方案详情
文
采用安东帕微波消解仪配备微波氧燃烧技术样品处理,采用ICP-OES检测,对含炭黑橡胶中国的Al, Fe, Mn, Sr 和Zn痕量元素进行检测。
方案详情

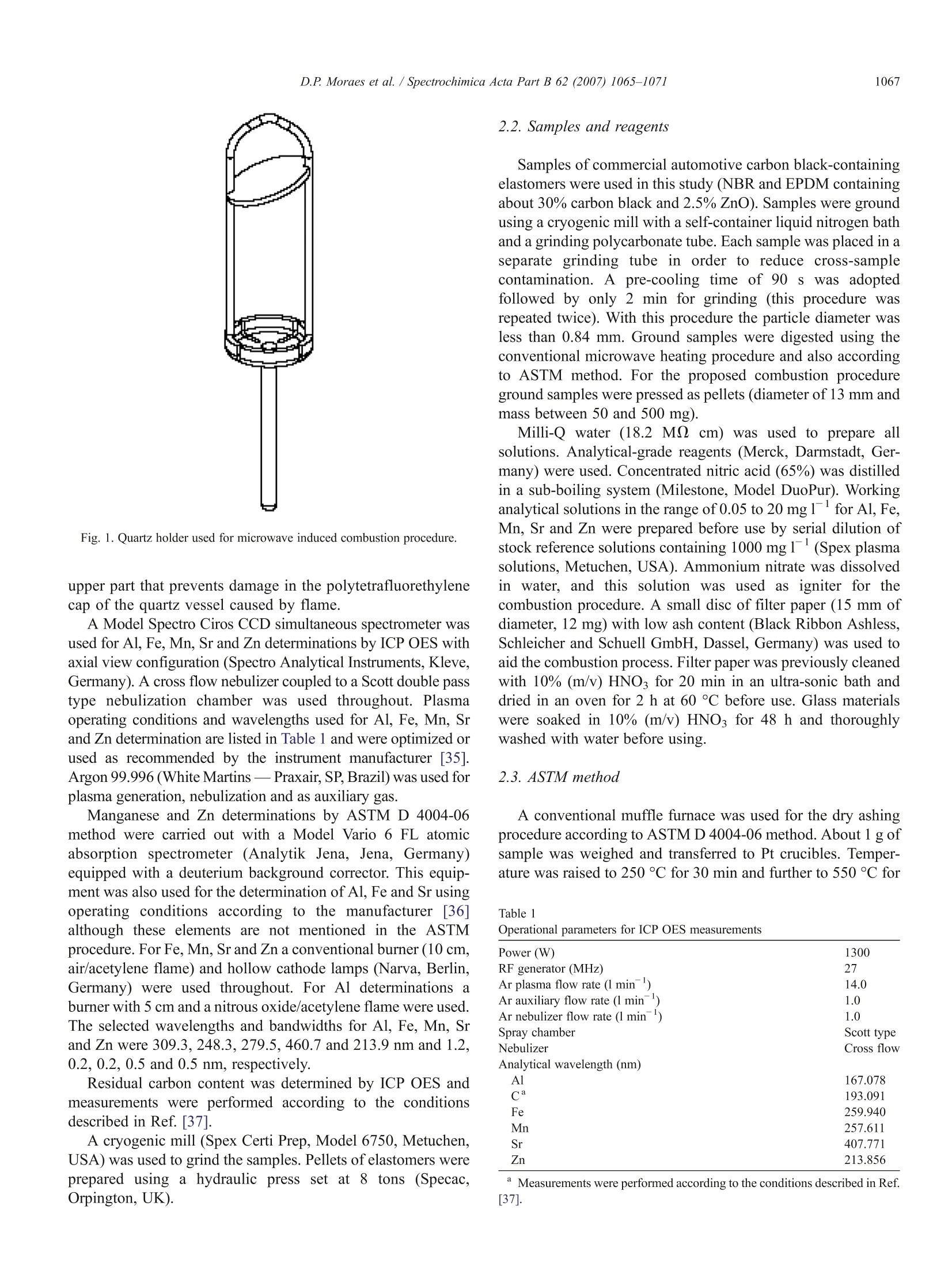
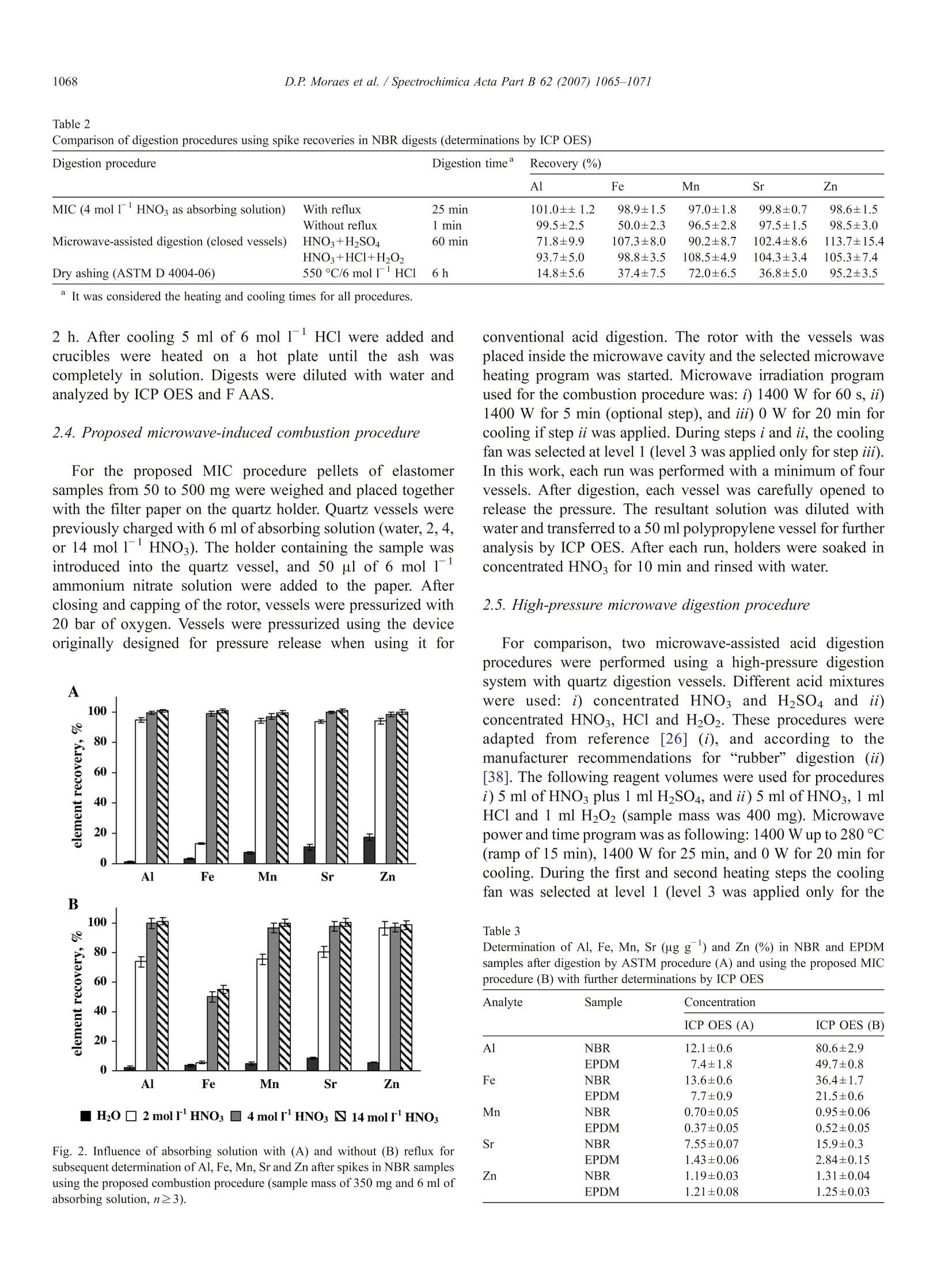
SPECTROCHIMICAACTAPARTB Spectrochimica Acta Part B 62 (2007)1065-1071www.elsevier.com/locate/sab 1066D.P. Moraes et al./ Spectrochimica Acta Part B 62 (2007) 1065-1071 doi:10.1016/j.sab.2007.03.011 Application of microwave induced combustion in closed vessels for carbonblack-containing elastomers decomposition Diogo P. Moraes , Márcia F. Mesko , Paola A. Mello , Jose N.G. Paniz, Valderi L. Dressler,Ginter Knapp, Erico M.M. Flores a.* Departamento de Quimica, Universidade Federal de Santa Maria 97105-900, Santa Maria, RS, Brazil Micro- and Radiochemistry, Graz University of Technology, Technikerstrasse 4, A-8010 Graz, Austria Received 24 December 2006; received in revised form 3 March 2007; accepted 12 March 2007Available online 24 March 2007 Abstract A rapid digestion procedure for the determination of Al, Fe, Mn, Sr and Zn in carbon black-containing elastomers (30%) has been developedusing sample combustion in closed quartz vessels. Microwave radiation was used for ignition. Combustion takes place in the presence of oxygenunder pressure using ammonium nitrate (50 ul of 6 mol1) as aid for ignition. Samples of nitrile-butadiene rubber and ethylenepropylene-dienemonomer were decomposed. A quartz device was used simultaneously as a sample holder and for the protection of vessel cap. The influence of theabsorption solution (nitric acid or water) and the necessity of an additional reflux step were evaluated. Determination of Al, Fe, Mn, Sr and Zn wasperormed byinducitcitively coupled plasma optical emission spectrometry. A reference method (ASTM D 4004-06) based on conventional dryashing and flame atomic absorption spectrometry was used for comparison (Mn and Zn). Results were also compared to those obtained by usingwet acid digestion in closed systems. Concentrated and diluted (4 mol1) nitric acid, with 5 min of reflux after the combustion, gave bestrecoveries for all analytes (from97 to 101%). For dry ashing quantitative recoveries were found only for Zn whereas for Al, Fe, Mn and Sr therecoveries were only 14, 37, 72 and 37%, respectively. With the proposed procedure the residual carbon content was below 0.5% and furtherdetermination of analytes was feasible with only the combustion step (for Fe a reflux with diluted HNO was necessary). Complete sampledigestion is obtained in less time using the proposed procedure than with other procedures and no concentrated acids were necessary.C 2007 Elsevier B.V. All rights reserved. Keywords: Microwave-induced combustion; Carbon black-containing elastomers; Sample digestion; Trace metals determination; ICP OES 1. Introduction Elastomers, or “rubbers”, are considered apart from otherpolymeric materials in view of their special properties aselasticity, strength, stiffness, abrasion and tear resistance [1,2].These properties may be enhanced by the addition of specificfillers to the elastomer before the vulcanization process. Carbonblack is the outstanding reinforcing filler currently used for bothnatural and synthetic elastomers and the only inexpensivematerial that improves all three of important properties: tensile ( This paper was presented at the 9t h Ri o Symposium on AtomicSpectrometry, held in Barquisimeto, Venezuela, 5-10 November 2006, and i s published i n the special i s sue of Spectrochimica Acta Par t B, dedicated to t h at conference. ) * Corresponding author. Fax: +55 55 3220 9445. ( E-mail address: f lores@quimi c a.u f sm.br ( E .M.M. Flores). ) ( 0584-8547/$- see front matter C 2 0 07 Elsevier B. V . Al l ri g hts reserved. ) strength, tear resistance and abrasion resistance [3,4]. Carbonblack content in rubbers varies widely and it may be present at50% m/m depending of the formulation [5]. In general,inorganic components or contaminants are present in a widerange ofconcentration in elastomers, usually from a few percentto ug g level, and there is a growing need to developanalytical methods that permit elemental analysis of polymericmaterials in a fast and reliable way [6,7]. Among the elements that need to be currently determined areAl, Fe, Mn, Sr and Zn. In general, the techniques currently usedfor polymer analysis are atomic absorption [8] and atomicspectrometry based on inductively coupled plasmas [6,7,9-12].Techniques with multielement capability such as inductivelycoupled plasma optical emission spectrometry (ICP OES) arethe most attractive ones for the routine analysis of inorganiccomposition of polymers due to their higher throughput [10,11,13]. In general, ICP OES requires analytes to be in anaqueous solution. However, the digestion procedure might be adifficult task if carbon black is used as polymer additive due tothe well-known difficulty to digest this kind of material byconventional techniques. In addition, literature data are veryscarce concerning elastomer sample preparation for inorganicanalysis and most of the available techniques do not complywith the modern requirements for trace analysis [8,14]. Ingeneral, sample preparation methods used to bring commonpolymers into solution include dry ashing using muffle furnace[8], conventional hot plate digestion using concentrated acids[15], open and closed vessel microwave-assisted acid digestion[12,13,16,], and combustion techniques in closed vessels [17-21]. Dry ashing procedures for polymers include generally foursteps: i) heating at 500 to 650 °℃ up to 18 h, depending on thepolymer type, ii) acid dissolution of the ash, iii) evaporation ofdigests to dryness and, iv) acid addition to complete dissolution.This procedure is not only time-consuming, but may result inanalyte losses for the most volatile elements during the ashingand/or the evaporation step [8,22]. In addition, this technique isstrongly prone to contamination by the laboratory atmosphereand also by the high consumption of reagents [23]. However,despite these drawbacks American Society for Testing andMaterials Standards (ASTM) recommends the use of a dryashing method for elastomer digestion (550 °C, with furtherdissolution of ashes using 6 mol1HCl) for the subsequentdetermination of Cu, Mn, Pb and Zn. Although this methodyields digests with very low residual carbon content (RCC) itmay not be applied for elastomers containing halogen fillersconsidering the risk of losses (for Zn and Pb determination,ASTM, Methods A and B) [8]. The use of microwave-assisted digestion using closedvessels has found wide-spread application in the last decadesas it combines high decomposition efficiency, low digestiontime and minimization of losses and contamination [24].However, the presence of high residual acid concentration inthe digests may not be supported by some analytical techniquesand a subsequent step to remove (or dilute) the excess acid maybe necessary [25]. Anyway, this technique has been applied fordifferent types of polymers [12,13,26]. Unfortunately, accord-ing to the experience of our laboratory these procedures are noteffective for digestion of some elastomers, mainly thosecontaining carbon black, even using conventional high-pressuremicrowave ovens. Among the digestion techniques for polymers, combustion isa very effective way to achieve complete matrix destruction(including organic additives) in view of the high temperatureachieved during the burning (about 1000 °℃) [27]. Combustionmay be performed in oxygen atmospheric pressure systemsusing the classical oxygen flask (Schoniger technique)[18,19,27] or using O2 pressurized combustion bombs[20,28,29]. Despite some advantages, these techniques havenot been widely used owing to some drawbacks as: i) only asingle sample may be processed each time causing lowthroughput, ii) depending on the analyte the cleaning of vesselwalls (and analyte dissolution) could be ineffective and it mustbe performed manually, iii) further trace determination of Cr, Ni, Fe and V is difficult due to contamination by vessel (forcombustion bomb), iv) sample mass may be limited for about50 mg (for Schoniger system) [14,30,31]. Recently, a novel decomposition technique that combinesthe advantages of classical combustion with those fromconventional closed systems heated by microwave radiationwas proposed [32]. In this system, a small quartz holder isplaced inside a high-purity quartz vessel and ignition isperformed only by microwave radiation [33]. As no metallicparts are present the contamination for a lot of elements isavoided. Moreover, the system allows an automatic refluxstep, if necessary, that is not available for other combustionsystems [34]. In this work the microwave-induced combustion(MIC) procedure is proposed for the decomposition of carbonblack-containing elastomers for the determination of Al, Fe,Mn, Sr and Zn by ICP OES. These elements (with exceptionof Zn) are considered contaminants for elastomers and theyneed to be controlled in industry in view of risks to changeelastomer properties; Mn, in particular, can catalyze theoxidative breakdown of rubber [8]. Ethylene-propylene-dienemonomer (EPDM) and nitrile-butadiene rubber (NBR) werechosen to evaluate the performance of the combustionprocedure. These samples are currently used as automotiveindustry components and contain about 30% of carbon black.The kind of absorbing solution (diluted or concentrated nitricacid, or water), the reflux step conditions, and residual carboncontent (RCC) were evaluated. For Mn and Zn, results werecompared with those using recommended digestion procedurefor elastomers and determination by flame atomic absorptionspectrometry (F AAS) following ASTM D 4004-06 method.However, as no certified reference materials are available formetals in elastomers, recovery tests had to be used for Al, Fe,Mn, Sr and Zn. In addition, wet digestion with high-pressuremicrowave vessels was used for comparison. 2. Materials and methods 2.1. Instrumentation A Model Multiwave 3000 microwave sample preparationsystem (Anton Paar, Graz, Austria) equipped with eight high-pressure quartz vessels was used in this study (volume of80 ml, maximum temperature and pressure of 280 °C and80 bar, respectively). This equipment was used for conven-tional high-pressure acid digestion and for the proposedcombustion procedure. The software version was v1.27-Syntand the microwave system was previously modified to runwith a maximum pressure rate of 3 bar s((and not 0.8 barsas in the original software). This change was necessary toprevent eventual interruption of the microwave irradiationbefore all the samples start the combustion. Pressure wasmonitored in each vessel all the runs. A specially designedquartz device was used as sample holders for the MICprocedure (Fig. 1). This device was developed to facilitate theoxygen diffusion by the pressed sample and to reduce the coolsurfaces that might cause carbon deposits in the quartzsurface. For additional safety, there is a quartz disc in the Fig. 1. Quartz holder used for microwave induced combustion procedure. upper part that prevents damage in the polytetrafluorethylenecap of the quartz vessel caused by flame. A Model Spectro Ciros CCD simultaneous spectrometer wasused for Al, Fe, Mn, Sr and Zn determinations by ICP OES withaxial view configuration (Spectro Analytical Instruments, Kleve,Germany). A cross flow nebulizer coupled to a Scott double passtype nebulization chamber was used throughout. Plasmaoperating conditions and wavelengths used for Al, Fe, Mn, Srand Zn determination are listed in Table 1 and were optimized orused as recommended by the instrument manufacturer [35].Argon 99.996 (White Martins - Praxair, SP, Brazil) was used forplasma generation, nebulization and as auxiliary gas. Manganese and Zn determinations by ASTM D 4004-06method were carried out with a Model Vario 6 FL atomicabsorption spectrometer (Analytik Jena, Jena, Germany)equipped with a deuterium background corrector. This equip-ment was also used for the determination of Al,Fe and Sr usingoperating conditions according to the manufacturer [36]although these elements are not mentioned in the ASTMprocedure. For Fe, Mn, Sr and Zn a conventional burner (10 cm,air/acetylene flame) and hollow cathode lamps (Narva, Berlin,Germany) were used throughout. For Al determinations aburner with 5 cm and a nitrous oxide/acetylene flame wereused.The selected wavelengths and bandwidths for Al, Fe, Mn, Srand Zn were 309.3,248.3,279.5,460.7 and 213.9 nm and 1.2,0.2, 0.2, 0.5 and 0.5 nm, respectively. Residual carbon content was determined by ICP OES andmeasurements were performed according to the conditionsdescribed in Ref. [37]. A cryogenic mill (Spex Certi Prep, Model 6750, Metuchen,USA) was used to grind the samples. Pellets of elastomers wereprepared using a hydraulic press set at 8ttons (Specac,Orpington,UK). 2.2. Samples and reagents Samples of commercial automotive carbon black-containingelastomers were used in this study (NBR and EPDM containingabout 30% carbon black and 2.5%ZnO). Samples were groundusing a cryogenic mill with a self-container liquid nitrogen bathand a grinding polycarbonate tube. Each sample was placed in aseparate grinding tube in order to reduce cross-samplecontamination. A pre-cooling time of 90 ss was adoptedfollowed by only 2 min for grinding (this procedure wasrepeated twice). With this procedure the particle diameter wasless than 0.84 mm. Ground samples were digested using theconventional microwave heating procedure and also accordingto ASTM method. For the proposed combustion procedureground samples were pressed as pellets (diameter of 13 mm andmass between 50 and 500 mg). Milli-Q water (18.2 M cm) was used to prepare allsolutions. Analytical-grade reagents (Merck, Darmstadt, Ger-many) were used. Concentrated nitric acid (65%) was distilledin a sub-boiling system (Milestone, Model DuoPur). Workinganalytical solutions in the range of 0.05 to 20 mg 1for Al, Fe,Mn, Sr and Zn were prepared before use by serial dilution ofstock reference solutions containing 1000 mg1(Spex plasmasolutions, Metuchen, USA). Ammonium nitrate was dissolvedin water, and this solution was used as igniter for thecombustion procedure. A small disc of filter paper (15 mm ofdiameter, 12 mg) with low ash content (Black Ribbon Ashless,Schleicher and Schuell GmbH, Dassel, Germany) was used toaid the combustion process. Filter paper was previously cleanedwith 10% (m/v) HNO3 for 20 min in an ultra-sonic bath anddried in an oven for 2 h at 60°C before use. Glass materialswere soaked in 10% (m/v) HNO for 48 h and thoroughlywashed with water before using. 2.3. ASTM method A conventional muffle furnace was used for the dry ashingprocedure according to ASTM D 4004-06 method. About 1 g ofsample was weighed and transferred to Pt crucibles. Temper-ature was raised to 250 °C for 30 min and further to 550°C for Table lOperational parameters for ICP OES measurements Power (W) 1300 RF generator (MHz) 27 Ar plasma flow rate (1 min) 14.0 Ar auxiliary flow rate(l min Ar nebulizer flow rate (l min n- Spray chamber Scott type Nebulizer Cross flow Analytical wavelength (nm) 167.078 193.091 259.940 Mn 257.611 407.771 213.856 Measurements were performed according to the conditions described in Ref.[37]. Table 2Comparison of digestion procedures using spike recoveries in NBR digests (determinations by ICP OES) Digestion procedure Digestion time Recovery (%) Al Fe Mn Sr Zn MIC (4 mo11HNO3 as absorbing solution) With reflux 25 min 101.0±±1.2 98.9±1.5 97.0±1.8 99.8±0.7 98.6±1.5 Without reflux 1 min 99.5±2.5 50.0±2.3 96.5±2.8 97.5±1.5 98.5±3.0 Microwave-assisted digestion (closed vessels) HNO3+HSO4 60 min 71.8±9.9 107.3±8.0 90.2±8.7 102.4±8.6 113.7±15.4 HNO:+HCl+H,O, 93.7±5.0 98.8±3.5 108.5±4.9 104.3±3.4 105.3±7.4 Dry ashing (ASTM D 4004-06) 550°C/6 mol1HCl 6h 14.8±5.6 37.4±7.5 72.0±6.5 36.8±5.0 95.2±3.5 It was considered the heating and cooling times for all procedures. 2 h. After cooling 5 ml of 6 mol 1 HCl were added andcrucibles were heated on a hot plate until the ash wascompletely in solution. Digests were diluted with water andanalyzed by ICP OES and F AAS. 2.4. Proposed microwave-induced combustion procedure For the proposed MIC procedure pellets of elastomersamples from 50 to 500 mg were weighed and placed togetherwith the filter paper on the quartz holder. Quartz vessels werepreviously charged with 6 ml of absorbing solution (water, 2,4,or 14 mol1 HNO3). The holder containing the sample wasintroduced into the quartz vessel, and 50 ul of 6 mol 1ammonium nitrate solution were added to the paper. Afterclosing and capping of the rotor, vessels were pressurized with20 bar of oxygen. Vessels were pressurized using the deviceoriginally designed for pressure release when using it for Fig. 2. Influence of absorbing solution with (A) and without (B) reflux forsubsequent determination of Al, Fe, Mn, Sr and Zn after spikes in NBR samplesusing the proposed combustion procedure (sample mass of 350 mg and 6 ml ofabsorbing solution, n≥3). conventional acid digestion. The rotor with the vessels wasplaced inside the microwave cavity and the selected microwaveheating program was started. Microwave irradiation programused for the combustion procedure was: i) 1400 W for 60 s, ii)1400 W for 5 min (optional step), and iii) 0 W for 20 min forcooling if step ii was applied. During steps i and ii, the coolingfan was selected at level 1 (level 3 was applied only for step iii).In this work,each run was performed with a minimum of fourvessels. After digestion, each vessel was carefully opened torelease the pressure. The resultant solution was diluted withwater and transferred to a 50 ml polypropylene vessel for furtheranalysis by ICP OES. After each run, holders were soaked inconcentrated HNO3 for 10 min and rinsed with water. 2.5. High-pressure microwave digestion procedure For comparison, two microwave-assisted acid digestionprocedures were performed using a high-pressure digestionsystem with quartz digestion vessels. Different acid mixtureswere used: i) concentrated HNOs and H2SO4 and ii)concentrated HNO3, HCl and H202. These procedures wereadapted from reference[26] (i), and according to themanufacturer recommendations for “rubber” digestion (ii)[38]. The following reagent volumes were used for proceduresi) 5 ml of HNO3 plus 1 ml H2SO4, and ii) 5 ml ofHNO3, 1 mlHCl and 1 ml H202 (sample mass was 400 mg). Microwavepower and time program was as following: 1400 W up to 280 °C(ramp of 15 min), 1400 W for 25 min, and 0 W for 20 min forcooling. During the first and second heating steps the coolingfan was selected at level 1 (level 3 was applied only for the Table 3 Determination of Al, Fe, Mn, Sr (ug g ) and Zn (%) in NBR and EPDMsamples after digestion by ASTM procedure (A) and using the proposed MICprocedure (B) with further determinations by ICP OES Analyte Sample Concentration ICP OES (A) ICP OES (B) Al NBR 12.1±0.6 80.6±2.9 EPDM 7.4±1.8 49.7±0.8 Fe NBR 13.6±0.6 36.4±1.7 EPDM 7.7±0.9 21.5±0.6 Mn NBR 0.70±0.05 0.95±0.06 EPDM 0.37±0.05 0.52±0.05 Sr NBR 7.55±0.07 15.9±0.3 EPDM 1.43±0.06 2.84±0.15 Zn NBR 1.19±0.03 1.31±0.04 EPDM 1.21±0.08 1.25±0.03 cooling step). This microwave program was applied for theprocedures i) and ii). After cooling digests were filtered toremove residues and diluted with water to 30 ml. Final solutionswere analyzed by ICP OES after dilution when necessary. 3. Results and discussion 3.1. Basic characterization of samples Initially, elastomers were characterized by elemental analysis(CHN) and infrared spectroscopy. Results of elemental analysisindicate 71% of carbon in NBR samples and 86.6% for EDPM. Anitrogen content of less than 4% was determined for both samplesand hydrogen concentration ranging from 6 to 9.5%. It isimportant to observe that about 30% of each elastomer iscomposed by black carbon (according to information ofmanufacturer). In addition, infrared spectroscopy was used forfunctional group identification. For NBR elastomer characteristicbands for nitrile groups and a very intense band at 1407 cmduethe hydrogen bonded carbon (C=C) were observed. This lastband was expected in view of the high content of aromaticcompounds due to the carbon black additive. For EPDM, bandswere observed at 2914 cm=and 2846 cm corresponding to C-H asymmetrical and symmetrical stretching, respectively, due themethylene group. These bands indicated bond characteristics ofethylene, propylene and dien (cyclopentadiene and cycloocta-diene) compounds for EPDM rubber. 3.2. ASTM digestion method For rubber decomposition American Society for Testing andMaterials Standards (ASTM D 4004-06) recommends the use ofa dry ashing procedure at 550°C with dissolution of the ash in6 mol1HCl and subsequent analyte determination by F AAS.In this procedure the temperature should be increased slowly inorder to avoid sample spattering. As a consequence a long timewas necessary to complete sample decomposition (usually,more than 6 h). After the ashing step, residual ZnO wasobserved for both samples. Residues were dissolved in hot6 mol1HCl before the determination by F AAS and ICP OES.Although the ASTM procedure is recommended only for Mnand Zn and restricted for determinations by F AAS, measure-ments by ICP OES were also performed in the same digests [8].For Zn results by F AAS were in agreement with those by ICPOES for NBR and EPDM samples (Student’s t-test). For NBR,Zn concentration was 1.25±0.04%and 1.19±0.03%, and forEPDM it was 1.17±0.05% and 1.21±0.08% by FAAS and ICPOES, respectively. However, in spite of the relatively highsample mass used for sample decomposition, Al, Fe, Mn and Srcould not be determined in digests by F AAS due to aninsufficient limit of quantification (LOQ) (6.7, 3.1,1.9 and1.6 ug g for Al, Fe, Mn and Sr, respectively) and only themeasurements by ICP OES were considered feasible. As nocertified reference rubbers were available, spikes were made indigests to evaluate possible interference in measurements byICP OES. Recoveries were 15, 37,72,48, and 95% for Al, Fe,Mn, Sr and Zn, respectively (Table 2). 3.3. Determination ofAl, Fe, Mn, Sr and Zn in elastomers aftermicrowave-induced combustion procedure The initial MIC conditions used in this work for elastomercombustion were the same as those used previously forbiological samples [33] with an O2 pressure of 15 bar [34].However, this pressure was effective only for sample masses upto 350 mg. During tests with higher sample masses (500 mg),incomplete combustion was observed in some cases with smallsample particles remaining before the reflux step. To preventthis drawback, the oxygen pressure was increased to 20 bar andthis condition was selected for subsequent studies. A major difference between the proposed MIC procedureand other closed vessel combustion systems (Schoniger flaskand combustion bombs) is the possibility to apply a reflux stepwithout opening the vessel. After combustion practically all theorganic material has been decomposed and the absorbingsolution is used to assure analyte removal from internal parts ofvessel. However, some analytes could remain adsorbed on thequartz surface or in solid residues and a more effective cleaningmight be necessary for complete removal of analytes. After thecombustion, when microwave radiation is continuously applied,the absorbing solution quickly refluxes and dissolves eventualresidues. Obviously, the leaching process and consequentanalyte recovery will depend on the chosen absorbing solution.In this work, a reflux step was applied for 5 min, immediatelyafter combustion. This time was selected according torecommendation of Ref.[34]. Tests were applied using waterand nitric acid (2, 4 and 14 mol1) as absorbing/refluxingsolutions. Spike recoveries for NBR samples are shown inFig. 2. It may beobserved that reflux using 4 mol1HNO3 wasenough to achieve quantitative recoveries for all analytes. With2 mol1HNO3, results for Fe were about 15% of the referencevalue and water, as expected, was inefficient for all analytes.However, testswithout reflux, but using 4 mol 1-orconcentrated HNO3, showed good results for Al, Mn, Sr andZn. For Fe, even using concentrated HNO3 recovery was nothigher than 58%. These results show that, with the exception ofFe, no reflux step would be necessary and digestion could beperformed in 1 min (only the time for the combustion step).However, if Fe must be determined in routine analysis a moregeneral procedure, using reflux with 4 mol1HNO3, should bechosen.Although concentrated HNO3 could also be applied, itsuse should be avoided in order to reduce acid consumption andto decrease the final acidity of digests. The same study wasperformed for EPDM samples and similar results were obtainedfor all analytes after the MIC procedure. Therefore, 4 mol1HNO3 was selected as absorbing solution with 5 min reflux.With this condition no residues were observed and digests wereobtained as completely clear and transparent solutions. 3.4. Wet digestion procedure in closed vessels Microwave-assisted digestion in closed vessels was per-formed in order to compare the results with those using theproposed procedure. For the wet digestion procedures (usingHNO3+H2SO4 and HNO3+HC1+H202 mixtures) a great amount of non-digested material remained in solution (deepblack color appearance). In addition, black solid particles alsoremain as suspension in the digests. After digestion thesolutions were filtered and the mass of solid particles wasdetermined. For digestion using closed vessels solid residueswere 11 and 27% (HNO3+H2SO4), and 8 and 25% (HNO3+HC1+H202) for NBR and EPDM samples, respectively.Composition of particles was basically black carbon. Filtereddigests were used for subsequent analysis by ICP OES. Table 2 presents the results of spikes in digests for the studieddigestion procedures. Using the closed vessels procedure withHNO3+H2SO4 mixture the recoveries for Fe and Zn werehigher than 100%, which was considered to be due to eventualcontamination by acids. However, for Mn and Al, maximumrecovery was about 90 and 72%, respectively. Moreover, valuesfor RSD were up to 13.7% for this procedure. It could besupposed that the black precipitates or soluble (but non-decomposed) substances could interfere in the determinationsby ICP OES. However, after filtering of digests almostquantitative recoveries were found for Fe, Sr and Zn using thedigestion procedure with HNO3+HCl+H2O2. For this mixture,the RSD was low for all analytes and recoveries were better thanthose using the dry ashing procedure or HNO3+HSO4 closedvessel digestion. However, values for Al presented again thelowest recoveries. Results for the MIC procedure are alsocompared in Table 2 and it is possible to observe that the RSDwas smaller than other procedures with and also without 4 mol1HNOs reflux step. In this work samples were ground toparticle size ≤0.84 mm and sample homogeneity wasconsidered suitable as the RSD has been always lower than2% using MIC procedure with reflux step. In addition, blanksfor the proposed procedure were always low, probably due tothe use of diluted nitric acid for the reflux step. A comparison of the digestion time for the studiedprocedures is also presented in Table 2. The time required fordry ashing is the highest, as already discussed. On the otherhand, using the proposed MIC procedure with reflux the time isabout 50% of that needed for high-pressure digestion in closedvessels (considering the cooling time for both procedures). Thisis important in industry routine analysis if results need to beobtained quickly. Samples of elastomers were analyzed by the proposedprocedure and results are shown in Table 3. Results for Al. Fe,Mn and Sr in the NBR sample were higher than those for theEPDM sample, whereas the values for Zn were practically thesame. This can be explained by the same amount of Zn-basedadditive that is added to both elastomers. The proposedprocedure could be applied for determination of tracecontaminants and also for high concentration additives. 3.5. Residual carbon content in digests The RCC has been used to evaluate the efficiency ofdigestion procedures [37]. Results for residual carbon betweendry ashing and MIC procedures were of the same level andvalues for the proposed procedure were the lowest related to theother studied digestion procedures. Residual carbon content values for these procedures were always below 0.5% for bothNBR and EPDM samples. For closed vessel microwavedigestion values for RCC were from 4 to 8%. It is importantto observe that digests (closed vessels) were filtered for non-digested particles removal before the RCC determination. The low RCC obtained for the proposed procedure could berelated to the high temperature (about 1300 C) during theelastomer combustion. This temperature is in the same level as thatcurrently determined by other polymers using the same system[39]. In this condition almost all the organic matter could becompletely decomposed resulting in a solution able to be analyzedeven using techniques where determinations are susceptible to beinfluenced by residual carbon or excessive acid concentration. 3.6. Safety considerations The advantages offered by the MIC procedure includeignition based only in the microwave radiation in closed vesselsunder oxygen atmosphere. The ignition step occurs between 6to 10 s at 1400 W of microwave power and total time ofcombustion will depend on the oxygen pressure, sample typeand sample mass [39]. For elastomer samples, even with samplemass up to 500 mg the maximum pressure does not exceed60 bar for few seconds. As the operational maximum pressurefor the equipment used is 80 bar, the proposed procedure couldbe considered safe in view of the risk of explosions. On theother hand, the temperature of absorbing solution does notexceed 60 °℃ if only the combustion step is applied and if refluxis used pressure and temperature are controlled by theequipment sensors. 4. Conclusions The proposed MIC procedure represents the association ofthe features of combustion techniques and microwave-assistedwet digestion in closed vessels for black carbon-containingelastomers digestion. Moreover, comparing to the use ofSchoniger combustion flask and combustion bombs, theadvantages of using the MIC procedure are the sample mass(Schoniger flask) and the higher throughput.With the proposedprocedure it is possible to digest up to eight samplessimultaneously using a sample mass of up to 500 mg. Thisvalue might be considered high in comparison with methodsbased on conventional closed vessel digestion. It is important topoint out that, despite the necessity of routine elastomers qualitycontrol, there is a lack of data in literature related to digestionprocedures for this matrix looking for further inorganic analysis.Even for other polymers few papers discuss aspects of samplepreparation related to inorganic analysis. On the other hand, theproposed procedure uses small amounts of reagents reducinghazardous waste during chemical analyses. Acknowledgements The authors are grateful to CNPq, CAPES and Fapergs andalso to Caribor Tecnologia da Borracha Ltda. (www.caribor.com.br) for donation of the elastomers samples. ( [1] J.E. Mark, R. Abou-Hussein, T.Z. Sen, A. Kloczkowski, Some s imulations on f iller reinforcement in elastomers, Polymer 46 ( 2 005) 8894-890 4 . ) ( [2] P. Sutanto, F. Picchioni, L.P.B.M. Janssen, K.A.J. Dijkhuis, W.K. Dierkes,J.W.M. N oordermeer, EPDM rubber reclaim from devulcanized E PDM, J. A ppl. P olym. S ci. 102 (2006) 5948-5957. ) ( [3] B.B. B oonstra, R ole o f particulate f illers i n elastomer reinforcement: a review, Polymer 20 ( 1979) 691-704. ) ( [4] F.W. Billmeyer, Textbook of Polymer S cience, 3rd ed., John Wiley &Sons, New York, 1 984, p p. 506-521. ) ( [5] E. Demirhan, F . Kandemirli, M. Kandemirli, The effects of furnace carbon blacks on t he m echanical a nd the rheological p roperties of SBR 1 5 02styrene butadiene rubber , Mater . Des. 28 (2007 ) 132 6- 1329. ) ( [6] A. R itter, E. Michel, M . S c himid, S. Af f olter, I n terlaboratory t e st o n polymers: determination of heavy m e tals i n polymer matrices, P o lym. Te s t. 23 (2004)467-474. ) ( [7] R.E. Wolf, C. Thomas, A . B o hlke, Analytical determination of metals in industrial polymer by laser ablatio n ICP-MS, Appl. Surf. Sci. 127-129 (1998) 299-303. ) ( [8] Annual Book of ASTM Sta n dards, Standard Test Methods for Rubber- Determination of Metals Content by Flame Atomic Absorption (AAS)Analysis, D 4004-06, 2 006. ) ( [9] K.G. D arrall, A. P indar, P . Quevauviller, Collaborative study f or the quality control of t race element determinations i n paint coatings. Part 1.Interlaboratory study , Fresenius’J. Anal. Chem. 357 (1997) 8 33-836. ) ( [10] A. L amberty, W . V an Borm, P. Q u evauviller, Collaborative stu d y toimprove the quality control of trace e lement determinations in p o lymers.Part 2. Certification of polyethylene reference materials (CRMs 680 and681) f or As, B r, Cd, Cl, Cr, Hg, Pb and S content, F r esenius’ J . Anal. Chem. 3 70 (2001) 81 1-8 18. ) ( [11] L . P erring, M. A lonso, D. Andrey, B. Bourqui, P. Zbinden, An eva l uationof a nalytical techniques f or determination of l e ad, c admium, ch r omium,and m ercury i n food-packaging materials, F r esenius’J. A n al. Chem. 37 0 (2001) 76-81. ) ( [12] K. D . B esecker, C .D. R hoades, B . T. Jones,K.W. Ba r nes, Cl o sed-vessel n itric acid microwave digestion of polymers, At. Spectr. 19 (1998) 55-59. ) ( [ 1 3] K.D. Besecker, C.D. R hoades, B.T. Jones, K . W. Barnes, A simple closed-vessel n itric acid digestion method f o r a po l yethylene/polypropylene polymer blend, At. Spectr. 19 (1998) 193-197. ) ( [14] Z. Mester, R. Sturgeon, S ample P r eparation f o r Trace Element Analysis,vol. XLI, Elsevier, Amsterdam, 2003, pp. 683 - 719. ) ( [ 1 5]T . E r nst, R . P o pp, R. va n Eldik, Quantification of h eavy meta l s for therecycling o f waste plastics from electrotechnical a pplications, T alanta 53 (2000)347-357. ) ( [16] H. Sakurai, J. Noro, A. Kawase, M . F ujinami, K . Oguma, Digestion of plastic materials for the determination of toxic metals w ith a microwave oven f or household use, Anal. Sci . 2 2 (2006)225-228. ) ( [17] D.R. B uefield, S.C. Ng, A simplified oxygen-flask combustion procedurefor polymer analysis, J. Chem. Edu. 6 1 ( 1 984) 917. ) ( [18] E.D. T ruscott, Determination of chlorine i n a p olyvinyl chlorine m atrixusing the Schoniger o xygen f l ask and a t omic absorption s p ectrometry, Anal. Chem. 42 (1970) 1657. ) ( [19] J. Haslam, J .B. H amilton, D.C.M. S q u irrell, A p plication o f the o x ygencombustion m ethod to th e de t ermination of chl o rine in po ly mers,plasticisers and organic compounds, J. Appl. Chem. 10 (1960) 97-10 0 . ) ( [20] W. S elig, Fluorine analysis of p l astic-bonded e x plosives and pl a stics, F resenius Z. Anal. Chem. 234 ( 1968) 261- 26 9. ) ( [21 ] R.A. A nduze, C o lorimetric d e termination o f titanium i n p o lyethylene, Anal. Chem.29 (1957) 9 0-91. ) ( [22] L . J orhem, D r y as h ing, so u rces of error, an d performance eval u ation inAAS, Mikrochim. A cta 1 19 (1995)211-218. ) ( [23] M .R e sano, M. Aramendia, W. Devos, F. Va n haecke, Direct multi-element analysis of a f luorocarbon p olymer via solid sampling-electrothermalvaporization-inductively c oupled plasma m a ss spectrometry, J. Anal. At. Spectrom. 21 (2006) 891-898. ) ( [24] H.M.S. K ingston, S.J. Haswell, Microwave- E nhanced C h emistry. Funda-mentals, Sample P r eparation and A p plications, A C S, Wa s hington, 199 7 , pp. 55-222,401- 4 22. ) ( [25] J . Tolodi, J. Mermet, Acid interferences in atomic spectrometry: analyte signal effects and subsequent reduction, Spectrochim. Acta P art B 5 4 (1999)895-929. ) ( [26] M . Z ischka, P . K e ttisch, P . K ainrath, M icrowave-assisted d i gestion ofplastic scrap: basic considerations and chemical approach, At. Spectr. 1 9 (1998) 223-227. ) ( [27] A.M.G. MacDonald, Th e oxygen flask method, Advances in Anal y tical Chemistry and I nstrumentation, John Wiley and Sons, New Y ork, 1 965, 87- 8 8 and 75- 1 16. ) ( [28] S . F ujiwara, H . N a rasaki, Determination of tra c e el e ments in o rg a nic material by the oxygen bomb method, Anal. C h em. 40 (1968) 2031-2032. ) ( [29] G.V. Iyengar, K.S. Subramanian, J . R .W. W oittiez, Element Analysis of Biological Samples Pr i nciples and Pr a ctice, C R C Pre s s, Boca Rat o n, 1997,pp. 116-117. ) ( [30] M . Stoeppler, Sampling and Sample Preparation, S p ringer-Verlag, Berlin, 1997, p. 192. ) ( [31] Z. Sulcek, P. Povondra, Methods of Decomposition in Inorganic Analysis, CRC Press, Florida, 1 989, p. 282. ) ( [32] E.M.M. F l ores, J .S. B arin, J.N.G. P aniz, J.A. Medeiros, G. K napp, Verfahren und Vorrichtung zum Verbrennen von Proben i n e inersauerstoffhaltig e n Atmosphare, Pat e nt Number WO2004092722,2004. ) ( [33] E.M.M. F lores, J.S. Barin, J.N.G. Paniz,J.A. Medeiros, G . K napp, M icrowave-assisted sample c o mbustion: a technique for sample prepara- t ion in trac e element determination, Anal. Chem. 76 (2004) 3525-3529. ) ( [34] M .F. M esko, D . P. M o raes, V . L. J . S.Barin, G . Dressler, E.M.M. K n app, Digestion of biological materials using the microwave-assisted sample combustion technique, Microchem.J. 8 2 ( 2 006) 183-1 88 . ) ( [35] Spectr o Ciros CCD- So f tware ve r sion 0 1 /March 2 0 03, S p ectro Analytical Instruments GmbH & Co. KG, Kleve, Germany. ) ( [36] Analytik Jena A G, Win A AS V 3. 1 3 . 0 Eng. Software, 1 9 98-2004, Je n a, Germany. ) ( [37] S.T . Gouveia, F .V. Silva, L.M. C o sta, A.R.A. N ogueira, J .A. N obrega, D etermination o f residual c a rbon by inductively-coupled plasma optical emission spectrometry w i th a x ial and r a dial view c o nfigurations, A n al.Chim. Acta 445 (2001) 269- 2 75. ) ( [38] Anton Paar GmbH, M u ltiwave 3000 microwave sa m ple pr e paration 、、 system, S oftware version v1 . 27-Synt, Graz, Austria (2003). ) ( [39] J .S. B arin, E.M.M. F l ores, G. Knapp, Tr e nds in s a mple preparation usingcombustion t echniques, in: M.A.Z. A rruda ( Ed.), Trends in S ample Preparation, Nova S cience P u blishers, H auppauge, 2 0 06, pp . 73 - 11 4, Chapter3. ) A rapid digestion procedure for the determination of Al, Fe, Mn, Sr and Zn in carbon black-containing elastomers (30%) has been developed using sample combustion in closed quartz vessels. Microwave radiation was used for ignition. Combustion takes place in the presence of oxygen under pressure using ammonium nitrate (50 μl of 6 mol l−1) as aid for ignition. Samples of nitrile-butadiene rubber and ethylenepropylene-diene monomer were decomposed. A quartz device was used simultaneously as a sample holder and for the protection of vessel cap. The influence of the absorption solution (nitric acid or water) and the necessity of an additional reflux step were evaluated. Determination of Al, Fe, Mn, Sr and Zn was performed by inductively coupled plasma optical emission spectrometry. A reference method (ASTM D 4004-06) based on conventional dry ashing and flame atomic absorption spectrometry was used for comparison (Mn and Zn). Results were also compared to those obtained by using wet acid digestion in closed systems. Concentrated and diluted (4 mol l−1) nitric acid, with 5 min of reflux after the combustion, gave best recoveries for all analytes (from 97 to 101%). For dry ashing quantitative recoveries were found only for Zn whereas for Al, Fe, Mn and Sr the recoveries were only 14, 37, 72 and 37%, respectively. With the proposed procedure the residual carbon content was below 0.5% and further determination of analytes was feasible with only the combustion step (for Fe a reflux with diluted HNO3 was necessary). Complete sample digestion is obtained in less time using the proposed procedure than with other procedures and no concentrated acids were necessary.
确定





还剩5页未读,是否继续阅读?
安东帕(上海)商贸有限公司为您提供《橡胶中Al, Fe, Mn, Sr, Zn元素检测方案(微波消解)》,该方案主要用于橡胶制品中Al, Fe, Mn, Sr, Zn元素检测,参考标准--,《橡胶中Al, Fe, Mn, Sr, Zn元素检测方案(微波消解)》用到的仪器有
相关方案
更多
该厂商其他方案
更多









