellant that has been studied a short time after its manufacture and itsdegradation followed until the end of the induction period. This renders an easy estimationof the remaining stable life possible at later occasions. With nosuchhiinitialmeasurementsavailable, thecase becomes more complex.Frey integrates the effect untilthe end of the induction period,assuming a constant decompo-sition velocity. From measure-ments at different temperaturesand by extrapolating the over-allreaction velocity to the storagetemperature, one can estimatethe remaining stable lifetime ofthe propellant. surements. From runs at different temperatures and the proposition where q is the power, and Q the total heat evolved by the propellant until time t, he de-rives activation energies, which he uses to calculate critical values according to Frank-Kamenetskii's thermal explosion theory. The application of thermal explosion theory is, of course, of great interest and thetheory in its simplest forms has been referred to widely. The practical cases, however,pres-ent some difficulties not taken into account by the simple Semenov and Frank-Kamenetskiitheories, as the explosives or propellants are usually parts of irregular ammunition itemsand are always packed in boxes and are surrounded by different kinds of more or less heatinsulating wrappings. An initial attempt to handle such problems has been made by Bod-dington, Griffiths, Scott and Hansson’ and shows an important influence of packing mate-rials on critical sizes, which should be taken into account when dealing with surveillanceproblems. REFERENCES 1. Siebert, Werner, Die Prufung der chemischen Bestandigkeit explosiver Stoffe, ins-besondere rauchschwacher Pulver, Berlin 1946, p. 5. .2. Frey, Max, CTI Einfuhrungssymposium vom 13. bis 15. Juni 1973, Swisttal-Heimer-zheim, p.352. 3. Van Gel,JL C, Self-ignition hazard of nitrate ester propellants,'s-Gravenhage 1969,p. 36. 4. Rat,Mauricette, Fifth Symp. Chem. Probl. Stab. Expl.(1979) p. 361,Application dela microcalorimetrie isotherme a 1'etude de la stabilite des poudres pour armes. 5 Dreyfus, M and Leveque, M, Fifth Symp. Chem. Probl. Stab. Expl. (1979) p. 381,Puissances calorifiques degagees par les poudres homogenes: resultats de mesuressur diverses lots de propergols neufs et anciens, par microcalorimetrie isotherme. .6 Frey, Max and Hansson, Jan, Foa Report C 20430-D1 (1981),Untersuchungen miteinem Prototyp eines neuen Mikrokalorimeters der Firma LKB. 7 Boddington, Terry, Griffiths, John, Scott, Stephen, and Hansson, Jan, Sixth Symp.Chem. Probl. Stab. Expl. (1982), Thermal explosion of dispersed media: criticalityfor discrete reactive particles in an inert matrix. M 一般炸药和推进剂的稳定性和兼容性的调查需要远远超出了讨论范围。在过去的100多年里,硝基一直被用作推进剂,设定了大量的测试来调查其稳定性。大多数的这些测试在本质上都很非常简单和实证的。然而,在过去10年左右的时间里,现代分析方法已被引入炸药领域,且常与旧方法一起使用。现代研究方法提供了有关该组分研究的更真实信息,但其结果是稳定的、兼容的且保持复杂混合物的稳定性,这在推进剂研究中是不容易的。
确定


还剩3页未读,是否继续阅读?
TA仪器为您提供《推进剂中微量热对推进剂性能影响检测方案(差示扫描量热)》,该方案主要用于其他中微量热对推进剂性能影响检测,参考标准--,《推进剂中微量热对推进剂性能影响检测方案(差示扫描量热)》用到的仪器有TA仪器+TAM IV+微量热仪
推荐专场
相关方案
更多
该厂商其他方案
更多
















