来自西北太平洋的北海狮中的有机氯农药残留量Organochlorines in Steller Sea Lions (Eumetopias jubatus) from the Western North Pacific
方案详情

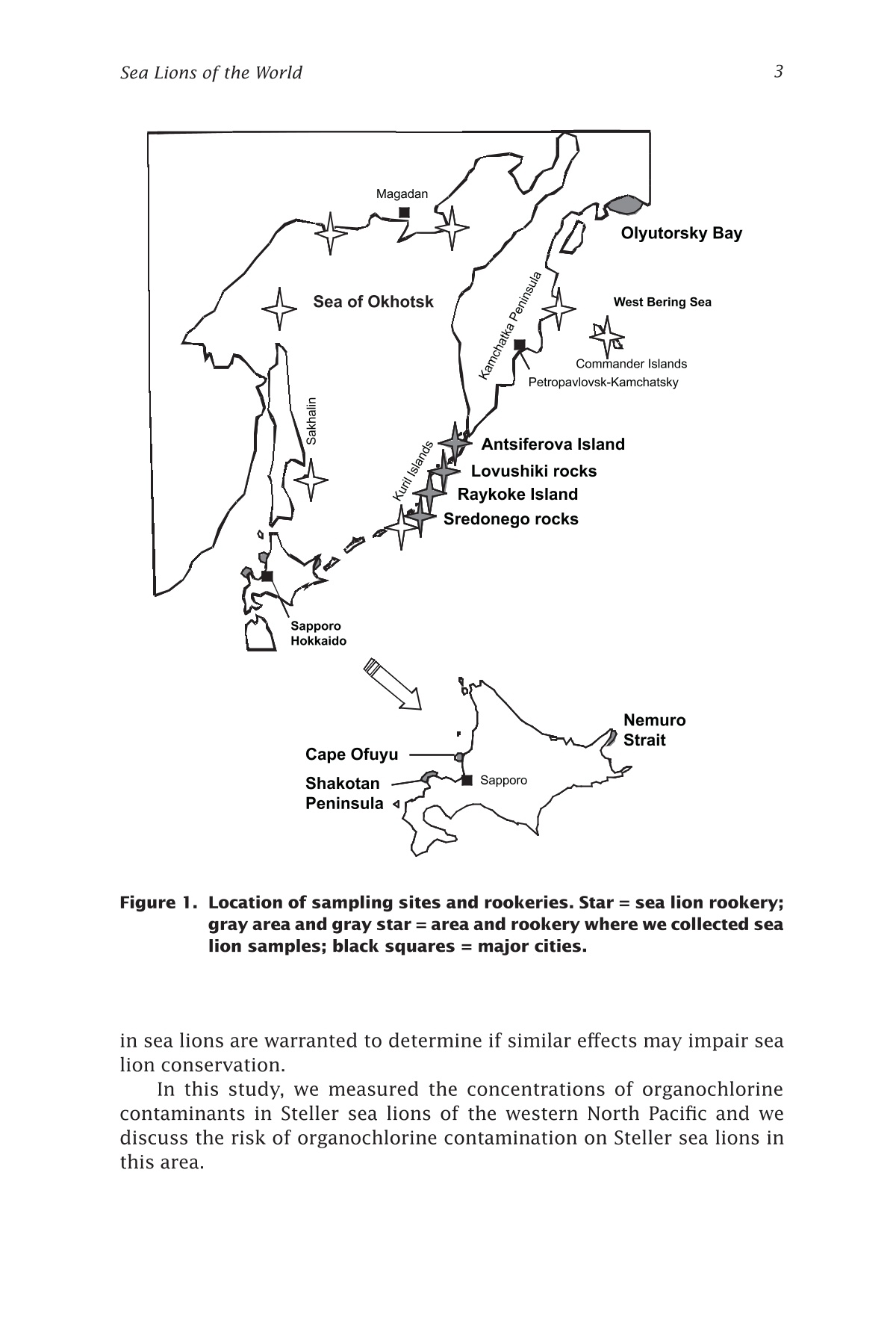
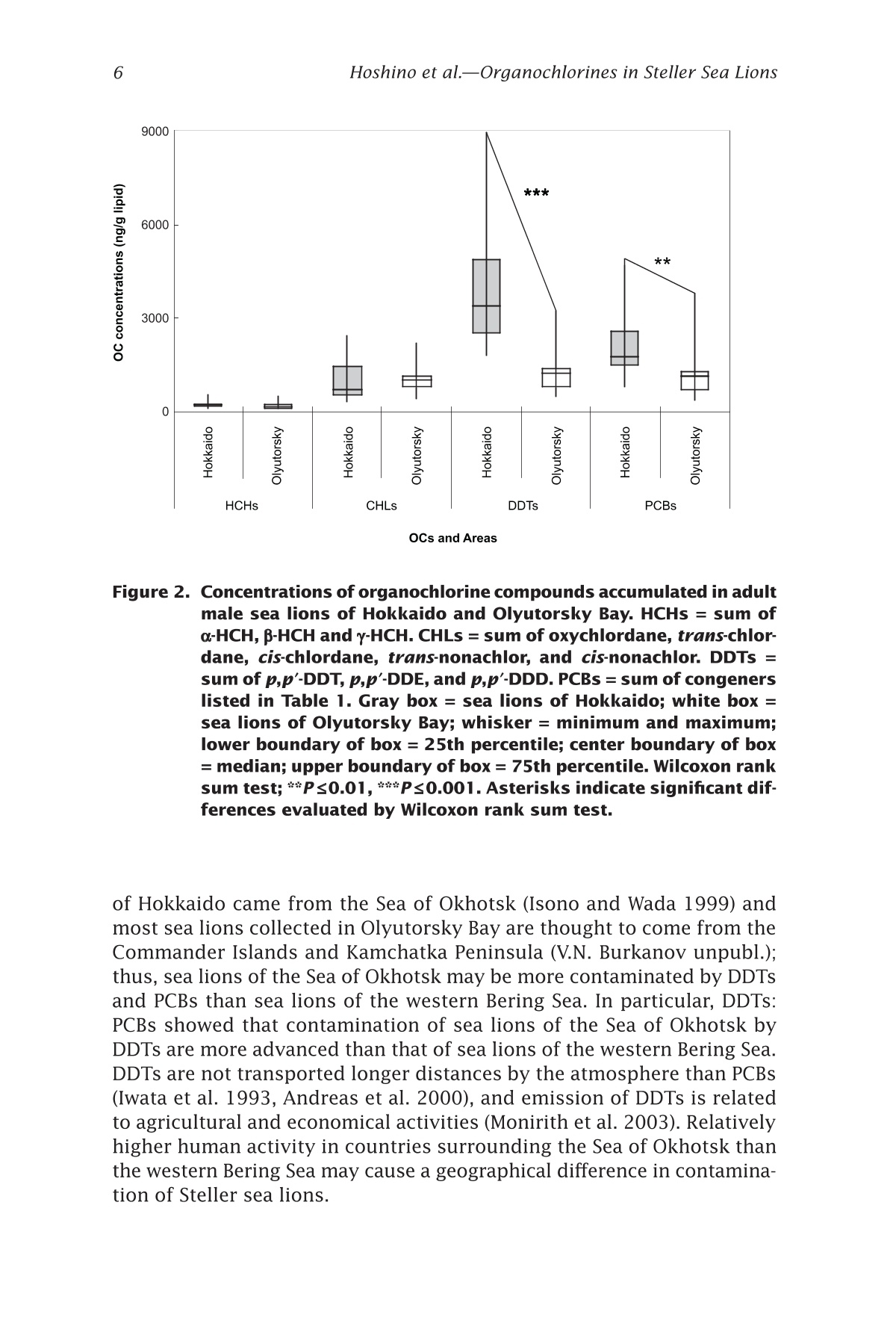
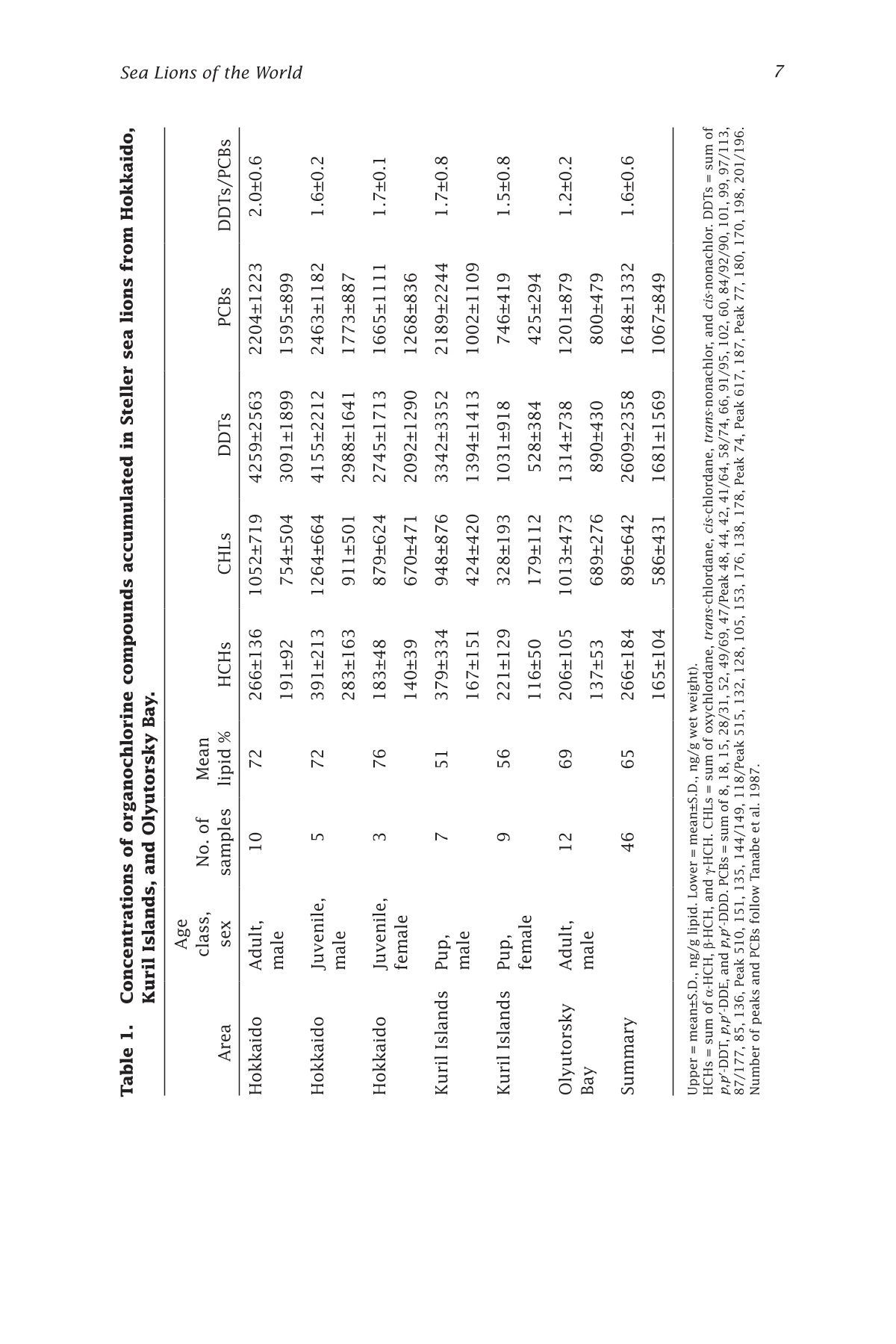
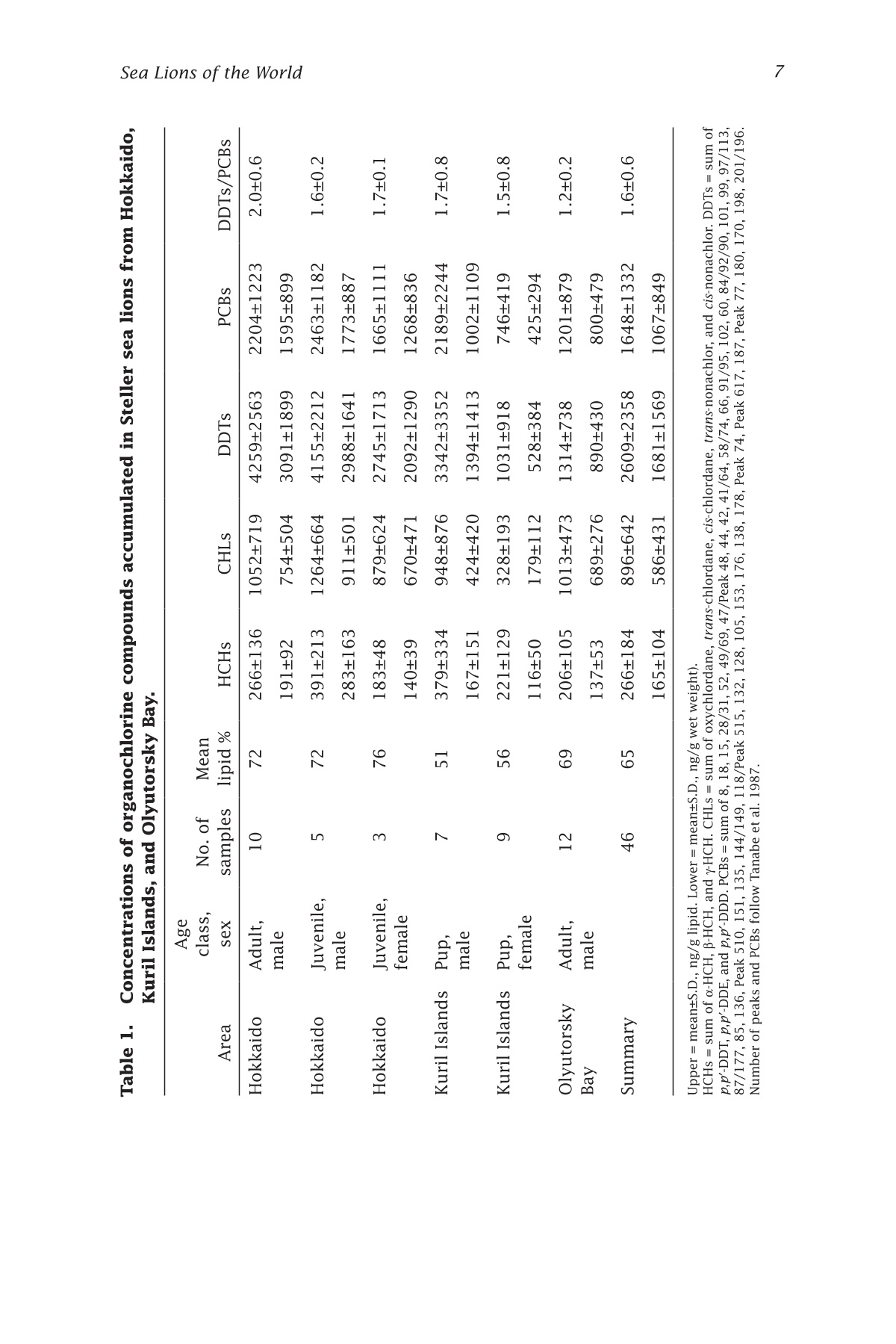
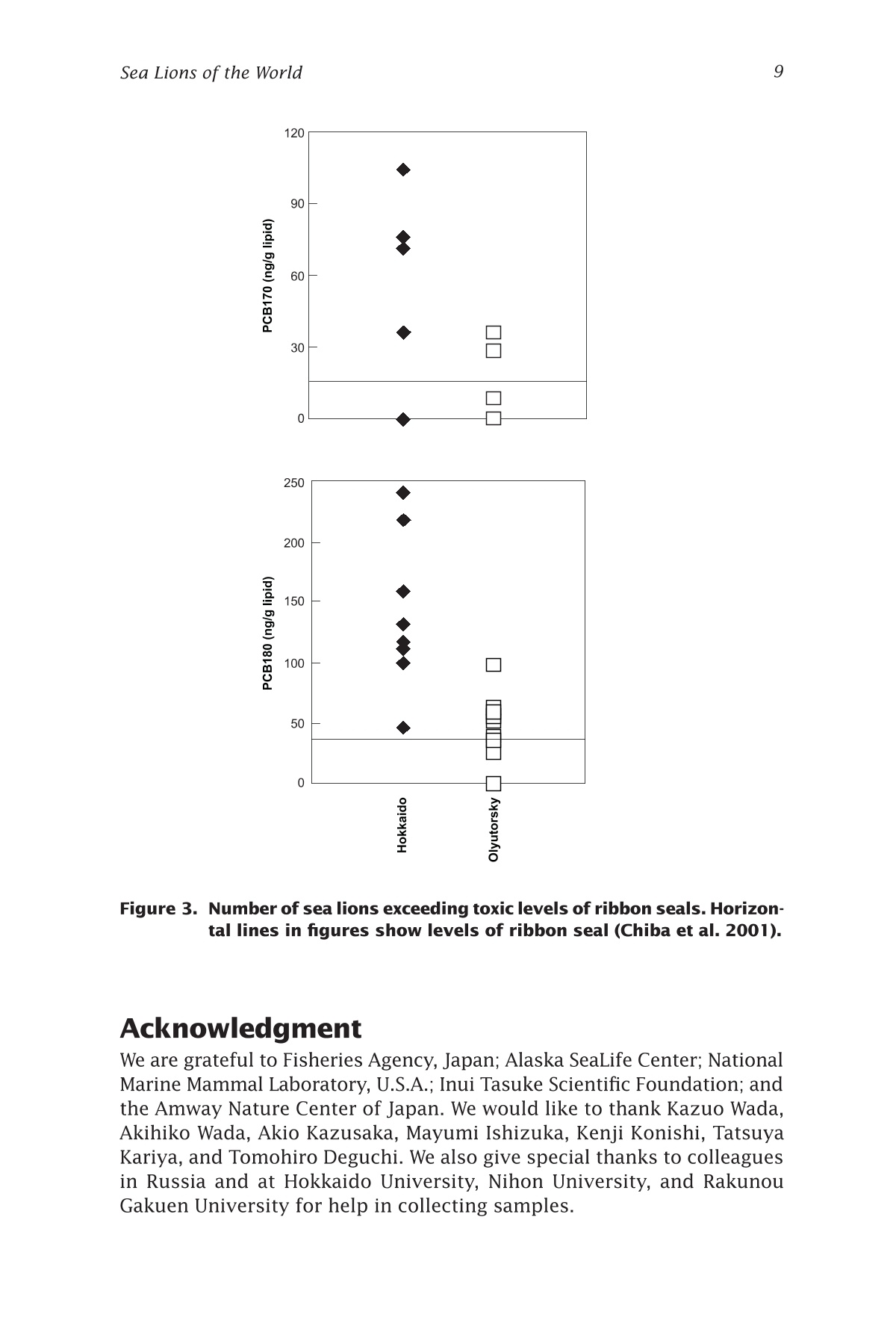
来自西北太平洋的北海狮中的有机氯农药残留量Organochlorines in Steller Sea Lions (Eumetopias jubatus) from the Western North PacificSea Lions of the WorldAlaska Sea Grant College Program • AK-SG-06-01, 2006 Hoshino et al.—Organochlorines in Steller Sea Lions2 来自西北太平洋的北海狮中的有机氯农药残留量 Organochlorines in Steller SeaLions (Eumetopias jubatus) from the Western North Pacifci Hiroshi Hoshino Hokkaido University, Division of Marine Environment and Resources,Graduate School of Fisheries Sciences, Hakodate, Japan 北海道大学渔业科学研究生院海洋环境与资源学部 Shouichi Fujita Hokkaido University, Laboratory of Toxicology, Graduate School of Veterinary Medicine, Sapporo, Japan 北海道大学兽医学研究生院毒理学实验室 Yoko Goto Hokkaido Kushiro Fisheries Experimental Station, Kushiro, Japan Takeomi Isono Econixe Co. Ltd., Sapporo, Japan Tsuyoshi Ishinazaka Nihon University, Laboratory of Theriogenology, College of Bioresource Sciences, Fujisawa, Japan Vladimir N. Burkanov Natural Resources Consultants Inc., and NOAA National Marine Fisheries Service, National Marine Mammal Laboratory, Seattle, Washington Yasunori Sakurai Hokkaido University, Division of Marine Environment and Resources,Graduate School of Fisheries Sciences, Hakodate, Japan Abstract The population of Steller sea lions, Eumetopias jubatus, has declined in the western North Pacifci since the late 1960s. Recently, the Sea of Okhotsk was reported to be polluted by organochlorine contaminants (OCs) including polychlorinated biphenyls (PCBs) and agrochemicals. OCs are known to have toxic efefcts on phocid species; thus, studies of OCs in sea lions are important for sea lion conservation. In this study, sea lion blubber samples were collected from the Kuril Islands and Olyutorsky Bay in Russia and Hokkaido in Japan from 1997to 2004, and OC concentrations in blubber were measured. 1,1'-(2,2,2-trichloroethylidene)bis(4-chlorobenzene) and its metabo-lites (DDTs) and PCBs were found to be the dominant compounds accu-mulated in sea lions of the western North Pacifci. The DDT and PCB levels were higher in Steller sea lions from Hokkaido than in sea lions from Olyutorsky Bay. The ratio of DDTs to PCBs in sea lions from Hokkaido was also higher than in sea lions from Olyutorsky Bay. A higher number of adult sea lions from Hokkaido exceeded the 2,2',3,4,4',5,5'-heptachlo-robiphenyl level reported to decrease circulating thyroid hormone in ribbon seals than sea lions from Olyutorsky Bay. DDTs and PCBs were the predominant OCs accumulated in Steller sea lions of the western North Pacifci measured in this study. In particular, Steller sea lions from Hok-kaido may have higher DDT accumulation than sea lions in the western Bering Sea and may have higher risks of toxicity. Introduction The Steller sea lion, Eumetopias jubatus, is the largest member of the family Otariidae living in the North Pacifci rim from California, U.S.A., to Hokkaido, Japan (Loughlin et al. 1987). Their world population has de-clined since the late 1960s, and the cause of the decline has been a matter of dispute among marine scientists (Loughlin et al. 1992, Loughlin and York 2002). In the western part of the North Pacifci, sea lion rookeries are located on the coast of the Sea of Okhotsk including the Kuril Islands and Kamchatka Peninsula, and the Commander Islands in the western Bering Sea (Fig. 1). The number of sea lions in these areas has declined from the 1960s to date (V.N. Burkanov and T.R. Loughlin unpubl.). Recently, the Sea of Okhotsk was reported to be polluted by runoff containing organochlorine compounds (OCs): hexachlorocyclohexanes (HCHs), chlordanes (CHLs), polychlorinated biphenyls (PCBs), and 1,1e-(2,2,2-trichloroethylidene)bis(4-chlorobenzene)(p,p(-DDT) and its me-tabolites (DDTs) (Chernyak et al. 1995, Zhulidov et al. 2000). Further,global transportation of pollutants from southeastern Asian countries to the high latitude regions may possibly have impacts on the Sea of Okhtosk (Iwata et al. 1993). These organochlorine compounds are used for the electronics industry and agriculture, and are known as the toxic and endocrine disrupting chemicals. They are highly lipophilic and tend to accumulate in higher trophic animals through the food web (Kawano et al. 1988). In phocid species, some toxic efefcts of OCs were reported,such as immune system suppression (de Swart et al. 1996) and a decrease in circulating thyroid hormone (Chiba et al. 2001); thus studies of OCs Figure 1. Location of sampling sites and rookeries. Star = sea lion rookery;gray area and gray star = area and rookery where we collected sea lion samples; black squares = major cities. in sea lions are warranted to determine if similar efefcts may impair sea lion conservation. In this study, we measured the concentrations of organochlorine contaminants in Steller sea lions of the western North Pacifci and we discuss the risk of organochlorine contamination on Steller sea lions in this area. Materials and methods We collected sea lion blubber samples from Hokkaido Island in Japan,the Kuril Islands, and Olyutorsky Bay in Russia from 1997 to 2004 (Fig.1). In the Kuril Islands, we collected sea lion pups found dead in rook-eries from late June to July. We collected fresh or slightly decomposed carcasses following the guideline of Geraci and Lounsbury (1993). Only three females and two males were fresh and the others were decomposed slightly. In Olyutorsky Bay, we collected sea lions taken accidentally by a Russian herring fsihery from October to December. In Hokkaido, we col-lected sea lions from licensed sea lion hunters from January to March.Blubber (200-500 g) was cut from the mid dorsal or breast region, frozen on a ship in Russia, transported by airplane from Russia, and stored at –25ºC until analysis. Blubber collected in Hokkaido was frozen at the feild station or laboratory as soon as possible after collection and stored at –25ºC too. The body length, body weight, blubber thickness on the xiphoid process, milk excretion, and pregnancy status of sea lions were checked when possible. Ages and reproductive status were determined in sea lions collected from Hokkaido following procedures described by Isono (1999) and Ishinazaka and Endo (1999). Male sea lions older than 3years or morphologically mature were classifeid as adults, and the other males were classifeid as juveniles. Female sea lions that were not preg-nant and younger than 4 years were classifeid as juveniles. Age of sexual maturity was determined by procedures described by Yamanaka (1986)and Ishinazaka and Endo (1999). OC analysis was carried out by the method described by Hoshino (2004). Several pieces of blubber (5 g) were cut vertically from the block of blubber to avoid difefrences in concentration between blubber layers (Severinsen et al. 2000). Pieces of blubber were ground with anhydrous Na2SO4,and organo-solvent compounds were extracted into a mixture of dichloromethane and n-hexane (13.5:1 vol:vol) by an automatic soxhlet extractor (Soxtherm S306A, Gerhardt, Bonn, Germany) for 3 hours. Ex-tracted OC solvent was concentrated by nitrogen gas and applied to Bio Beads SX-3 gel permeation chromatography (Norstrom et al. 1986), eluted OC solvent was washed with sulfuric acid and then applied to silica gel high performance liquid chromatography. The lipid concentration was determined gravimetrically using an aliquot of the extracted OCs (Lee et al. 1996). An equivalent mixture of dichloromethane and hexane was used for the mobile phase of gel permeation chromatography, and a mixture of dichloromethane and hexane (2:8 vol: vol) was used for the mobile phase of high performance liquid chromatography. Concentrations of OCs were measured by a Chromatopack 3800 gas chromatography linked to a Saturn 2000 ion trap mass spectrometer (Varian, Walnut Creek, Cali-fornia). The Chromatopack 3800 was equipped with a fused silica capil-lary column (30 m length ××0.25 mm inner diameter) coated with ZB-1 (Phenomenex, Torrance, California, 0.25 um bonded phase thickness). We determined the concentrations of 55 polychlorinated biphenyl congeners and 11 organochlorine pesticides. The concentrations of OCs were quanti-feid by comparison with corresponding external standards. An equivalent mixture of Kanechlors 300, 400, 500, and 600 (GL Sciences, Shinjuku,Tokyo, Japan) was used as the external standard for quantifciation of PCBs (Tanabe et al. 1987). The concentrations of total PCBs were the sum of 55 congeners. The unit of organochlorine concentrations (ng per g lipid)quantifeis the number of the nanograms of organochlorine contained in 1g of lipid extracted from the blubber. Recoveries of all PCBs and the other OCs through the analytical procedure were examined by spiking 4 og of PCBs and 100-200 ng of the other organochlorine standards into about 0.7 g of sea lion lipid. Average recovery efcfieincies were 97±13%for the PCB congeners, and 103±16% for the other OCs. And limits of detection were determined followed by the ofcfiail method (Kankyochou Suishitsu Hozenkyoku Suishitsu Kanrika 1998) and 0.1-10 ng per g lipid for PCBs and 2.3-14 ng per g lipid for others. The nonparametric multiple comparisons test (Steel-Dwass test) was used to compare OC concentra-tions in sea lions. The Wilcoxon rank sum test was used to compare OC concentrations in sea lions from Hokkaido with those in sea lions from Olyutorsky. Welch’s test was used to compare p,p'-DDE and PCBs in juve-nile sea lions investigated in this study with the data reported by Barron et al. (2004). The 2-sample test for equality of proportions without conti-nuity correction was used to compare the proportion of sea lions exceed-ing toxic levels in Hokkaido with those from Olyutorsky. The Steel-Dwass test and Wilcoxon rank sum test were conducted with Kyplot (KyensLab Inc., www.kyenslab.com/en/index.html), Welch’s test was conducted with web statistic tools (www.clg.niigata-u.ac.jp/~takagi/takagi.html), and the 2-sample test for equality of proportions without continuity correction was conducted with R2.2.0 (CRAN, www.r-project.org). Results and discussion In Steller sea lions from the western North Pacifci, DDTs (2609±2358 ng per g lipid) and PCBs (1648±1332 ng per g lipid) are signifciantly higher than chlordanes (CHLs) (896±642 ng per g lipid) and hexachlorocyclohex-anes (HCHs) (266±184 ng per g lipid) (Steel-Dwass test, P ≤ 0.05) (Table 1).This suggests that DDTs and PCBs are the predominant OCs accumulated in sea lions of the western North Pacifci measured in this study. Among adult male sea lions, DDTs and PCBs in sea lions of Hokkaido were signifciantly higher than those in sea lions of Olyutorsky Bay (Wil-coxon rank sum test, DDTs; P ≤ 0.001, PCBs; P ≤ 0.01) (Fig. 2). In addition,the ratios of DDTs to PCBs (DDTs:PCBs) in sea lions of Hokkaido were also signifciantly higher than those in sea lions of Olyutorsky (Table 1, Wilcoxon rank sum test, P < 0.01). Sea lions collected of fthe coast OCs and Areas Figure 2. Concentrations of organochlorine compounds accumulated in adult male sea lions of Hokkaido and Olyutorsky Bay. HCHs = sum of o-HCH, B-HCH and y-HCH. CHLs = sum of oxychlordane, trans-chlor-dane, cis-chlordane, trans-nonachlor, and cis-nonachlor. DDTs =sum of p,p'-DDT, p,p'-DDE, and p,p'-DDD. PCBs = sum of congeners listed in Table 1. Gray box = sea lions of Hokkaido; white box =sea lions of Olyutorsky Bay; whisker = minimum and maximum;lower boundary of box = 25th percentile; center boundary of box = median; upper boundary of box = 75th percentile. Wilcoxon rank sum test; **P ≤ 0.01, ***P ≤ 0.001. Asterisks indicate signifciant dif-ferences evaluated by Wilcoxon rank sum test. of Hokkaido came from the Sea of Okhotsk (Isono and Wada 1999) and most sea lions collected in Olyutorsky Bay are thought to come from the Commander Islands and Kamchatka Peninsula (V.N. Burkanov unpubl.);thus, sea lions of the Sea of Okhotsk may be more contaminated by DDTs and PCBs than sea lions of the western Bering Sea. In particular, DDTs:PCBs showed that contamination of sea lions of the Sea of Okhotsk by DDTs are more advanced than that of sea lions of the western Bering Sea.DDTs are not transported longer distances by the atmosphere than PCBs (Iwata et al. 1993, Andreas et al. 2000), and emission of DDTs is related to agricultural and economical activities (Monirith et al. 2003). Relatively higher human activity in countries surrounding the Sea of Okhotsk than the western Bering Sea may cause a geographical difefrence in contamina-tion of Steller sea lions. Table 1. CHLs o uu o os ooouo oo 1052±719 1264±664 Concentrations of organochlorine compounds accumulated in Steller sea lions from Hokkaido,om9Kuril Islands, and Olyutorsky Bay.6a6O Age O6N s class, No. of Mean 6Area sexsamples lipid % HCHs Oo0Hokkaido Adult, 10 72 266±13666NNO male O寸6MoM 191±92寸 寸'a Hokkaido Juvenile,N 5 72O 391±213O寸 male 寸N9oa 283±16o 3Hokkaido Juvenile, 3 76 183±48female o6寸寸 140±39's mmm寸 Kuril Islands Pup, 一寸十寸 751 379±334寸N寸 male 寸66O9167±151寸9寸 ao一 Kuril Islands Pup, 9 56 221±129寸寸'N O female ONMN卜寸寸寸寸 116±50c寸寸m Olyutorsky Adult, 寸 寸 12 69 6 206±105寸r Bay male 6寸Oo 137±53m 'Summary 46 65 266±18s 4寸6o9165±10u 4寸 Upper = mean±S.D., ng/g lipi oN卜 d. Lower = mean±S.D., ng/g wet weight).m HCHs = sum of -HCH, -HCH, and -HCH. CHLs = sum of oxychlordane 3, trans-chlordane, cis-chlordane, trans-nonachlor, and cis-nonachlor. DDTs = sum of p,p-DDT, p,p-DDE, and p,p-DDD. PCBs = sum of 8, 18, 15, 28/31, 52, 49/69, 47/Peak 48, 44, 42, 41/64, 58/74, 66, 91/95, 102, 60, 84/92/90, 101, 99, 97/113,>87/177, 85, 136, Peak 510, 151, 135, 144/149, 118/Peak 515, 132, 1283ox , o 105, 153, 176, 138, 178, Peak 74, Peak 617, 187, Peak 77, 180, 170, 198, 201/196.Number of peaks and PCBs follow Tanabe et al. 1987.u o 之 >6寸O o艹w 一寸 o 二 LO M 导 寸工 3am o ①工o 口工 a a工工u mo ooc N ca 工Oa工 1013±473 Table 1. CHLs o uu o os ooouo oo 1052±719 1264±664 Concentrations of organochlorine compounds accumulated in Steller sea lions from Hokkaido,om9Kuril Islands, and Olyutorsky Bay.6a6O Age O6N s class, No. of Mean 6Area sexsamples lipid % HCHs Oo0Hokkaido Adult, 10 72 266±13666NNO male O寸6MoM 191±92寸 寸'a Hokkaido Juvenile,N 5 72O 391±213O寸 male 寸N9oa 283±16o 3Hokkaido Juvenile, 3 76 183±48female o6寸寸 140±39's mmm寸 Kuril Islands Pup, 一寸十寸 751 379±334寸N寸 male 寸66O9167±151寸9寸 ao一 Kuril Islands Pup, 9 56 221±129寸寸'N O female ONMN卜寸寸寸寸 116±50c寸寸m Olyutorsky Adult, 寸 寸 12 69 6 206±105寸r Bay male 6寸Oo 137±53m 'Summary 46 65 266±18s 4寸6o9165±10u 4寸 Upper = mean±S.D., ng/g lipi oN卜 d. Lower = mean±S.D., ng/g wet weight).m HCHs = sum of -HCH, -HCH, and -HCH. CHLs = sum of oxychlordane 3, trans-chlordane, cis-chlordane, trans-nonachlor, and cis-nonachlor. DDTs = sum of p,p-DDT, p,p-DDE, and p,p-DDD. PCBs = sum of 8, 18, 15, 28/31, 52, 49/69, 47/Peak 48, 44, 42, 41/64, 58/74, 66, 91/95, 102, 60, 84/92/90, 101, 99, 97/113,>87/177, 85, 136, Peak 510, 151, 135, 144/149, 118/Peak 515, 132, 1283ox , o 105, 153, 176, 138, 178, Peak 74, Peak 617, 187, Peak 77, 180, 170, 198, 201/196.Number of peaks and PCBs follow Tanabe et al. 1987.u o 之 >6寸O o艹w 一寸 o 二 LO M 导 寸工 3am o ①工o 口工 a a工工u mo ooc N ca 工Oa工 1013±473 In addition, we compared the level of p,p'-DDE in juvenile Steller sea lions of the western North Pacifci with that in Steller sea lions of South-east Alaska and Prince William Sound reported by Barron et al. (2003).The major content of DDTs accumulated in sea lions is p,p'-DDE (Lee et al. 1996). This is a preliminary comparison because difefrent analytical methods were used; however, p,p'-DDE levels in juvenile sea lions from Hokkaido are slightly higher than those in juvenile sea lions from Prince William Sound (PWS = 1.3±0.6 ug per g wet weight, 19 individuals) (Welch’s test, P = 0.095). The level of PCBs in sea lions is similar among all areas of the north Pacifci. It is possible that more DDTs accumulated in Steller sea lions living in the Sea of Okhotsk than in Steller sea lions in other North Pacifci areas. Inter-laboratory studies are needed to clarify contamination in sea lions from all habitats. In phocid species, immune suppression and a decrease of circulating thyroid hormone due to OCs have been reported. Immunosuppression was observed in harbor seals, Phoca vitulina, that had accumulated 2.4ug per g lipid of p,p'-DDT and 16.5 ug per g lipid of PCBs in their blub-ber (de Swart et al. 1996). No sea lions in this study, however, exceeded the levels of PCBs and p,p'-DDT in the harbor seal. In addition, two species of congeners, 2,2',3,3',4,4',5-heptachlorobiphenyl (PCB170) and 2,2',3,4,4',5,5'-heptachlorobiphenyl (PCB180), were suggested to decrease the circulating thyroid hormone in ribbon seals, Phoca fasciata, that had accumulated 14 ng per g lipid of PCB170 and 43 ng per g lipid of PCB180in their blubber (Chiba et al. 2001; calculated from mean fat = 92%). We compared the numbers of adult sea lions exceeding the PCB170 and PCB180 levels of ribbon seals in Hokkaido with those in Olyutorsky (Fig.3). For PCB170, 4 of 10 sea lions in Hokkaido and 2 of 12 sea lions in Olyutorsky exceeded the level of ribbon seals. For PCB 180, all sea lions of Hokkaido and 5 of 12 sea lions of Olyutorsky exceeded the level of ribbon seals. The proportion of sea lions of Hokkaido exceeding the toxic level is signifciantly higher than that of sea lions of Olyutorsky (2-sample test for equality of proportions without continuity correction, P ≤ 0.01).These data suggest that sea lions of the Sea of Okhotsk have higher toxic risks than sea lions of the western Bering Sea, although sea lions may not have the same sensitivity to toxicity as ribbon seals. We concluded that DDTs and PCBs are the predominant organochlo-rines accumulated in Steller sea lions of the western North Pacifci in this study. Steller sea lions from the Sea of Okhotsk may have higher DDT accumulations than sea lions of the western Bering Sea and the eastern North Pacifci and may have higher toxic risks, such as decrease of circu-lating thyroid hormone. Figure 3. Number of sea lions exceeding toxic levels of ribbon seals. Horizon-tal lines in fgiures show levels of ribbon seal (Chiba et al. 2001). Acknowledgment We are grateful to Fisheries Agency, Japan; Alaska SeaLife Center; National Marine Mammal Laboratory, U.S.A.; Inui Tasuke Scientifci Foundation; and the Amway Nature Center of Japan. We would like to thank Kazuo Wada,Akihiko Wada, Akio Kazusaka, Mayumi Ishizuka, Kenji Konishi, Tatsuya Kariya, and Tomohiro Deguchi. We also give special thanks to colleagues in Russia and at Hokkaido University, Nihon University, and Rakunou Gakuen University for help in collecting samples.
确定





还剩8页未读,是否继续阅读?
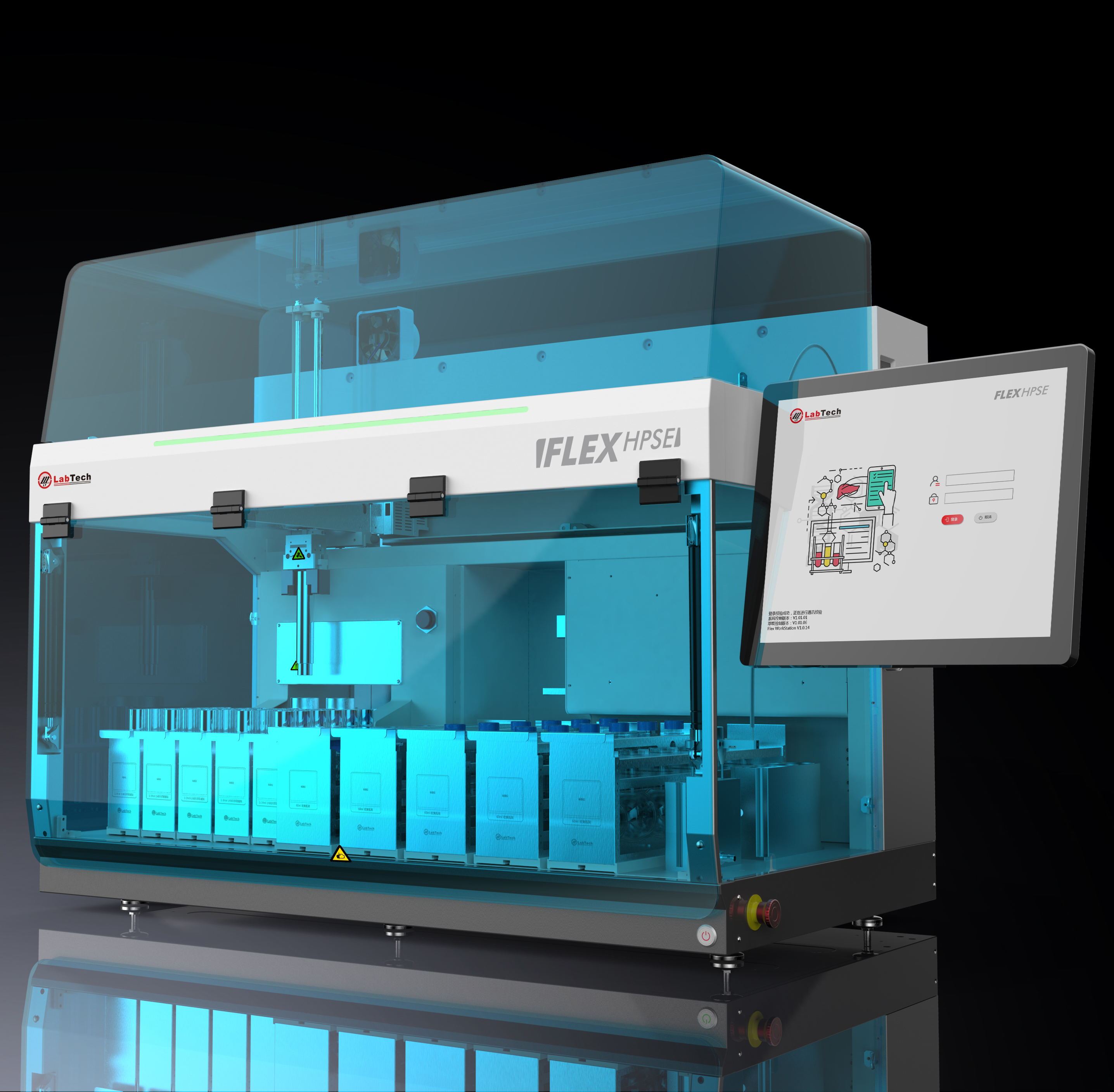
中国格哈特为您提供《来自西北太平洋的北海狮中的有机氯农药残留量》,该方案主要用于其他水产制品中农药残留检测,参考标准--,《来自西北太平洋的北海狮中的有机氯农药残留量》用到的仪器有格哈特全自动快速溶剂萃取仪Sox416、格哈特快速干燥仪STL56、德国加液器MM、棕色避光防紫外线萃取杯
推荐专场
该厂商其他方案
更多