方案详情
文
无机锡及其化合物会使肠道吸收不良,并造成身体的持久危害。同时,少量的金属就能影响饮品的口味和储藏。传统的火焰原子吸收光谱法测定锡的灵敏度低,定量低浓度的锡比较困难。本方法采用石墨炉原子吸收光谱法测定锡既能保证分析的灵敏度,同时,大大缩短分析周期,提高了工作效率。
方案详情

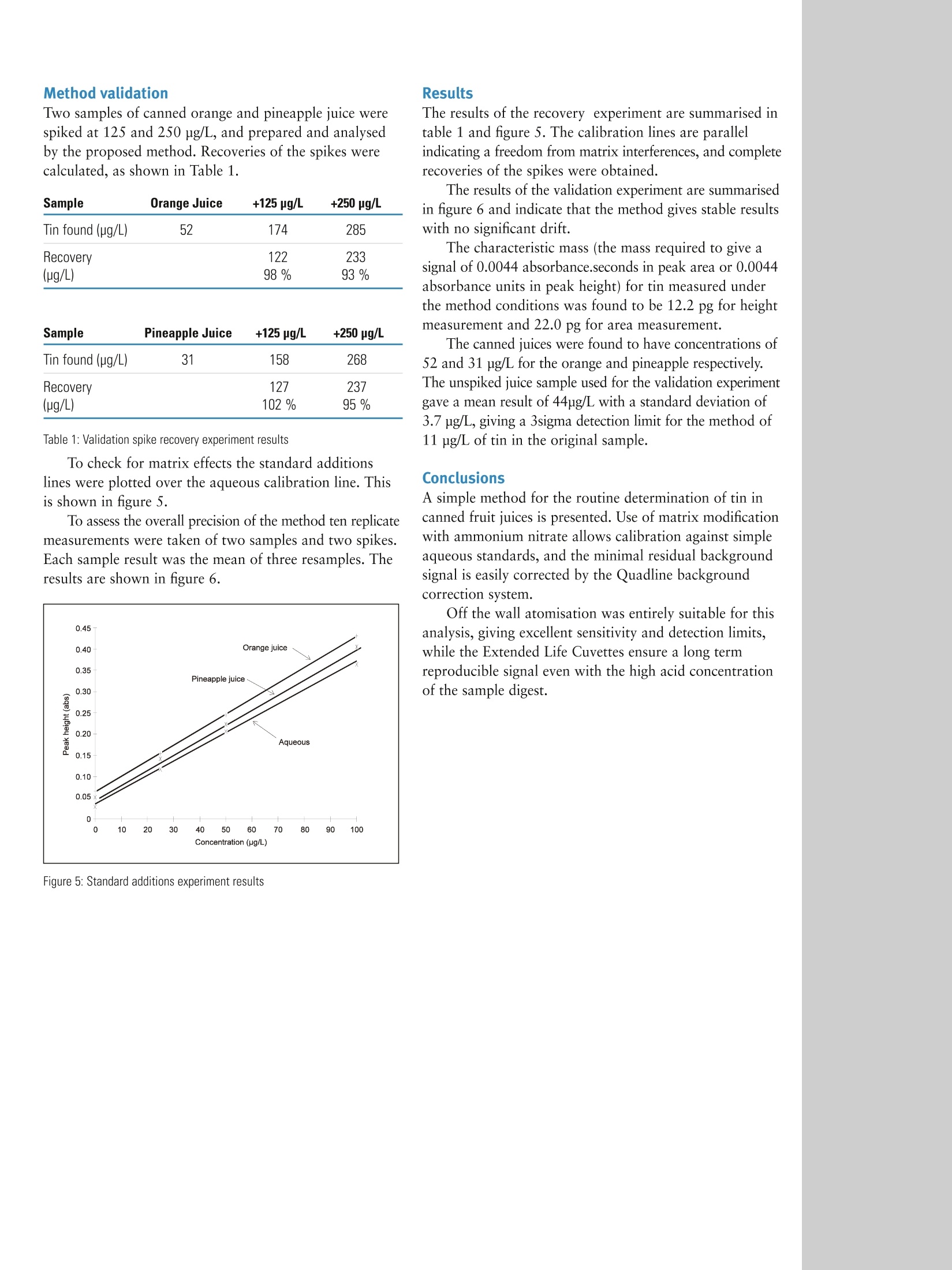
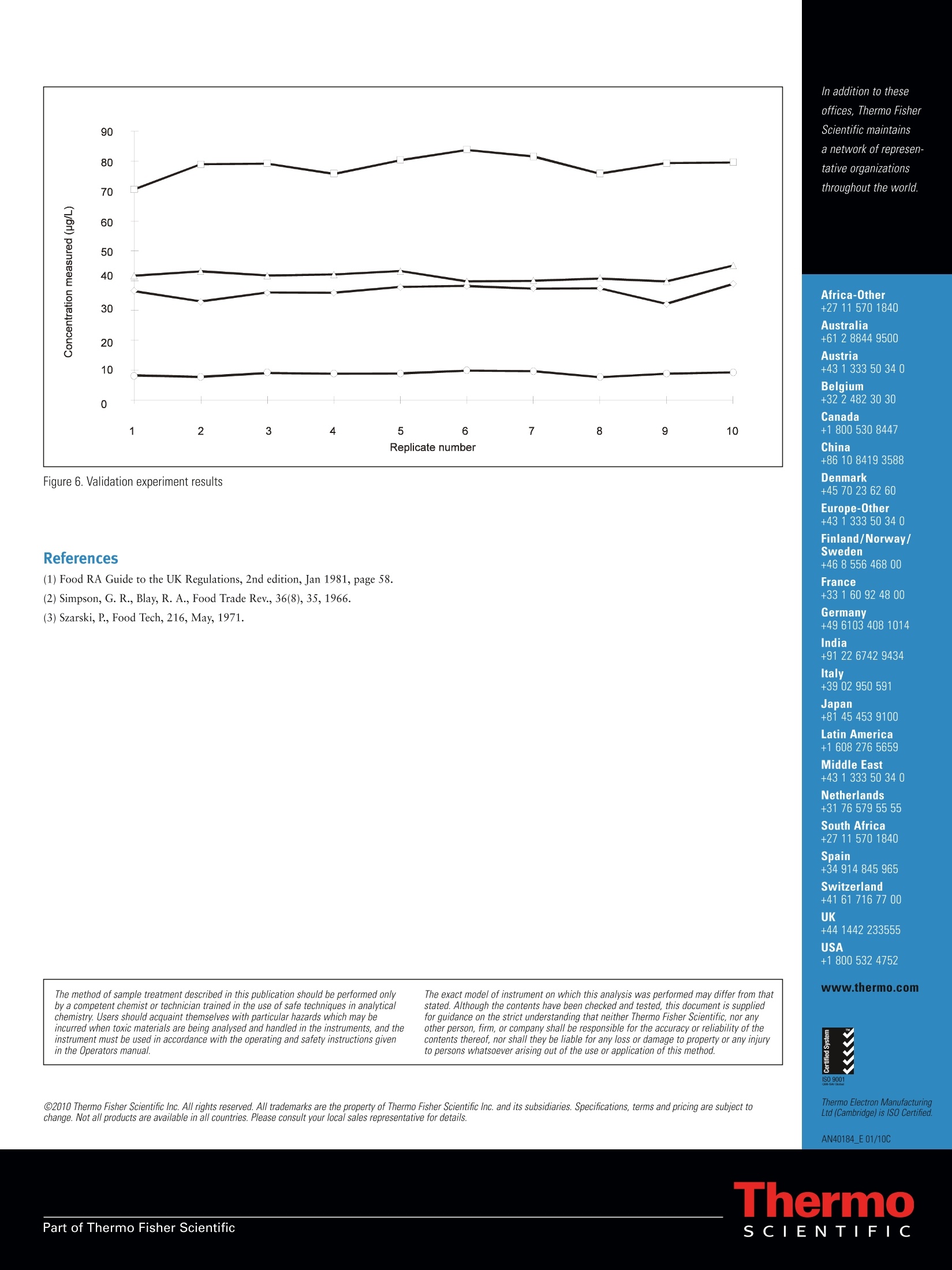
C2010 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific Inc. and its subsidiaries. Specifications, terms and pricing are subject tochange. Not all products are available in all countries. Please consult your local sales representative for details.Part of Thermo Fisher Scientific Method Guide: 40184 Atomic Absorption Full MethodSn in Canned Fruit Juice Introduction Inorganic tin and its compounds are poorly absorbed inthe intestinal tract and lasting harmful effects have notbeen documented. However even small amounts of themetal can adversely affect the flavour and storage propertiesof food products. The metal is introduced as a contaminant duringprocessing of food products and can accumulate duringstorage due to leaching from the containers. The United Kingdom food regulations permit a maximumguideline limit of 250 mg/kg in canned foods(1). Howeverimprovements to the processing and use of new materialsfor canning mean food manufacturers are required tomeasure significantly lower concentrations. The traditional flame atomic absorption spectrometricdetermination of tin is relatively insensitive, and accuratequantification at low concentrations is difficult. Tin can be successfully determined by Graphite FurnaceAtomic Absorption Spectrometry using an accuratebackground correction system provided care is taken tominimise losses during the program cycle. Analytical range A method for the determination of tin in canned fruit juicesis presented. The 3 sigma method detection limit is 11 ug/Lin the original sample, and concentrations up to 500 pg/Lcan be measured using the method described. Samples withhigher levels of tin could be analysed after dilution. Principle Tin is extracted from the sample with hydrochloric acidand is determined by direct calibration against aqueousstandards with Quadline background correction.Ammonium nitrate is used as a matrix modifier withconventional off-the-wall atomisation from an ExtendedLife Cuvette. Reagents: Hydrochloric acid (Spectrosol grade). Tin master standard (1000 mg/L Spectrosol or equivalent). Ammonium nitrate (Aristar grade or equivalent). All reagent examples available from: Fisher Scientific Bishop Meadow Rd Loughborough, LE11 5RG UK. Sample collection Tin can be easily extracted from food products byhydrolysis with hydrochloric acid(2,3). Samples should bewell mixed by shaking before analysis. Pipette 20.0 mL of fruit juice into a 100 mL beakerand add 10 mL of hydrochloric acid. Heat the mixture toboiling point and after allowing it to cool transfer it to a100 mL volumetric flask and make up to the mark withdeionised water. An aliquot of this solution should becentrifuged prior to analysis, and the clear supernatanttransferred to the autosampler cup. Standard solutions containing 25, 50, and 100 ug/L of tinin 10 % v/v hydrochloric acid and an acid blank wereprepared. A canned orange juice sample spiked at 125 ug/Lwas used for the method development experiments. Ridged Extended Lifetime Cuvettes were used throughout. Figure 1: Analysis parameters Figure 2: Ash/atomise plot for orange juice with matrix modifier For these reasons 10 pL of a 2 % m/v solution ofammonium nitrate was used as a matrix modifier. This hasthe advantage of volatilising the chloride present in thesample as ammonium chloride while stabilising the tin. Figure 4: Correction with Zeeman background correction An ash/atomise plot for the spiked juice was automaticallygenerated (figure 2). This showed that the tin was stable up to a temperatureof 1000 ℃ in the presence of the matrix modifier, whileoptimum signals were obtained by atomising at 2500℃C..Clean well defined peak shapes were obtained with minimalbackground signals for both QuadLine (figure3) and Zeeman(figure 4) background correction systems, both of whichwere capable compensating accurately for the small residualbackground. The Quadline system was used because ofthe superior sensitivity and baseline noise levels obtained. Two samples of canned orange and pineapple juice werespiked at 125 and 250 pg/L, and prepared and analysedby the proposed method. Recoveries of the spikes werecalculated, as shown in Table 1. Sample Orange Juice +125 pg/L +250 pg/L Tin found (ug/L) 52 174 285 Recovery (ug/L) 122 233 98% 93% Sample Pineapple Juice +125 pg/L +250 pg/L Tin found (ug/L) 31 158 268 Recovery (ug/L) 127 237 102% 95% Table 1: Validation spike recovery experiment results To check for matrix effects the standard additionslines were plotted over the aqueous calibration line. Thisis shown in figure 5. To assess the overall precision of the method ten replicatemeasurements were taken of two samples and two spikes.Each sample result was the mean of three resamples. Theresults are shown in figure 6. Figure 5: Standard additions experiment results Results The results of the recovery experiment are summarised intable 1 and figure 5. The calibration lines are parallelindicating a freedom from matrix interferences, and completerecoveries of the spikes were obtained. The results of the validation experiment are summarisedin figure 6 and indicate that the method gives stable resultswith no significant drift. The characteristic mass (the mass required to give asignal of 0.0044 absorbance.seconds in peak area or 0.0044absorbance units in peak height) for tin measured underthe method conditions was found to be 12.2 pg for heightmeasurement and 22.0 pg for area measurement. The canned juices were found to have concentrations of52 and 31 pg/L for the orange and pineapple respectively.The unspiked juice sample used for the validation experimentgave a mean result of 44ug/L with a standard deviation of3.7 ug/L, giving a 3sigma detection limit for the method of11 ug/L of tin in the original sample. Conclusions A simple method for the routine determination of tin incanned fruit juices is presented. Use of matrix modificationwith ammonium nitrate allows calibration against simpleaqueous standards, and the minimal residual backgroundsignal is easily corrected by the Quadline backgroundcorrection system. Off the wall atomisation was entirely suitable for thisanalysis, giving excellent sensitivity and detection limits,while the Extended Life Cuvettes ensure a long termreproducible signal even with the high acid concentrationof the sample digest. In addition to theseoffices, Thermo FisherScientific maintainsa network of represen-tative organizationsthroughout the world. Africa-Other +27 11 570 1840 Australia +61 2 8844 9500 Austria +43 1 333 50 34 0 Belgium +32 2 482 30 30 Canada +1 800 530 8447 China +86 10 8419 3588 Figure 6. Validation experiment results Denmark +45 70 23 62 60 Europe-Other +43 1 333 50 340 Finland/Norway/ Sweden (1) Food RA Guide to the UK Regulations, 2nd edition, Jan 1981, page 58. France (2) Simpson, G. R., Blay, R. A., Food Trade Rev., 36(8),35,1966. +33 1 60 92 48 00 (3) Szarski, P., Food Tech, 216, May, 1971. Germanv +49 6103 408 1014 India +91 22 6742 9434 Italy +39 02 950 591 Japan +81 45 453 9100 Latin America +1 608 276 5659 Middle East +43 1 333 50 340 Netherlands +31 76 579 55 55 South Africa +27 11 570 1840 Spain +34 914 845 965 Switzerland +41 61 716 77 00 UK +44 1442 233555 USA +1 800 532 4752 The method of sample treatment described in this publication should be performed onlyby a competent chemist or technician trained in the use of safe techniques in analyticalchemistry. Users should acquaint themselves with particular hazards which may beincurred when toxic materials are being analysed and handled in the instruments, and theinstrument must be used in accordance with the operating and safety instructions givenin the Operators manual. The exact model of instrument on which this analysis was performed may differ from thatstated. A/though the contents have been checked and tested, this document is suppliedfor guidance on the strict understanding that neither Thermo Fisher Scientific, nor anyother person, firm, or company shall be responsible for the accuracy or reliability of thecontents thereof, nor shall they be liable for any loss or damage to property or any injuryto persons whatsoever arising out of the use or application of this method. www.thermo.com Thermo Electron ManufacturingLtd (Cambridge) is ISO Certified. AN40184 E 01/10C
确定


还剩2页未读,是否继续阅读?
赛默飞色谱与质谱为您提供《果汁中锡检测方案 》,该方案主要用于果蔬汁类及其饮料中重金属检测,参考标准--,《果汁中锡检测方案 》用到的仪器有赛默飞 iCE 3500 原子吸收光谱仪
推荐专场
该厂商其他方案
更多
















