
在受控制的实验室条件下,土壤类型、水分含量和生物固体应用对农业土壤中大肠杆菌命运的影响Influence of soil type moisture content and biosolids application on the fate of Escherichia coli in agricultural soil under controlled laboratory conditions
方案详情

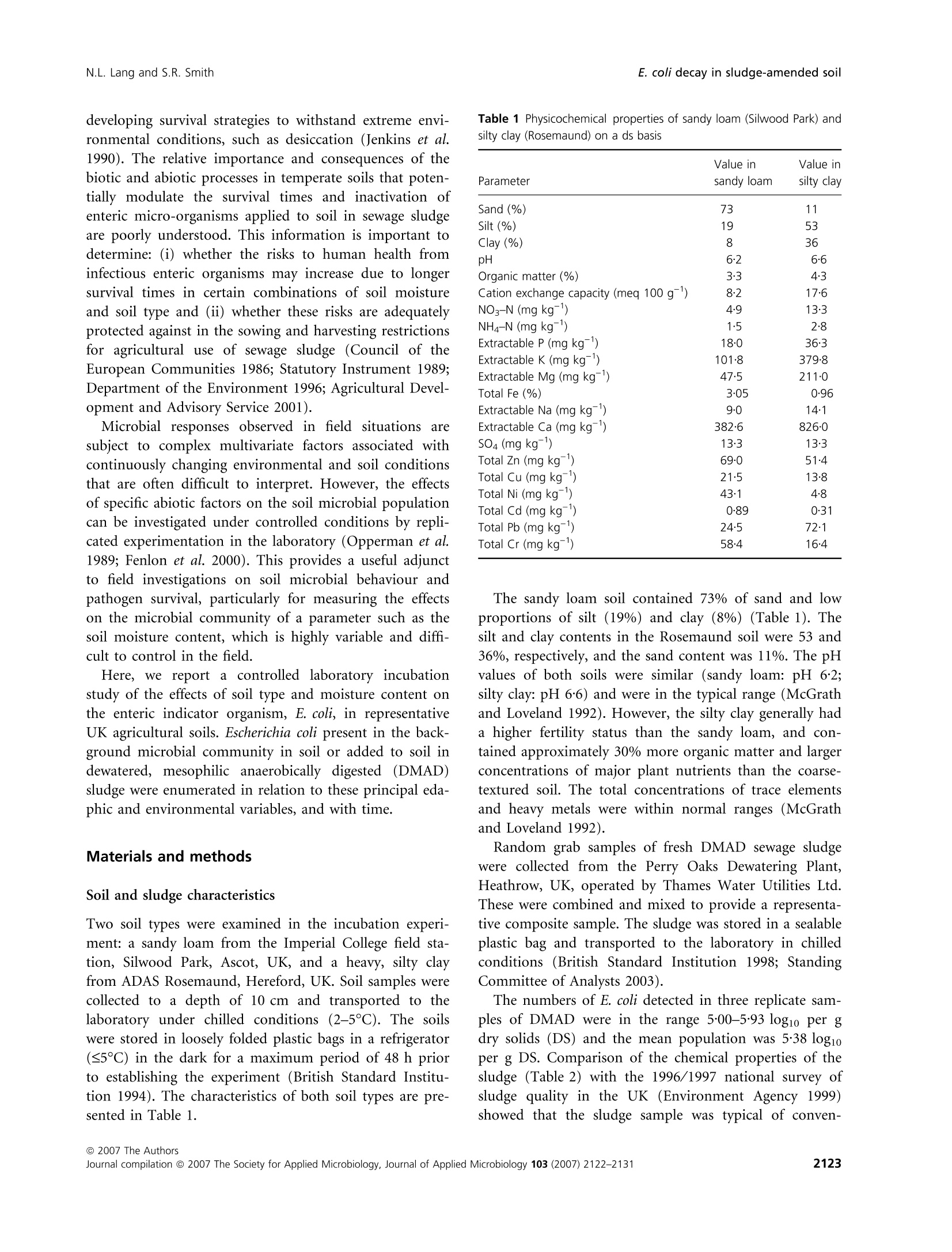
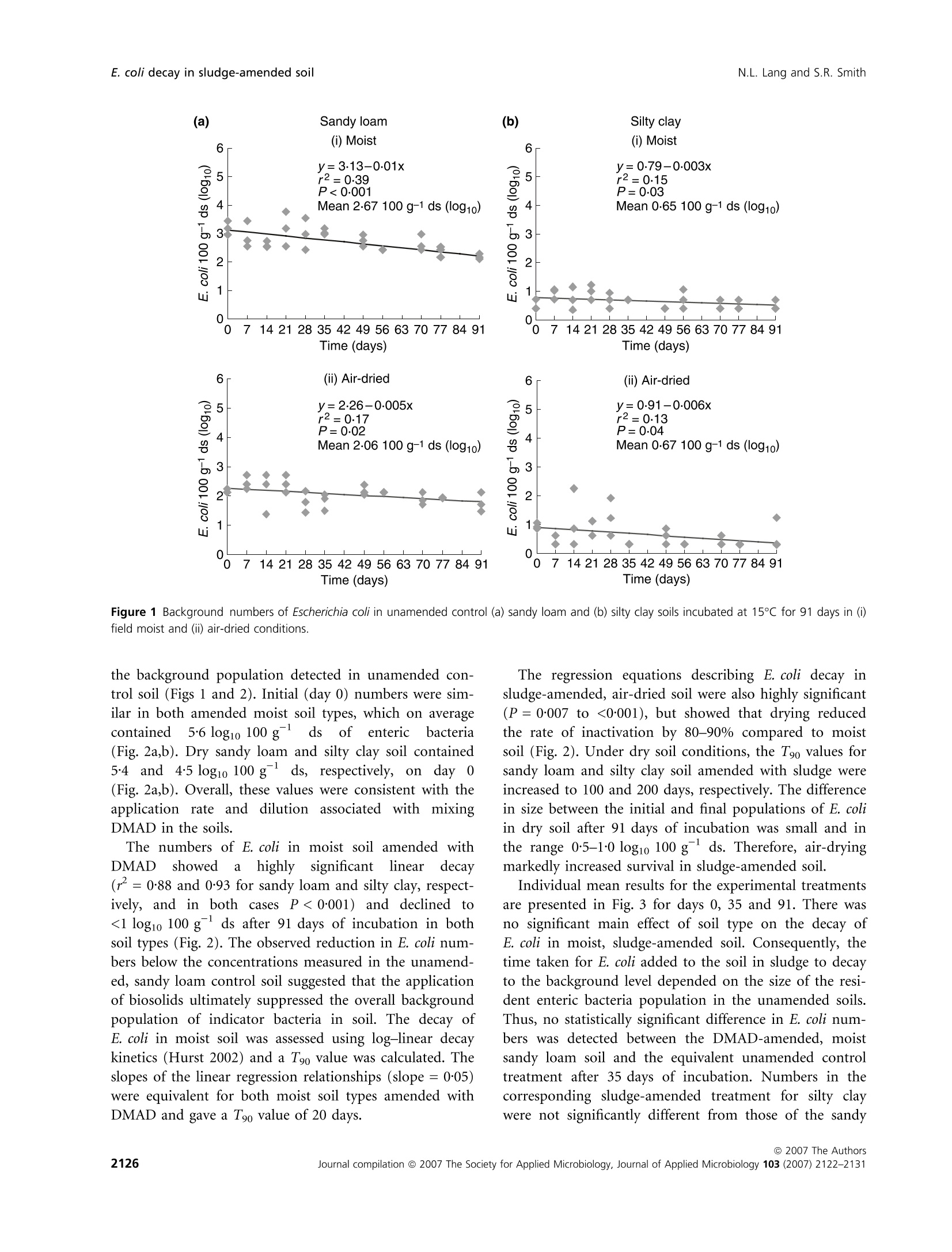
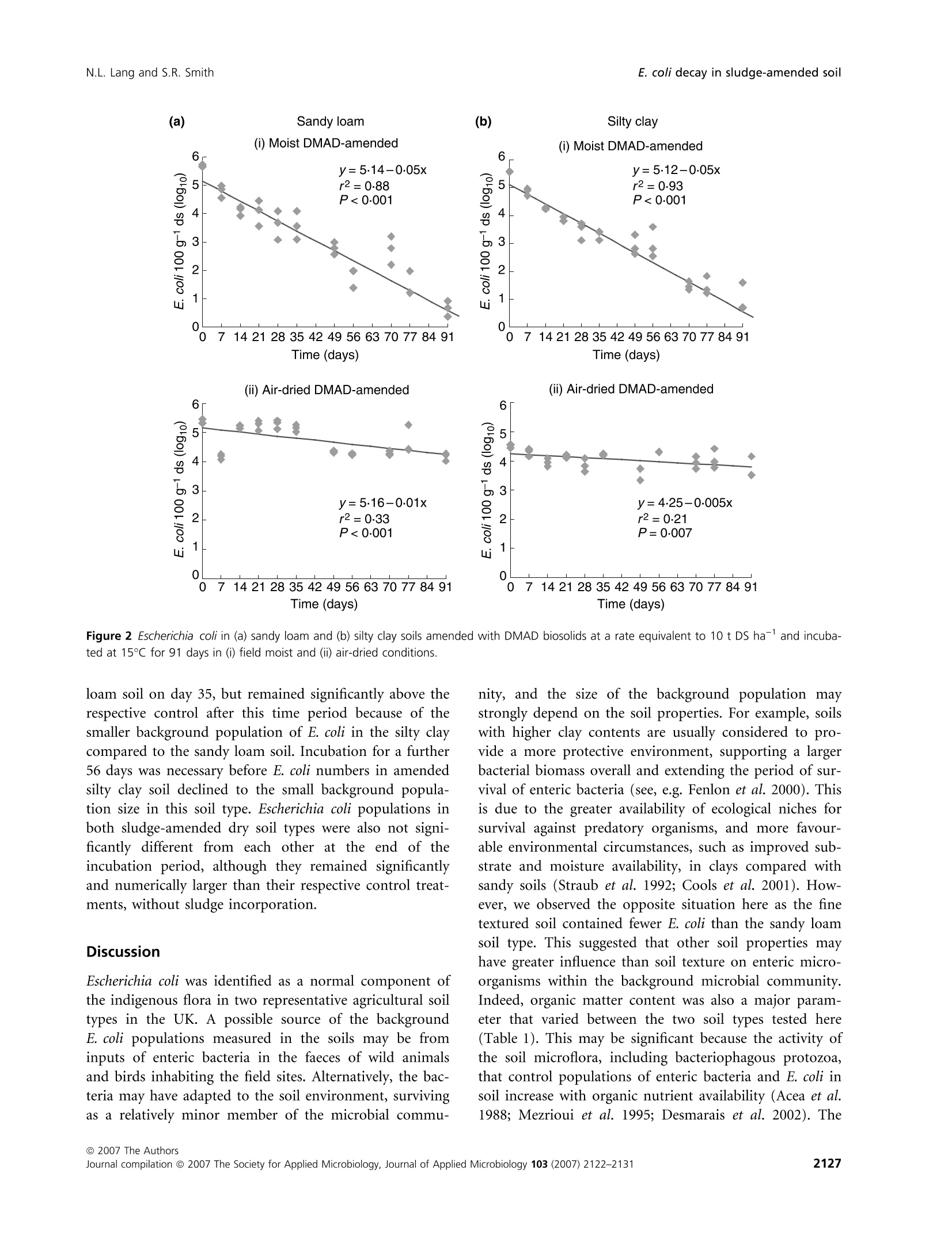
在受控制的实验室条件下,土壤类型、水分含量和生物固体应用对农业土壤中大肠杆菌命运的影响Influence of soil type moisture content and biosolids application on the fate of Escherichia coli in agricultural soil under controlled laboratory conditionsJournal of Applied Microbiology ISSN 1364-5072 N.L. Lang and S.R. SmithE. coli decay in sludge-amended soil ORIGINAL ARTICLE 在受控制的实验室条件下,土壤类型、水分含量和生物固体应用对农业土壤中大肠杆菌命运的影响 Influence of soil type, moisture content and biosolids application on the fate of Escherichia coli in agricultural soil under controlled laboratory conditions N.L. Lang and S.R. Smith Centre for Environmental Control and Waste Management, Department of Civil and Environmental Engineering, Imperial College London, UK Keywords agriculture, biosolids, decay, E. coli , moisture, sewage sludge, soil. Correspondence S.R. Smith, Centre for Environmental Control and Waste Management, Department of Civil and Environmental Engineering, Skempton Building, Imperial College London, South Kensington Campus, London SW7 2AZ, UK. E-mail: s.r.smith@imperial.ac.uk 2006⁄ 0067: received 19 January 2006, revised 29 March 2007 and accepted 19 April 2007 doi:10.1111/j.1365-2672.2007.03490.x Abstract Aims: To determine the fate of the enteric indicator organism, Escherichia coli , in sewage sludge (biosolids)-amended agricultural soil in relation to soil type and moisture status under controlled conditions. Methods and Results:We enumerated Escherichia coli in soil by membrane fil-tration and most probable number techniques. The background concentration of E. coli was higherinsandyloam than in siltyclaysoil.E. coli numbers increased in soil following addition of dewatered, mesophilic anaerobically digested sludge. Escherichia coli declined to a small extent with time in both moist and air-dried unamended control soils, although decay was only highly significant (P < 0Æ 001) in moist sandy loam (T 90 = 100 days). Removal rates were high in sludge-treated moist soil (T 90 = 20 days), but were significantly reduced in amended air-dried soil. Conclusions: Slow removal of E. coli in air-dried soil as against their rapid decay in moist soil after sludge application indicated that the soil biota are involved in pathogen reduction processes in sludge-amended soil. Significance and Impact of the Study: Soil ecological mechanisms are implica-ted as having a critical role in the fate of enteric organisms introduced into temperate agricultural soil in sewage sludge. Introduction Land application of treated sewage sludge is widely acknowledged as the optimummethod of sludge manage-ment as a beneficialfertilizer andsoilconditioner for crop production. The natural decay of enteric micro-organisms in soilrepresentsanimportantstageinthe multi-barrier approach to protect human health from the residual numbers of potentially infectious pathogens that may be present in sewage sludge recycled to agricultural land (World Health Organisation 1981; Pike and Carring-ton 1986). Thedecayofentericorganisms is influencedbysoil andenvironmentalvariables,includingsoiltextureand organic matter content, pH value, temperature and mois-ture content. Soil moisture content, in particular, is iden-tified as a principal factor influencing pathogen survival in the field (Gerba and Bitton 1984). It is generally accep-ted that declining soil moisture accelerates and has a direct impact on pathogen decay due to desiccation and autolysis of bacterial cells (Bergner-Rabinowitz 1956; Fen-lon et al . 2000). Also, sandy soils are considered to be more aggressive to the decay of enteric organisms than are clay soil types because they are more susceptible to moisturestress (Bergner-Rabinowitz 1956;Fenlon et al .2000). The moisture status of soil may also impact micro-bial pathogens indirectly by modulating soil predatory or antagonistic processes, but in contrast to the direct adverse effects of low soil moisture on enteric microbes, these biotic mechanisms increase and potentially reduce survival in moist conditions as compared to dry soil (ds) conditions (Vargas and Hattori 1986). However, enteric bacteria may adaptto thesoilenvironment, becoming part of the indigenous soil microbial community by developing survival strategies to withstand extreme envi-ronmental conditions, such as desiccation (Jenkins et al .1990). The relative importance and consequences of the biotic and abiotic processes in temperate soils that poten-tially modulate the survival times and inactivation of enteric micro-organisms applied to soil in sewage sludge are poorly understood. This information is important to determine: (i) whether the risks to human health from infectious enteric organisms may increase due to longer survivaltimes in certaincombinationsofsoil moisture and soil type and (ii) whether these risks are adequately protected against in the sowing and harvesting restrictions for agricultural use of sewage sludge (Council of the European Communities 1986; Statutory Instrument 1989; Department of the Environment 1996; Agricultural Devel-opment and Advisory Service 2001). Microbial responses observed in field situations are subjecttocomplex multivariate factorsassociated with continuously changing environmental and soil conditions that are often difficult to interpret. However, the effects of specific abiotic factors on the soil microbial population can be investigated under controlled conditions by repli-cated experimentation in the laboratory (Opperman et al.1989; Fenlon et al . 2000). This provides a useful adjunct to fieldinvestigationsonsoil microbialbehaviourand pathogen survival, particularly for measuring the effects on the microbial community of a parameter such as the soil moisture content, which is highly variable and diffi-cult to control in the field. Here, we report a controlled laboratory incubation study of the effects of soil type and moisture content on theentericindicatororganism,E. coli ,inrepresentative UK agricultural soils. Escherichia coli present in the back-ground microbial community in soil or added to soil in dewatered, mesophilic anaerobically digested (DMAD) sludge were enumerated in relation to these principal eda-phic and environmental variables, and with time. Materials and methods Soil and sludge characteristics Two soil types were examined in the incubation experi-ment: a sandy loam from the Imperial College field sta-tion,Silwood Park,Ascot, UK, andaheavy,siltyclay from ADAS Rosemaund, Hereford, UK. Soil samples were collectedto adepth of 10 cm andtransportedtothe laboratory under chilled conditions(2–5so C).The soils were stored in loosely folded plastic bags in a refrigerator (£5℃℃C) in the dark for a maximum period of 48 h prior to establishing the experiment (British Standard Institu-tion 1994). The characteristics of both soil types are pre-sented in Table 1. Table 1 Physicochemical properties of sandy loam (Silwood Park) and silty clay (Rosemaund) on a ds basis Parameter Value in sandy loam Value in silty clay Sand (%) 73 11 Silt (%) 19 53 Clay (%) 8 36 pH 6Æ2 6Æ6 Organic matter (%) 3Æ3 4Æ3 Cation exchange capacity (meq 100 g)1) 8Æ2 17Æ6 NO3–N (mg kg)1) 4Æ9 13Æ3 NH4–N (mg kg)1) 1Æ5 2Æ8 Extractable P (mg kg)1) 18Æ0 36Æ3 Extractable K (mg kg)1) 101Æ8 379Æ8 Extractable Mg (mg kg)1) 47Æ5 211Æ0 Total Fe (%) 3Æ05 0Æ96 Extractable Na (mg kg)1) 9Æ0 14Æ1 Extractable Ca (mg kg)1) 382Æ6 826Æ0 SO4 (mg kg)1) 13Æ3 13Æ3 Total Zn (mg kg)1) 69Æ0 51Æ4 Total Cu (mg kg)1) 21Æ5 13Æ8 Total Ni (mg kg)1) 43Æ1 4Æ8 Total Cd (mg kg)1) 0Æ89 0Æ31 Total Pb (mg kg)1) 24Æ5 72Æ1 Total Cr (mg kg)1) 58Æ4 16Æ4 The sandy loam soil contained 73% of sand and low proportions of silt (19%) and clay (8%) (Table 1). The silt and clay contents in the Rosemaund soil were 53 and 36%, respectively, and the sand content was 11%. The pH values ofbothsoils were similar (sandyloam: pH 6Æ 2; silty clay: pH 6Æ 6) and were in the typical range (McGrath and Loveland 1992). However, the silty clay generally had a higher fertilitystatus than the sandy loam, and con-tained approximately 30%more organic matter and larger concentrations of major plant nutrients than the coarse-textured soil. The total concentrations of trace elements and heavy metals were within normal ranges (McGrath and Loveland 1992). Random grab samples of fresh DMAD sewage sludge were collected from the Perry Oaks Dewatering Plant, Heathrow, UK, operated by Thames Water Utilities Ltd. These were combined and mixed to provide a representa-tive composite sample. The sludge was stored in a sealable plastic bag and transported to the laboratory in chilled conditions (British StandardInstitution 1998; Standing Committee of Analysts 2003). The numbers of E. coli detected in three replicate sam-ples of DMAD were in the range 5Æ 00–5Æ 93 log10 per g dry solids (DS) and the mean population was 5Æ 38 log10per g DS. Comparison of the chemical properties of the sludge (Table 2) with the 1996⁄1997 national survey of sludge quality in the UK (Environment Agency 1999) showed thatthesludgesample was typicalofconven- Table 2 Nutrient and heavy metal contents (DS basis) of DMAD bio-solids Parameter Value Dry solids (%) 24Æ5 Organic matter (%) 60Æ9 Total N (%) 5Æ89 Total P (%) 2Æ66 Total K (%) 0Æ24 NH4–N (mg kg)1) 4233Æ0 NO3–N (mg kg)1) <0Æ01 Total Mg (%) 0Æ37 Total Ca (%) 5Æ24 Total S (%) 1Æ14 Total Fe (%) 17Æ9 Total Zn (mg kg)1) 566Æ0 Total Cu (mg kg)1) 498Æ0 Total Ni (mg kg)1) 28Æ2 Total Cd (mg kg)1) 6Æ06 Total Pb (mg kg)1) 155Æ0 Total Cr (mg kg)1) 51Æ4 tional, anaerobically digested biosolids applied to agricul-tural land in the UK. Sample preparation and soil incubation procedure Experimental soils were sieved to pass 5Æ 6 mm in a field freshconditiontoremovestonesandorganicdetritus. Approximately 10 kgofeachsoil type was air-driedat room temperature (20℃℃C) for 3 days, and a further 10 kg was maintained under refrigerated conditions (£5°°C) at field moisture. Field moist and air-dried soils were further subdividedtoprovidetwo 5 kgportions. One portion was amended with DMAD at a rate equivalent to 10 t DS ha )1 (assuming an incorporationdepth of 10 cm), and the other control sample was maintained in an unamend-ed condition. The DMADwas mixed with sterile, de-ion-ized water to form a slurry paste, and sludge-amended soil was mixed and re-sieved twice to ensure homogen-eous incorporation. Triplicate, 100 g sub-samples of the soiltreatments(i)control moist,(ii)controlair-dried,(iii) amended moist and (iv) amended air-dried were placed in partially sealed plastic bags (to allow gas exchange)foreachsoiltypeandsamplingdateinthe incubation time series. The bags of soil were transferred to a temperature-controlled incubator (M ⁄R-253 Cooled Incubator,Sanyo Gallenkamp, Loughborough, UK) and maintained at 15°°C in the dark. Sampling and enumeration of Escherichia coli Escherichia coli were enumerated in DMAD by a mem-brane filtration (MF) method (Humphrey 1999) prior to soil amendment. Sludge-amendedsoil was assessed for E. coli content by MF, and unamended control treatments were enumerated by a most probable number (MPN) procedure (Humphrey 1999). As E. coli concentrations in sludge-amended soil approached the limit of detection of MF (<3 log10 100 g )1),enumeration was performed by MPN. The moresensitive detectionlimit ofthe MPN assay (<0Æ 3 log10 100 g )1) enabled continuity of measure-ments of the time series data for small E. coli populations in the experimental treatments. Alternating between dif-ferent standardized detection methods is a recommended practice during time-series analysis of microbial decay when there is a transition between population sizes of a target organism from the detection range of one test pro-tocol to another, thus permitting enumeration over the full detectable range of the organism (Fenlon et al . 2000; Vinten et al . 2002). Escherichia coli concentrations in the soil treatments were measured on the day of sludge incorporation (day 0) and subsequently at weekly intervals for a period of 91 days (13 weeks). Sample preparation Biosolids were homogenized by aseptically transferring a 10 gsub-sample of sludgeintoa sterilestomacher bag with 90 ml of maximum recovery diluent (Oxoid, Basing-stoke, UK) and stomaching (400 circulator, Sewards, Thetford, UK) at 230 rev min )1for2 min (Humphrey 1999). For soil, sub-samples (10 g) were aseptically trans-ferred into Duran @ bottles (Schott Glass, Stafford, UK) containing 90 ml of maximum recovery diluent and approximately 10 g of glass beads (5 mm, RossLab, Macclesfield, Cheshire, UK) for homogenization using a laboratory shaker (Laboshake, Gerhardt, Ko¨nigswinter, Germany) set at 200 rev min )1 for 4 min. For all sample types, further dilutions were prepared in maximum recovery diluent and each dilution was mixed by vortexing(Whirlimixer,FisherScientific, Loughbor-ough, UK). MF technique Sludge and amended soil suspensions were filtered in 1 ml aliquots under vacuum through a 0Æ 45 l m, gridded sterile membrane (47 mm, Pall Gelman, Portsmouth, UK). A 20 ml aliquot of sterile de-ionized water was added to improve the uniformity of dispersion over the membrane surface. Membranes were transferred to pre-dried membrane-lactose glucuronide agar plates (Sartory and Howard 1992; Humphrey 1999), which were inverted and maintained at for 4 ± 0Æ 5 h, followed by 44 ± 0Æ 5℃℃C for 14 h in a temperature-controlled incuba-tor (M ⁄R-253 Cooled Incubator, Sanyo Gallenkamp plc., Loughborough, UK). Green colonies were counted as presumptive E. coli and a selection of typical colonies (approximately three) were confirmed by growth and gas productioninbrilliantgreenbilebroth(Lab M, Bury, UK) and a positive indole reaction in tryptone water (Lab M, Bury, UK) using Kovac’s reagent (BDHMerck, Poole, UK). Numbers of E. coli were reported on a DS basis for sludge or in dry soil (ds). MPN technique A 5 · 4 MPN matrix was prepared for the examination of soil suspensions using a dilution series in lauryl tryp-tose broth (Oxoid, Basingstoke, UK) containing bromoc-resol purple (BDHMerck, Poole, UK) (Humphrey 1999). The tubes were incubated at 36 ± 1Æ 0°°C and were exam-ined for the presence of acid due to growth and fermen-tation by E. coli after 24 and 48 h. Tubes showing acid production were identified by a change in broth colour from purple to yellow. These were recorded as presump-tively positive for E. coli and were confirmed using brilli-antgreenbilebrothandtryptone water.The MPN of organisms was estimated using standard probability tables (American Public Health Association 1998) from the number of tubes in a series of consecutive dilutions that confirmed positive for E. coli. The MPN value was calcu-lated based on three consecutive series of tubes using the dilution providing the most positive tubes, followed by the next succeeding highest dilutions. Numbers of E. coli were adjusted for moisturecontentofthesampleand reported on a ds basis. Moisture content The moisturecontent was measuredgravimetrically by drying 5 g sub-samples of soil or sludge overnight in a forced-air oven (Hotbox oven, OVB-300 series, Sanyo Gallenkamp, Loughborough, UK) set at (British Standard Institution 1994). The gravimetric moisture contents of the experimental soil treatments are presented in Table 3. Statistical analysis Thelog10ofsurviving E. coli was plottedagainsttime and the decimal reduction time (T 90) was estimated by Table 3 Gravimetric moisture contents (%) of the experimental soils Moisture Unamended Sludge- amended Soil type status control Sandy loam Field moist 17Æ3 21Æ3 Sandy loam Air-dried 2Æ34 6Æ83 Silty clay Field moist 27Æ5 32Æ0 Silty clay Air-dried 4Æ52 8Æ60 linear regression analysis (Microsoft Excel 2000 Profes-sional Version 5) from the reciprocal of the slope (Hurst 2002). Multiway anova (Genstat 5, version 4.1, Lawes Agri-cultural Trust, Rothamsted Experimental Station) was performed for each set of data in the incubation time ser-ies and, in almost all circumstances, the F probabilities of the main treatment effects and interactions were highly statistically significant (P < 0Æ 001). A number of the time points (days 0, 35 and 91) were selected and scrutinized furthertoexamine andcomparetheindividual(third-order interaction) treatment means in more detail using the least significant difference (LSD0Æ 05) test (Dytham 2003), adjusted for the number of means being compared according to the studentized range. Results Indigenous Escherichia coli in unamended control soil Sandy loam soil contained a larger background popula-tion of E. coli than the silty clay. The mean number of E. coli (for all sampling times) in moist sandy loam soil was 2Æ 67 log10 100 g )1 ds, and the moist silty clay con-tained 0Æ 65 log10 100 g )1 ds (Fig. 1a,b). There was no sta-tistically significant effect of drying soil on the mean numbers of E. coli in the silty clay (0Æ 67 log10 100 g )1 ds) compared with the moist condition. For sandy loam soil, however, desiccation significantly lowered the background mean (for all sampling times) log10 numbers of E. coli by0Æ 6 log10 100 g )1 ds relative to the field moist condition, equivalent to a reduction in the E. coli population of 75%calculated using untransformed data. Linear regression analysis indicated that there was sig-nificant decay (slope = 0Æ 01, r 2 = 0Æ 39, P < 0Æ 001) in log10numbers of indigenous E. coli in relation to incubation time for moist, unamended sandy loam soil (Fig. 1a) and the decimal decay rate (T 90) was calculated as 100 days. The linear regression models of E. coli decay in the other unamended soil treatments were within statistical signifi-cance (P = 0Æ 02–0Æ 04); however, the r 2 values were small and in the range 0Æ 13–0Æ 17, indicating that they were less reliable than the predictions for moist sandy loam. This was explained,inpart,byvariationamong the experi-mental replicates, and also because the observed trends in these data suggested that the rates of decay were much slower overall compared to moist sandy loam soil (Fig. 1). DMAD-amended soil As expected, numbers of E. coli in soil were significantly increased(P < 0Æ 001)by DMAD applicationrelativeto Figure 1 Background numbers of Escherichia coli in unamended control (a) sandy loam and (b) silty clay soils incubated at 15℃℃C for 91 days in (i)field moist and (ii) air-dried conditions. the background population detected in unamended con-trol soil (Figs 1 and 2). Initial (day 0) numbers were sim-ilar in both amended moist soil types, which on average contained 5Æ 6 log10 100 g )1 ds of enteric bacteria (Fig. 2a,b). Dry sandy loam and silty clay soil contained 5Æ 4 and4Æ 5 log10 100 g )1ds, respectively, on day 0(Fig. 2a,b). Overall, these values were consistent with the application rate and dilution associated with mixing DMAD in the soils. Thenumbers of E. coli in moist soilamended with DMAD showed a highly significant linear decay (r 2 = 0Æ 88 and 0Æ 93 for sandy loam and silty clay, respect-ively, and in both cases P < 0Æ 001) and declined to <1 log10 100 g )1 ds after 91 days of incubation in both soil types (Fig. 2). The observed reduction in E. coli num-bers below the concentrations measured in the unamend-ed, sandy loam control soil suggested that the application of biosolids ultimately suppressed the overall background population of indicator bacteria insoil. The decay of E. coli in moist soil was assessed using log–linear decay kinetics (Hurst 2002) and a T 90 value was calculated. The slopes of the linear regression relationships (slope = 0Æ 05) were equivalent for both moist soil types amended with DMAD and gave a T 90 value of 20 days. The regression equations describing E. coli decay in sludge-amended, air-dried soil were also highly significant (P = 0Æ 007 to <0Æ 001), but showed that drying reduced therateofinactivationby 80–90% compared to moist soil (Fig. 2). Under dry soil conditions, the T 90 values for sandy loam and silty clay soil amended with sludge were increased to 100 and 200 days, respectively. The difference in size between the initial and final populations of E. coli in dry soil after 91 days of incubation was small and in therange0Æ 5–1Æ 0 log10 100 g )1ds. Therefore,air-drying markedly increased survival in sludge-amended soil. Individual mean results for the experimental treatments are presented in Fig. 3 for days 0, 35 and 91. There was no significant main effect ofsoil type on the decay of E. coli in moist, sludge-amended soil. Consequently, the time taken for E. coli added to the soil in sludge to decay to the background level depended on the size of the resi-dent enteric bacteria population in the unamended soils. Thus, no statistically significant difference in E. coli num-bers was detectedbetweenthe DMAD-amended, moist sandy loam soil and the equivalent unamended control treatmentafter35 daysofincubation. Numbersinthe corresponding sludge-amended treatment for silty clay were not significantly different from those of the sandy Figure 2 Escherichia coli in (a) sandy loam and (b) silty clay soils amended with DMAD biosolids at a rate equivalent to 10 t DS ha )1 and incuba-ted at 15℃℃C for 91 days in (i) field moist and (ii) air-dried conditions. loam soil on day 35, but remained significantly above the respective controlafter this time period because of the smaller background population of E. coli in the silty clay compared to the sandy loam soil. Incubation for a further 56 days was necessary before E. coli numbers in amended silty clay soil declined to the small background popula-tion size in this soil type. Escherichia coli populations in both sludge-amended dry soil types were also not signi-ficantly different from each other at the end of the incubationperiod,althoughtheyremainedsignificantly and numerically larger than their respective control treat-ments, without sludge incorporation. Discussion Escherichia coli was identified as a normal component of the indigenous flora in two representative agricultural soil typesinthe UK. A possiblesourceofthebackground E. coli populations measuredinthesoils may befrom inputs of enteric bacteria in the faeces of wild animals and birds inhabiting the field sites. Alternatively, the bac-teria may have adapted to the soil environment, surviving as a relatively minor member of the microbial commu- nity, and the size of the background population may strongly depend on the soil properties. For example, soils with higher clay contents are usually considered to pro-vide a more protective environment, supporting a larger bacterial biomass overall and extending the period of sur-vival of enteric bacteria (see, e.g. Fenlon et al . 2000). This is due to the greater availability of ecological niches for survival against predatory organisms, and more favour-able environmental circumstances, such as improved sub-strate and moisture availability, in clays compared with sandy soils (Straub et al . 1992; Cools et al . 2001). How-ever, we observed the opposite situation here as the fine textured soil contained fewer E. coli than the sandy loam soil type. This suggested that other soil properties may have greater influence than soil texture on enteric micro-organisms within the background microbial community. Indeed, organic matter content was also a major param-eterthatvariedbetween thetwo soil typestestedhere (Table 1). This may be significant because the activity of the soil microflora, including bacteriophagous protozoa, that control populations of enteric bacteria and E. coli in soil increase with organic nutrient availability (Acea et al .1988; Mezrioui et al . 1995; Desmarais et al . 2002). The Soil moisture status and type Soil moisture status and type Figure 3 Mean numbers of Escherichia coli in control (unamended;) sandy loam and silty clay soil and in soil amended with DMAD bio-solids at a rate equivalent to 10 t DS ha )1 ( ) at day 0, and after incubation at 15CC C for 35 and 91 days in field moist and air-dried conditions. LSD0Æ 05 is the least significant difference between mean values at P = 0Æ 05. silty clay soil examined here contained 30%more organic matter compared to the sandy loam (Table 1). Therefore, the smaller background numbers of E. coli measured in the clay soil could be explained byincreased intrinsic suppression of the enteric bacteria in this soil type than in the sandy loam as a result of greater microbial predat-oryandantagonisticactivitysupportedbytheelevated organic matter content. The data reported here also demonstrated that indigen-ous E. coli remain viable and survive extreme desiccation in air-dried soil in the laboratory in the absence of exter-nal inputs of bacteria from wildlife reservoirs (Fig. 1a,b). Thus,as with otherbacteria,theseresultssuggestthat E. coli may exhibit varying degrees of adaptation-acquired tolerance to potentially extreme drying conditions in the temperate edaphic environment (Stanlake 1977; Van Overbeek et al . 1995). Production of extracellular polysac-charides could also be a survival mechanism adopted in response to drying soil conditions (Chenu 1993; Or and Friedman 2002). In contrast to the effects of soil type on background populations of E. coli , the patterns of decay observed for E. coli introduced into moist soil in DMAD were inde-pendent of soil type (Figs 2 and 3). Natvig et al . (2002) also found that E. coli and spiked Salmonellla Typhimu-rium in bovine manure decayed at approximately equival-ent rates inamended soil types similar tothoseused here. These observations suggest that the intrinsic physic-ochemical and biological properties of soil may be relat-ively unimportant in defining the inactivation kinetics of enteric bacteria when consolidated organic amendments, such as farmyard manure or dewatered sludge cake, are applied to agricultural soil. A plausible explanation for this behaviour is that bulky organic materials, such as DMAD, supply large bacterial andorganic substrateinputsto soilthatstimulatethe growth of the indigenous soil microflora, including bac-terial feeding protozoa and nematodes (Stotzky and Nor-man 1964; Alexander 1981; Acea et al . 1988; Opperman etal . 1989;Recorbert etal . 1992; Turpin etal .1993; Griffiths et al . 1998; Sidhu et al . 2001; Trevisan et al . 2002; Marschner etal .2003).Thisislikelytohavea major impact on the survival of bacterial pathogens applied to agricultural soil in sludge and manure. For example, Acea et al. (1988)presented evidence linkingtheperiod of maximum decline of a range of bacterial species including Rhizobium spp., Agrobacterium tumefaciens , Micrococcus flavus , Corynebacterium spp. and Pseudomonas spp. with a one to two magnitude increase in the protozoan popula-tion in a loam soil. More recently, Sørensen et al . (1999) measured a 10- to 100-fold multiplication of protozoa, an increase in protozoa activity and a rise in indigenous soil flagellates and ciliates following the addition of E. coli to soil,and found that that this wasresponsible for the declineinnumbersoftheenteric bacteriainthesoil. Direct measurements of soil predator activity are outside thescope of the workpresentedhere. However,these results suggest that predation processes are likely to be an important pathogen inactivation mechanism in temperate, biosolids-amended agriculturalsoiland warrantfurther investigation. The potential role of soil ecological processes in enteric decay was further emphasized by the population dynamics of the indicator bacteria in air-dried, DMAD-amended soil(Fig. 2a,b).Theprocesses responsiblefor thedecay of E. coli in moist soil were almostentirely suppressedunderdrysoilconditions,stronglyimplica-ting the involvement of biological mechanisms in the inactivationofentericbacteriaaddedto soilinsewage sludge. In dry soil, we postulate that the bacteria were protected within the particles of sludge cake, presumably because drying restricted predatory activity and ⁄or pro-vided a barrier to invasion of the sludge particles by soil predators (Vargas and Hattori 1986). This behaviour is consistent with the increased survival of inoculated bacteria observed in sterile soil compared with nonsterile conditions. For example, Jiang et al . (2002) showed the inactivation of E. coli O157:H7 inoculated into sandy loam soil and in soil amended with cattle manure occurred rapidly in control (unautoclaved) samples com-pared to sterile (autoclaved) soil incubated at 5, 15 and Concentrations of E. coli inoculated into sterile manure amended soil were also maintained in the absence ofnatural predatoryand competitive microbial activity in the earlier work reported by Tate (1978). Cellular decay andtheintrinsic loss of viability are considered to be the principal mechanisms of decay of enteric bacteria introduced into soil with sewage sludge (Gerba and Bitton 1984; Sørensen et al . 1999). However, the results presented here implicate soil ecological proces-ses in the decay of E. coli in sludge-amended soil, which are active under moist conditions but suppressed in dry soil (Recorbert et al . 1992; Jiang et al . 2002). Thus, con-trary to the generally accepted view that pathogen survival is usually extended in moist compared to dry soil (Sedgwick and Winslow 1902, cited in Rudolfs et al .1950; Kligler 1921; Rudolfs et al . 1950; Van Donsel et al .1967; Reddy et al . 1981), this work has shown that, in temperate soil conditions, survival times of enteric bacteria applied in bulky types of biosolids (such as mechanically dewatered sludge cake) may be shorter in moist soils and prolonged by dry conditions. Acknowledgements The work described here formed part of a programme of research on Predicting Agricultural Benefit of Novel Bio-solids Products funded by the Environment Agency, Sev-ern Trent Water, Thames Water, Yorkshire Water, Scottish Water and the EPSRC. References Acea, M.J., Moore, C.R. and Alexander, M. (1988) Survival and growth of bacteria introduced into soil. Soil Biol Bio-chem 20, 509–515. Agricultural Development and Advisory Service (2001) The Safe Sludge Matrix: Guidelines for the Application of Sewage Sludge to Arable and Horticultural Crops , 3rd edn (www. adas.co.uk). Alexander, M. (1981) Why microbial predators and parasites do not eliminate their prey and hosts. Annu Rev Microbiol 25, 113–133. American Public Health Association (1998) Standard Methods for the Examination of Water and Wastewater , 20th edn. Baltimore: United Book Press. Bergner-Rabinowitz, S. (1956) The survival of coliforms, Strep-tococcus faecalis and Salmonella tennessee in the soil and climate of Israel. J Appl Microbiol 4, 101–106. British Standard Institution (1994) Soil quality – Part 3: Chem-ical methods – Section 3.1. Determination of dry matter and water content on a mass basis by a gravimetric method. BS 7755-3.1:1994 ISO 11465:1993. London: British Standards Institution. British Standard Institution (1998)Water quality – Sampling –Part 13: Guidance on sampling of sludges from sewage and water treatment works. BS EN ISO 5667-13:1998⁄ BS 6068-6.13:1998. London: British Standards Institution. Chenu, C. (1993) Clay or sand polysaccharide associations as models for the interface between microorganisms and soil:water related properties and microstructure.Geoderma 56,143–156. Cools, D., Merckx, R., Vlassak, K. and Verhaegen, J. (2001) Survival of E. coli and Enterococcus spp. derived from pig slurry in soils of different texture. Appl Soil Ecol 417, 53–62. Council of the European Communities (1986) Council Direct-ive of 12 June 1986 on the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture (86⁄ 278⁄ EEC). Official Journal of the European Communities, No. L181⁄ 6-12. Department of the Environment (1996) Code of Practice for Agricultural Use of Sewage Sludge . London: Her Majesty’s Stationery Office. Desmarais, T.R., Solo-Gabriele, H.M. and Palmer, C.J. (2002) Influence of soil on faecal indicator organisms in a tidally influenced subtropical environment. Appl Environ Micro-biol 68, 1165–1172. Dytham, C. (2003) Choosing and Using Statistics – A Biologist’s Guide . Oxford: Blackwell Science Publishing. Environment Agency (1999)UK Sewage Sludge Survey 1996⁄ 97– National Presentation . Research and Development Tech-nical Report P165. Bristol: The Environment Agency. Fenlon, D.R., Ogden, I.D., Vinten, A. and Svoboda, I. (2000) The fate of Escherichia coli and E. coli O157 in cattle slurry after application to land. J Appl Microbiol Symp Suppl 88,149S–156S. Gerba, C.P. and Bitton, G. (1984) Microbial pollutants: their survival and transport in groundwater. In Groundwater Pollution Microbiology ed. Bitton, G. and Gerba, C. pp. 65–88 New York: Wiley. Griffiths, B.S., Wheately, R.E., Olesen, T., Henriksen, K., Ekelund, F. and Ronn, R. (1998) Dynamics of nematodes and protozoa following the experimental addition of cattle or pig slurry to soil. Soil Biol Biochem 30, 1379–1387. Humphrey, N. (1999) A Survey of E. coli in UK Sludges . Sewage Sludge-99⁄ SL ⁄ 06⁄ 3. London: UK Water Industry Research. Hurst, C.J. (2002) Modelling the fate of microorganisms in water, wastewater, and soil. In Manual of Environmental Microbiology 2nd edn. ed. Hurst, C.J., Crawford, R.L., Knudsen, G.R., McInerney, M.J. and Stetzenbach, L.D. pp. 300–308 Washington, DC: ASM Press. Jenkins, D.E., Chaisson, S.A. and Matin, A. (1990) Starvation-induced cross protection against osmotic challenge in Escherichia coli . J Bacteriol 172, 2779–2781. Jiang, X., Morgan, J. and Doyle, M.P. (2002) Fate of Escheri-chia coli O157:H7 in manure amended soil. Appl Environ Microbiol 68, 2605–2609. Kligler, I.J. (1921) Investigations of soil pollution and the rela-tion of the various types of privies to the spread of intestinal infections . International Health Board, Monograph 15. Princeton: Rockefeller Institute of Medical Research. Marschner, P., Kandeler, E. andMarschner, B. (2003) Structure and function of the soilmicrobial community in a long-term fertilizer experiment. Soil Biol Biochem 35, 453–461. McGrath, S.P. and Loveland, P.J. (1992) The Soil Geochemical Atlas of England and Wales . London: Blackie Academic &Professional. Mezrioui, N., Baleux, B. and Troussellier, M. (1995) A micro-cosm study of the survival of Escherichia coli and Salmonel-lla typhimurium in brackish water.Water Res 29, 459–465. Natvig, E.E., Ingham, S.C., Ingham, B.H., Cooperband, L.R. and Roper, T.R. (2002) Salmonella enterica Serovar typhimurium and Eschericha coli contamination of root and leaf vegetables grown in soil with incorporated bovine manure. Appl Environ Microbiol 68, 2737–2744. Opperman, M.H., Wood, M. and Harris, P.J. (1989) Changes in microbial population following the application of cattle slurry to soil at two temperatures. Soil Biol Biochem 21,263–268. Or, D. and Friedman, S. (2002) Physical processes affecting microbial habitats and activity in unsaturated porous media. In Proceedings of the 17th World Congress of Soil Sciences, Thailand, 14–21 August 2002. Pike, E.B. and Carrington, E.G. (1986) Stabilization of sludge by conventional and novel processes – a healthy future. In The Agricultural Use of Sewage Sludge – Is there a Future? London: The Institute of Water Pollution Control. Recorbert, G., Steinberg, C. and Faurie, G. (1992) Survival in soil of genetically engineered Escherichia coli as related to inoculum density, predation and competition. FEMS Microbiol Ecol 101, 251–260. Reddy, K.R., Khaleel, R. and Overcash, M.R. (1981) Behaviour and transport of microbial pathogens and indicator organ- isms in soils treated with organic wastes. J Environ Qual 10, 22–266. Rudolfs, W., Falk, L.L. and Ragotzkie, R.A. (1950) Literature review on the occurrence and survival of enteric, patho-genic, and related organisms in soil, water, sewage and sludges and on vegetation. I. Bacterial and virus diseases. Sewage Ind Wastes 22, 1261–1281. Sartory, D.P. and Howard, L. (1992) A medium detecting b -glucuronidase for the simultaneous membrane filtration enumeration of Eschericha coli and coliforms from drink-ing water. Lett Appl Microbiol 15, 273–276. Sedgwick, W.T. and Winslow, C.E.A. (1902) Am Acad Arts Sci 12, 508. Cited in Rudolfs et al. (1950). Sidhu, J., Gibbs, R.A., Ho, G.E. and Unkovich, I. (2001) The role of indigenous microorganisms in suppression of Sal-monella regrowth in composted biosolids.Water Res 35,913–920. Sørensen, S.J., Schyberg, T. and Rønn, R. (1999) Predation by protozoa on Escherichia coli K12 in soil and transfer of resistance plasmid RP4 to indigenous bacteria in soil. Appl Soil Ecol 11, 79–90. Standing Committee of Analysts (2003) The Microbiology of Sludge – Part 2 – Practices and procedures for sampling and sample preparation .Methods for the Examination of Waters and Associated Materials No. 189. London: Her Majesty’s Stationery Office. Stanlake, G.J. (1977) A comparison of the predominate soil microflora in eight vegetative communities in Okalahoma. Proc Oklahoma Acad Sci 57, 86–90. Statutory Instrument (1989) The Sludge (Use in Agriculture) Regulations SI 1263 as amended (1990) SI 880. London:Her Majesty’s Stationery Office. Stotzky, G. and Norman, A.G. (1964) Factors limiting micro-bial growth activities in soil. III. Supplementary substrate additions. Can J Microbiol 10, 143–147. Straub, T.M., Pepper, I.L. and Gerba, C.P. (1992) Persistence of viruses in desert soils amended with anaerobically diges-ted sewage sludge. Appl Environ Microbiol 58, 636–641. Tate, R.L. (1978) Cultural and environmental factors affecting the longevity of Escherichia coli in histosols. Appl Environ Microbiol 35, 925–929. Trevisan, D., Vansteelant, J.Y. and Dorioz, J.M. (2002) Survival and leaching of faecal bacteria after slurry spreading on mountain hay meadows: consequences for the manage-ment of water contamination risk.Water Res 36, 275–283. Turpin, P.E., Maycroft, K.A., Rowlands, C.L. and Wellington, E.M.H. (1993) Viable but non-culturable salmonellas in soil. J Appl Bacteriol 74, 421–427. Van Donsel, D.J., Geldreich, E.E. and Clarke, N.A. (1967) Sea-sonal variations in survival of indicator bacteria in soil and their contribution to stormwater pollution. Appl Environ Microbiol 15, 1361–1370. Van Overbeek, L.S., Eberl, L., Givskov, M., Molin, S. and Van Elsas, J.D. (1995) Survival of, and induced stress resistance in, carbon-starved Pseudomonas fluorescens cells residing in soil. Appl Environ Microbiol 61, 4202–4208. Vargas, R. and Hattori, T. (1986) Protozoan predation of bac-terial cells in soil aggregates. FEMS Microbiol Ecol 38, 233–242. Vinten, A.J.A., Lewis, D.R., Fenlon, D.R., Leach, K.A., Howard, R., Svoboda, I. and Ogden, I. (2002) Fate of Escherichia coli and Escherichia coli O157 in soils and drainage water following cattle slurry application at 3 sites in southern Scotland. Soil Use Manag 18, 223–231. World Health Organisation (1981) The Risk to Health of Microbes in Sewage Sludge Applied to Land . Report on a WHOWorking Group, 6–9 January, Stevenage. Copenha-gen: World Health Organisation.
确定






还剩8页未读,是否继续阅读?
中国格哈特为您提供《农业土壤和淤泥中大肠杆菌检测的样品振荡》,该方案主要用于土壤中大肠杆菌检测,参考标准--,《农业土壤和淤泥中大肠杆菌检测的样品振荡》用到的仪器有格哈特强力高重现振荡器LS500/RO500、格哈特快速干燥仪STL56、德国移液器MM
推荐专场
快速干燥仪
更多
相关方案
更多
该厂商其他方案
更多

















