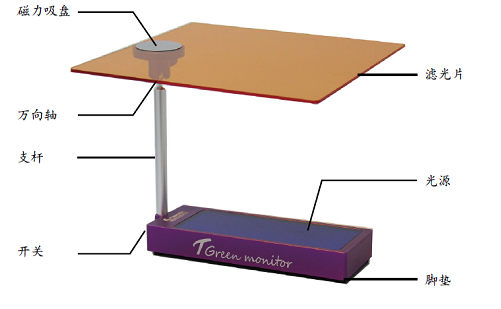
?近年来,基因治疗载体相关的研发和临床应用将腺相关病毒 (AAV) 的研究推向了更加火热的程度。衣壳滴度、基因组滴度以及空/实心比是表征AAV非常重要的参数,目前常用的检测方法例如荧光定量PCR (qPCR)、酶联免疫分析 (ELISA)、透射电子显微镜 (TEM) 和分析型超速离心 (AUC) 等技术,都存在一定的局限性;来自Charles River和Unchained Labs的科学家们近期在GEN Biotechnology发表了名为“Rapid Quality Control Assessment of Adeno-Associated Virus Vectors Via Stunner”《通过Stunner快速进行AAV载体的质量控制》的文章。科学家们通过Stunner测定AAV衣壳滴度、空/实心比、粒径和聚集程度等参数,并将其与qPCR、ELISA、TEM和AUC等方法的数据进行比较。结果表明,Stunner仅采用微量的样品便可极其快速地评估不同血清型AAV的理化性质。
方案详情

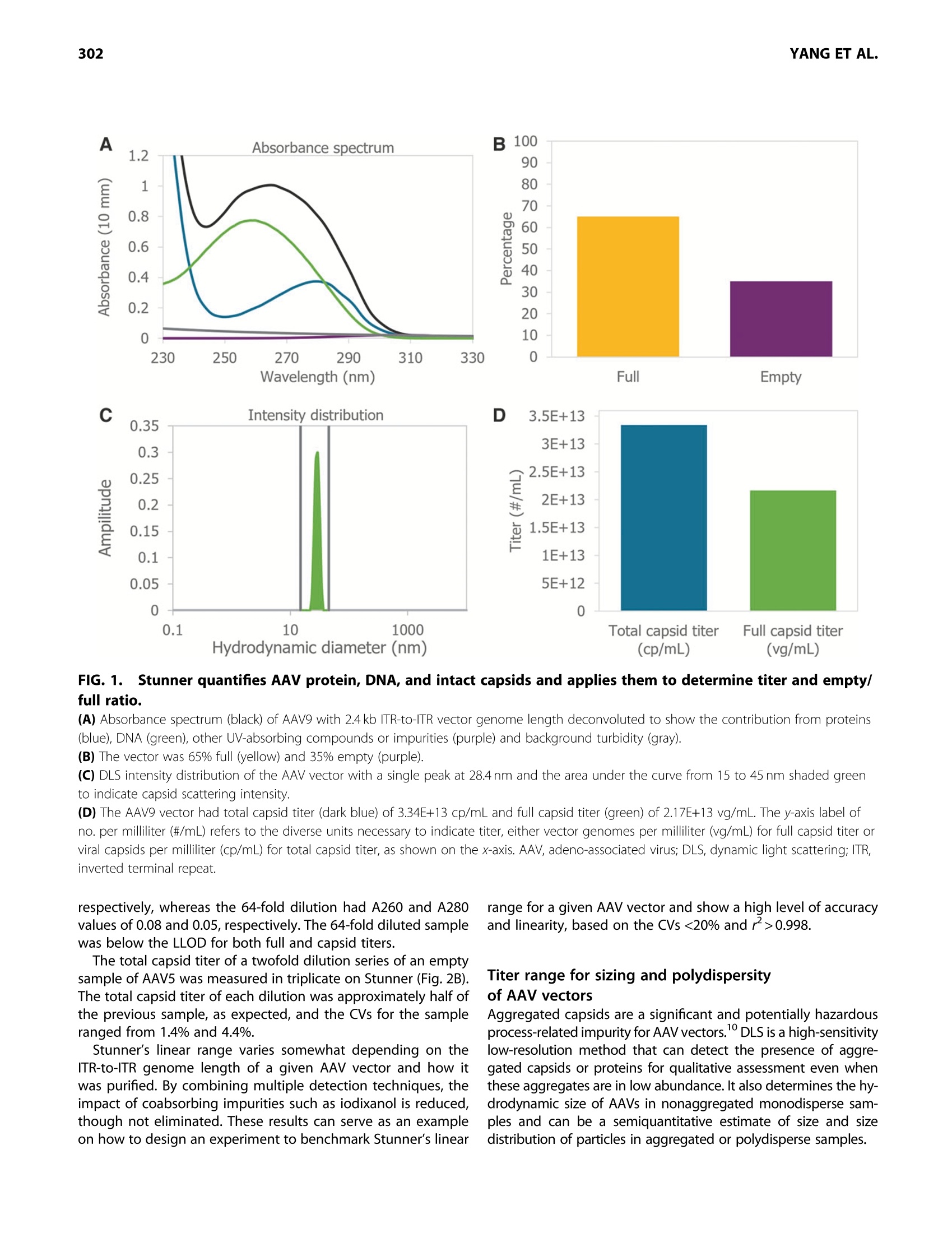
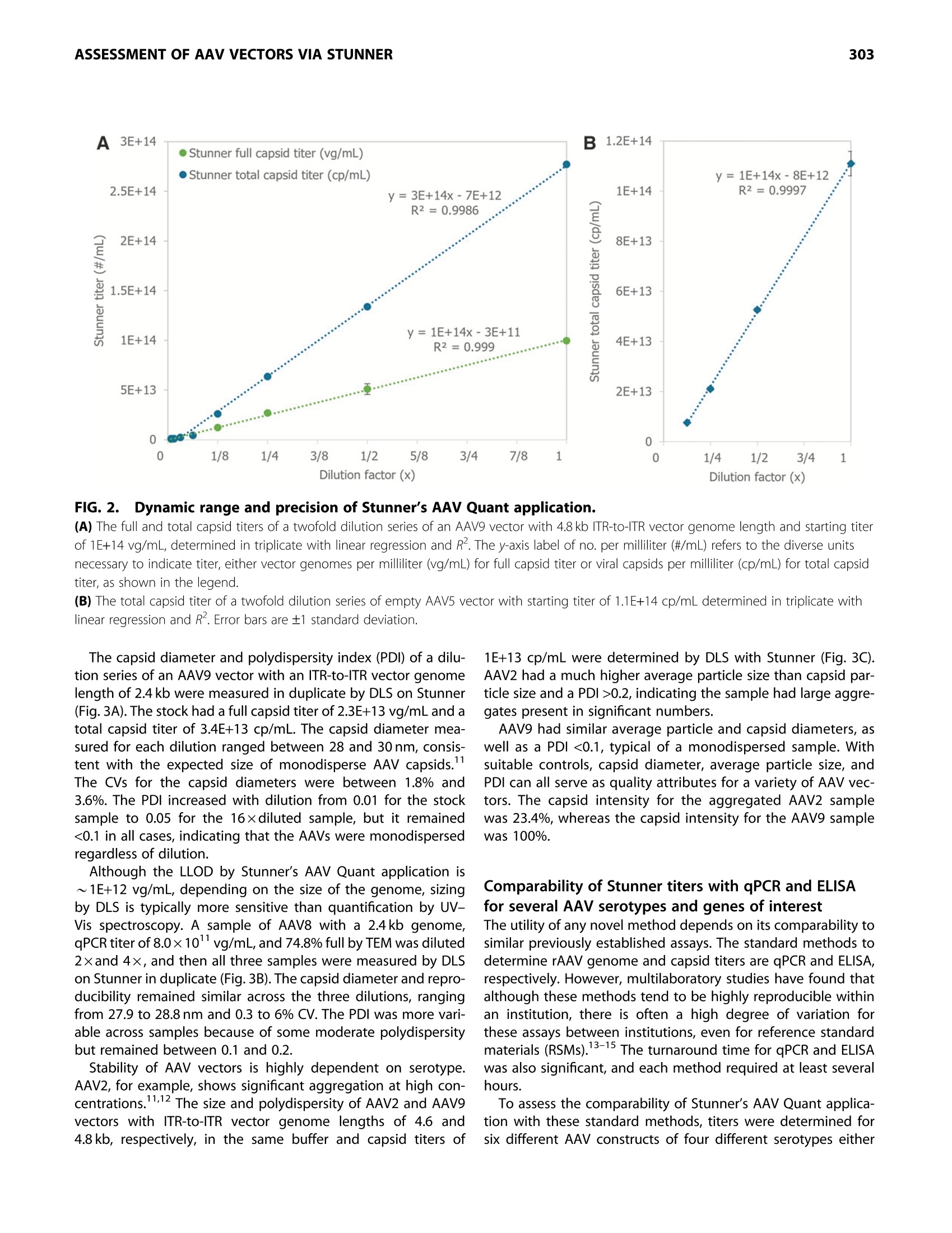
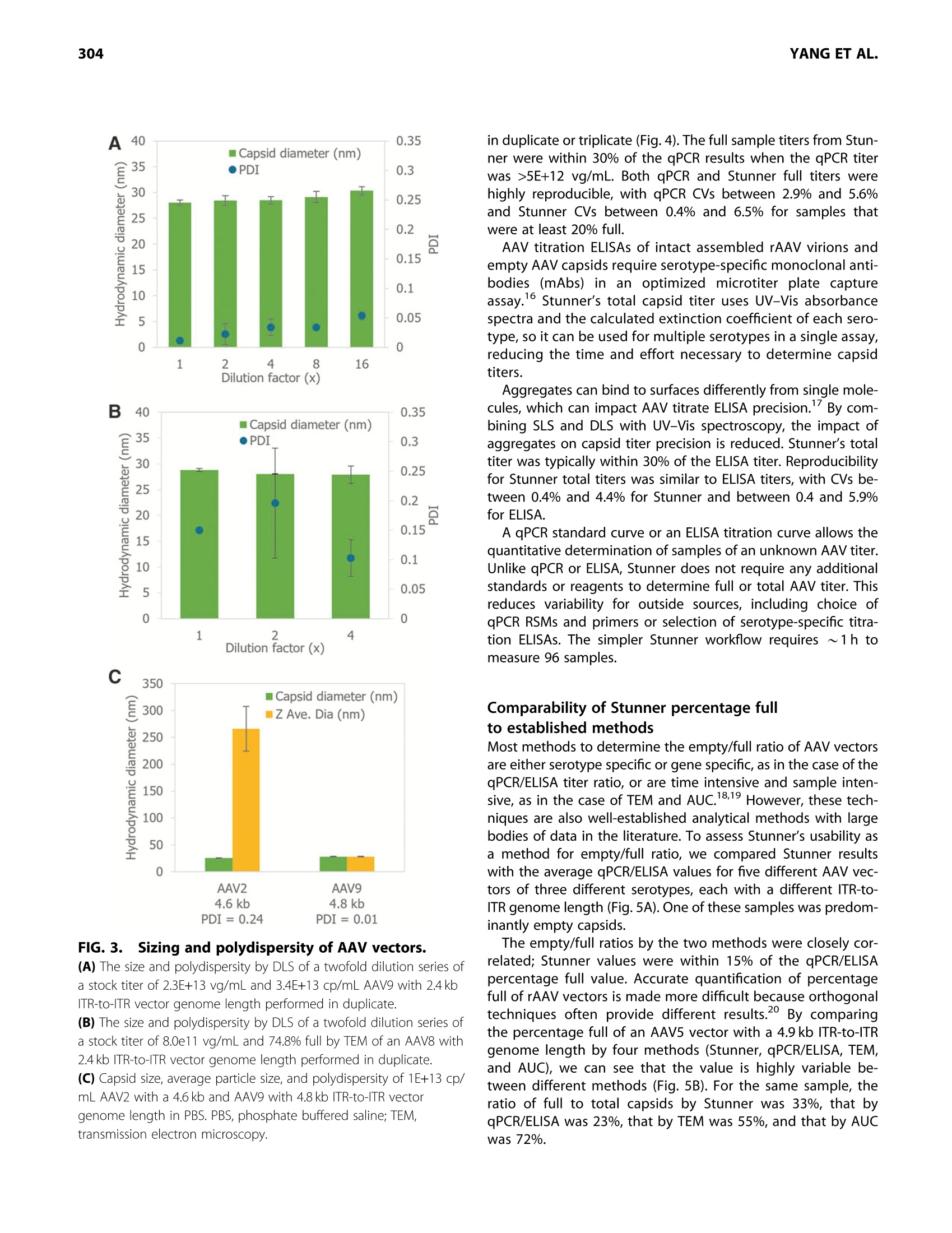
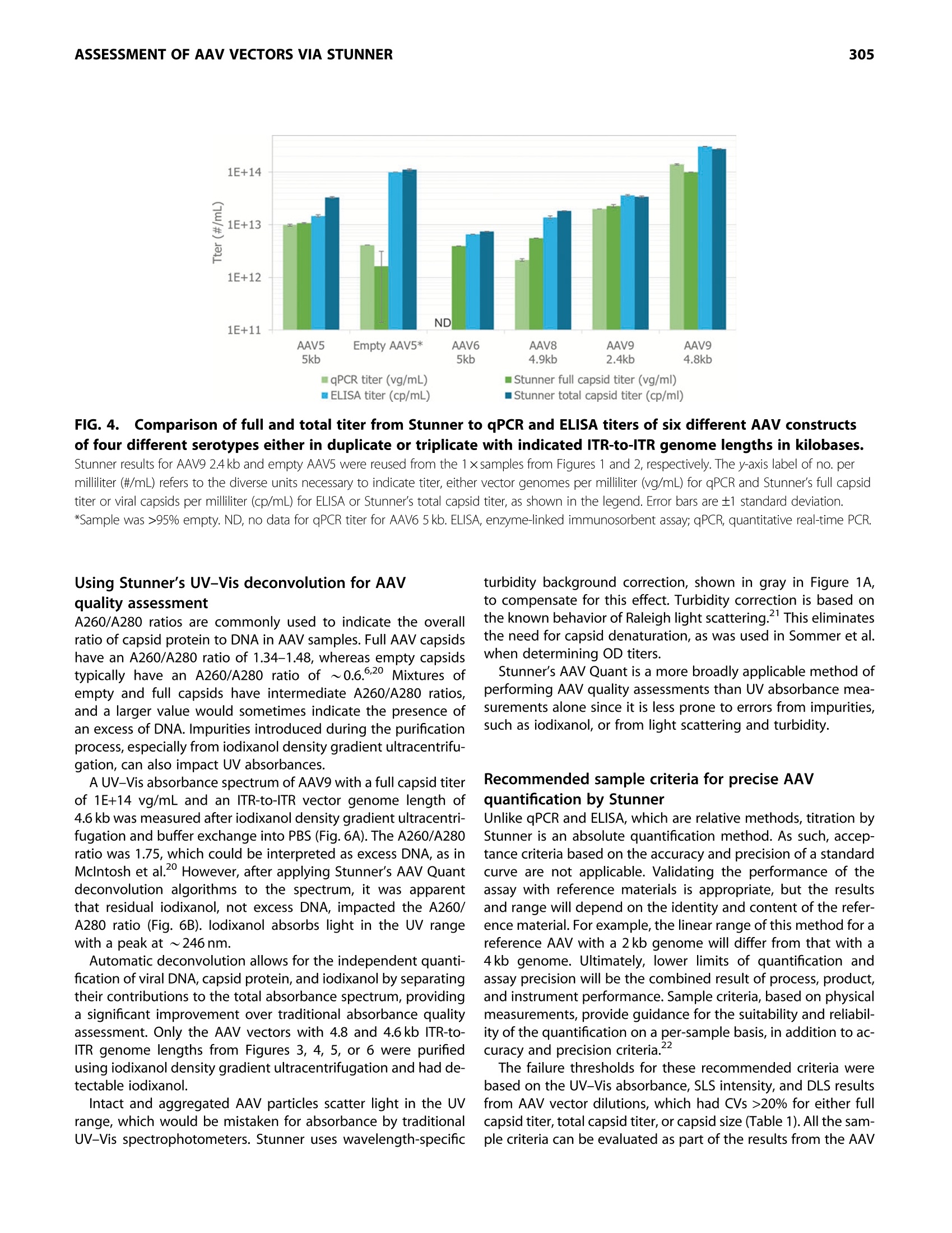
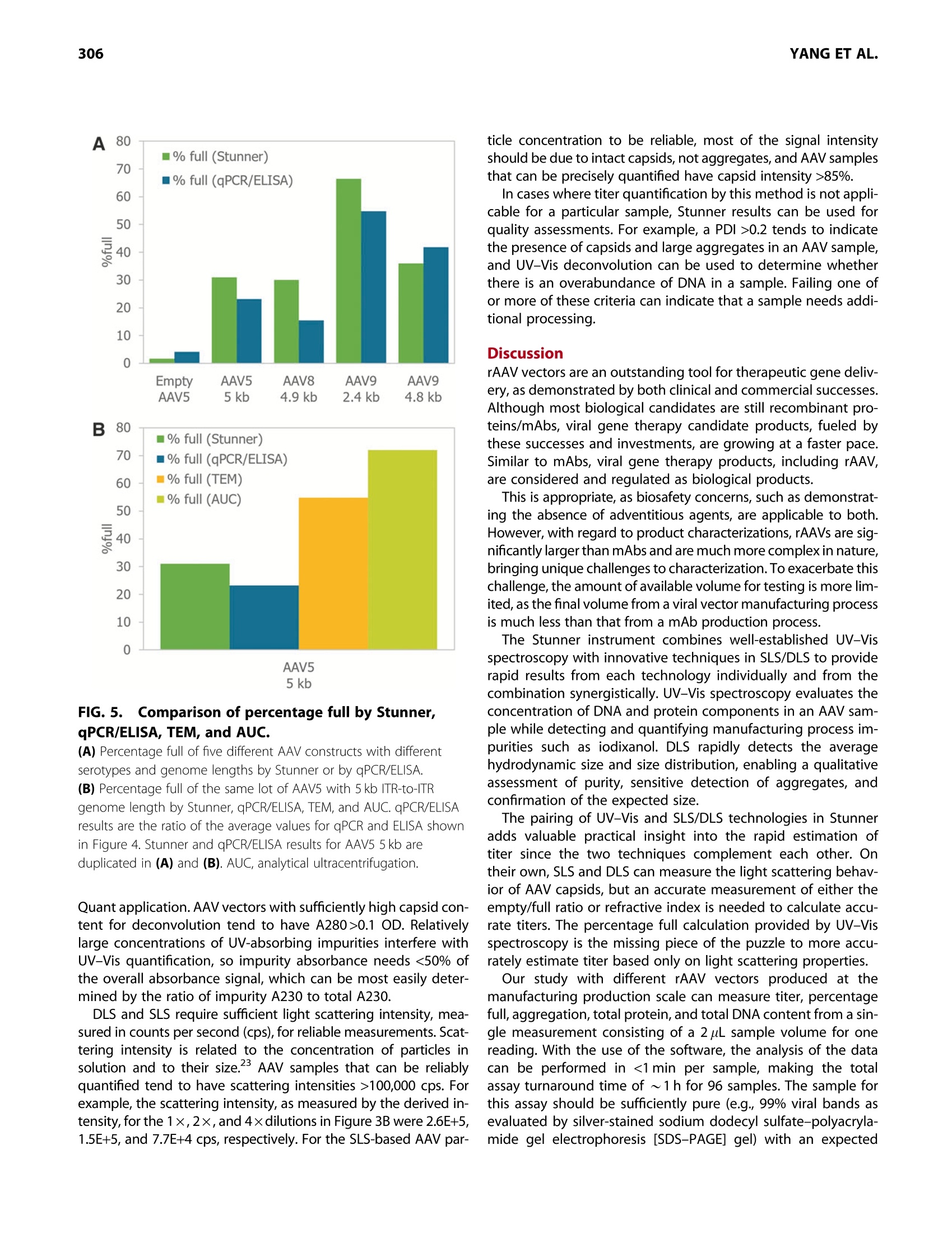
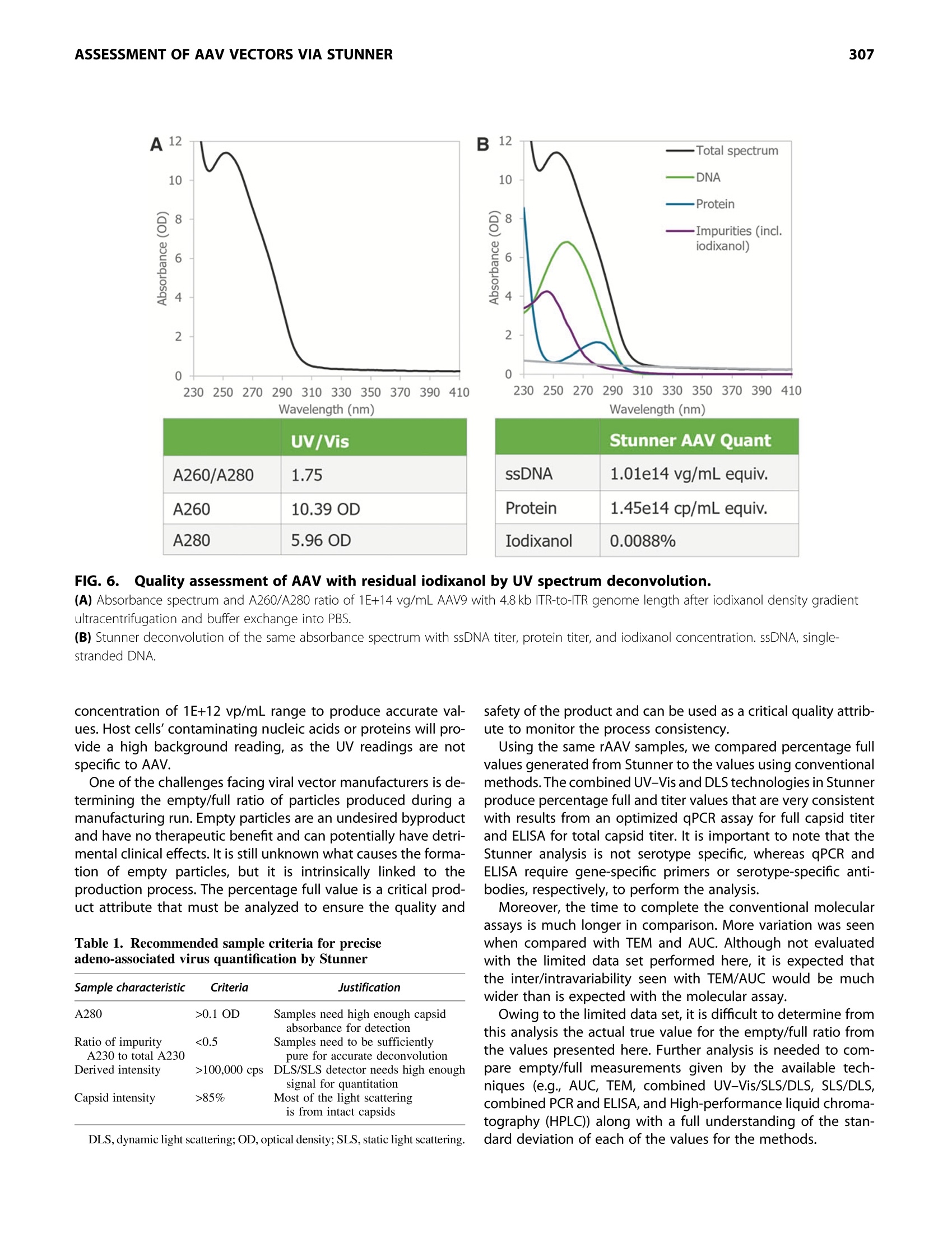
GEN BiotechnologyVolume 1, Number 3, 2022Mary Ann Liebert, Inc.DOI: 10.1089/genbio.2022.0007 ASSESSMENT OF AAV VECTORS VIA STUNNER301 300 RESEARCH ARTICLE Rapid Quality Control Assessment of Adeno-AssociatedVirus Vectors Via Stunner Quan-En Yang,Ross W. Walton,Tarana Kudrolli, Nelis Denys, Kevin Lance, and Audrey Chang1* Abstract As recombinant adeno-associated virus (AAV) vectors expand the availability of gene therapy; high-speed low-volumeanalytical methods become more vital for manufacturing. Current approaches for characterization are time consumingand sample consuming. Stunner combines ultraviolet-visible (UV-Vis) spectroscopy, static light scattering (SLS), anddynamic light scattering (DLS) to characterize up to 96 samples in <1 h, using 2 uL per sample. These data can beused to precisely quantify critical AAV titer, empty/full ratio, size, and aggregation. This study compares this techniquewith quantitative real-time PCR (qPCR), enzyme-linked immunoassay (ELISA), transmission electron microscopy, andanalytical ultracentrifugation using AAV vectors with multiple serotypes, genome sizes, and purification strategies. Rec-ommended sample criteria were established to provide guidelines on the suitability of Stunner analysis on a per-sample basis. The data presented herein lay the groundwork in establishing the use ofcombined UV-Vis, SLS, andDLS as a powerful and flexible analytical tool for AAV gene therapy vector development and manufacturing. herapeutic adeno-associated virus (AAV) vectors are rap-Tidly progressing as highly efficient gene delivery tools,with applications in many different human diseases. Theadvantages of the viral vector platform include easily manipu-lated genomes, low immunogenicity, broad infectivity, highyield from relatively simple production processes, and the po-tential for long-term expression. These features of the AAV-based viral vector make it the most commonly used viral vectorbeing investigated and manufactured in the past two decades. In general, recombinant AAV (rAAV) vectors are produced inmammalian or insect cells by cotransfecting the cell line withmultiple plasmids that carry either vector DNA or helper DNA.After cell lysis, the intracellularly matured virions are purifiedand enriched by brief centrifugation clarification. Commonlyused purification procedures include (1) density gradient ultra-centrifugation of virions using either cesium chloride (CsCl) oriodixanol; (2) pore-size controlled ultrafiltration (UF) for targetretention or filter out, such as parallel tangential flow filtration(TFF), UF, and buffer exchange dialysis filtration (also known asdiafiltration [DF]), or column-based CIMmultus monolithic filtra-tion (CMF); or (3) chromatography-based preparation, includingion exchange size-exclusion chromatography and antibody-based affinity chromatography.23 Each purification method has its own advantages and draw-backs. Usually, two to three different purification methods arecombined to optimize final drug quality for in vivo use.The bal-ance between the yield and purity of the final products varies foreach individual purification strategy, and each method must bepaired with analytical methods to confirm performance. Currently, a suite of assays has been established by the na-tional and international authorities as "ad hoc"procedures forviral titer quantification, ratio of fully packaged versus empty/partially packaged virions, vector integrity, and impurity. Eachindividual assay has a specific purpose for in-process and finaldrug monitoring and evaluation, such as quantitative real-timePCR (qPCR) for vector genome (vg) copies, enzyme-linkedimmunoassay (ELISA) for viral particle quantification, transmis-sion electron microscopy (TEM) or analytical ultracentrifugation(AUC) for empty/full assessments, chromatography for multipleviral characteristics, and dynamic light scattering (DLS) for sizeand size distribution analysis. However, performing the full suite of assays in a manufactur-ing environment is not only time consuming but also labor in-tensive. The burden of running a high number of assays fordifferent quality assurance purposes has also increased thecost of drug development and manufacturing. Importantly, ( *Address correspondence to:Audrey Chan g , Vigene Biosc i ences, A Ch a rles River Laboratory Company, 5 Rese a rch Court, Rockvi l le, MD 20850, USA, E - mail: audrey . chang@crl.com ) ( @ Quan-En Ya n g et al., 2022; Published by M ary Ann Liebert, Inc. T his Open Access article is distr i buted under the te r ms o f the Cre a tiv e Commons License [CC-BY](htt p :// creativecommons.org/licenses/by/4.0), w h ich permits unrestricted use, d i stribution, and r eproduction in any medium, pr o vided the original wo r k is properly ci t ed. ) extended delays for analytical results on in-process samples maycause deviations in viral titer and, ultimately,gene expression ef-ficiency between stored, processed, and analyzed batches. Stunner (Unchained Labs) is a combination ultraviolet-visible(UV-Vis) spectrophotometer, static light scattering (SLS), andDLS instrument designed to rapidly assess genomic and viralcapsid (cp) titers, empty/full ratio, and detect aggregation witha minimum of time, labor, and sample consumption." The use of UV-Vis spectroscopy to estimate the AAV titer andempty/full ratio is a well-established, simple, and reliable meth-od. 6 However, the use of UV-Vis spectroscopy alone is limitedby its inability to confirm whether light is absorbed from intactAAV capsids or unassembled protein and nucleic acids.UV-Visspectroscopy analysis can also be impacted by turbidity fromAAV capsids scattering light in the UV region, where absorbanceis used for DNA/protein quantification of AAV. Advanced algo-rithmic fitting, such as that done on Stunner, can determinethe turbidity of a sample to remove turbidity as a source of error. Static and DLS are complementary methods to UV-Vis spec-troscopy, previously used to measure the concentration of AAVcapsids and other viral vectors in a sample.8However, light scat-tering methods require information about the molecular weight(MW) and refractive index of the particle to accurately determinethe capsid concentration. In this approach,UV-Vis spectroscopycontributes information used to estimate the refractive indexbased on the ratio of protein and nucleic acids in a sample. By combining multiple detection technologies for the same sam-ple through spectroscopy and light scattering techniques, Stunnerprovides a complementary analysis of the properties of an AAVsample. In the past, these concepts have been used individuallyfor the evaluation of AAV vectors. In this article, we evaluate thecombination of these technologies for the rapid assessment ofAAV vectors with different serotypes and compare them with clas-sical quantification and quality assessment methods. Results Stunner quantifies protein, DNA, and intact capsidsin AAV samples and applies them to determine titerand empty/full ratio The UV-Vis absorbance spectrum of a purified AAV is the com-bination of the absorbance from the viral capsid, single-stranded DNA (ssDNA) genome, and any impurities that mightbe present. Using the amino acid sequence of the viral capsidproteins, the ratio between VP1:VP2:VP3,and the inverted termi-nal repeat (ITR)-to-ITR length of the vector genome, StunnerAnalysis deconvolutes, or separates, the overall absorbancespectrum into the contribution from proteins, DNA, other UV-absorbing compounds or impurities,and background turbidity.The absorbance spectrum of a CsCl-purified AAV9 vector with a2.4 kb ITR-to-ITR genome length was measured and deconvo-luted using Stunner's AAV Quant application (Fig. 1A). After deconvolution, the ssDNA titer of 2.2E+13 vg/mL equiva-lents was calculated from A260 of the DNA absorbance spectrumand the 752 kDa MW of a single genome. The protein titer of3.3E+13 cp/mL equivalents was calculated from the A280 of theprotein absorbance spectrum and the 4.0 MDa MW of the com- plete capsid. Protein titer is the maximum number of capsidsthat could possibly be assembled from the proteins in a sample. The ssDNA titer is the maximum number of viral genomesif all the DNA measured by UV-Vis spectroscopy is encapsulatedinto assembled nonaggregated AAV particles. For a purifiedsample of AAV, the ratio of protein and DNA present is relatedto the viral capsid empty/full ratio. The AAV9 vector was deter-mined to be 65% full by dividing the ssDNA titer by the proteintiter (Fig. 1B). The vector was calculated to be 35% empty. Intact AAV capsids have an expected hydrodynamic diameterof ~25 nm. In purified AAV samples, anything larger is typicallyviral aggregates, and anything smaller is most likely small parti-cles, including unassembled capsid proteins. To determine theproportion of light scattering that was attributable to intact cap-sids, the area under the DLS intensity distribution curve between15 and 45 nm was divided by the total area under the curve andreported as the "capsid intensity."The AAV9 vector had 100% cap-sid intensity, indicating there were no large aggregates and all thecapsid proteins were assembled into intact capsids (Fig. 1C). By combining all information from Stunner, the total numberof intact AAV9 capsids was determined to be 3.34E+13 cp/mL(Fig. 1D). The full capsid titer of 2.17E+13 vg/mL was calculatedusing the sample's percentage full from Figure 1B and the totalcapsid titer. In some cases, UV-Vis spectroscopy will measuremore protein in the sample than the total capsid titer or moreDNA in the sample than the full capsid titer. UV-Vis spectroscopy measures the total amounts of pro-tein and ssDNA in a sample, whereas the total capsid titerincludes information from SLS and DLS to calculate a titer for as-sembled nonaggregated AAV capsids. Differences between pro-tein titer and total capsid titer are reported as a “"free &aggregated protein" metric. Similarly, the difference betweenssDNA titer and full capsid titer is reported as "free & aggregatedssDNA." Both values were 0 for this nonaggregated AAV9 vector. Dynamic range and precision of full and total titerof purified AAV vectors and empty capsids Determining the dynamic range and precision of Stunner'squantification of titers and empty/full ratios using purified AAVsamples of different serotypes and capsid contents is key toestablishing its usefulness as a rapid high-throughput analyticalmethod. A twofold dilution series of a purified AAV9 vectorwith a 4.8 kb ITR-to-ITR vector genome length in phosphate buff-ered saline (PBS) was measured in triplicate using Stunner's AAVQuant application (Fig. 2A). The stock sample had a full capsidtiter of 1E+14 vg/mL and a total capsid titer of 2.8E+14 cp/mL. Dilutions with full capsid titers and total capsid titers abovethe lower limits of detection (LLOD) of 1E+12 vg/mL and2E+12 cp/mL, respectively, had coefficients of variation (CVs) be-tween 0.5% and 11.8%. The 16-and 32-fold diluted samples hadfull capsid titers close to 1/2 the previous sample's concentra-tion, but Stunner indicated both samples had 100% full capsids,most likely due to residual iodixanol interfering with the ssDNAdeconvolution. The full capsid percentage for samples down toan eightfold dilution ranged from 36% to 47% full. The 32-folddiluted sample had A260 and A280 values of 0.23 and 0.14, FIG.1.Stunner quantifies AAV protein, DNA, and intact capsids and applies them to determine titer and empty/full ratio. (A) Absorbance spectrum (black) of AAV9 with 2.4 kb ITR-to-ITR vector genome length deconvoluted to show the contribution from proteins(blue), DNA (green),other UV-absorbing compounds or impurities (purple) and background turbidity (gray). (B) The vector was 65% full (yellow) and 35% empty (purple). (C) DLS intensity distribution of the AAV vector with a single peak at 28.4 nm and the area under the curve from 15 to 45 nm shaded greento indicate capsid scattering intensity. (D) The AAV9 vector had total capsid titer (dark blue) of 3.34E+13 cp/mL and full capsid titer (green) of 2.17E+13 vg/mL. The y-axis label ofno. per milliliter (#/mL) refers to the diverse units necessary to indicate titer, either vector genomes per milliliter (vg/mL) for full capsid titer orviral capsids per milliliter (cp/mL) for total capsid titer, as shown on the x-axis. AAV, adeno-associated virus; DLS, dynamic light scattering; ITR,inverted terminal repeat. respectively, whereas the 64-fold dilution had A260 and A280values of 0.08 and 0.05, respectively. The 64-fold diluted samplewas below the LLOD for both full and capsid titers. range for a given AAV vector and show a high level of accuracyand linearity, based on the CVs <20% and >0.998. The total capsid titer of a twofold dilution series of an emptysample of AAV5 was measured in triplicate on Stunner (Fig.2B).The total capsid titer of each dilution was approximately half ofthe previous sample, as expected, and the CVs for the sampleranged from 1.4% and 4.4%. Stunner’s linear range varies somewhat depending on theITR-to-ITR genome length of a given AAV vector and how itwas purified. By combining multiple detection techniques, theimpact of coabsorbing impurities such as iodixanol is reduced,though not eliminated. These results can serve as an exampleon how to design an experiment to benchmark Stunner's linear Titer range for sizing and polydispersityof AAV vectors Aggregated capsids are a significant and potentially hazardousprocess-related impurity for AAV vectors.DLS is a high-sensitivitylow-resolution method that can detect the presence of aggre-gated capsids or proteins for qualitative assessment even whenthese aggregates are in low abundance. It also determines the hy-drodynamic size of AAVs in nonaggregated monodisperse sam-ples and can be a semiquantitative estimate of size and sizedistribution of particles in aggregated or polydisperse samples. FIG. 2. Dynamic range and precision of Stunner's AAV Quant application. (A) The full and total capsid titers of a twofold dilution series of an AAV9 vector with 4.8 kb ITR-to-ITR vector genome length and starting titerof 1E+14 vg/mL, determined in triplicate with linear regression and R. The y-axis label of no. per milliliter (#/mL) refers to the diverse unitsnecessary to indicate titer, either vector genomes per milliliter (vg/mL) for full capsid titer or viral capsids per milliliter (cp/mL) for total capsidtiter, as shown in the legend. (B) The total capsid titer of a twofold dilution series of empty AAV5 vector with starting titer of 1.1E+14 cp/mL determined in triplicate withlinear regression and R2. Error bars are ±1 standard deviation. The capsid diameter and polydispersity index (PDI) of a dilu-tion series of an AAV9 vector with an ITR-to-ITR vector genomelength of 2.4 kb were measured in duplicate by DLS on Stunner(Fig.3A). The stock had a full capsid titer of 2.3E+13 vg/mL and atotal capsid titer of 3.4E+13 cp/mL. The capsid diameter mea-sured for each dilution ranged between 28 and 30 nm, consis-tent with the expected size of monodisperse AAV capsids.The CVs for the capsid diameters were between 1.8% and3.6%. The PDI increased with dilution from 0.01 for the stocksample to 0.05 for the 16×diluted sample, but it remained<0.1 in all cases, indicating that the AAVs were monodispersedregardless of dilution. Although the LLOD by Stunner's AAV Quant application is~1E+12 vg/mL, depending on the size of the genome, sizingby DLS is typically more sensitive than quantification by UV-Vis spectroscopy. A sample of AAV8 with a 2.4kb genome,qPCR titer of 8.0×10"vg/mL, and 74.8% full by TEM was diluted2xand 4×, and then all three samples were measured by DLSon Stunner in duplicate (Fig.3B). The capsid diameter and repro-ducibility remained similar across the three dilutions, rangingfrom 27.9 to 28.8 nm and 0.3 to 6% CV. The PDI was more vari-able across samples because of some moderate polydispersitybut remained between 0.1 and 0.2. Stability of AAV vectors is highly dependent on serotype.AAV2, for example, shows significant aggregation at high con-.11,12-centrations.1112 The size and polydispersity of AAV2 and AAV9vectors with ITR-to-ITR vector genome lengths of 4.6 and4.8 kb, respectively, in the same buffer and capsid titers of 1E+13 cp/mL were determined by DLS with Stunner (Fig. 3C).AAV2 had a much higher average particle size than capsid par-ticle size and a PDI>0.2, indicating the sample had large aggre-gates present in significant numbers. AAV9 had similar average particle and capsid diameters, aswell as a PDI <0.1, typical of a monodispersed sample. Withsuitable controls, capsid diameter, average particle size, andPDI can all serve as quality attributes for a variety of AAV vec-tors. The capsid intensity for the aggregated AAV2 samplewas 23.4%, whereas the capsid intensity for the AAV9 samplewas 100%. Comparability of Stunner titers with qPCR and ELISAfor several AAV serotypes and genes of interest The utility of any novel method depends on its comparability tosimilar previously established assays. The standard methods todetermine rAAV genome and capsid titers are qPCR and ELISA,respectively. However, multilaboratory studies have found thatalthough these methods tend to be highly reproducible withinan institution, there is often a high degree of variation forthese assays between institutions, even for reference standardmaterials (RSMs).13-15 The turnaround time for gPCR and ELISAwas also significant, and each method required at least severalhours. To assess the comparability of Stunner’s AAV Quant applica-tion with these standard methods, titers were determined forsix different AAV constructs of four different serotypes either Dilution factor (x) C FIG.3.Sizing and polydispersity of AAV vectors. (A) The size and polydispersity by DLS of a twofold dilution series ofa stock titer of 2.3E+13 vg/mL and 3.4E+13 cp/mL AAV9 with 2.4 kb ITR-to-ITR vector genome length performed in duplicate.(B) The size and polydispersity by DLS of a twofold dilution series ofa stock titer of 8.0e11 vg/mL and 74.8% full by TEM of an AAV8 with2.4 kb ITR-to-ITR vector genome length performed in duplicate. (C) Capsid size, average particle size, and polydispersity of 1E+13 cp/mL AAV2 with a 4.6 kb and AAV9 with 4.8 kb ITR-to-ITR vectorgenome length in PBS. PBS, phosphate buffered saline; TEM,transmission electron microscopy. in duplicate or triplicate (Fig. 4). The full sample titers from Stun-ner were within 30% of the qPCR results when the qPCR titerwas >5E+12 vg/mL. Both qPCR and Stunner full titers werehighly reproducible, with qPCR CVs between 2.9% and 5.6%and Stunner CVs between 0.4% and 6.5% for samples thatwere at least 20% full. AAV titration ELISAs of intact assembled rAAV virions andempty AAV capsids require serotype-specific monoclonal anti-bodies (mAbs) in an optimized microtiter plate captureassay.16 Stunner’s total capsid titer uses UV-Vis absorbancespectra and the calculated extinction coefficient of each sero-type, so it can be used for multiple serotypes in a single assay,reducing the time and effort necessary to determine capsidtiters. Aggregates can bind to surfaces differently from single mole-cules, which can impact AAV titrate ELISA precision.By com-bining SLS and DLS with UV-Vis spectroscopy, the impact ofaggregates on capsid titer precision is reduced. Stunner’s totaltiter was typically within 30% of the ELISA titer. Reproducibilityfor Stunner total titers was similar to ELISA titers, with CVs be-tween 0.4% and 4.4% for Stunner and between 0.4 and 5.9%for ELISA. A qPCR standard curve or an ELISA titration curve allows thequantitative determination of samples of an unknown AAV titer.Unlike qPCR or ELISA, Stunner does not require any additionalstandards or reagents to determine full or total AAV titer. Thisreduces variability for outside sources, including choice ofqPCR RSMs and primers or selection of serotype-specific titra-tion ELISAs. The simpler Stunner workflow requires ~1 h tomeasure 96 samples. Comparability of Stunner percentage fullto established methods Most methods to determine the empty/full ratio of AAV vectorsare either serotype specific or gene specific, as in the case of theqPCR/ELISA titer ratio, or are time intensive and sample inten-sive, as in the case of TEM and AUC.18,19 However, these tech-niques are also well-established analytical methods with largebodies of data in the literature. To assess Stunner's usability asa method for empty/full ratio, we compared Stunner resultswith the average qPCR/ELISA values for five different AAV vec-tors of three different serotypes, each with a different ITR-to-ITR genome length (Fig. 5A). One of these samples was predom-inantly empty capsids. The empty/full ratios by the two methods were closely cor-related; Stunner values were within 15% of the qPCR/ELISApercentage full value. Accurate quantification of percentagefull of rAAV vectors is made more difficult because orthogonaltechniques often provide different results.2 By comparingthe percentage full of an AAV5 vector with a 4.9 kb ITR-to-ITRgenome length by four methods (Stunner, qPCR/ELISA, TEM,and AUC), we can see that the value is highly variable be-tween different methods (Fig.5B). For the same sample, theratio of full to total capsids by Stunner was 33%, that byqPCR/ELISA was 23%, that by TEM was 55%, and that by AUCwas 72%. FIG. 4l.. Comparison of full and total titer from Stunner to qPCR and ELISA titers of six different AAV constructsof four different serotypes either in duplicate or triplicate with indicated ITR-to-ITR genome lengths in kilobases. Stunner results for AAV9 2.4kb and empty AAV5 were reused from the 1 ×samples from Figures 1 and 2, respectively. The y-axis label of no. permilliliter (#/mL) refers to the diverse units necessary to indicate titer, either vector genomes per milliliter (vg/mL) for qPCR and Stunner's full capsidtiter or viral capsids per milliliter (cp/mL) for ELISA or Stunner's total capsid titer, as shown in the legend. Error bars are ±1 standard deviation.*Sample was >95% empty. ND, no data for qPCR titer for AAV6 5 kb. ELISA, enzyme-linked immunosorbent assay; qPCR, quantitative real-time PCR. Using Stunner's UV-Vis deconvolution for AAVquality assessment A260/A280 ratios are commonly used to indicate the overallratio of capsid protein to DNA in AAV samples. Full AAV capsidshave an A260/A280 ratio of 1.34-1.48, whereas empty capsidstypically have an A260/A280 ratio of ~0.6.6,20 Mixtures ofempty and full capsids have intermediate A260/A280 ratios,and a larger value would sometimes indicate the presence ofan excess of DNA. Impurities introduced during the purificationprocess, especially from iodixanol density gradient ultracentrifu-gation, can also impact UV absorbances. AUV-Vis absorbance spectrum of AAV9 with a full capsid titerof 1E+14 vg/mL and an ITR-to-ITR vector genome length of4.6 kb was measured after iodixanol density gradient ultracentri-fugation and buffer exchange into PBS (Fig.6A). The A260/A280ratio was 1.75, which could be interpreted as excess DNA, as inMclntosh et al.20 However, after applying Stunners AAV Quantdeconvolution algorithms to the spectrum, it was apparentthat residual iodixanol, not excess DNA, impacted the A260/A280 ratio (Fig.6B). lodixanol absorbs light in the UV rangewith a peak at~246 nm. Automatic deconvolution allows for the independent quanti-fication of viral DNA, capsid protein, and iodixanol by separatingtheir contributions to the total absorbance spectrum, providinga significant improvement over traditional absorbance qualityassessment. Only the AAV vectors with 4.8 and 4.6kb ITR-to-ITR genome lengths from Figures 3, 4, 5, or 6 were purifiedusing iodixanol density gradient ultracentrifugation and had de-tectable iodixanol. Intact and aggregated AAV particles scatter light in the UVrange, which would be mistaken for absorbance by traditionalUV-Vis spectrophotometers. Stunner uses wavelength-specific turbidity background correction, shown in gray in Figure 1A,to compensate for this effect. Turbidity correction is based onthe known behavior of Raleigh light scattering.This eliminatesthe need for capsid denaturation, as was used in Sommer et al.when determining OD titers. Stunner's AAV Quant is a more broadly applicable method ofperforming AAV quality assessments than UV absorbance mea-surements alone since it is less prone to errors from impurities,such as iodixanol, or from light scattering and turbidity. Recommended sample criteria for precise AAVquantification by Stunner Unlike qPCR and ELISA, which are relative methods, titration byStunner is an absolute quantification method. As such, accep-tance criteria based on the accuracy and precision of a standardcurve are not applicable. Validating the performance of theassay with reference materials is appropriate, but the resultsand range will depend on the identity and content of the refer-ence material. For example, the linear range of this method for areference AAV with a 2 kb genome will differ from that with a4 kb genome. Ultimately, lower limits of quantification andassay precision will be the combined result of process, product,and instrument performance. Sample criteria, based on physicalmeasurements, provide guidance for the suitability and reliabil-ity of the quantification on a per-sample basis, in addition to ac-curacy and precision criteria." The failure threshold for these recommended criteria werebased on the UV-Vis absorbance, SLS intensity, and DLS resultsfrom AAV vector dilutions, which had CVs >20% for either fullcapsid titer, total capsid titer, or capsid size (Table 1). All the sam-ple criteria can be evaluated as part of the results from the AAV B80 FIG.5.. Comparison of percentage full by Stunner,qPCR/ELISA,TEM, and AUC. (A) Percentage full of five different AAV constructs with differentserotypes and genome lengths by Stunner or by qPCR/ELISA. (B) Percentage full of the same lot of AAV5 with 5 kb ITR-to-ITRgenome length by Stunner, qPCR/ELISA, TEM, and AUC. qPCR/ELISAresults are the ratio of the average values for qPCR and ELISA shownin Figure 4. Stunner and qPCR/ELISA results for AAV5 5 kb areduplicated in (A) and (B). AUC, analytical ultracentrifugation. Quant application. AAV vectors with sufficiently high capsid con-tent for deconvolution tend to have A280>0.1 OD. Relativelylarge concentrations of UV-absorbing impurities interfere withUV-Vis quantification, so impurity absorbance needs <50% ofthe overall absorbance signal, which can be most easily deter-mined by the ratio of impurity A230 to total A230 DLS and SLS require sufficient light scattering intensity, mea-sured in counts per second (cps), for reliable measurements. Scat-tering intensity is related to the concentration of particles insolution and to their size.3 AAV samples that can be reliablyquantified tend to have scattering intensities >100,000 cps. Forexample, the scattering intensity, as measured by the derived in-tensity, for the 1×,2×,and 4×dilutions in Figure 3B were 2.6E+5,1.5E+5, and 7.7E+4 cps, respectively. For the SLS-based AAV par- ticle concentration to be reliable, most of the signal intensityshould be due to intact capsids, not aggregates, and AAV samplesthat can be precisely quantified have capsid intensity >85%. In cases where titer quantification by this method is not appli-cable for a particular sample, Stunner results can be used forquality assessments. For example, a PDI>0.2 tends to indicatethe presence of capsids and large aggregates in an AAV sample,and UV-Vis deconvolution can be used to determine whetherthere is an overabundance of DNA in a sample. Failing one ofor more of these criteria can indicate that a sample needs addi-tional processing. Discussion rAAV vectors are an outstanding tool for therapeutic gene deliv-ery, as demonstrated by both clinical and commercial successes.Although most biological candidates are still recombinant pro-teins/mAbs, viral gene therapy candidate products, fueled bythese successes and investments, are growing at a faster pace.Similar to mAbs, viral gene therapy products, including rAAv,are considered and regulated as biological products. This is appropriate,as biosafety concerns, such as demonstrat-ing the absence of adventitious agents, are applicable to both.However, with regard to product characterizations, rAAVs are sig-nificantly larger than mAbs and are much more complex in nature,bringing unique challenges to characterization. To exacerbate thischallenge,the amount of available volume for testing is more lim-ited, as the final volume from a viral vector manufacturing processis much less than that from a mAb production process. The Stunner instrument combines well-established UV-Visspectroscopy with innovative techniques in SLS/DLS to providerapid results from each technology individually and from thecombination synergistically. UV-Vis spectroscopy evaluates theconcentration of DNA and protein components in an AAV sam-ple while detecting and quantifying manufacturing process im-purities such as iodixanol. DLS rapidly detects the averagehydrodynamic size and size distribution, enabling a qualitativeassessment of purity, sensitive detection of aggregates, andconfirmation of the expected size. The pairing of UV-Vis and SLS/DLS technologies in Stunneradds valuable practical insight into the rapid estimation oftiter since the two techniques complement each other. Ontheir own, SLS and DLS can measure the light scattering behav-ior of AAV capsids, but an accurate measurement of either theempty/full ratio or refractive index is needed to calculate accu-rate titers. The percentage full calculation provided by UV-Visspectroscopy is the missing piece of the puzzle to more accu-rately estimate titer based only on light scattering properties. Our study with different rAAV vectors produced at themanufacturing production scale can measure titer, percentagefull,aggregation, total protein, and total DNA content from a sin-gle measurement consisting of a 2 pL sample volume for onereading. With the use of the software, the analysis of the datacan be performed in <1 min per sample, making the totalassay turnaround time of ~1 h for 96 samples. The sample forthis assay should be sufficiently pure (e.g., 99% viral bands asevaluated by silver-stained sodium dodecyl sulfate-polyacryla-mide gel electrophoresis [SDS-PAGE] gel) with an expected FIG. 6i.. Quality assessment of AAV with residual iodixanol by UV spectrum deconvolution. (A) Absorbance spectrum and A260/A280 ratio of 1E+14 vg/mL AAV9 with 4.8 kb ITR-to-ITR genome length after iodixanol density gradientultracentrifugation and buffer exchange into PBS. concentration of 1E+12 vp/mL range to produce accurate val-ues. Host cells' contaminating nucleic acids or proteins will pro-vide a high background reading, as the UV readings are notspecific to AAV. One of the challenges facing viral vector manufacturers is de-termining the empty/full ratio of particles produced during amanufacturing run. Empty particles are an undesired byproductand have no therapeutic benefit and can potentially have detri-mental clinical effects. It is still unknown what causes the forma-tion of empty particles, but it is intrinsically linked to theproduction process. The percentage full value is a critical prod-uct attribute that must be analyzed to ensure the quality and Table 1. Recommended sample criteria for preciseadeno-associated virus quantification by Stunner Sample characteristic Criteria Justification A280 >0.1 OD Samples need high enough capsid absorbance for detection Samples need to be sufficiently Ratio of impurity <0.5 A230 to total A230 pure for accurate deconvolution Derived intensity >100,000 cps DLS/SLS detector needs high enough Capsid intensity >85% signal for quantitation Most of the light scattering is from intact capsids DLS, dynamic light scattering; OD, optical density; SLS, static light scattering. safety of the product and can be used as a critical quality attrib-ute to monitor the process consistency. Using the same rAAV samples, we compared percentage full2nYevalues generated from Stunner to the values using conventionalmethods.The combined UV-Vis and DLS technologies in Stunnerproduce percentage full and titer values that are very consistentwith results from an optimized qPCR assay for full capsid titerand ELISA for total capsid titer.It is important to note that theStunner analysis is not serotype specific, whereas qPCR andELISA require gene-specific primers or serotype-specific anti-bodies, respectively, to perform the analysis. Moreover, the time to complete the conventional molecularassays is much longer in comparison. More variation was seenwhen compared with TEM and AUC. Although not evaluatedwith the limited data set performed here, it is expected thatthe inter/intravariability seen with TEM/AUC would be muchwider than is expected with the molecular assay. Owing to the limited data set, it is difficult to determine fromthis analysis the actual true value for the empty/full ratio fromthe values presented here. Further analysis is needed to com-pare empty/full measurements given by the available tech-niques (e.g., AUC, TEM, combined UV-Vis/SLS/DLS, SLS/DLS,combined PCR and ELISA, and High-performance liquid chroma-tography (HPLC)) along with a full understanding of the stan-dard deviation of each of the values for the methods. With titer, percentage full,size, aggregation, total protein, andtotal DNA content from a single assay, the potential applicationsfor the assay performed by Stunner span research, manufactur-ing, and QC needs for downstream samples. Conclusions The use of high-throughput low-volume analytical methods is in-creasingly central in conserving time and sample when assayingAAV titer, empty/full ratio, size, and aggregation. Stunner's ap-D-proach to combine UV-Vis spectroscopy, SLS, and DLS makes fora promising technology that delivers multiple data points on a min-imal sample volume and in a minimal time. This approach is appli-cable to multiple serotypes, genome constructs, and purificationstrategies and adds a complementary and orthogonal approachto other current techniques such as qPCR, ELISA, TEM and AUC. Future study will be needed to explore the advantages anddisadvantages of each method and to elucidate the optimal ap-proaches for any given sample and purification strategy. In thisarticle, we have taken the first step in that direction by recom-mending a set of sample criteria guidelines that inform whethersamples are suitable for Stunner analysis. Combined UV/Vis, SLS,and DLS is a rapid and powerful analytical tool that fulfills a needfor rapid and low-volume AAV gene therapy vector quantifica-tion, characterization, and quality assessment in developmentand manufacturing environments. The Bigger Picture The molecular composition of a biological product dictates itsspecific light absorption, scatter, and refraction behavior. Bycombining different optical technologies together, the opticalprofile of rAAV can be collected simultaneously and cross-analyzed, generating an "optical signature" unique to the biolog-ical product. This "optical signature" generated from a small sam-ple volume can be useful for evaluating process developmentand formulation studies. The same product-specific "optical sig-nature" can be used to monitor the consistency performance ofGood Manufacturing Practices (GMP) manufacturing runs whilemore resource-intensive assays are being performed. Having a more detailed understanding of how the process im-pacts the product will allow the manufacturer to set relevantand meaningful release specifications.Regulatory bodies acknowl-edge that some information is limited during the early phases ofdevelopment, and some release specifications may be establishedduring the later phase after more information is obtained. Unlike the maturemAb manufacturingprocess, AAVmanufacturing is not yet standardized, and many critical as-pects of the process are still being elucidated. These decisionsmust be derived from data. Prospectively collecting and com-paring data to conventional methods is the first step in estab-lishing a new innovative assay for implementation andvalidation in a GMP process. We herein present the first com-parability data from a combined UV-Vis and SLS/DLS instru-ment for rAAV vector characterization. Materials and Methods Manufacture of AAV vector products Specific detail on manufacturing conditions is available in Supplemen-tary Table S1. In general, 293T cells in suspension were cotransfectedwith plasmid DNA that contains either genetically modified AAV withthe desired gene fragment insertion or helper viral DNA for the repand cap of AAV and E1/E4 of adenovirus (double or triple transfection)by polyethylenimine. The transfected cells were grown and selected insuspension culture, and finally expanded in a bioreactor for 48-72 hunder optimal culture conditions. The accumulated intracellular AAV vector virions were harvestedand released from the cells by repeated freeze-thaws and mildnonionic detergent treatment and separated from cell debris byhigh-speed centrifugation (12000×grcf in Beckman centrifuge for60 min). Clarified virion solutions were purified by established purifica-tion methods for individual serotypes or assigned combinationsaccording to customer requirements. The methods used for vector purification include (1) TFF, (2) UF/DF,(3)CMF, (4) CsCI density gradient ultracentrifugation, (5) iodixanol den-sity gradient ultracentrifugation, and (6) size exclusion or ion exchangechromatography. The purified final AAV drug substance (raw material)and product (ready to use) were aliquoted and cryopreserved below-65℃ for single use. Viral particle quantification by sandwich ELISA The serotype-specific capsid sandwich ELISA kits for AAV2, AAV3,AAV5, AAV6, AAV8, and AAV9 were purchased from Progen (Cat No.:PRAAV2, PRAAV3, PRAAV5, PRAAV6, PRAAV8,PRAAV9). For each AAVserotype, only assembled AAV viral particles from the test samplewere captured by a conformational epitope-specific anti-AAV mono-clonal antibody coated on the ELISA plate. The addition of a biotin-conjugated serotype-specific monoclonal antibody results in an im-mune complex. The immune complex is detected by a streptavidin-horseradish per-oxidase (HRP) conjugate upon binding of streptavidin to biotin. Addi-tion of an HRP-specific substrate solution results in a color-changereaction whose absorbance measured at 450 nm is directly proportionalto the amount of specifically bound AAV viral particles. Each kit contains its unique serotype-specific AAV as a standard. Seri-ally diluted AAV standards were used to generate a standard curve forviral particle quantification. To ensure the accuracy of the quantifica-tion, the test samples were also serially diluted proportionally to the op-timal detection range ofthe assay. Viral genomic copy quantification by qPCR qPCR was used to determine AAV vector genome titer, amplifying eitheran AAV-specific gene fragment or the inserted gene of interest (GOl)fragment. For AAV-specific qPCR, a 62 base pair long fragment locatedin the ITR site was amplified (forward primer: 5'-GGAACCCCTAGTGATGGAGTT-3', and reverse primer: 5'-CGGCCTCAGTGAGCGA-3). ForGOl-specific qPCR, the primer pairs were designed and provided bythe customer. For genomic copy quantification,a linearized plasmidDNA specific for the individual rAAV vector was diluted to generate astandard curve [from 1×10° genome copies (gc)/pL to 1×10gc/uLon a log1o scale]. In brief, the individual samples were pretreated with DNase I (NEB) at37°C for 30 min to clear out unassembled extravirion DNA and contam-inated host cell DNA. The assembled vector DNA was further releasedfrom the virion by proteinase K digestion (Thermo Fisher or NEB) at37-56℃ for another 10-30 min. The samples were then subjected to additional DNA purification by a Qiagen DNA purification kit or directlyused after Proteinase K digestion for qPCR quantification. For accuratequantification, the samples were serially diluted to optimal concentra-tions to fall within the range of the standard curve. At least two tothree dilutions within the detection range were used to determinethe final concentration. The PCRs were performed in 0.2 mL96-well PCR plates by mixingsamples with diluted primers and 2×SYBR green PCR master mix(Thermo Fisher). The PCR was carried out on an Applied BiosystemsQuantStudio 5 Thermal Cycler. The thermal cycling conditions in-cluded a hold at 50°C for 2 min, hold at 95℃ for 10-15 min, 40 PCR cy-cles of denaturation at 95°C for 15 s, and annealing/extension at 56-60℃ for 1 min, according to individual primer pairs. The thermal cy-cling was followed by a melt curve: 95℃for 15 s/60℃ for 1 min/95°C for 1 s. Vector genome titers were calculated by multiplying the genomecopies per well with the dilution factors and assuming that one copyof double-stranded plasmid DNA is equivalent to two single-strandedvector genomes, therefore, multiplying further by a factor of 2 forfinal vector genome per milliliter. Viral vector purity evaluation by silver staining The purity of the drug substance or final drug product was checked andconfirmed by silver staining (Supplementary Figs. S1 and S2). Viral par-ticles (1×101° to 5×1019) were serially diluted with gel loading bufferand/or drug formulation buffer, denatured at 95℃ for 10 min and sep-arated by SDS-PAGE on NuPAGE 4-12% Bis-Tris gels (Thermo Fisher)under reducing conditions. The AAV2 reference virus from ATCC (CatNo.: ATCC 1616) was used as a positive control. After gel fixation (40% methanol and 10% acetic acid in deionizedwater) and washing (30% ethanol solution in deionized water), thegel was sequentially primed with sodium thiosulfate (12.65 mM,1 min), stained with silver nitrate (6 mM, 20 min), and developed (so-dium carbonate, 0.57 M for 20 s to 10 min). The visible protein bandswere fixed and imaged for data analysis by densitometry. The size ofeach protein band was calculated by comparing the individual proteinmigration rate in the gel with a protein ladder (Mark12, Thermo Fisher).Densitometric analysis for entire gel lane band intensities (Band%) wasperformed with Bio-Rad Image Lab software. AAV-specific protein identification by Western blot AAV-specific capsid proteins (VP1,VP2, and VP3) in drug substances orfinal drug products were identified by specific rabbit polyclonal anti-bodies against VP1/VP2/VP3 (Cat. No.: 61084; Progen). Since all threecapsid proteins are transcribed from the same DNA sequence andshare homology, we confirmed that the capsid proteins from differentserotypes of AAV, including AAV2, AAV3,AAV5, AAV6, AAV8, andAAV9, can all be recognized by this antibody. Viral particles (1×10° to1×10°) were serially diluted with gel loadingbuffer and/or drug formulation buffer, denatured at 95C for 10 min andseparated by SDS--PAGE on NuPAGE 4-12% Bis-Tris gels (ThermoFisher) under reducing conditions. The separated proteins were electri-cally transferred onto a PVDF membrane. After blocking the membranewith 5% nonfat dry milk (BLOTTO, Rockland) in 1xtris-buffered salineTween 20 (TBST), it was developed with rabbit anti-VP1/VP2/VP3antibody and HRP-conjugated goat antirabbit lgG H&L (Cat. No.:ab205718; Abcam). The VP1/VP2/VP3 bands were visualized by the addition of Western-Sure PREMIUM Chemiluminescent Substrate (Cat. No.: 926-95000;LICOR) and captured by a Li-Cor imaging system. The band sizes weredetermined on the membrane by comparing their migration rates with WesternSure Prestained Chemiluminescent Protein Ladder (Cat.No.: 926-98000; LICOR). Consecutive UV-Vis light absorption and SLS/DLSanalysis of the AAV vector samples by Stunner Stunner (Unchained Labs) combines UV-Vis spectroscopy (from 230 to750 nm) with DLS using a 660 nm laser. Using the built-in AAV Quant ap-plication, multiple parameters, including titer, empty/full ratio, size, andpolydispersity, and protein and DNA quantification of AAV particles, canbe obtained simultaneously. The instrument uses a specificallydesigned 96-well plate (Cat. No. 701-2025;Unchained Labs) to performthe assay. In brief, 2 uL undiluted or serially diluted sample in PBS wasloaded in the wells in duplicate or triplicate. The AAV Quant application was selected in the Lunatic & Stunner Cli-ent software using four DLS acquisitions of 5s each. Autoblanking orwater blanking was used to avoid the risk of overcorrection by bufferblanking during UV-Vis deconvolution. The UV-Vis and DLS resultswere analyzed with Lunatic & Stunner Analysis software using analytesof the indicated AAV serotypes and ITR-to-ITR genome lengths. In brief,SLS is used to assay the intensity of light scatter from a sample, whichcorresponds to a possible range of titers. DLS was next used to reducethe total SLS intensity to only the proportion attributable to AAV capsidsand not particles larger or smaller than capsids. Finally, UV-Vis spectroscopy results were used to determine anempty/full ratio that identifles the best titer result out of the range iden-tified by SLS and DLS. The DLS results reported were z-average diame-ter, derived intensity, and PDI, which is square of the z-average standarddeviation divided by the z-average. PDIs >0.2 indicate highly polydis-persed samples, whereas PDIs ≤0.1 are typical of monodispersed sam-ples.24Results were evaluated against defined criteria to confirmsamples were within appropriate detection limits for the assay. AAV vector characterization by other methods Additional AAV vector product characterization was performed in con-tract testing laboratories to identify and confirm the drug qualities andquantities. Empty versus full viral particle assays by TEM were per-formed by Charles River Laboratories, Vironova, or Creative Biostructure.AUC was performed by Wuxi Biologics. Statistical analysis of the data Means and standard deviations were calculated for technical duplicatesor triplicates. Empty/full ratio of vector particles was calculated by intra-virion genomic copy numbers (qPCR)/assembled virion particle numbers(ELISA)×100%. Student’s t test was used for statistical analysis of the data. Authors' Contributions Q.Y., R.W.W., K.L., and A.C. conceived the idea and designedexperiments. Q.Y, and T.K. performed the experiments and char-acterized the assay protocols. R.W.W., N.D., K.L. and A.C. eval-uated analytical protocols. All authors discussed the resultsand wrote the manuscript. Author Disclosure Statement Q.-E.Y.,T.K., and A.C. are all employees of Vigene Biosciences, ACharles River Laboratory Company. R.W.W., N.D., and K.L. are allemployees of Unchained Labs. Funding Information This study was supported by Vigene Biosciences, A Charles RiverLaboratory Company, and Unchained Labs. Supplementary Material Supplementary Figure S1Supplementary Figure S2Supplementary Figure S3Supplementary Figure S4Supplementary Figure S5Supplementary Figure S6Supplementary Figure S7Supplementary Figure S8Supplementary Figure S9Supplementary Figure S10Supplementary Figure S11Supplementary Table S1 References ( 1 . Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev D rug Discov.2 0 19;18:358-378. DOI: 10.1038/ s41573-019-0012-9. ) ( 2. Blessing D, Deglon N, Schneider BL. Scalable p roduction and purification of adeno-associated viral vectors (AAV). Methods Mol Biol.2018;1850:259-274. DOI: 10.1007/978-1-4939-8730-6 _ 17. ) ( 3. S trobel B, Miller FD, Rist W, et al. Comparative analysis of cesium chloride- andiodixanol-based purification of recombinant adeno-associated viral vectors for preclinical applications. Hum Gene Ther Methods. 2015;26:147-157. DOI: 10.1089/hgtb.2015.051. ) ( 4. Trivedi PD, Yu C, Chaudhuri P, et al. Comparison o f highly pure rAAV9 v e ctor stocks produced in suspension by PEl transfection or H SV infection reveals striking quantitative and qualitative differences. Mol Ther Methods C l in Dev. 2022;24:154-170. DOI: 10.1016/J.OMTM.2021.12.006. ) ( 5. Porterfield JZ, Zlotnick A. A simple and g eneral method for determining theprotein a nd nucleic acid c o ntent of viruses by UV absorbance. Virology. 2 010;407:281-288. DOI: 1 0 .1016/j.virol.2010.08.015. ) ( 6. Sommer JM, Smith PH, Parthasarathy S, et al. Quantification of adeno- associated v irus particles and empty capsids b y optical density m e asure- m ent. Mol T h er. 2 003;7:122-128. DOI: 1 0 .1016/S152 5 -0016(02)00019-9. ) ( 7. Zhang X, W ang W, Kenrick S. AN5007: Characterization o f AAV-based vi r al vectors by DynaPro DLS/SLS in s truments. 2020. Available from: ht t ps:// www.wyatt.com/files/literature/app-notes/dls-plate/AN5007-AAV-quantitation-and-stability-analysis-by-batch-DLS.pdf Accessed May 31, 2022 ) ( 8. M akra l, Terejanszky P, Gyurcsanyi RE. A method based on light s c attering to estimate the concentration o f v i rus particles without the need for virus particle standards.MethodsX. 2015;2:91-99.DOI: 10.1016/ i.mex.2015.02.003. ) ( 9. W right JF. P roduct-related i m purities in clinical-grade recombinant AAV vectors: characterization and r isk assessment. Biomedicines. 2014;2:80-97. DOI: 10.3390/biomedicines2010080. ) 10. Wright JF, Le T, Prado J, et al.Identification of factors that contribute to re-combinant AAV2 particle aggregation and methods to prevent its occur- rence during vector purification and formulation. Mol Ther.2005;12:171-178.DOI: 10.1016/j.ymthe.2005.02.021. 11. Rayaprolu V, Kruse S, Kant R, et al. Comparative analysis of adeno-associatedvirus capsid stability and dynamics. J Virol. 2013;87:13150-13160. DOI:10.1128/jvi.01415-13. 12. Penaud-Budloo M, Broucque F, Harrouet K, et al. Stability of the adeno-associated virus 8 reference standard material. Gene Ther. 2019;26:211-215.DOI: 10.1038/s41434-019-0072-9. 13. Lock M, McGorray S, Auricchio A, et al. Characterization of a recombinantadeno-associated virus type 2 reference standard material. Hum Gene Ther.2010;21:1273-1285. DOI: 10.1089/hum.2009.223. 14. Ayuso E, Blouin V,Lock M, et al. Manufacturing and characterization of arecombinant adeno-associated virus type 8 reference standard material.Hum Gene Ther. 2014;25:977-987. DOI: 10.1089/hum.2014.057. 15. Kuck D, Kern A, Kleinschmidt JA. Development of AAV serotype-specificELISAs using novel monoclonal antibodies. J Virol Methods. 2007;140:17-24.DOI: 10.1016/j.jviromet.2006.10.005. 16. Gibbs J, Vessels M, Rothenberg M. Optimizing the immobilization of proteinand other biomolecules for ELISA assays (Application Note). Life Sci.2017;10:1-8. 17. Dobnik D, Kogovsek P, Jakomin T, et al. Accurate quantification and charac-terization of adeno-associated viral vectors. Front Microbiol. 2019;10:1570.DOI: 10.3389/fmicb.2019.01570. 18. Burnham B, Nass S, Kong E, et al. Analytical ultracentrifugation as an ap-proach to characterize recombinant adeno-associated viral vectors. HumGene Ther Methods. 2015;26:228-242. DOI: 10.1089/hgtb.2015.048. 19. Tomono T, Hirai Y, Okada H,et al. Highly efficient ultracentrifugation-free chromatographic purification of recombinant AAV serotype 9. MolTher Methods Clin Dev. 2018;11:180-190. DOI: 10.1016/j.omtm.2018.10.015. 20. Mcintosh NL, Berguig GY, Karim OA, et al. Comprehensive characterizationand quantification of adeno associated vectors by size exclusion chroma-tography and multi angle light scattering. Sci Rep. 2021;11:3012. DOI:10.1038/s41598-021-82599-1. ( 21. Walton RW, Finan D. A look under the hood of Lun a tic. 2020. Available from: https://www.unchainedlabs.com/wp-content/uploads/2021/11/ T ech _ Note_A _ l o ok_under_the_hood_of _ Lunatic.pdf Accessed May 31, 2022. ) ( 22. Robinson C J , Sadick M, Deming SN, et al. A s say acceptance criteria for multiwell-plate-based biological potency a s says draft for consultation. BioProcess International 2014;12:30-41. ) ( 23. S tetefeld J, McKenna SA, Patel TR. D y namic lig h t scattering: a p ractical g u ide and applications in biomedical sciences. Biophys Rev. 2 016;8:409. D OI: 10.1007/S12551-016-0218-6. ) ( 24. I SO22412. Particle size analysis—Dynamic ligh t scattering (DLS). BritishStandard. 2 008;34. Available from: https://www.iso.org/obp/ui/#iso:std:iso: 22412:ed-2:v1:en.Accessed March 1 3, 2022. ) ( Received: March 1 , 2022 ) Accepted: May 17, 2022 /ssue Publication Date: June 15, 2022
确定





还剩9页未读,是否继续阅读?
非链(上海)贸易有限公司为您提供《腺相关病毒(AAV)中衣壳滴度,基因组滴度及空实心比检测方案(紫外分析仪)》,该方案主要用于其他中表征检测,参考标准--,《腺相关病毒(AAV)中衣壳滴度,基因组滴度及空实心比检测方案(紫外分析仪)》用到的仪器有Unchained Labs Stunner 高通量浓度粒度分析仪
推荐专场
相关方案
更多