
方案详情
文
Like ITC, DSC is a valuable approach for studying binding between a biological macromolecule and a ligand such as another biopolymer or a drug. Unlike ITC, DSC allows the thermodynamics that drive binding to be correlated, at least to a degree, with conformational changes in the macromolecule caused by the binding reaction. DSC is particularly useful for characterizing very tight or slow binding interactions. DSC also allows characterization of binding reactions that are incompatible with the organic solvent requirements of some ITC experiments (i.e., where ligand solubility for an ITC experiment requires concentrations of organic solvent not tolerated by the protein).?
方案详情

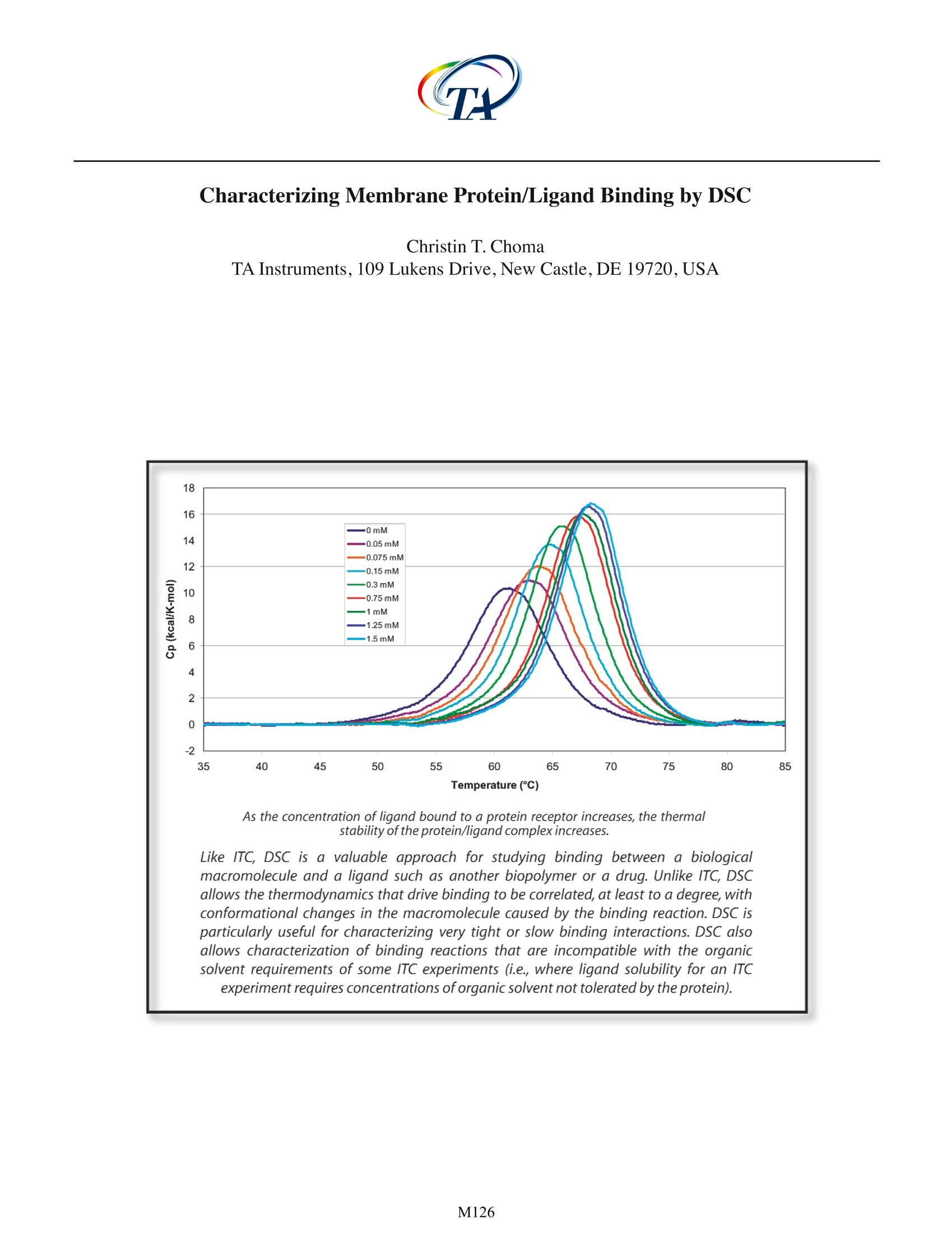
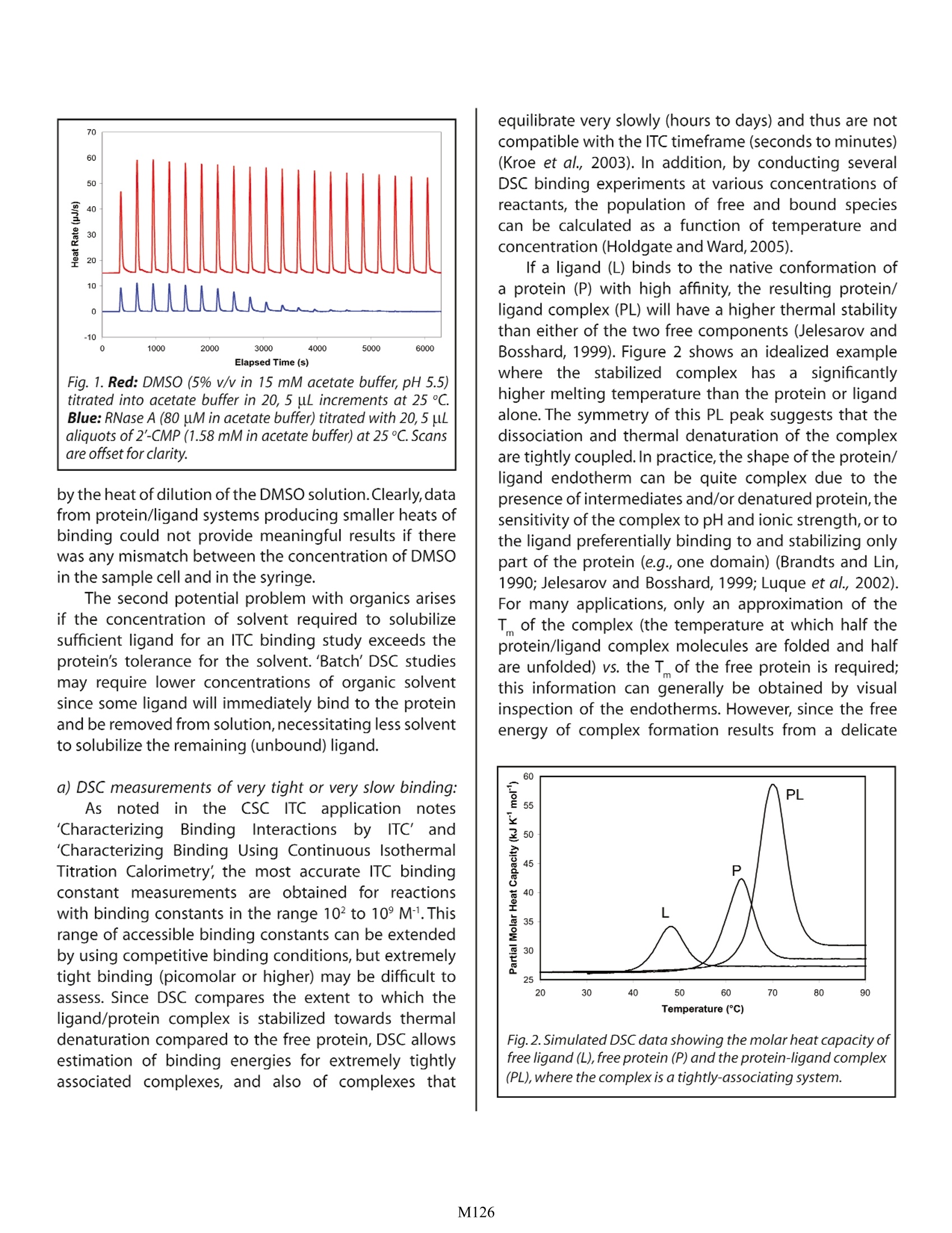
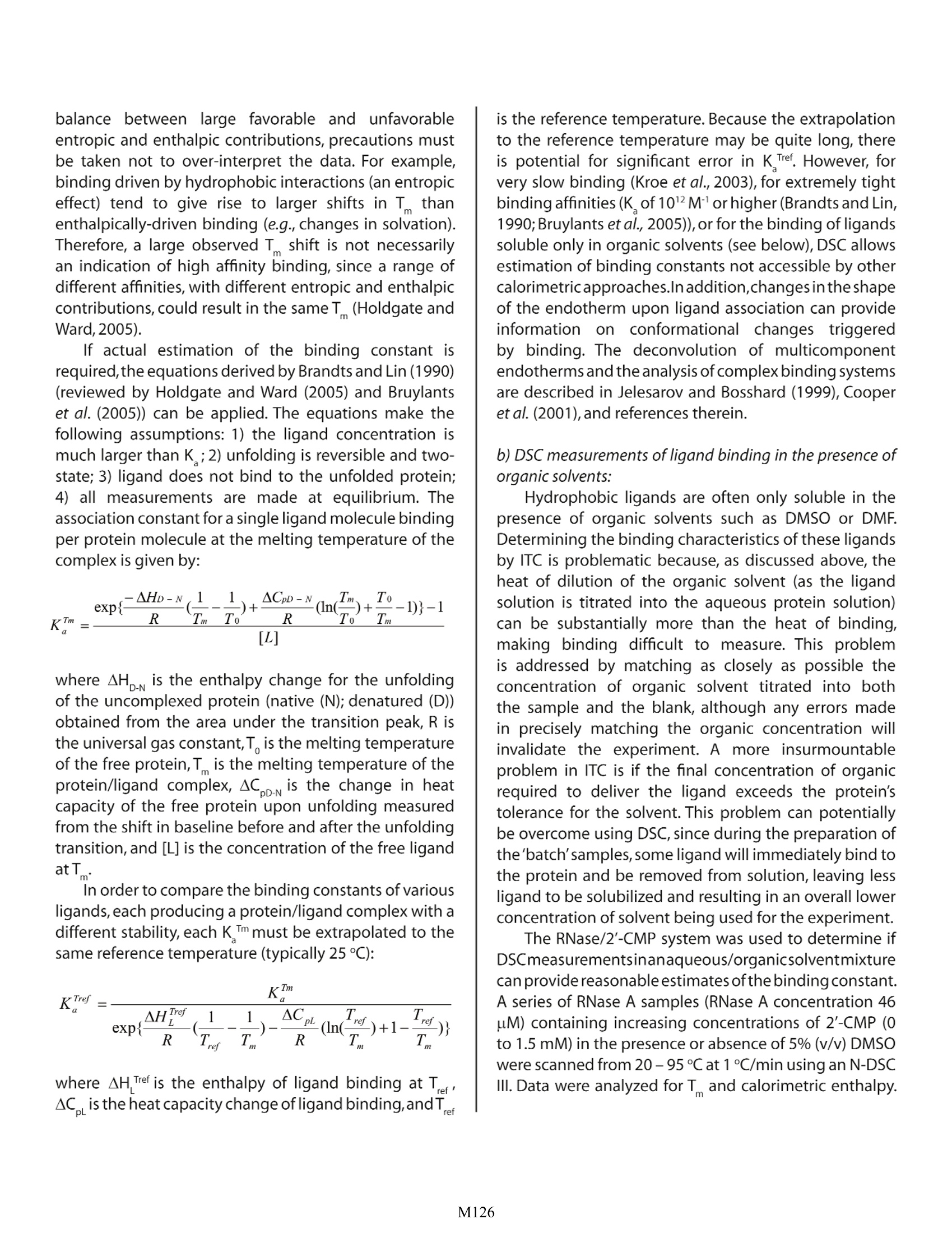
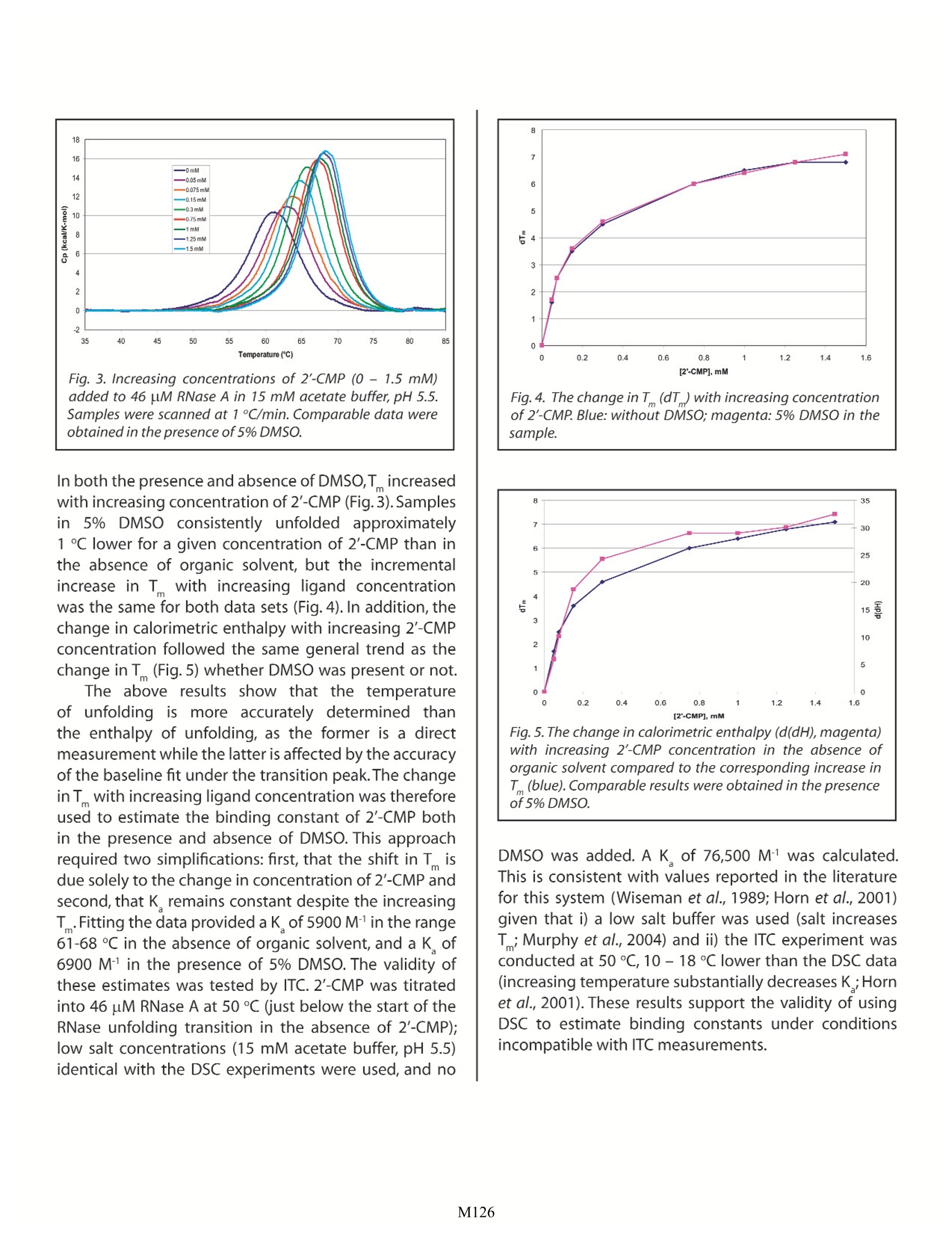
Characterizing Membrane Protein/Ligand Binding by DSC Christin T. Choma TA Instruments, 109 Lukens Drive, New Castle, DE 19720,USA experiment requires concentrations of organic solvent not tolerated by the protein). ll proteinsare capable of recognizing andAbinding specific molecules such as other proteins,IcCofactors, prosthetic groups or drugs. Effortsto understand the mechanisms controlling selectivebinding were initially prompted by the realization thatrecognition and binding are universal features of allbiochemical processes. These efforts have intensifiedwith the awareness that knowledge-based drug designrequires not only high-quality structural data on both theprotein and the drug candidate, but also a quantitativeunderstanding of the thermodynamics driving binding.A ligand will bind to a protein (or other macromolecule)only if the resulting complex is more stable than theoriginal, non-liganded protein. Binding can occur tothe native, folded protein (stabilizing the native state),or it can bind preferentially to the denatured protein, inwhich case the ligand will destabilize the native protein.In either case,binding triggers changes in intramolecularand intermolecular interactions, and in the dynamics ofboth the protein and the ligand. Since the degree ofstabilization or destabilization of the native proteindepends on the magnitude of the binding energy,comparison of the stability of the complex with thestability of the ligand-free protein allows the bindingenergy to be estimated. Differential scanning calorimetry (DSC) is particularlysuited to studying the thermodynamics controllingconformational transitions in macromolecules such asproteins. As explained in CSC's overview note entitled'Life Science Applications of DSC, DSC is generally usedto measure the partial molar heat capacity of a proteinover a temperature range of approximately 80℃. lf aligand binds preferentially to the native state of theprotein, the temperature at which the protein-ligandcomplex denatures will be higher compared to thetemperature at which the free protein unfolds. DSC thusprovides a direct measure of whether ligand bindingto a protein is stabilizing or destabilizing, and so cancomplement studies of binding equilibria obtained byisothermal titration calorimetry (ITC). For a discussionof the utility of ITC in binding studies, please see CSC'sapplication note entitled 'Characterizing protein/ligandbinding by ITC. Assessing ligand binding by DSC Proteins are large and flexible, and constantlysample the continuum of conformational states from partially folded to fully native.The native state itself is notone conformation, but a rapidly-changing ensemble ofclosely related structures (Ringe and Petsko,1985;Palmer1997).The binding between a flexible protein and a smallligand therefore produces a complex energy profileinvolving many favorable and unfavorablecontributions.The thermodynamic assessment of a binding event isfurther complicated by the fact that ligands such as drugmolecules often contain hydrophobic moieties whichinteract with hydrophobic patches on the surface (or inthe binding crevice) of the protein.Water is more orderedadjacent to hydrophobic surfaces (Shinoda,1977); whenthe protein and ligand bind and hydrophobic surfacesinteract, bound water at these surfaces is transferredinto the bulk solvent. Since the free energy of the entiresystem must decrease if binding is to occur, these solventeffects must be accounted for in addition to the energychanges resulting from direct noncovalent interactionsbetween the protein and ligand (Jelesarov and Bosshard,1999). ITC is the most direct approach for assessing bindinginteractions: several rapid incremental or continuousITC experiments can provide a direct determination ofthe binding constant, the stoichiometry of binding, theenthalpy and entropy of the reaction,and the change inheat capacity due to binding. In contrast, DSC indirectlyestimates the binding constant from measurementsof the equilibrium between the folded and unfoldedprotein. However, characterizing binding by DSC isdistinctly advantageous if a) the binding constant isextremely high, or binding is extremely slow and cannotbe measured by ITC or b) if the ligand is only solublein organic solvents such as dimethyl sulfoxide (DMSO)or dimethylformamide (DMF). Organic solvents areproblematic in ITC experiments in two circumstances.The first is if formation of the ligand/protein complexproduces only a small heat of binding. This heat canbe masked by the high heat of dilution of the organicduring an ITC experiment, although careful matching oforganic concentrations in the blank and sampletitrationscan often (but not always) overcome this obstacle. Forexample, Fig. 1 shows the heat of dilution of a 5% DMSOsolution titrated into actetate buffer, plotted abovethe heat released when2'-CMP binds to RNase A. The2'-CMP/RNase systemyieldsheatsSoffbindingsubstantially higher than that obtained from manyprotein/ligand systems, but even so its heats are dwarfed Fig. 1. Red: DMSO (5% v/v in 15 mM acetate buffer, pH 5.5)titrated into acetate buffer in 20, 5 uL increments at 25 ℃.Blue: RNase A (80 uM in acetate buffer) titrated with 20, 5 uLaliquots of 2'-CMP (1.58 mM in acetate buffer) at 25 C. Scansare offset for clarity. by the heat of dilution of the DMSO solution.Clearly,datafrom protein/ligand systems producing smaller heats ofbinding could not provide meaningful results if therewas any mismatch between the concentration of DMSOin the sample cell and in the syringe. The second potential problem with organics arisesif the concentration of solvent required to solubilizesufficient ligand for an ITC binding study exceeds theprotein's tolerance for the solvent. 'Batch' DSC studiesmay require lower concentrations of organic solventsince some ligand will immediately bind to the proteinand be removed from solution, necessitating less solventto solubilize the remaining (unbound) ligand. a) DSC measurements of very tight or very slow binding:As noted in the CSC ITC application notes'CharacterizingBindingInteractions by ITC'and'Characterizing Binding Using Continuous IsothermalTitration Calorimetry, the most accurate ITC bindingconstant measurements are obtained for reactionswith binding constants in the range 10 to 10° M1. Thisrange of accessible binding constants can be extendedby using competitive binding conditions, but extremelytight binding (picomolar or higher) may be difficult toassess. Since DSC compares the extent to which theligand/protein complex is stabilized towards thermaldenaturation compared to the free protein, DSC allowsestimation of binding energies for extremely tightlyassociated complexes, and also of complexes that equilibrate very slowly (hours to days) and thus are notcompatible with the ITC timeframe (seconds to minutes)(Kroe et al., 2003). In addition, by conducting severalDSC binding experiments at various concentrations ofreactants, the population of free and bound speciescan be calculated as a function of temperature andconcentration (Holdgate and Ward,2005). lf a ligand (L) binds to the native conformation ofa protein (P) with high affinity, the resulting protein/ligand complex (PL) will have a higher thermal stabilitythan either of the two free components (Jelesarov andBosshard, 1999). Figure 2 shows an idealized examplewhere the stabilized complex has a significantlyhigher melting temperature than the protein or ligandalone. The symmetry of this PL peak suggests that thedissociation and thermal denaturation of the complexare tightly coupled.In practice,the shape of the protein/ligand endotherm can be quite complex due to thepresence of intermediates and/or denatured protein,thesensitivity of the complex to pH and ionic strength, or tothe ligand preferentially binding to and stabilizing onlypart of the protein (e.g., one domain) (Brandts and Lin,1990; Jelesarov and Bosshard, 1999; Luque et al., 2002).For many applications, only an approximation of theT of the complex (the temperature at which half theprotein/ligand complex molecules are folded and halfare unfolded) vs. the T of the free protein is required;this information can generally be obtained by visualinspection of the endotherms. However, since the freeenergy of complex formation results from a delicate (PL), where the complex is a tightly-associating system. balance between large favorable and unfavcentropic and enthalpic contributions, precautions mustbe taken not to over-interpret the data. For example,binding driven by hydrophobic interactions (an entropiceffect) tend to give rise to larger shifts in T thanenthalpically-driven binding (e.g.,changes in solvation).Therefore, a large observed T shift is not necessarilyan indication of high affinity binding, since a range ofdifferent affinities, with different entropic and enthalpiccontributions, could result in the same T(Holdgate andWard,2005). If actual estimation of the binding constant isrequired,the equations derived by Brandts and Lin (1990)(reviewed by Holdgate and Ward (2005) and Bruylantset al. (2005)) can be applied.The equations make thefollowing assumptions: 1) the ligand concentration ismuch larger than K ;2) unfolding is reversible and two-state; 3) ligand does not bind to the unfolded protein;4) all measurements are made at equilibrium. Theassociation constant for a single ligand molecule bindingper protein molecule at the melting temperature of thecomplex is given by: where AHp is the enthalpy change for the unfoldingof the uncomplexed protein (native (N); denatured (D))obtained from the area under the transition peak, R isthe universal gas constant,T, is the melting temperatureof the free protein,T is the melting temperature of theprotein/ligand complex,AC pn is the change in heatcapacity of the free protein upon unfolding measuredfrom the shift in baseline before and after the unfoldingtransition, and [L] is the concentration of the free ligandatT. In order to compare the binding constants of variousligands,each producing a protein/ligand complex with adifferent stability, each K Immust be extrapolated to thesame reference temperature (typically 25℃): where AH Tef is the enthalpy of ligand binding at Te,AC。is the heat capacity change ofligand binding,andT is the reference temperature. Because the extrapolationto the reference temperature may be quite long, thereis potential for significant error in K Tref. However, forvery slow binding (Kroe et al., 2003), for extremely tightbinding affinities (K of 1012M"orhigher (Brandts and Lin,1990;Bruylants et al., 2005)), or for the binding of ligandssoluble only in organic solvents (see below), DSC allowsestimation of binding constants not accessible by othercalorimetricapproaches.Inaddition,changes intheshapeof the endotherm upon ligand association can provideinformation on conformational changesttriggeredby binding. The deconvolution of multicomponentendotherms and the analysis of complex binding systemsare described in Jelesarov and Bosshard (1999), Cooperet al.(2001),and references therein. b) DSC measurements of ligand binding in the presence oforganic solvents: Hydrophobic ligands are often only soluble in thepresence of organic solvents such as DMSO or DMF.Determining the binding characteristics of these ligandsby ITC is problematic because, as discussed above, theheat of dilution of the organic solvent (as the ligandsolution is titrated into the aqueous protein solution)can be substantially more than the heat of binding,making binding difficult to measure. This problemis addressed by matching as closely as possible theconcentration of organic solvent titrated into boththe sample and the blank, although any errors madein precisely matching the organic concentration willinvalidate the experiment. A more insurmountableproblem in ITC is if the final concentration of organicrequired to deliver the ligand exceeds the protein'stolerance for the solvent. This problem can potentiallybe overcome using DSC, since during the preparation ofthe'batch'samples,some ligand will immediately bind tothe protein and be removed from solution, leaving lessligand to be solubilized and resulting in an overall lowerconcentration of solvent being used for the experiment. The RNase/2'-CMP system was used to determine ifDSCmeasurementsinanaqueous/organicsolventmixturecan provide reasonable estimates ofthe binding constant.A series of RNase A samples (RNase A concentration 46uM) containing increasing concentrations of 2'-CMP (0to 1.5 mM) in the presence or absence of 5% (v/v) DMSOwere scanned from 20-95℃at1C/min using an N-DSCⅢ. Data were analyzed for T and calorimetric enthalpy. Fig. 3. Increasingconcentrations of 2'-CMP(0 - 1.5 mM)added to 46 uM RNase A in 15 mM acetate buffer, pH 5.5.Samples were scanned at 1 C/min. Comparable data wereobtained in the presence of 5% DMSO. In both the presence and absence of DMSO,T increasedwith increasing concentration of 2'-CMP(Fig.3). Samplesin 5%DMSO consistently unfolded approximately1℃lower for a given concentration of 2'-CMP than inthe absence of organic solvent, but the incrementalincrease in T with increasing ligand concentrationwas the same for both data sets (Fig. 4). In addition, thechange in calorimetric enthalpy with increasing 2'-CMPconcentration followed the same general trend as thechange in T (Fig.5) whether DMSO was present or not. The above results show that the temperatureof unfolding is more accurately determined thanthe enthalpy of unfolding, as the former is a directmeasurement while the latter is affected by the accuracyof the baseline fit under the transition peak.The changeinT with increasing ligand concentration was thereforeused to estimate the binding constant of 2'-CMP bothin the presence and absence of DMSO. This approachrequired two simplifications: first, that the shift in Tisdue solely to the change in concentration of 2'-CMP andsecond,that K remains constant despite the increasingT.Fitting the data provided a K of5900 M in the range61-68 ℃ in the absence of organic solvent, and a K of6900 M in the presence of 5% DMSO. The validity ofthese estimates was tested by ITC. 2'-CMP was titratedinto 46 pM RNase A at 50℃ (just below the start of theRNase unfolding transition in the absence of 2'-CMP);low salt concentrations (15 mM acetate buffer,pH 5.5)identical with the DSC experiments were used, and no Fig.4. The change in T (dT) with increasing concentrationof 2'-CMP. Blue: without DMSO; magenta: 5% DMSO in thesample. Fig.5. The change in calorimetric enthalpy (d(dH), magenta)with increasing 2'-CMP concentration in the absence oforganic solvent compared to the corresponding increase inT(blue). Comparable results were obtained in the presenceof 5% DMSO. DMSO was added. A K of 76,500 M-1 was calculated.This is consistent with values reported in the literaturefor this system (Wiseman et al., 1989; Horn et al., 2001)given that i) a low salt buffer was used (salt increasesT;Murphy et al., 2004) and ii) the ITC experiment wasconducted at 50 C,10-18℃ lower than the DSC data(increasing temperature substantially decreases K ;Hornet al., 2001). These results support the validity of usingDSC to estimate binding constants under conditionsincompatible with ITC measurements. Summary: Although ITC remains the method of choice fordetermining binding constants, DSC can provideestimates of K where binding occurs very slowly, is tootightto bemeasured by ITC,or where high concentrationsof organic solvent are required. In addition, whencombined with structural and ITC data, DSC provides anindependent approach to elucidating the free energychanges accompanying the formation of protein/ligand complexes, which in turn can shed light on themechanism of the association reaction. Used together,ITC and DSC provide the most direct, comprehensiveapproach for monitoring and interpreting bindinginteractions involving biological macromolecules. References: (Preference has been given to current references.Citation doesnot imply that a paper is necessarily the original reference to astudy.) ( Brandts, J. F . and L-N Lin. (1990) Study of strong t o ultratight protein interactions u sing differentialscanning c alorimetry. Biochemistry 29,6927-6940. ) Cooper,A.,M.A.Nutley and A.Wadood.(2001) Differentialscanning calorimetry.p.287-318.In S.E. Harding andB. Z. Chowdry (Eds.) Protein-Ligand Interactions:hydrodynamics and calorimetry. Oxford UniversityPress,Oxford. Holdgate, G. A. and W. H. J. Ward. (2005) Measurementsof binding thermodynamics in drug discovery.DrugDiscov. Today 10,1543-1550. ( Horn, J. R., D. Russell, E . A . Lewis and K . P . Murphy.(2001) van't Hoff and calorimetric enthalpies fromisothermal titration calorimetry: are there significant discrepancies? Biochemistry 40,1774-1778. ) ( Jelesarov,l.and H.R.Bosshard.(1999) Isothermal titrationcalorimetry and differential scanning calorimetry as complementary tools to investigate the energetics of biomolecular recognition.J.Mol.Recognit. 1 2,3-18. ) ( Kroe, R. R. e t al.(2003) Thermal denaturation: A method to rank slow binding, high-affinity P38o MAP k inase inhibitors.J.Med.Chem.46,4669-4675. ) ( Luque, I., S. A. L eavitt and E. Freire. (2002) T helinkage between protein folding a nd functional cooperativity: two sides of the same coin? Annu. Rev. Biophys. Biomol.Struct.31,235-256. ) Murphy, K. P., T. T. Waldron and G. L. Schrift. (2004) Salteffects in ribonuclease-ligand interactions:screeningor competitive binding? p. 93-105. In J. E. Ladburyand M. L. Doyle (Eds.) Biocalorimetry 2: applicationsof calorimetry in the biological sciences. Wiley,Chichester,UK. Palmer, A. G. (1997) Probing molecular motion by NMR.Curr.Opin. Struct. Biol.7,732-737. Ringe, D. and G. A. Petsko. (1985) Mapping proteindynamics by X-ray diffraction.Prog. Biophys.Mol. Biol.45,197-235. Shinoda, K. (1977) 'lceberg'formation and solubility. J.Phys.Chem.81,8069-8072. Tame,J.R.H.,R.O'Brien and J.E.Ladbury.(1998)Isothermaltitration calorimetry of biomolecules. p. 27-38.In J.E.Ladbury and B. Z. Chowdhry (Eds.) Biocalorimetry:applications of calorimetry in the biological sciences.Wiley, Chichester,UK. ( Wiseman, T., S. Williston, J. F. Brandts and L -N Lin. (1 989) Rapid measurements of binding constants and heatsof binding u sing a new titration calorimeter. A nal. Biochem. 179,131-137. ) M Like ITC, DSC is a valuable approach for studying binding between a biological macromolecule and a ligand such as another biopolymer or a drug. Unlike ITC, DSC allows the thermodynamics that drive binding to be correlated, at least to a degree, with conformational changes in the macromolecule caused by the binding reaction. DSC is particularly useful for characterizing very tight or slow binding interactions. DSC also allows characterization of binding reactions that are incompatible with the organic solvent requirements of some ITC experiments (i.e., where ligand solubility for an ITC experiment requires concentrations of organic solvent not tolerated by the protein).
确定


还剩4页未读,是否继续阅读?
TA仪器为您提供《膜蛋白和配体中结合检测方案(差示扫描量热)》,该方案主要用于其他中结合检测,参考标准--,《膜蛋白和配体中结合检测方案(差示扫描量热)》用到的仪器有NANO等温差示扫描量热仪
推荐专场
相关方案
更多















